“Stop, Little Pot” as the Motto of Suppressive Management of Various Microbial Consortia
Abstract
:1. Introduction
2. Various Compounds and Their Combinations against Polymicrobial Consortia
2.1. Effects of Non-Peptide Antimicrobial Compounds of Plant and Animal Origin on Mixed Consortia
- -
- haemolysin genes (hla and hld) in S. aureus, several biofilm-related genes in E. coli (csgAB, fimH and flhD) and hypha cell wall gene HWP1 in C. albicans [28];
- -
- genes responsible for the synthesis of substances involved in the formation of QS in P. aeruginosa [29];
- -
- genes responsible for the motility of Klebsiella pneumoniae bacteria, their adhesion and biofilm formation (genes mrkA, FKS1, ERG11 and ALS5), as well as the gene ERG11 participating in the ergosterine synthesis and gene FKS1 dealing with β-1,3-glucane synthase which is a key enzyme participating in the production of main polysaccharide component of cell walls [30];
- -
- genes responsible for the formation of hyphae and biofilms (genes ECE1, HGT10, HWP1 and UME6) and regulation of transport functions (genes CDR4, CDR11 and TPO2) [33].
| Consortia | Form and Site (Reason) of Presence | Suppressive Compounds | Effects |
|---|---|---|---|
| C. albicans/S. aureus, E. coli [28] | Biofilm host and environmental surfaces | Saw palmetto oil (100 µg/mL), lauric acid and myristic acid (20 µg/mL) with dimethyl sulfoxide (DMSO) | 90% inhibition of bacteria/yeast biofilm formation without affecting planktonic cell growth |
| Pseudomonas aeruginosa/Aspergillus fumigatus or Scedosporium apiospermum [29] | Biofilms, patients with chronic infections | Pompia and grapefruit essential oils (10 mg/L) with DMSO | 70% inhibition of biofilm formation |
| Candida auris/Klebsiella pneumoniae [30] | Biofilms in the urinary tract, bronchi, liver | Myrtenol (50 μg/mL) with DMSO | 90% inhibition of biofilm formation |
| Candida albican/E. coli [31] | Biofilm, mucosal surfaces | Aqueous garlic extract (50 mg/mL) | 70.2% decrease in cell concentration in biofilm |
| C. albicans/Klebsiella pneumoniae [32] | Biofilm, urinary tract, device-related infections | Allium ursinum and Allium oschaninii methanol extracts (75 μg/mL) | Up to 99% death of microorganisms in biofilms |
| C. albicans/S. aureus or Acinetobacter baumannii [33] | Biofilm, silicon catheter | Nepodin (10–20 µg/mL) | 75–85% inhibition of biofilm formation |
| C.tropicalis/S. aureus [34] | Biofilm, nosocomial infections | Glycyrrhiza glabra extract (1.5 mg/mL) and Manuka honey (37.5%) | Decrease of cell amount inside biofilm to 1.0–3.5 log CFU/mL |
| C. parapsilosis/Exophiala dermatitidis [35] | Biofilm, infection of toenail | Ethanol extract of propolis (1675 μg/mL) | Total eradication of E. dermatitidis in biofilms and 14% reduction of C. parapsilosis cells |
| C. albicans/E. faecalis [36] | Biofilm, tongue mucosal infections, sputum, sepsis, and root canal infections | Luteolin (256 μg/mL) with DMSO | 78% death of microbial cells in biofilms and destruction of biofilm matrix components (mainly polysaccharides and proteins) |
| C. albicans/E. coli [37] | Biofilm, urinary catheters and endotracheal tubes | Lawsone (100 µg/mL) with DMSO | Reduced curli production in E. coli and C. albican hyphal formation |
| A. niger, Aureobasidium pullulans, Chaetomium globosu, Cladosporium cladosporioides Alternaria alternata, and Penicillium citrinum [38] | Microbial colonization, biofilm, modern painting; cultural heritage | Citrus aurantium hydrolate (99.97% v/v) and Cinnamomum zeylanicum essential oil (0.03 v/v), 5 h (28 μL/cm2) | Complete killing of the fungal species; inhibition of fungal ATPases and cell wall biosynthesis by altering the membrane structure and integrity |
| Aspergillus sp., Penicillium sp. and Mucor sp. [39] | Microbial colonization, cultural heritage | Ocimum basilicum hydro-alcoholic and water extracts and essential oils (15 µL/paper discs) | Inhibition of fungal growth (100%) up to 144 h incubation |
| Hormoconis, Aspergillus, Fusarium, Trichosporon [40] | Biofilms, oil industry | Essential oil of Lippia gracilis Schauer (20 μg/L) | Completely inhibition of the fungal growth |
| Combinations of antimicrobial non-peptide compounds of plant origin with antibacterials or antifungals | |||
| C. albicans/S. mutans [41] | Biofilm, dental plaque | Eugenol (12.5 μg/mL with azithromycin (128 μg/mL) with DMSO | Eradication of mixed biofilms; Fractional Inhibitory Concentration Index (FICI-0.14) |
| C. albicans/S. aureus [42] | Biofilm, skin wounds, denture stomatitis and bloodstream infections | Berberine (128 μg/mL) with amphotericin B (4 μg/mL) with DMSO | 96% death of C. albicans, 76% death of S. aureus cells |
2.2. Antimicrobial Peptides (AMP) against the Polymicrobial Consortia
| Consortia | Form and Site (Reason) of Presence | Suppressive Compounds | Effects |
|---|---|---|---|
| C. albicans/P. aeruginosa, Acinetobacter baumannii, E. coli, K. pneumoniae [43] | Biofilm, medical devices (catheters, endotracheal tubes, contact lenses); infection of the oral cavity, cystic fibrosis lungs, wounds, abdominal cavity and urinary tract | Synthetic mimic AMP–ceragenins (CSA-13, CSA-90) synthesized from a cholic acid scaffold technique) or natural AMP: magainin, cecropin A and LL-37 (100 µg/mL) | CSA-13, CSA-90 provided 100-fold decrease of cell concentrations in multispecies biofilms. All other AMPs were ineffective except magainin against only Candida and E. coli with log reduction of cell concentrations |
| C. albicans/Achromobacter xylosoxidans or Stenotrophomonas maltophilia [44] | Biofilm, cystic fibrosis | Synthetic AMP (mimic of myxinidin)—WMR (4–20 μM) | 40–50% disruption of biofilms |
| C. albicans/S. aureus [45] | Biofilm, catheter-related bloodstream infections | Synthetic AMP (mimic of arginine, and alanine) guanylated polymethacrylates (128 mg/mL) | 99% death of S. aureus and 82% death of C. albicans |
| C. albicans/K.pneumoniae [46] | Biofilm, intravascular or urinary catheters, oral infections | Synthetic AMP (mimic of glycoprotein H of herpes simplex virus type 1) gH625-M (50 µM) | 50% inhibition of cell growth and eradication of polymicrobial biofilm |
| AMP in suppressive combinations | |||
| Carbapenem-resistant Pseudomonas aeruginosa and Candida sp. [47] | Biofilm, wound infection, chronic lung disease, pneumonia associated with mechanical ventilation, and bloodstream infections | Natural AMP polymyxin B (2 μg/mL) with caspofungin (32–64 μg/mL) with DMSO | Decrease of the cell concentration to 1–2 log CFU/mL and loss of total biofilm biomass |
| Aspergillus fumigatus/carbapenem-resistant P. aeruginosa [48] | Biofilm, cystic fibrosis | Combination of natural AMP—polymyxin B (2 μg/mL) with caspofungin (64 μg/mL) and DMSO | Alteration of hyphae morphology and death of bacterial cells with a decrease of bacterial cells to 2.0–3.0 log CFU/mL |
| Fusarium oxysporum/C. albicans [49] | Biofilm, mycoses | Synthetic mimic AMP (modified myxinidin)—WMR (12.5 μM) with fluconazole (30 μM) | 60% decrease of biomass |
2.3. Inhibition of Polymicrobial Consortia by Commercial Antimicrobials and Organic Solvents
| Consortia | Form and Site (Reason) of Presence | Suppressive Compounds | Effects |
|---|---|---|---|
| Airborne bacteria (Staphylococcus, Bacillus) and fungi (Cladosporioides, Rhodotorula sp.) [50] * | Bacterial-fungal aerosols, wastewater treatment plants | Amoxycillin, ampicillin, ceftazidime, cefalotin, cefuroxime sodium, nalidixic acid, amikacin, doxycycline, erythromycin, gentamicin, kanamycin, neomycin, streptomycin, tobramycin, tetracycline, trimethoprim, rifampicin, chloramphenicol, nitrofurantoin and novobiocin (5–200 μg) | 12% decrease in concentrations of bacterial cells was reached, and Bacillus mycoides demonstrated the highest resistance to antibiotics |
| C. albicans/Klebsiella pneumoniae or S. aureus [51] | Biofilms, oral infections, respiratory diseases | Tetrazole oteseconazole VT-1161 (2.0 µg/mL) with DMSO | Inhibition of fungal CYP51 and 90% eradication of biofilms |
| F. solani/S. aureus or S. epidermidis [52] | Biofilms, human cadaveric cornea | Amikacin, gentamicin, tobramycin, ampicillin, cephalosporins, cefuroxime, ceftriaxone, cefepine, cefazolin, gatifloxacin, moxifloxacin, ciprofloxacin, ofloxacin, chloramphenicol, azithromycin, metronidazole, clindamycin, lincomycin, monocycline (12–1024 µg/mL) with ethanol | Minimum biofilm eradication concentration (MBEC) (µg/mL) for: biofilm S. aureus/F. solani chloramphenicol −128 monocycline −128 ampicillin −256 other antibiotics ≥480 biofilm S. epidermidis/F. solani ciprofloxacin −64 tobramycin −256 chloramphenicol −256 other antibiotics ≥512 |
| C. parapsilosis/S. aureus [53] | Biofilm, bloodstream infections | Resolvin D1 (25 µg/mL) with ethanol | 80% suppression of expression of the genes, participating in the formation of biofilms |
| C. albicans/S. aureus [54] | Biofilm, periodontitis, cystic fibrosis, stomatitis, urinary tract, burn wound infections | Quaternary ammonium compound based on terbinafine and pyridoxine (KFU-127) (400–800 mg/L) with methanol | Inhibition of growth of both bacterial and fungal cells inside the biofilm |
| C. albicans/S. epidermidis [55] | Biofilm, catheters | 50% solution EDTA (Ethylenediaminetetraacetic acid) in ethanol | Dramatically reduced mass of biofilms |
| C. albicans/S. aureus/ P. aeruginosa [56] | Biofilms, wound infections | G21-cholic acid derived amphiphile (8 μg/mL) with methanol | Decrease concentration of cells in biofilms from 9–10 log CFU/mL down to 4–5 log CFU/mL |
| C. albican or C. auris/S. aureus [57] | Biofilms, mycotic infections | 100% surface disinfectant NSSD | 99.9% death of cells |
| C. parapsilosis/S. aureus [58] | Biofilm, nosocomial infections | Orthophthalaldehyde (0.55% solution) | Bacteriostatic and fungicidal activity decreases the concentration of yeasts from 7 log CFU/mL down to 2 log CFU/mL and bacteria from 8 log CFU/mL down to 4 log CFU/mL |
| Alternarla, Cladosporium, Fusarium, Pénicillium, Phoma, Trichoderma and Ulocladium genera [59] * | Co-colonization, wooden constructions | Commercial wood preservatives: “Borolitas”, “WT Sodium Hypochlorite; “Anti-mould liquid”, “Boramon”, “Arlitas”, “Complete Wood Treatment” | Alternaria and Fusarium were the most tolerant to wood preservatives, whereas growth of Penicillium cells was suppressed |
| 41 fungal isolates including Aspergillus fumigatus, Fusarium oxysporum, and Candida sp. [60] * | Consortia, wastewater treatment plants | Fluconazole, ketoconazole, itraconazole, and voriconazole (0.06–64 μg/mL) | MIC ** values for all tested antifungals against Candida krusei and A. fumigatus ≤ 1 μg/mL except fluconazole (64 μg/mL). MIC values for F. oxysporum were ≥4 μg/mL |
| A. fumigatus, A. lentulus [61] | Mixed infection, cystic fibrosis | Voriconazole (10 mg/kg) | The invasive growth of A. lentulus was observed in mixed infections after antifungal treatment |
| Microbial communities (Firmicutes, Proteobacteria, Neocallimastigomycota, Basidiomycota, Bacteroidetes, Ascomycota etc.) [62] | Consortia, aerobic composting | Tetracycline hydrochloride (50–300 mg/kg) | Low concentrations of antibiotic promote Chytridiomycota growth, while high concentrations inhibit fungal activity |
| Acinetobacter sp., E. coli, Pseudomonas sp., Staphylococcus sp., Desulfovibrio sp., Clostridium sp. Penicillium sp., Fusarium sp., Cladosporium sp., Rhizopus sp., Aspergillus sp., Candida sp. [63] | Consortia, biofilms, oil and gas industry | 3.3′-metylenebis[5-methyloksazolidine] (MBO) (2%) | 100% death of cells in biofilms |
| Anaerobic sludge [64] | Consortia, process of biogas production | Dibenzothiophene sulfone (0.45 mM) | 30% decrease in the metabolic activity of cells |
| Suppressive combinations | |||
| C. albicans/S. aureus/E.coli [65] | Biofilms, medical devices | Combination of moxifloxacin (6 mg/L) with caspofungin (12.5 mg/L) or meropenem (30 mg/L) with caspofungin (12.5 mg/L) in DMSO | Both combinations were able to reduce the cells in biofilms, and a C.albicans cells were dead after incubation with meropenem-caspofungin |
| C. albicans/S. aureus [66] | Biofilms, medical devices, skin, mucosal, and bloodstream infections | Combination of chalcone-based derivative (53 μM) with polycyclic anthracene-maleimide (4 μM) in DMSO | Up to 64% biomass reduction in biofilm |
| A. fumigatus/ Stenotrophomonas maltophilia [67] | Biofilms, cystic fibrosis | Amphotericin B (64 μg/mL) with levofloxacin (4 μg/mL) with DMSO | Biomass inhibition −90% S. maltophilia increased the antifungal susceptibility of A. fumigatus to amphotericin B, whereas A. fumigatus protected S. maltophilia from levofloxacin |
2.4. Heavy Metals and Nanoparticles (NPs) against Mixed Consortia of Microorganisms
| Consortia | Form and Site (Reason) of Presence | Suppressive Compounds | Effects |
|---|---|---|---|
| Superficial deposits-bacteria (Bacteroidetes, Proteobacteria, Actinobacteria) and dark-colored fungi (Alternaria alternata, Cladosporium cladosporioides, Coniosporium sp., Phoma herbarum, Aureobasidium pullulans) and A. niger [25] | Consortia, stone monuments | Fe, Mn, Zn, Cu, Pb, and Cd (8.5–32,280.6 μg/g) | Resistance of consortia to the action of heavy metals |
| Chlorella vulgaris/Aspergillus oryzae [68] | Consortium, wastewater treatment plants | Cu (II) (0.7–1.0 mg/L) | Decrease of metabolic activity of cells |
| Aspergillus lentulus, A. terreus and Rhizopus oryzae [69] | Consortium, wastewater treatment | Cr(VI) and Cu(II) (75 mg/L) | No inhibition |
| C. albicans/S. aureus [70] | Biofilm, human infections | Ag NP (32 μg/mL) | Eradication of biofilm |
| Rhodotorula sp., Debaryomyces hansenii and Hanseniaspora valbyensis [71] | Consortium, bioremediation processes | NP of ZnO—(3.0 g/L) | Decrease of metabolic activity of cells |
| Diversispora versiformis, Funneliformis dimorphicus and Glomus indicum [72] | Symbiosis, agriculture, arbuscular mycorrhizal fungi | NP of TiO2—(100 mg/plant) | Inhibition of fungal growth due to the binding of TiO2 with plant roots or increases in internal concentration of TiO2 in root tissue |
| Glomus versiforme and G. caledonium [73] * | Symbiosis, agriculture, arbuscular mycorrhizal fungi | NP of ZnO—(800 mg/kg) | Inhibition of fungal colonization of plant roots |
| Combinations with metal NP | |||
| Lithobiotic microbial community (bacteria, microscopic fungi, algae, and lichens) [26] | Consortium, stone monuments | Sol-gel-derived epoxysiloxane coatings with 0.3–0.5 wt% NP of TiO2 P25 in combination with nanodiamond powder | Inhibition of the micromycetes growth |
| C. albicans/S. aureus or Streptococcus mutans [74] | Biofilms, oral cavity infection, otitis, chronic lung infection, burn wounds, urinary tract infection | Fucoidan-AuNP (Fu-Au NP) (2048 µg/mL) | Decrease of cell concentration down to 2.4–5.8 log CFU/mL as a result of inhibition of expression of genes involved in the biofilm formation, altering of membrane penetration and ROS generation |
| C. albicans/S. aureus [75] | Biofilm, skin, mucosal, and bloodstream infections | β-caryophyllene-Au NP (1024 μg/mL) | Decrease of S. aureus and C. albicans cells in the mixed biofilm by 1.4 log CFU/mL and 2.1 log CFU/mL, respectively. |
| C. albicans/S. aureus [76] | Biofilm, nosocomial infection | Phloroglucinol-Au NP (1024 μg/mL) | Decrease of cells in mixed-biofilm down to 2.8 log CFU/mL. |
| C. albicans/S. aureus [77] | Biofilm, vaginitis | Ag NP and L-carnitine (1000 ppm) | 90% inhibition of biofilm growth |
| C. albicans/S. mutans/ E. faecalis [78] | Biofilm, oral cavity infections | Sulfonated lignin-with Pd NP (SLS-Pd) (1.65 mg/mL) in combination with near-infrared (NIR) irradiation (808 nm, 1W cm−2) | 50% eradication of biofilm |
2.5. Physicochemical Methods for Suppression of Polymicrobial Consortia
| Consortia | Form and Site (Reason) of Presence | Suppressive Compounds | Effects |
|---|---|---|---|
| C. albicans/E. faecalis [79] | Biofilm, endodontic infections | Zn(II)chlorin e6 methyl ester (0.1 mg/L) with DMSO and light activation for 60–90 s | 60% Removal of biomass from biofilm |
| C. albicans/P. aeruginosa [80] | Biofilm, burn wound, chronic lung infections | Blue Light irradiation (405 nm), 90 min | Inactivation of P. aeruginosa 2.58-log10 CFU/mL and C. albicans 1.71-log10 CFU/mL |
| Soil bacteria and fungi [81] | Consortium, soil | pH 4.0–8.0 | Negative interactions between bacteria and fungi at alkaline pH |
| Activated sludge (Trichoderma, Cutaneotrichosporon, Nitrosomonas, Nitrospira, Dechoromonas, Rhodanobacter) [82] | Consortium, wastewater treatment plants | pH 5.5 | Growth of fungi and inhibition of nitrogen removal by bacterial cells |
| Citrobacter freundii/Sphingobacterium multivorum/ Coniochaeta sp. [83] * | Consortium, degradation of wheat straw | pH 5.2–7.2 or shaking speed (60–180 rpm) | Suppression of fungal growth at pH 5.2–6.2 and 180 rpm in the presence of bacterial cells |
| Aureobasidium sp., Cladosporium sp., Penicillium sp. [84] | Biofilms, consortia, water plants | Filtration with ozonation or chlorination | 90% decrease in fungal cells |
2.6. Microbial Cells and Their Metabolites as Means of Suppression of Polymicrobial Consortia
2.6.1. Enzymes as Antimicrobial Agents
| Consortia | Form and Site (Reason) of Presence | Suppressive Compounds | Effects |
|---|---|---|---|
| C. albicans/S. aureus or E.coli or P. aeruginosa or K. pneumoniae [85] | Biofilms, bloodstream infections | Bovhyaluronidase azoximer Longidaza® (750 IU) | 30–40% decrease of biomass in biofilm |
| C. albicans/S. aureus [86] | Biofilm, bloodstream infections | Combination of cellobiose dehydrogenase and deoxyribonuclease I (co-immobilization on chitosan NP (1–2 mM)) | Penetration through the biofilm matrix and 90.5%inhibition of the biofilm formation |
| S. cerevisiae/Flavimonas oryzihabitans, Lactobacillus brevis, Leuconostoc mesenteroides [87] | Biofilms, dispense equipment | Combination of enzymes: α-amylase (10 U/m), β-glucuronidase (10 U/mL), glucose oxidase (10 U/mL), dextranase (1 U/mL), protease (10 U/mL) and pectinase (60 U/mL) | Removal of L. brevis and L.mesenteroides cells from biofilms, but not of S. cerevisiae and F. oryzihabitans |
| Fusarium sp./Alternaria sp. [88] | Symbiont of pathogens, banana fungal diseases | Combination of chitinase and β-1,3-glucanase from Penicillium sp. and Bacillus sp. | 60% decrease in banana disease |
| Fusarium spp., Alternaria sp., Cladosporium sp. [89] | Fungal communities, olive tree twigs | Combination of antibiotics and fungal cell wall degrading enzymes from Pseudomonas savastanoi pv. savastanoi | Preventing fungal colonization and proliferation on the surface |
| Trichoderma sp. [90] * | Consortia, mushroom farms | Bacillus subtilis (5 × 108 CFU/mL) producing antibiotics, β-1,3-glucanase, chitinase, protease, lipase, amylase siderophores, pyrazine, 2, 3-dimethyl-5-(1-methylpropyl)] | 48–52% inhibition of fungal growth |
| C. albicans/S. epidermidis [91] | Biofilm, catheter-associated urinary tract infection | DNase I or marine bacterial DNase from Vibrio alginolyticus (5 µg/mL) with biosurfactant from Bacillus subtilis (300 μg/mL) | 79–85% inhibition of biofilm formation due to inhibition of C. albicans hyphal appearance |
| Grape must consortium [92] | Consortia, wine making | Recombinant β-glucanase as a yeast killer toxin (LrKpkt) from Tetrapisispora phaffi (2 AU/mL) | 90% decrease in cell viability |
| Anaerobic sludges [23] | Consortia, landfills | His6-OPH ** (5 mg/L) in combination with bacitracin (100 mg/L), potassium humate modified with naphthoquinone (CHP-NQ 5 g/L), and K2S2O8 (4 g/L) | 34% decrease in biogas production and content of CH4 in it |
2.6.2. Quorum Molecules in the Regulation of Polymicrobial Consortia
| Consortia | Form and Site (Reason) of Presence | Suppressive Compounds | Effects |
|---|---|---|---|
| C. tropicalis/C. krusei/C. parapsilosis [93] | Biofilms, oral infections | Cell free supernatants of Lactobacillus gasseri and Lactobacillus rhamnosus (30% v/v) | 56–67% decrease in biomass of surface-associated biofilm |
| C. albicans/S. aureus [94] | Biofilm, bloodstream infections | DMSO extract of Limosilactobacillus fermentum metabolites (406 µg/mL) | 90% inhibition of biofilm formation and decrease in cell concentration |
| C. albicans/S. mutans [95] | Biofilm, caries | Supernatant of cultural broth after cultivation of Lactobacillus plantarum cells | 91.5% and 43.7% decrease of S. mutans and C. albicans cells in the biofilm |
| C. albicans/C. tropicalis, S. salivarius, Rothia dentocariosa, S. epidermidis [96] | Biofilms, medical silicone devices | Cell free supernatant of Lactobacillus rhamnosus (40% v/v) | 91% inhibition of cell adhesion to silicone and 58% decrease in cells viability |
| Aspergillus niger/Bacillus subtilis [27] | Co-culture, marble and limestone monuments | Fungal and bacterial metabolites secreted by microorganisms to the broth | Significant effect on metabolic activity of cells |
| Pythium debaryanum, Fusarium oxysporum lycopersici, F. moniliforme and Rhizoctonia solani [97] | Consortium, disease of tomato seedlings | Extracts of compost containing Anabaena variabilis, A. ocillarioides, and vermiculite | 10–15% decrease in diseases of tomato seedlings |
| C. albicans/E. coli [98] | Biofilm, mucosal infections, vulvovaginal candidiasis | Farnesol (600 µM) in DMSO | Degradation of biofilm |
| C. albicans/S. mutans [99] | Biofilm, caries | Streptococcus salivarius LAB813 cells (107 CFU/mL) | 90% death of cells |
| Activated sludge with Zoophagus sp [100] * | Consortium, wastewater treatment plants | Clonostachys rosea cells | C. rosea cells penetrate to the interior of the Zoophagus mycelium and use the cytoplasm as a nutrition medium |
| Combinations | |||
| C. albicans/S. aureus [101] | Biofilm, catheters | RLmix_Arg—biosurfactants produced by P. aeruginosa with Pluronic F-127 | Decrease of cell viability |
| Rhodotorula mucilaginosa/Candida tropicalis, C. krusei, C. kefyr, Listeria monocytogenes, Salmonella enterica, Escherichia coli [102] | Biofilm, apple juice processing lines | Combination of natamycin (10 µM) and farnesol (600 µM) in DMSO | Inhibition of biofilm growth due to decrease of filamentous formation by yeast cells, destabilization with defragmentation of 3D-structure of biofilm and concentration decrease of bacterial cells |
| C. albicans/Providencia stuartii/S. aureus or C. albicans/Acinetobacter baumannii/S. aureus [103] | Biofilms, nosocomial bloodstream infection | Combination of carvacrol (0.5 mg/mL) with farnesol (0.7 mg/mL) in DMSO | 75% inhibition of biofilm formation |
| C.albicans/S.aureus [104] | Biofilm, central venous catheters, urinary catheters, cardiovascular devices | Combination of farnesol (300 μM) with oxacillin (2 mg/mL) in ethanol | 80% inhibition of biofilm formation |
2.6.3. Living Cells of Microorganisms and Their Complex Metabolites in the Regulation of Polymicrobial Consortia
3. Viruses in the Regulation of the Functioning of Consortia
3.1. Bacteriophages
| Consortia | Form and Site (Reason) of Presence | Suppressive Compounds | Effects |
|---|---|---|---|
| C. albicans/ P. aeruginosa [124] | Biofilms, cystic fibrosis | Lytic Pseudomonas phage Motto (109 PFU (plaque forming units)/mL) combined with cefotaxime, ciprofloxacin, gentamicin, meropenem and tetracycline (128 μg/mL), fuconazole (64 μg/mL) | Decrease of P. aeruginosa cells in the biofilm without notable influence on the C. albicans cells |
| C. albicans/ S. aureus [125] | Biofilm, bloodstream infections | Bacteriophages vB_SauM-A (A) and vB_SauM-D (D) (107 PFU/mL) combined with ciprofloxacin (32 μg/mL) | 82% decrease in S. aureus cells in the presence of C. albicans in the biofilm |
3.2. Mycoviruses as Biological Control Agents
| Target Fungi for Mycovirus Action | Negative Effect of the Fungal Cells | Mycovirus [Reference] | Mycovirus Family |
|---|---|---|---|
| Cryphonectria parasitica | Causative agent of chestnut blight | CHV-1 Cryphonectria hypovirus 1 [135] | Hypoviridae dsRNA |
| Sclerotinia sclerotiorum | Infecting over 400 plant species found worldwide—Sclerotinia stem rot or white mold | SsHADV-1 Sclerotinia sclerotiorum negative-stranded RNA virus 1 [137] | Sclerotiniaceae ssDNA |
| Magnaporthe oryzae (Pyricularia oryzae) | Rice blast | MoCV1-A Magnaporthe oryzae chrysovirus 1-A [138] | Chrysoviridae dsRNA |
| Botrytis cinerea (Botryotinia fuckeliana) | Grey mould with a necrotrophic lifestyle attacking over 200 crop hosts | BcMV10 Botrytis cinerea mitovirus 10 [139] | Mitoviridae + ssRNA |
| Fusarium oxysporum | Fusarium wilt | FodV1 Fusarium oxysporum f. sp. dianthi virus 1 [140] | Chrysoviridae dsRNA |
| Botryosphaeria dothidea | Causing the canker and dieback of fruit trees: pear, poplar, apple, walnut, and jujube trees | BdCV1 Botryosphaeria dothidea chrysovirus 1 [141] | Chrysoviridae dsRNA |
| BdRV1 Botryosphaeria dothidea RNA virus 1 [142] | Polymycoviridae dsRNA | ||
| BdPV1 Botryosphaeria dothidea partitivirus 1 [141,143] | Partitiviridae dsRNA | ||
| BdPV2 Botryosphaeria dothidea partitivirus 2 [144] | Partitiviridae dsRNA | ||
| Rosellinia necatrix | White root rot disease (apple, avocado, pears) | RnPV10 Rosellinia necatrix partitivirus 10 and RnVLV Rosellinia necatrix virga-like virus [145] | Partitiviridae dsRNA Virgaviridae-like +ssRNA |
4. Analysis of Wide-Spread Polymicrobial Consortia with Fungi and the Approaches to Their Suppression and Elimination
- -
- Today, AMPs are studied exclusively for medical purposes to suppress the discussed consortia with pathogens.
- -
- Physical and chemical methods are being studied for use in regulating water purification processes, as well as in medicine.
- -
- Agriculture turned out to be focused on studying the negative impact of metal NPs on the vital activity of cells in mixed consortia.
- -
- Microbial cells and enzymes are traditionally of interest for suppressing spoilage processes in the food industry.
- -
- All of the approaches to suppressing microbial consortia covered in the review have been tested in medicine, including many of them used to preserve cultural heritage sites, prevent their degradation, and for environmental purposes (to free water purification systems from biofilms).
- -
- Metal NPs and physicochemical methods are not popular for suppressing biofilms for medical purposes, probably due to the fact that NPs are toxic to any cells, and physicochemical methods are characterized by low efficiency; the use of such means and methods is considered for sanitary purposes.
- -
- Combinations of different antimicrobial agents are widely studied and have a greater suppressive effect in various fields of application against consortia of different compositions in comparison with individual compounds. However, combinations, as well as physicochemical methods of suppression, have been studied very little in the interests of agriculture and combating biocorrosion. Obviously, the potential for using possible combinations has not yet been revealed, and there is a wide field for scientific activity and experimental research.
- (1)
- This is due to the use of several antibiotics in reduced concentrations to overcome their total toxicity problem [162].
- (2)
- A combination of antibiotics is used when one of them is already ineffective, and the start of treatment with a combination of antibiotics is delayed due to the presence of pathogens in high concentrations and the QS state. For example, a combination of several antibiotics (cefazolin, gentamicin and vancomycin) suppressed biofilm formation on an orthopedic implant during the first 3 days after infection but did not have a positive effect if the same combination was used after 7 days of infection development [163].
- (3)
- There are known risks of the combined use of two or more antibiotics due to the occurrence of undesirable interference between them and the manifestation of drug-drug antagonism [164]. Thus, during clinical trials, it was shown that combinations consisting of antibiotics of group I (penicillin, streptomycin, bacitracin, neomycin and polymixin) and group II (chloramphenicol, tetracyclins and erythromycin) are often antagonists. Observations of individual cells confirmed the antagonism between bacteriostatic and bactericidal antibiotics [165]. At the same time, it is also known that the effectiveness of a combination of antibiotics can be antagonistic in one concentration range but synergistic in another, however, this should be established experimentally [166].
- (4)
- Often, the use of combinations of antibiotics leads to their appearance in the environment since the degradation efficiency of combined antibiotic variants is quite low in wastewater treatment plants [7].
- -
- The results of the analysis of complications in a significant number of patients after the COVID-19 pandemic indicate that fungi of the genera Candida, Aspergillus, Mucor cause opportunistic infections in patients with weakened immunity [167,168]. At the same time, fungi are often associated with bacteria and show high resistance to antimicrobial drugs;
- -
- In patients with Parkinson’s disease, immunohistochemistry and specific antibodies allowed the revealing of both bacteria (genera Streptococcus and Pseudomonas) and fungi (genera Botrytis, Candida, Fusarium and Malassezia) in brain tissues [169] that initiates the need to correct treatment strategies for such patients;
- -
- In dental laboratories, it was found that even after disinfection of pumice, there remains a high probability of cross-infection for technicians, dentists and patients with both bacteria (Acinetobacter lowffi, Bacillus cereus, Staphylococcus aureus, Pseudomonas aeruginosa, Diphteroids, Serratia mercescens, Enterobacter aerogenes, Morganella morgani, Providencia rettgeri, Staphy-lococcus albidus and Streptococcus sanguis), and fungi (Candida sp., Aspergillus niger, Fusarium sp., Aspergillus flavous, Cephalosporium sp. and Pencillium sp.) [170,171];
- -
- Soil bacterial (Streptomyces spp.) and fungal cells (Trichoderma spp.) can cause human skin diseases and a number of other dangerous diseases [172];
- -
- Fungi of the genera Aspergillus, Alternaria, Penicillium, Aureobasidium are capable of causing bio-damage to construction sites [173,174]. Fungal spores and metabolites (mycotoxins) that cause the development of upper and lower respiratory tract infections, allergic reactions and poisoning can cause bronchial irritation and allergy, broncho pulmonary mycoses, and hypersensitivity pneumonitis [175]. In chronic rhinosinusitis, fungal and bacterial biofilms are usually found, which can cause a chronic and antimicrobial-resistant stage of the disease [176]; the presence of mycotoxins in food and feed is dangerous due to their toxicity and carcinogenesis development [109,111];
- -
- Bacteria, as the main microorganisms present in the human body, are studied more widely, whereas databases of complete genomes of fungi present in the human body are less well provided [177]; this lack of information underlies the development of resistant dysbiosis;
- -
- In the process of observed climatic changes, viral and bacterial diseases as a potential cause of epidemics and pandemics may fade into the background and give way to fungi since it is fungi that can pose an equal or even greater threat: there are no vaccines against fungal pathogens yet, the arsenal of antifungal drugs is extremely limited, including even AMPs [109,178,179], and fungi can live saprotrophic, producing a large number infectious spores, without requiring direct contact with the affected object, have a unique ability to adapt to new conditions, including temperature conditions [180]. Taking all this into account, fungal pathogens have recently been included for the first time in the “World Health Organization (WHO) fungal priority pathogens list”, compiled from 19 groups of fungal microorganisms associated with a serious risk of human mortality or morbidity [181].
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Alonso, V.P.; Furtado, M.M.; Iwase, C.H.; Brondi-Mendes, J.Z.; Nascimento, M.D. Microbial resistance to sanitizers in the food industry. Crit. Rev. Food Sci. Nutr. 2024, 64, 654–669. [Google Scholar] [CrossRef] [PubMed]
- Puvača, N.; Ljubojević Pelić, D.; Pelić, M.; Bursić, V.; Tufarelli, V.; Piemontese, L.; Vuković, G. Microbial resistance to antibiotics and biofilm formation of bacterial isolates from different carp species and risk assessment for public health. Antibiotics 2023, 12, 143. [Google Scholar] [CrossRef] [PubMed]
- Freeland, G.; Hettiarachchy, N.; Atungulu, G.G.; Apple, J.; Mukherjee, S. Strategies to combat antimicrobial resistance from farm to table. Food Rev. Int. 2023, 39, 27–40. [Google Scholar] [CrossRef]
- Salam, M.A.; Al-Amin, M.Y.; Salam, M.T.; Pawar, J.S.; Akhter, N.; Rabaan, A.A.; Alqumber, M.A. Antimicrobial resistance: A growing serious threat for global public health. Healthcare 2023, 11, 1946. [Google Scholar] [CrossRef] [PubMed]
- Kulshrestha, A.; Gupta, P. Combating polymicrobial biofilm: Recent approaches. Folia Microbiol. 2023, 68, 495–505. [Google Scholar] [CrossRef] [PubMed]
- Efremenko, E.; Senko, O.; Stepanov, N.; Aslanli, A.; Maslova, O.; Lyagin, I. Quorum sensing as a trigger that improves characteristics of microbial biocatalysts. Microorganisms 2023, 11, 1395. [Google Scholar] [CrossRef] [PubMed]
- Efremenko, E.; Stepanov, N.; Senko, O.; Maslova, O.; Lyagin, I.; Aslanli, A. Progressive biocatalysts for the treatment of aqueous systems containing pharmaceutical pollutants. Life 2023, 13, 841. [Google Scholar] [CrossRef] [PubMed]
- Kiki, C.; Qin, D.; Liu, L.; Qiao, M.; Adyari, B.; Ifon, B.E.; Adeoye, A.B.E.; Zhu, L.; Cui, L.; Sun, Q. Unraveling the role of microalgae in mitigating antibiotics and antibiotic resistance genes in photogranules treating antibiotic wastewater. Environ. Sci. Technol. 2023, 57, 16940–16952. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zheng, L.; Wang, S.; Zhao, Y.; Xu, X.; Han, B.; Hu, T. Quorum sensing bacteria in the phycosphere of HAB microalgae and their ecological functions related to cross-kingdom interactions. Int. J. Environ. Res. Public Health 2022, 19, 163. [Google Scholar] [CrossRef] [PubMed]
- Kulshrestha, A.; Gupta, P. Polymicrobial interaction in biofilm: Mechanistic insights. Pathog. Dis. 2022, 80, ftac010. [Google Scholar] [CrossRef]
- Zupančič, J.; Raghupathi, P.K.; Houf, K.; Burmølle, M.; Sørensen, S.J.; Gunde-Cimerman, N. Synergistic interactions in microbial biofilms facilitate the establishment of opportunistic pathogenic fungi in household dishwashers. Front. Microbiol. 2018, 9, 328552. [Google Scholar] [CrossRef] [PubMed]
- Gliźniewicz, M.; Miłek, D.; Olszewska, P.; Czajkowski, A.; Serwin, N.; Cecerska-Heryć, E.; Dołęgowska, B.; Grygorcewicz, B. Advances in bacteriophage-mediated strategies for combating polymicrobial biofilms. Front. Microbiol. 2024, 14, 1320345. [Google Scholar] [CrossRef] [PubMed]
- MacAlpine, J.; Robbins, N.; Cowen, L.E. Bacterial-fungal interactions and their impact on microbial pathogenesis. Mol. Ecol. 2023, 32, 2565–2581. [Google Scholar] [CrossRef] [PubMed]
- Ponde, N.O.; Lortal, L.; Ramage, G.; Naglik, J.R.; Richardson, J.P. Candida albicans biofilms and polymicrobial interactions. Crit. Rev. Microbiol. 2021, 47, 91–111. [Google Scholar] [CrossRef]
- Rodrigues, M.E.; Gomes, F.; Rodrigues, C.F. Candida spp./bacteria mixed biofilms. J. Fungi 2020, 6, 5. [Google Scholar] [CrossRef]
- Simões, D.; de Andrade, E.; Sabino, R. Fungi in a one health perspective. Encyclopedia 2023, 3, 900–918. [Google Scholar] [CrossRef]
- Shay, R.; Wiegand, A.A.; Trail, F. Biofilm formation and structure in the filamentous fungus Fusarium graminearum, a plant pathogen. Microbiol. Spectr. 2022, 10, e00171-22. [Google Scholar] [CrossRef] [PubMed]
- Costa-Orlandi, C.B.; Sardi, J.C.O.; Pitangui, N.S.; De Oliveira, H.C.; Scorzoni, L.; Galeane, M.C.; Medina-Alarcón, K.P.; Melo, W.C.M.A.; Marcelino, M.Y.; Braz, J.D.; et al. Fungal biofilms and polymicrobial diseases. J. Fungi 2017, 3, 22. [Google Scholar] [CrossRef] [PubMed]
- Alanzi, A.; Elhawary, E.A.; Ashour, M.L.; Moussa, A.Y. Aspergillus co-cultures: A recent insight into their secondary metabolites and microbial interactions. Arch. Pharm. Res. 2023, 46, 273–298. [Google Scholar] [CrossRef]
- Ye, R.; Xu, S.; Wang, Q.; Fu, X.; Dai, H.; Li, W. Fungal diversity and its mechanism of community shaping in the milieu of sanitary landfill. Front. Environ. Sci. Eng. 2021, 15, 77. [Google Scholar] [CrossRef]
- Niu, L.; Li, Y.; Xu, L.; Wang, P.; Zhang, W.; Wang, C.; Cai, W.; Wang, L. Ignored fungal community in activated sludge wastewater treatment plants: Diversity and altitudinal characteristics. Environ. Sci. Pollut. Res. 2017, 24, 4185–4193. [Google Scholar] [CrossRef] [PubMed]
- Krohn, I.; Bergmann, L.; Qi, M.; Indenbirken, D.; Han, Y.; Perez-Garcia, P.; Katzowitsch, E.; Hägele, B.; Lübcke, T.; Siry, C.; et al. Deep (meta)genomics and (meta)transcriptome analyses of fungal and bacteria consortia from aircraft tanks and kerosene identify key genes in fuel and tank corrosion. Front. Microbiol. 2021, 12, 722259. [Google Scholar] [CrossRef] [PubMed]
- Efremenko, E.; Stepanov, N.; Senko, O.; Maslova, O.; Volikov, A.; Zhirkova, A.; Perminova, I. Strategies for variable regulation of methanogenesis efficiency and velocity. Appl. Microbiol. Biotechnol. 2022, 106, 6833–6845. [Google Scholar] [CrossRef] [PubMed]
- Vlasov, D.Y.; Parfenov, V.A.; Zelenskaya, M.S.; Plotkina, Y.V.; Geludova, V.M.; Frank-Kamenetskaya, O.V.; Marugin, A.M. Methods of monument protection from damage and their performance. In The Effect of the Environment on Saint Petersburg’s Cultural Heritage. Geoheritage, Geoparks and Geotourism; Frank-Kamenetskaya, O., Vlasov, D., Rytikova, V., Eds.; Springer: Cham, Switzerland, 2019; pp. 161–178. [Google Scholar] [CrossRef]
- Sazanova, K.V.; Zelenskaya, M.S.; Vlasov, A.D.; Bobir, S.Y.; Yakkonen, K.L.; Vlasov, D.Y. Microorganisms in superficial deposits on the stone monuments in Saint Petersburg. Microorganisms 2022, 10, 316. [Google Scholar] [CrossRef] [PubMed]
- Shilova, O.A.; Vlasov, D.Y.; Khamova, T.V.; Zelenskaya, M.S.; Frank-Kamenetskaya, O.V. Microbiologically induced deterioration and protection of outdoor stone monuments. In Biodegradation and Biodeterioration at the Nanoscale; Iqbal, H.M.N., Nguen, T.A., Bilal, M., Yasin, G., Eds.; Elsevier: Amsterdam, The Netherlands, 2022; pp. 339–367. [Google Scholar] [CrossRef]
- Sazanova, K.V.; Frank-Kamenetskaya, O.V.; Vlasov, D.Y.; Zelenskaya, M.S.; Vlasov, A.D.; Rusakov, A.V.; Petrova, M.A. Carbonate and oxalate crystallization by interaction of calcite marble with Bacillus subtilis and Bacillus subtilis–Aspergillus niger association. Crystals 2020, 10, 756. [Google Scholar] [CrossRef]
- Kim, Y.G.; Lee, J.H.; Park, S.; Kim, S.; Lee, J. Inhibition of polymicrobial biofilm formation by saw palmetto oil, lauric acid and myristic acid. Microb. Biotechnol. 2022, 15, 590–602. [Google Scholar] [CrossRef] [PubMed]
- Pekmezovic, M.; Aleksic, I.; Barac, A.; Arsic-Arsenijevic, V.; Vasiljevic, B.; Nikodinovic-Runic, J.; Senerovic, L. Prevention of polymicrobial biofilms composed of Pseudomonas aeruginosa and pathogenic fungi by essential oils from selected Citrus species. Pathog. Dis. 2016, 74, ftw102. [Google Scholar] [CrossRef] [PubMed]
- Maione, A.; La Pietra, A.; de Alteriis, E.; Mileo, A.; De Falco, M.; Guida, M.; Galdiero, E. Effect of myrtenol and its synergistic interactions with antimicrobial drugs in the inhibition of single and mixed biofilms of Candida auris and Klebsiella pneumoniae. Microorganisms 2022, 10, 1773. [Google Scholar] [CrossRef]
- Ashrit, P.; Sadanandan, B.; Shetty, K.; Vaniyamparambath, V. Polymicrobial biofilm dynamics of multidrug-resistant Candida albicans and ampicillin-resistant Escherichia coli and antimicrobial inhibition by aqueous garlic extract. Antibiotics 2022, 11, 573. [Google Scholar] [CrossRef] [PubMed]
- Galdiero, E.; Di Onofrio, V.; Maione, A.; Gambino, E.; Gesuele, R.; Menale, B.; Ciaravolo, M.; Carraturo, F.; Guida, M. Allium ursinum and Allium oschaninii against Klebsiella pneumoniae and Candida albicans mono- and polymicrobic biofilms in in vitro static and dynamic models. Microorganisms 2020, 8, 336. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Kim, Y.G.; Khadke, S.K.; Yamano, A.; Watanabe, A.; Lee, J. Inhibition of biofilm formation by Candida albicans and polymicrobial microorganisms by nepodin via hyphal-growth suppression. ACS Infect. Dis. 2019, 5, 1177–1187. [Google Scholar] [CrossRef]
- Gomes, F.; Dias, M.I.; Rodrigues, M.E.; Barros, L.; Henriques, M. Glycyrrhiza glabra and manuka honey extracts alone and in combination inhibit bacterial and fungal planktonic cells and biofilms. Phytomed. Plus 2024, 4, 100561. [Google Scholar] [CrossRef]
- Fu, Y.; Wang, W.; Zeng, Q.; Wang, T.; Qian, W. Antibiofilm efficacy of luteolin against single and dual species of Candida albicans and Enterococcus faecalis. Front. Microbiol. 2021, 12, 715156. [Google Scholar] [CrossRef] [PubMed]
- Salvador, A.; Veiga, F.F.; Svidzinski, T.I.E.; Negri, M. Case of mixed infection of toenail caused by Candida parapsilosis and Exophiala dermatitidis and in vitro effectiveness of propolis extract on mixed biofilm. J. Fungi 2023, 9, 581. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Kim, Y.G.; Lee, J. Antibiofilm activity of lawsone against polymicrobial enterohemorrhagic Escherichia coli O157: H7 and Candida albicans by suppression of curli production and hyphal growth. Phytomedicine 2024, 124, 155306. [Google Scholar] [CrossRef] [PubMed]
- Di Vito, M.; Vergari, L.; Mariotti, M.; Proto, M.R.; Barbanti, L.; Garzoli, S.; Sanguinetti, M.; Sabatini, L.; Peduzzi, A.; Bellardi, M.G.; et al. Anti-Mold effectiveness of a green emulsion based on Citrus aurantium hydrolate and Cinnamomum zeylanicum essential oil for the modern paintings restoration. Microorganisms 2022, 10, 205. [Google Scholar] [CrossRef] [PubMed]
- Fierascu, I.; Ion, R.M.; Radu, M.; Bunghez, I.R.; Avramescu, S.M.; Fierascu, R.C. Comparative study of antifungal effect of natural extracts and essential oils of Ocimum basilicum on selected artefacts. Rev. Roum. Chim. 2014, 59, 207–211. [Google Scholar]
- Viana, M.G.; Lutterbach, M.T.S.; da Silva, D.R.; de Albuquerque, C.C.; dos Santos, F.J.N.; dos Santos, E.S. Antimicrobial and antibiofilm activity of essential oil of Lippiagracilis Schauer on Clostridium bifermentans and fungal-containing biofilms. Appl. Chem. Eng. 2019, 2, 1–12. [Google Scholar] [CrossRef]
- Jafri, H.; Banerjee, G.; Khan, M.S.A.; Ahmad, I.; Abulreesh, H.H.; Althubiani, A.S. Synergistic interaction of eugenol and antimicrobial drugs in eradication of single and mixed biofilms of Candida albicans and Streptococcus mutans. AMB Express 2020, 10, 185. [Google Scholar] [CrossRef] [PubMed]
- Gao, S.; Zhang, S.; Zhang, S. Enhanced in vitro antimicrobial activity of amphotericin B with berberine against dual-species biofilms of Candida albicans and Staphylococcus aureus. J. Appl. Microbiol. 2021, 130, 1154–1172. [Google Scholar] [CrossRef]
- Hacioglu, M.; Oyardi, O.; Bozkurt-Guzel, C.; Savage, P.B. Antibiofilm activities of ceragenins and antimicrobial peptides against fungal-bacterial mono and multispecies biofilms. J. Antibiot. 2020, 73, 455–462. [Google Scholar] [CrossRef] [PubMed]
- Bellavita, R.; Maione, A.; Braccia, S.; Sinoca, M.; Galdiero, S.; Galdiero, E.; Falanga, A. Myxinidin-derived peptide against biofilms caused by cystic fibrosis emerging pathogens. Int. J. Mol. Sci. 2023, 24, 3092. [Google Scholar] [CrossRef] [PubMed]
- Qu, Y.; Locock, K.; Verma-Gaur, J.; Hay, I.D.; Meagher, L.; Traven, A. Searching for new strategies against polymicrobial biofilm infections: Guanylated polymethacrylates kill mixed fungal/bacterial biofilms. J. Antimicrob. Chemother. 2016, 71, 413–421. [Google Scholar] [CrossRef] [PubMed]
- Maione, A.; de Alteriis, E.; Carraturo, F.; Galdiero, S.; Falanga, A.; Guida, M.; Di Cosmo, A.; Maselli, V.; Galdiero, E. The membranotropic peptide gH625 to combat mixed Candida albicans/Klebsiella pneumoniae biofilm: Correlation between in vitro anti-biofilm activity and in vivo antimicrobial protection. J. Fungi 2021, 7, 26. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, L.; Fortes, B.N.; Lincopan, N.; Ishida, K. Caspofungin and polymyxin B reduce the cell viability and total biomass of mixed biofilms of carbapenem-resistant Pseudomonas aeruginosa and Candida spp. Front. Microbiol. 2020, 11, 573263. [Google Scholar] [CrossRef]
- Fortes, B.N.; Scheunemann, G.; de Azevedo Melo, A.S.; Ishida, K. Caspofungin alone or combined with polymyxin B are effective against mixed biofilm of Aspergillus fumigatus and carbapenem-resistant Pseudomonas aeruginosa. Res. Microbiol. 2023, 174, 103993. [Google Scholar] [CrossRef] [PubMed]
- Falanga, A.; Maione, A.; La Pietra, A.; de Alteriis, E.; Vitale, S.; Bellavita, R.; Carotenuto, R.; Turrà, D.; Galdiero, S.; Galdiero, E.; et al. Competitiveness during dual-species biofilm formation of Fusarium oxysporum and Candida albicans and a novel treatment strategy. Pharmaceutics 2022, 14, 1167. [Google Scholar] [CrossRef] [PubMed]
- Kowalski, M.; Wolany, J.; Pastuszka, J.S.; Płaza, G.; Wlazło, A.; Ulfig, K.; Malina, A. Characteristics of airborne bacteria and fungi in some Polish wastewater treatment plants. Int. J. Environ. Sci. Technol. 2017, 14, 2181–2192. [Google Scholar] [CrossRef]
- Maione, A.; Mileo, A.; Pugliese, S.; Siciliano, A.; Cirillo, L.; Carraturo, F.; de Alteriis, E.; De Falco, M.; Guida, M.; Galdiero, E. VT-1161—A Tetrazole for management of mono- and dual-species biofilms. Microorganisms 2023, 11, 237. [Google Scholar] [CrossRef] [PubMed]
- Shivaji, S.; Nagapriya, B.; Ranjith, K. Differential susceptibility of mixed polymicrobial biofilms involving ocular coccoid bacteria (Staphylococcus aureus and S. epidermidis) and a filamentous fungus (Fusarium solani) on ex vivo human corneas. Microorganisms 2023, 11, 413. [Google Scholar] [CrossRef] [PubMed]
- Maione, A.; Buonanno, A.; Galdiero, M.; de Alteriis, E.; Petrillo, F.; Reibaldi, M.; Guida, M.; Galdiero, E. A Re-Purposing Strategy: Sub-Lethal concentrations of an eicosanoid derived from the omega-3-polyunsaturated fatty acid resolvin D1 affect dual species biofilms. Int. J. Mol. Sci. 2023, 24, 12876. [Google Scholar] [CrossRef] [PubMed]
- Garipov, M.R.; Sabirova, A.E.; Pavelyev, R.S.; Shtyrlin, N.V.; Lisovskaya, S.A.; Bondar, O.V.; Laikov, A.V.; Romanova, J.G.; Bogachev, M.I.; Kayumov, A.R.; et al. Targeting pathogenic fungi, bacteria and fungal-bacterial biofilms by newly synthesized quaternary ammonium derivative of pyridoxine and terbinafine with dual action profile. Bioorg. Chem. 2020, 104, 104306. [Google Scholar] [CrossRef] [PubMed]
- Lagudas, M.F.G.; Bureros, K.J.C. Inhibition of Candida albicans and Staphylococcus epidermidis mixed biofilm formation in a catheter disk model system treated with EtOH–EDTA solution. Lett. Appl. Microbiol. 2023, 76, ovac074. [Google Scholar] [CrossRef] [PubMed]
- Arora, A.; Singh, M.; Saini, V.; Mehta, D.; Safwan, S.M.; Pandey, N.; Verma, V.; Bajaj, A. Cholic acid-derived gemini amphiphile can eradicate interkingdom polymicrobial biofilms and wound infections. ACS Infect. Dis. 2023, 10, 138–154. [Google Scholar] [CrossRef] [PubMed]
- Cox, C.A.; Manavathu, E.K.; Wakade, S.; Myntti, M.; Vazquez, J.A. Efficacy of biofilm disrupters against Candida auris and other Candida species in monomicrobial and polymicrobial biofilms. Mycoses 2024, 67, e13684. [Google Scholar] [CrossRef] [PubMed]
- Castro, V.D.P.; Thomaz, D.Y.; Vieira, K.D.L.; Lopes, L.G.; Rossi, F.; Del Negro, G.M.; Benard, G.; Pires, R.H. In vitro activity of sanitizers against mono-and polymicrobial biofilms of C. parapsilosis and S. aureus. Antimicrob. Agents Chemother. 2023, 67, e00534-23. [Google Scholar] [CrossRef] [PubMed]
- Bridziuviene, D.; Raudoniene, V. Fungi surviving on treated wood and some of their physiological properties. Mater. Sci. 2013, 19, 43–50. [Google Scholar] [CrossRef]
- Assress, H.A.; Selvarajan, R.; Nyoni, H.; Ogola, H.J.O.; Mamba, B.B.; Msagati, T.A. Azole antifungal resistance in fungal isolates from wastewater treatment plant effluents. Environ. Sci. Pollut. Res. 2021, 28, 3217–3229. [Google Scholar] [CrossRef] [PubMed]
- Alcazar-Fuoli, L.; Buitrago, M.; Gomez-Lopez, A.; Mellado, E. An alternative host model of a mixed fungal infection by azole susceptible and resistant Aspergillus spp strains. Virulence 2015, 6, 376–384. [Google Scholar] [CrossRef] [PubMed]
- Cui, H.; Ou, Y.; Wang, L.; Yan, B.; Bao, M. Tetracycline hydrochloride-stressed succession in microbial communities during aerobic composting: Insights into bacterial and fungal structures. Chemosphere 2022, 289, 133159. [Google Scholar] [CrossRef] [PubMed]
- Turkiewicz, A.; Brzeszcz, J.; Kapusta, P. The application of biocides in the oil and gas industry. Nafta-Gaz 2013, 69, 103–111. [Google Scholar] [CrossRef]
- Senko, O.; Maslova, O.; Gladchenko, M.; Gaydamaka, S.; Akopyan, A.; Lysenko, S.; Karakhanov, E.; Efremenko, E. Prospective approach to the anaerobic bioconversion of benzo- and dibenzothiophene sulfones to sulfide. Molecules 2019, 24, 1736. [Google Scholar] [CrossRef]
- Ruiz-Sorribas, A.; Poilvache, H.; Van Bambeke, F. Pharmacodynamics of moxifloxacin, meropenem, caspofungin, and their combinations against in vitro polymicrobial interkingdom biofilms. Antimicrob. Agents Chemother. 2022, 66, e02149-21. [Google Scholar] [CrossRef] [PubMed]
- Bonvicini, F.; Belluti, F.; Bisi, A.; Gobbi, S.; Manet, I.; Gentilomi, G.A. Improved eradication efficacy of a combination of newly identified antimicrobial agents in C. albicans and S. aureus mixed-species biofilm. Res. Microbiol. 2021, 17, 103873. [Google Scholar] [CrossRef] [PubMed]
- Roisin, L.; Melloul, E.; Woerther, P.L.; Royer, G.; Decousser, J.W.; Guillot, J.; Dannaoui, E.; Botterel, F. Modulated response of Aspergillus fumigatus and Stenotrophomonas maltophilia to antimicrobial agents in polymicrobial biofilm. Front. Cell. Infect. Microbiol. 2020, 10, 574028. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Zhu, L. Copper regulates degradation of typical antibiotics by microalgal-fungal consortium in simulated swine wastewater: Insights into metabolic routes and dissolved organic matters. Water Res. 2023, 245, 120654. [Google Scholar] [CrossRef] [PubMed]
- Mishra, A.; Malik, A. Metal and dye removal using fungal consortium from mixed waste stream: Optimization and validation. Ecol. Eng. 2014, 69, 226–231. [Google Scholar] [CrossRef]
- Dorgham, R.A.; Abd Al Moaty, M.N.; Chong, K.P.; Elwakil, B.H. Molasses-silver nanoparticles: Synthesis, optimization, characterization, and antibiofilm activity. Int. J. Mol. Sci. 2022, 23, 10243. [Google Scholar] [CrossRef] [PubMed]
- Mandal, S.K.; Ojha, N.; Das, N. Process optimization of benzo [ghi] perylene biodegradation by yeast consortium in presence of ZnO nanoparticles and produced biosurfactant using Box-Behnken design. Front. Biol. 2018, 13, 418–424. [Google Scholar] [CrossRef]
- Priyanka, K.P.; Harikumar, V.S.; Balakrishna, K.M.; Varghese, T. Inhibitory effect of TiO2 NPs on symbiotic arbuscular mycorrhizal fungi in plant roots. IET Nanobiotechnol. 2017, 11, 66–70. [Google Scholar] [CrossRef]
- Wang, F.; Liu, X.; Shi, Z.; Tong, R.; Adams, C.A.; Shi, X. Arbuscular mycorrhizae alleviate negative effects of zinc oxide nanoparticle and zinc accumulation in maize plants–a soil microcosm experiment. Chemosphere 2016, 147, 88–97. [Google Scholar] [CrossRef]
- Tabassum, N.; Khan, F.; Kang, M.-G.; Jo, D.-M.; Cho, K.-J.; Kim, Y.-M. Inhibition of polymicrobial biofilms of Candida albicans–Staphylococcus aureus/Streptococcus mutans by fucoidan–gold nanoparticles. Mar. Drugs 2023, 21, 123. [Google Scholar] [CrossRef] [PubMed]
- Khan, F.; Tabassum, N.; Jeong, G.-J.; Jung, W.-K.; Kim, Y.-M. Inhibition of mixed biofilms of Candida albicans and Staphylococcus aureus by β-caryophyllene-gold nanoparticles. Antibiotics 2023, 12, 726. [Google Scholar] [CrossRef] [PubMed]
- Tabassum, N.; Jeong, G.J.; Jo, D.M.; Khan, F.; Kim, Y.M. Treatment of Staphylococcus aureus and Candida albicans polymicrobial biofilms by phloroglucinol-gold nanoparticles. Microb. Pathog. 2023, 185, 106416. [Google Scholar] [CrossRef] [PubMed]
- Fatahi Dehpahni, M.; Chehri, K.; Azadbakht, M. Effect of silver nanoparticles and L-carnitine supplement on mixed vaginitis caused by Candida albicans/Staphylococcus aureus in mouse models: An experimental study. Curr. Microbiol. 2021, 78, 3945–3956. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Peng, M.; Li, H.; Zhou, J.; He, W.; Hu, R.; Ye, F.; Li, Y.; Shi, L.; Liu, Y. Metal-phenolic network with Pd nanoparticle nodes synergizes oxidase-like and photothermal properties to eradicate oral polymicrobial biofilm-associated infections. Adv. Mater. 2024, 36, 2306376. [Google Scholar] [CrossRef]
- Diogo, P.; Fernandes, C.; Caramelo, F.; Mota, M.; Miranda, I.M.; Faustino, M.A.; Neves, M.G.; Uliana, M.P.; de Oliveira, K.T.; Santos, J.M.; et al. Antimicrobial photodynamic therapy against endodontic Enterococcus faecalis and Candida albicans mono and mixed biofilms in the presence of photosensitizers: A comparative study with classical endodontic irrigants. Front. Microbiol. 2017, 8, 246588. [Google Scholar] [CrossRef] [PubMed]
- Ferrer-Espada, R.; Liu, X.; Goh, X.S.; Dai, T. Antimicrobial blue light inactivation of polymicrobial biofilms. Front. Microbiol. 2019, 10, 437888. [Google Scholar] [CrossRef] [PubMed]
- Rousk, J.; Brookes, P.C.; Bååth, E. Investigating the mechanisms for the opposing pH relationships of fungal and bacterial growth in soil. Soil Biol. Biochem. 2010, 42, 926–934. [Google Scholar] [CrossRef]
- Lu, X.; Feng, Z.; Cui, B.; Zhou, D. Low pH induces simultaneous fungal bulking and nitrogen removal deterioration in an activated sludge reactor: Mechanisms based on microbial transboundary coevolution. J. Clean. Prod. 2023, 389, 136047. [Google Scholar] [CrossRef]
- Wang, Y.; Elzenga, T.; van Elsas, J.D. Effect of culture conditions on the performance of lignocellulose-degrading synthetic microbial consortia. Appl. Microbiol. Biotechnol. 2021, 105, 7981–7995. [Google Scholar] [CrossRef]
- De Marchi, R.; Koss, M.; Ziegler, D.; De Respinis, S.; Petrini, O. Fungi in water samples of a full-scale water work. Mycol. Prog. 2018, 17, 467–478. [Google Scholar] [CrossRef]
- Gatina, A.; Trizna, E.; Kolesnikova, A.; Baidamshina, D.; Gorshkova, A.; Drucker, V.; Bogachev, M.; Kayumov, A. The bovhyaluronidase azoximer (Longidaza®) disrupts Candida albicans and Candida albicans-bacterial mixed biofilms and increases the efficacy of antifungals. Medicina 2022, 58, 1710. [Google Scholar] [CrossRef]
- Tan, Y.; Ma, S.; Leonhard, M.; Moser, D.; Ludwig, R.; Schneider-Stickler, B. Co-immobilization of cellobiose dehydrogenase and deoxyribonuclease I on chitosan nanoparticles against fungal/bacterial polymicrobial biofilms targeting both biofilm matrix and microorganisms. Mater. Sci. Eng. C 2020, 108, 110499. [Google Scholar] [CrossRef]
- Walker, S.L.; Fourgialakis, M.; Cerezo, B.; Livens, S. Removal of microbial biofilms from dispense equipment: The effect of enzymatic pre-digestion and detergent treatment. J. Inst. Brew. 2007, 113, 61–66. [Google Scholar] [CrossRef]
- Win, T.T.; Bo, B.; Malec, P.; Fu, P. The effect of a consortium of Penicillium sp. and Bacillus spp. in suppressing banana fungal diseases caused by Fusarium sp. and Alternaria sp. J. Appl. Microbiol. 2021, 131, 1890–1908. [Google Scholar] [CrossRef] [PubMed]
- Gomes, T.; Pereira, J.A.; Lino-Neto, T.; Bennett, A.E.; Baptista, P. Bacterial disease induced changes in fungal communities of olive tree twigs depend on host genotype. Sci. Rep. 2019, 9, 5882. [Google Scholar] [CrossRef] [PubMed]
- Milijašević-Marčić, S.; Stepanović, M.; Todorović, B.; Duduk, B.; Stepanović, J.; Rekanović, E.; Potočnik, I. Biological control of green mould on Agaricus bisporus by a native Bacillus subtilis strain from mushroom compost. Eur. J. Plant Pathol. 2017, 148, 509–519. [Google Scholar] [CrossRef]
- Srikanth, R.; Banu, S.F.; Sowndarya, J.; Parveen, J.H.S.; Rubini, D.; Wilson, A.; Nithyanand, P. Biosurfactant synergized with marine bacterial DNase disrupts polymicrobial biofilms. Folia Microbiol. 2021, 66, 831–842. [Google Scholar] [CrossRef] [PubMed]
- Carboni, G.; Fancello, F.; Zara, G.; Zara, S.; Ruiu, L.; Marova, I.; Pinna, G.; Budroni, M.; Mannazzu, I. Production of a lyophilized ready-to-use yeast killer toxin with possible applications in the wine and food industries. Int. J. Food Microbiol. 2020, 335, 108883. [Google Scholar] [CrossRef] [PubMed]
- Tan, Y.; Leonhard, M.; Moser, D.; Ma, S.; Schneider-Stickler, B. Inhibitory effect of probiotic lactobacilli supernatants on single and mixed non-albicans Candida species biofilm. Arch. Oral Biol. 2018, 85, 40–45. [Google Scholar] [CrossRef] [PubMed]
- Rather, I.A.; Wani, M.Y.; Kamli, M.R.; Sabir, J.S.M.; Hakeem, K.R.; Firoz, A.; Park, Y.H.; Hor, Y.Y. Limosilactobacillus fermentum KAU0021 abrogates mono- and polymicrobial biofilms formed by Candida albicans and Staphylococcus aureus. Pharmaceutics 2023, 15, 1079. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, N.; Ellepola, K.; Venkiteswaran, N.; Chai, L.Y.A.; Ohshima, T.; Seneviratne, C.J. Lactobacillus plantarum 108 inhibits Streptococcus mutans and Candida albicans mixed-species biofilm formation. Antibiotics 2020, 9, 478. [Google Scholar] [CrossRef] [PubMed]
- Tan, Y.; Leonhard, M.; Moser, D.; Schneider-Stickler, B. Inhibition activity of Lactobacilli supernatant against fungal-bacterial multispecies biofilms on silicone. Microb. Pathog. 2017, 113, 197–201. [Google Scholar] [CrossRef] [PubMed]
- Chaudhary, V.; Prasanna, R.; Nain, L.; Dubey, S.C.; Gupta, V.; Singh, R.; Jaggi, S.; Bhatnagar, A.K. Bioefficacy of novel cyanobacteria-amended formulations in suppressing damping off disease in tomato seedlings. World J. Microbiol. Biotechnol. 2012, 28, 3301–3310. [Google Scholar] [CrossRef] [PubMed]
- Yılmaz Öztürk, B.; Yenice Gürsu, B.; Dağ, İ. In vitro effect of farnesol on planktonic cells and dual biofilm formed by Candida albicans and Escherichia coli. Biofouling 2022, 38, 355–366. [Google Scholar] [CrossRef] [PubMed]
- Gong, S.G.; El-Shennawy, S.; Choudhary, P.; Dufour, D.; Lévesque, C.M. Antimicrobial activity of probiotic Streptococcus salivarius LAB813 on in vitro cariogenic biofilms. Arch. Oral Biol. 2023, 154, 105760. [Google Scholar] [CrossRef]
- Turnau, K.; Pajdak-Stós, A.; Korzh, Y.; Domka, A.; Bień-Kostycz, P.; Fiałkowska, E. Biological control of predatory fungi inhabiting activated sludge in wastewater treatment. J. Environ. Manag. 2024, 356, 120572. [Google Scholar] [CrossRef] [PubMed]
- da Silva, C.R.; Sá, L.G.D.A.V.; de Andrade Neto, J.B.D.; Barroso, F.D.D.; Cabral, V.P.D.F.; Rodrigues, D.S.; da Silva, L.J.; Lima, I.S.P.; Perez, L.; da Silva, A.R.; et al. Antimicrobial potential of a biosurfactant gel for the prevention of mixed biofilms formed by fluconazole-resistant C. albicans and methicillin-resistant S. aureus in catheters. Biofouling 2024, 40, 165–176. [Google Scholar] [CrossRef]
- del Rosario Agustín, M.; Tarifa, M.C.; Vela-Gurovic, M.S.; Brugnoni, L.I. Application of natamycin and farnesol as bioprotection agents to inhibit biofilm formation of yeasts and foodborne bacterial pathogens in apple juice processing lines. Food Microbiol. 2023, 109, 104123. [Google Scholar] [CrossRef]
- Touil, H.F.Z.; Boucherit, K.; Boucherit-Otmani, Z.; Kohder, G.; Madkour, M.; Soliman, S.S.M. Optimum inhibition of amphotericin-B-resistant Candida albicans strain in single- and mixed-species biofilms by Candida and Non-Candida terpenoids. Biomolecules 2020, 10, 342. [Google Scholar] [CrossRef] [PubMed]
- Gaálová-Radochová, B.; Kendra, S.; Jordao, L.; Kursawe, L.; Kikhney, J.; Moter, A.; Bujdáková, H. Effect of quorum sensing molecule farnesol on mixed biofilms of Candida albicans and Staphylococcus aureus. Antibiotics 2023, 12, 441. [Google Scholar] [CrossRef] [PubMed]
- Mishra, S.; Gupta, A.; Upadhye, V.; Singh, S.C.; Sinha, R.P.; Häder, D.P. Therapeutic strategies against biofilm infections. Life 2023, 13, 172. [Google Scholar] [CrossRef]
- Ai, L.; Fu, S.; Li, Y.; Zuo, M.; Huang, W.; Jin, Z.; Chen, Y. Natural products-based: Synthesis and antifungal activity evaluation of novel L-pyroglutamic acid analogues. Front. Plant Sci. 2022, 13, 1102411. [Google Scholar] [CrossRef] [PubMed]
- Moussa, H.; El Omari, B.; Chefchaou, H.; Tanghort, M.; Mzabi, A.; Chami, N.; Remmal, A. Action of thymol, carvacrol and eugenol on Penicillium and Geotrichum isolates resistant to commercial fungicides and causing postharvest citrus decay. Can. J. Plant Pathol. 2021, 43, 26–34. [Google Scholar] [CrossRef]
- Clerck, C.D.; Maso, S.D.; Parisi, O.; Dresen, F.; Zhiri, A.; Jijakli, M.H. Screening of antifungal and antibacterial activity of 90 commercial essential oils against 10 pathogens of agronomical importance. Foods 2020, 9, 1418. [Google Scholar] [CrossRef] [PubMed]
- Efremenko, E.; Aslanli, A.; Stepanov, N.; Senko, O.; Maslova, O. Various biomimetics, including peptides as antifungals. Biomimetics 2023, 8, 513. [Google Scholar] [CrossRef] [PubMed]
- Aslanli, A.; Domnin, M.; Stepanov, N.; Efremenko, E. Synergistic antimicrobial action of lactoferrin-derived peptides and quorum quenching enzymes. Int. J. Mol. Sci. 2023, 24, 3566. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Culebras, P.V.; Gandía, M.; Garrigues, S.; Marcos, J.F.; Manzanares, P. Antifungal peptides and proteins to control toxigenic fungi and mycotoxin biosynthesis. Int. J. Mol. Sci. 2021, 22, 13261. [Google Scholar] [CrossRef] [PubMed]
- Struyfs, C.; Cammue, B.P.A.; Thevissen, K. Membrane-interacting antifungal peptides. Front. Cell Dev. Biol. 2021, 9, 649875. [Google Scholar] [CrossRef] [PubMed]
- Masuda, Y. Bioactive 3D structures of naturally occurring peptides and their application in drug design. Biosci. Biotechnol. Biochem. 2021, 85, 24–32. [Google Scholar] [CrossRef] [PubMed]
- Bezerra, L.P.; Silva, A.F.; Santos-Oliveira, R.; Alencar, L.M.; Amaral, J.L.; Neto, N.A.; Silva, R.G.G.; Belem, M.O.; de Andrade, C.R.; Oliveira, J.T.A.; et al. Combined antibiofilm activity of synthetic peptides and antifungal drugs against Candida spp. Future Microbiol. 2022, 17, 1133–1146. [Google Scholar] [CrossRef]
- Wang, X.; Wang, D.; Lu, H.; Wang, X.; Wang, X.; Su, J.; Xia, G. Strategies to Promote the Journey of Nanoparticles Against Biofilm-Associated Infections. Small 2024, 20, 2305988. [Google Scholar] [CrossRef] [PubMed]
- Mohanta, Y.K.; Chakrabartty, I.; Mishra, A.K.; Chopra, H.; Mahanta, S.; Avula, S.K.; Patowary, K.; Ahmed, R.; Mishra, B.; Mohanta, T.P.; et al. Nanotechnology in combating biofilm: A smart and promising therapeutic strategy. Front. Microbiol. 2023, 13, 1028086. [Google Scholar] [CrossRef] [PubMed]
- Guo, Z.; Chen, Y.; Wang, Y.; Jiang, H.; Wang, X. Advances and challenges in metallic nanomaterial synthesis and antibacterial applications. J. Mater. Chem. B 2020, 8, 4764–4777. [Google Scholar] [CrossRef]
- Efremenko, E.; Stepanov, N.; Maslova, O.; Senko, O.; Aslanli, A.; Lyagin, I. “Unity and struggle of Opposites” as a basis for the functioning of synthetic bacterial immobilized consortium that continuously degrades organophosphorus pesticides. Microorganisms 2022, 10, 1394. [Google Scholar] [CrossRef] [PubMed]
- Efremenko, E.; Aslanli, A.; Domnin, M.; Stepanov, N.; Senko, O. Enzymes with lactonase activity against fungal quorum molecules as effective antifungals. Biomolecules 2024, 14, 383. [Google Scholar] [CrossRef] [PubMed]
- Efremenko, E.; Stepanov, N.; Aslanli, A.; Lyagin, I.; Senko, O.; Maslova, O. Combination of enzymes with materials to give them antimicrobial features: Modern trends and perspectives. J. Funct. Biomater. 2023, 14, 64. [Google Scholar] [CrossRef]
- Mehmood, A.; Liu, G.; Wang, X.; Meng, G.; Wang, C.; Liu, Y. Fungal quorum-sensing molecules and inhibitors with potential antifungal activity: A Review. Molecules 2019, 24, 1950. [Google Scholar] [CrossRef] [PubMed]
- Meroni, G.; Panelli, S.; Zuccotti, G.; Bandi, C.; Drago, L.; Pistone, D. Probiotics as therapeutic tools against pathogenic biofilms: Have we found the perfect weapon? Microbiol. Res. 2021, 12, 916–937. [Google Scholar] [CrossRef]
- Chen, Z.; Schlafer, S.; Göstemeyer, G.; Schwendicke, F. Probiotic effects on multispecies biofilm composition, architecture, and caries activity in vitro. Microorganisms 2020, 8, 1272. [Google Scholar] [CrossRef] [PubMed]
- Manohar, P.; Loh, B.; Nachimuthu, R.; Leptihn, S. Phage-antibiotic combinations to control Pseudomonas aeruginosa-Candida two-species biofilms. Sci. Rep. 2024, 14, 9354. [Google Scholar] [CrossRef] [PubMed]
- Roszak, M.; Dołȩgowska, B.; Cecerska-Heryć, E.; Serwin, N.; Jabłońska, J.; Grygorcewicz, B. Bacteriophage-ciprofloxacin combination effectiveness depends on Staphylococcus aureus-Candida albicans dual-species communities’ growth model. Microb. Drug Resist. 2022, 28, 613–622. [Google Scholar] [CrossRef]
- Hough, B.; Steenkamp, E.; Wingfield, B.; Read, D. Fungal viruses unveiled: A comprehensive review of mycoviruses. Viruses 2023, 15, 1202. [Google Scholar] [CrossRef] [PubMed]
- Ghabrial, S.A.; Castón, J.R.; Jiang, D.; Nibert, M.L.; Suzuki, N. 50-plus years of fungal viruses. Virology 2015, 479, 356–368. [Google Scholar] [CrossRef] [PubMed]
- García-Pedrajas, M.D.; Cañizares, M.C.; Sarmiento-Villamil, J.L.; Jacquat, A.G.; Dambolena, J.S. Mycoviruses in biological control: From basic research to field implementation. Phytopathology 2019, 109, 1828–1839. [Google Scholar] [CrossRef] [PubMed]
- Kotta-Loizou, I. Mycoviruses and their role in fungal pathogenesis. Curr. Opin. Microbiol. 2021, 63, 10–18. [Google Scholar] [CrossRef] [PubMed]
- Khan, H.A.; Nerva, L.; Bhatti, M.F. The good, the bad and the cryptic: The multifaceted roles of mycoviruses and their potential applications for a sustainable agriculture. Virology 2023, 585, 259–269. [Google Scholar] [CrossRef] [PubMed]
- Tonka, T.; Walterová, L.; Čurn, V. Biological control of pathogenic fungi: Can mycoviruses play an important role? J. Cent. Eur. Agric. 2022, 23, 540–551. [Google Scholar] [CrossRef]
- Özkan, S.; Coutts, R.H.A. Aspergillus fumigatus mycovirus causes mild hypervirulent effect on pathogenicity when tested on Galleria mellonella. Fungal Genet. Biol. 2015, 76, 20–26. [Google Scholar] [CrossRef] [PubMed]
- Das, T.; Prasad, A.; Dey, A. Mycoviral gene-incorporating phytopathogenic fungi: A biocontrol agent. Trends Plant Sci. 2023, 28, 864–866. [Google Scholar] [CrossRef] [PubMed]
- Myers, J.M.; James, T.Y. Mycoviruses. Curr. Biol. 2022, 32, R150–R155. [Google Scholar] [CrossRef] [PubMed]
- Romon-Ochoa, P.; Forster, J.; Chitty, R.; Gorton, C.; Lewis, A.; Eacock, A.; Kupper, Q.; Rigling, D.; Pérez-Sierra, A. Canker development and biocontrol potential of CHV-1 infected english isolates of Cryphonectria parasitica is dependent on the virus concentration and the compatibility of the fungal inoculums. Viruses 2022, 14, 2678. [Google Scholar] [CrossRef] [PubMed]
- Ko, Y.H.; So, K.K.; Chun, J.; Kim, D.H. Distinct roles of two DNA methyltransferases from Cryphonectria parasitica in fungal virulence, responses to hypovirus infection, and viral clearance. MBio 2021, 12, e02890-20. [Google Scholar] [CrossRef] [PubMed]
- Ding, F.; Cheng, J.; Fu, Y.; Chen, T.; Li, B.; Jiang, D.; Xie, J. Early transcriptional response to DNA virus infection in Sclerotinia sclerotiorum. Viruses 2019, 11, 278. [Google Scholar] [CrossRef] [PubMed]
- Urayama, S.I.; Sakoda, H.; Takai, R.; Katoh, Y.; Le, T.M.; Fukuhara, T.; Arie, T.; Teraoka, T.; Moriyama, H. A dsRNA mycovirus, Magnaporthe oryzae chrysovirus 1-B, suppresses vegetative growth and development of the rice blast fungus. Virology 2014, 448, 265–273. [Google Scholar] [CrossRef]
- Wang, Q.; Zou, Q.; Dai, Z.; Hong, N.; Wang, G.; Wang, L. Four novel mycoviruses from the hypovirulent Botrytis cinerea SZ-2-3y isolate from Paris polyphylla: Molecular characterisation and mitoviral sequence transboundary entry into plants. Viruses 2022, 14, 151. [Google Scholar] [CrossRef]
- Lemus-Minor, C.G.; Cañizares, M.C.; García-Pedrajas, M.D.; Pérez-Artés, E. Horizontal and vertical transmission of the hypovirulence-associated mycovirus Fusarium oxysporum f. sp. dianthi virus 1. Eur. J. Plant Pathol. 2019, 153, 645–650. [Google Scholar] [CrossRef]
- Li, S.; Zhu, H.; He, Y.; Hong, N.; Wang, G.; Wang, L. BdCV1-encoded P3 silencing suppressor identification and its roles in Botryosphaeria dothidea, causing pear ring rot disease. Cells 2023, 12, 2386. [Google Scholar] [CrossRef]
- Zhai, L.; Xiang, J.; Zhang, M.; Fu, M.; Yang, Z.; Hong, N.; Wang, G. Characterization of a novel double-stranded RNA mycovirus conferring hypovirulence from the phytopathogenic fungus Botryosphaeria dothidea. Virology 2016, 493, 75–78. [Google Scholar] [CrossRef]
- Wang, L.; Jiang, J.; Wang, Y.; Hong, N.; Zhang, F.; Xu, W.; Wang, G. Hypovirulence of the phytopathogenic fungus Botryosphaeria dothidea: Association with a coinfecting chrysovirus and a partitivirus. J. Virol. 2014, 88, 7517–7527. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhao, H.; Cao, J.; Yin, X.; Guo, Y.; Guo, L.; Wu, H.; Zhang, M. Characterization of a novel mycovirus from the phytopathogenic fungus Botryosphaeria dothidea. Viruses 2022, 14, 331. [Google Scholar] [CrossRef] [PubMed]
- Arjona-López, J.M.; Telengech, P.; Suzuki, N.; López-Herrera, C.J. Coinfection of Rosellinia necatrix by a partitivirus and a virga-like virus is associated with hypovirulence. Eur. J. Plant Pathol. 2020, 158, 111–119. [Google Scholar] [CrossRef]
- Selim, M.T.; Salem, S.S.; Mohamed, A.A.; El-Gamal, M.S.; Awad, M.F.; Fouda, A. Biological treatment of real textile effluent using Aspergillus flavus and Fusarium oxysporium and their consortium along with the evaluation of their phytotoxicity. J. Fungi 2021, 7, 193. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Hai, D.; Mu, F.; Yu, X.; Zhao, Y.; He, B.; Xie, J.; Jiang, D.; Liu, H. Molecular characterization of a novel fusarivirus infecting the plant-pathogenic fungus Botryosphaeria dothidea. Arch. Virol. 2020, 165, 1033–1037. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Wang, G.; Wang, J.; Zhai, H.; Xue, X. Inhibitory effect and underlying mechanism of cinnamon and clove essential oils on Botryosphaeria dothidea and Colletotrichum gloeosporioides causing rots in postharvest bagging-free apple fruits. Front. Microbiol. 2023, 14, 1109028. [Google Scholar] [CrossRef] [PubMed]
- Law, J.W.F.; Ser, H.L.; Khan, T.M.; Chuah, L.H.; Pusparajah, P.; Chan, K.G.; Goh, B.H.; Lee, L.H. The potential of Streptomyces as biocontrol agents against the rice blast fungus, Magnaporthe oryzae (Pyricularia oryzae). Front. Microbiol. 2017, 8, 215552. [Google Scholar] [CrossRef] [PubMed]
- Surovy, M.Z.; Rahman, S.; Rostás, M.; Islam, T.; von Tiedemann, A. Suppressive effects of volatile compounds from Bacillus spp. On Magnaporthe oryzae Triticum (MoT) pathotype, causal agent of wheat blast. Microorganisms 2023, 11, 1291. [Google Scholar] [CrossRef] [PubMed]
- AbuQamar, S.; Moustafa, K.; Tran, L.S. Mechanisms and strategies of plant defense against Botrytis cinerea. Crit. Rev. Biotechnol. 2017, 37, 262–274. [Google Scholar] [CrossRef] [PubMed]
- Orozco-Mosqueda, M.d.C.; Kumar, A.; Fadiji, A.E.; Babalola, O.O.; Puopolo, G.; Santoyo, G. Agroecological management of the grey mould fungus Botrytis cinerea by plant growth-promoting bacteria. Plants 2023, 12, 637. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Xie, J.; Cheng, J.; Fu, Y.; Li, G.; Yi, X.; Jiang, D. Fungal negative-stranded RNA virus that is related to bornaviruses and nyaviruses. Proc. Natl. Acad. Sci. USA 2014, 111, 12205–12210. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.P.; Keswani, C.; Singh, S.P.; Sansinenea, E.; Hoat, T.X. Trichoderma spp. mediated induction of systemic defense response in brinjal against Sclerotinia sclerotiorum. Curr. Res. Microb. Sci. 2021, 2, 100051. [Google Scholar] [CrossRef]
- Sawant, S.S.; Song, J.; Seo, H.-J. Characterization of Bacillus velezensis RDA1 as a biological control agent against white root rot disease caused by Rosellinia necatrix. Plants 2022, 11, 2486. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Wang, H.; Liao, X.L.; Gao, B.; Lu, X.; Sun, D.; Gong, W.; Zhong, J.; Zhu, H.; Pan, X.; et al. Mycoviral gene integration converts a plant pathogenic fungus into a biocontrol agent. Proc. Natl. Acad. Sci. USA 2022, 119, e2214096119. [Google Scholar] [CrossRef] [PubMed]
- Schaffrath, R.; Meinhardt, F.; Klassen, R. Yeast killer toxins: Fundamentals and applications. In Physiology and Genetics. The Mycota; Anke, T., Schüffler, A., Eds.; Springer: Cham, Switzerland, 2018; pp. 87–118. [Google Scholar] [CrossRef]
- Mannazzu, I.; Domizio, P.; Carboni, G.; mZara, S.; Zara, G.; Comitini, F.; Budroni, M.; Ciani, M. Yeast killer toxins: From ecological significance to application. Crit. Rev. Biotechnol. 2019, 39, 603–617. [Google Scholar] [CrossRef] [PubMed]
- Solieri, L. The revenge of Zygosaccharomyces yeasts in food biotechnology and applied microbiology. World J. Microbiol. Biotechnol. 2021, 37, 96. [Google Scholar] [CrossRef] [PubMed]
- Abu-Mejdad, N.M.J.A.; Al-Badran, A.I.; Al-Saadoon, A.H.; Minati, M.H. A new report on gene expression of three killer toxin genes with antimicrobial activity of two killer toxins in Iraq. Bull. Natl. Res. Cent. 2020, 44, 162. [Google Scholar] [CrossRef]
- Zhang, H.; Xie, J.; Fu, Y.; Cheng, J.; Qu, Z.; Zhao, Z.; Cheng, S.; Chen, T.; Li, B.; Wang, Q.; et al. A 2-kb mycovirus converts a pathogenic fungus into a beneficial endophyte for Brassica protection and yield enhancement. Mol. Plant 2020, 13, 1420–1433. [Google Scholar] [CrossRef]
- Kim, S.; Lieberman, T.D.; Kishony, R. Alternating antibiotic treatments constrain evolutionary paths to multidrug resistance. Proc. Natl. Acad. Sci. USA 2014, 111, 14494–14499. [Google Scholar] [CrossRef] [PubMed]
- Tomizawa, T.; Nishitani, K.; Ito, H.; Okae, Y.; Morita, Y.; Doi, K.; Saito, M.; Ishie, S.; Yoshida, S.; Murata, K.; et al. The limitations of mono-and combination antibiotic therapies on immature biofilms in a murine model of implant-associated osteomyelitis. J. Orthop. Res. 2021, 39, 449–457. [Google Scholar] [CrossRef] [PubMed]
- Coates, A.R.; Hu, Y.; Holt, J.; Yeh, P. Antibiotic combination therapy against resistant bacterial infections: Synergy, rejuvenation and resistance reduction. Expert Rev. Anti-Infect. Ther. 2020, 18, 5–15. [Google Scholar] [CrossRef] [PubMed]
- Ocampo, P.S.; Lázár, V.; Papp, B.; Arnoldini, M.; Abel zur Wiesch, P.; Busa-Fekete, R.; Fekete, G.; Pál, C.; Ackermann, M.; Bonhoeffer, S. Antagonism between bacteriostatic and bactericidal antibiotics is prevalent. Antimicrob. Agents Chemother. 2014, 58, 4573–4582. [Google Scholar] [CrossRef] [PubMed]
- Singh, N.; Yeh, P.J. Suppressive drug combinations and their potential to combat antibiotic resistance. J. Antibiot. 2017, 70, 1033–1042. [Google Scholar] [CrossRef] [PubMed]
- Kubin, C.J.; McConville, T.H.; Dietz, D.; Zucker, J.; May, M.; Nelson, B.; Istorico, E.; Bartram, L.; Small-Saunders, J.; Magdalena, E.; et al. Characterization of bacterial and fungal infections in hospitalized patients with coronavirus disease 2019 and factors associated with health care-associated infections. Open Forum Infect. Dis. 2021, 8, ofab201. [Google Scholar] [CrossRef] [PubMed]
- Meawed, T.E.; Ahmed, S.M.; Mowafy, S.M.; Samir, G.M.; Anis, R.H. Bacterial and fungal ventilator associated pneumonia in critically ill COVID-19 patients during the second wave. J. Infect. Public Health 2021, 14, 1375–1380. [Google Scholar] [CrossRef] [PubMed]
- Pisa, D.; Alonso, R.; Carrasco, L. Parkinson’s disease: A comprehensive analysis of fungi and bacteria in brain tissue. Int. J. Biol. Sci. 2020, 16, 1135. [Google Scholar] [CrossRef] [PubMed]
- Vojdani, M.; Zibaei, M. Frequency of bacteria and fungi isolated from pumice in dental laboratories. J. Res. Health Sci. 2023, 6, 33–38. [Google Scholar]
- Firoozeh, F.; Zibaei, M.; Zendedel, A.; Rashidipour, H.; Kamran, A. Microbial contamination of pumice used in dental laboratories. Healthc. Low-Resour. Settings 2013, 1, 5. [Google Scholar] [CrossRef]
- Brevik, E.C.; Slaughter, L.; Singh, B.R.; Steffan, J.J.; Collier, D.; Barnhart, P.; Pereira, P. Soil and human health: Current status and future needs. Air Soil Water Res. 2020, 13, 1178622120934441. [Google Scholar] [CrossRef]
- Di Carlo, E.; Chisesi, R.; Barresi, G.; Barbaro, S.; Lombardo, G.; Rotolo, V.; Sebastianelli, M.; Travagliato, G.; Palla, F. Fungi and bacteria in indoor cultural heritage environments: Microbial-related risks for artworks and human health. Environ. Ecol. Res. 2016, 4, 257–264. [Google Scholar] [CrossRef]
- Thrasher, J.D. Fungi, bacteria, nano-particulates, mycotoxins and human health in water-damaged indoor environments. J. Community Public Health Nurs. 2016, 2, 2. [Google Scholar] [CrossRef]
- Khan, A.H.; Karuppayil, S.M. Fungal pollution of indoor environments and its management. Saudi J. Biol. Sci. 2012, 19, 405–426. [Google Scholar] [CrossRef] [PubMed]
- Shin, S.-H.; Ye, M.-K.; Lee, D.-W.; Geum, S.-Y. Immunopathologic role of fungi in chronic rhinosinusitis. Int. J. Mol. Sci. 2023, 24, 2366. [Google Scholar] [CrossRef] [PubMed]
- Lapiere, A.; Richard, M.L. Bacterial-fungal metabolic interactions within the microbiota and their potential relevance in human health and disease: A short review. Gut Microbes 2022, 14, 2105610. [Google Scholar] [CrossRef] [PubMed]
- Buda De Cesare, G.; Cristy, S.A.; Garsin, D.A.; Lorenz, M.C. Antimicrobial peptides: A New frontier in antifungal therapy. mBio 2020, 11, e02123-20. [Google Scholar] [CrossRef]
- Fontanot, A.; Ellinger, I.; Unger, W.W.J.; Hays, J.P. A Comprehensive review of recent research into the effects of antimicrobial peptides on biofilms—January 2020 to September 2023. Antibiotics 2024, 13, 343. [Google Scholar] [CrossRef] [PubMed]
- Nnadi, N.E.; Carter, D.A. Climate change and the emergence of fungal pathogens. PLoS Pathog. 2021, 17, e1009503. [Google Scholar] [CrossRef] [PubMed]
- Fisher, M.C.; Denning, D.W. The WHO fungal priority pathogens list as a game-changer. Nat. Rev. Microbiol. 2023, 21, 211–212. [Google Scholar] [CrossRef] [PubMed]
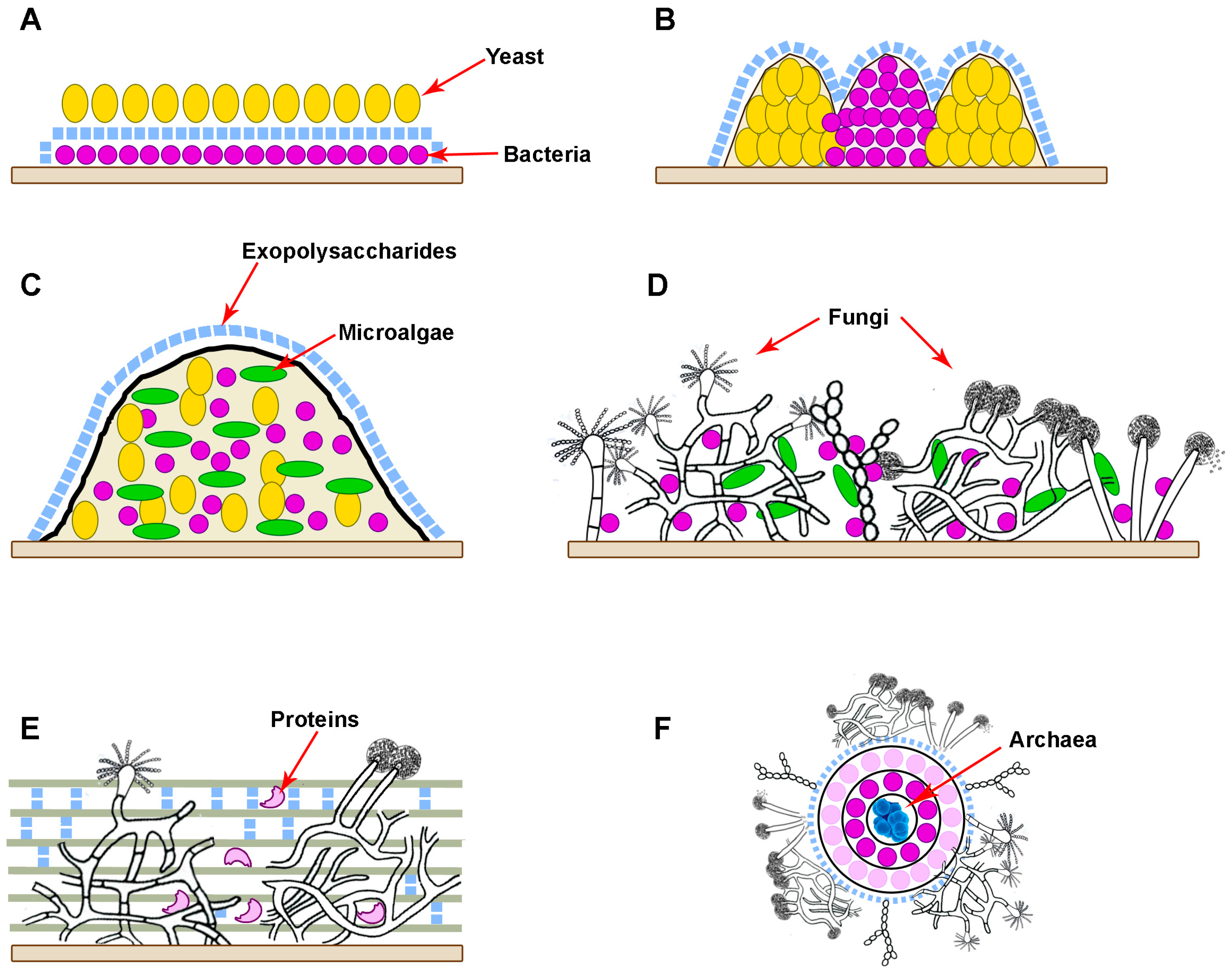
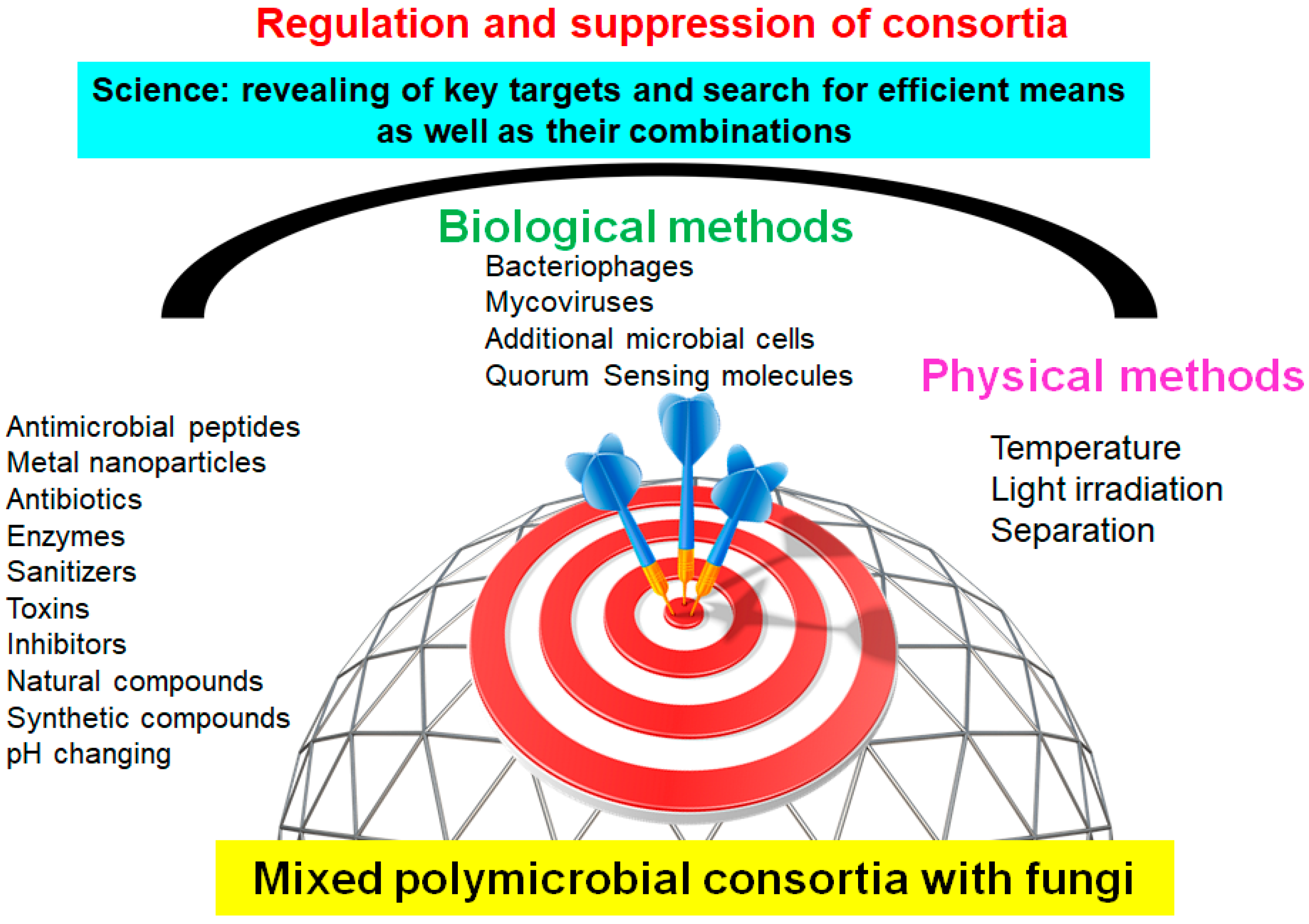

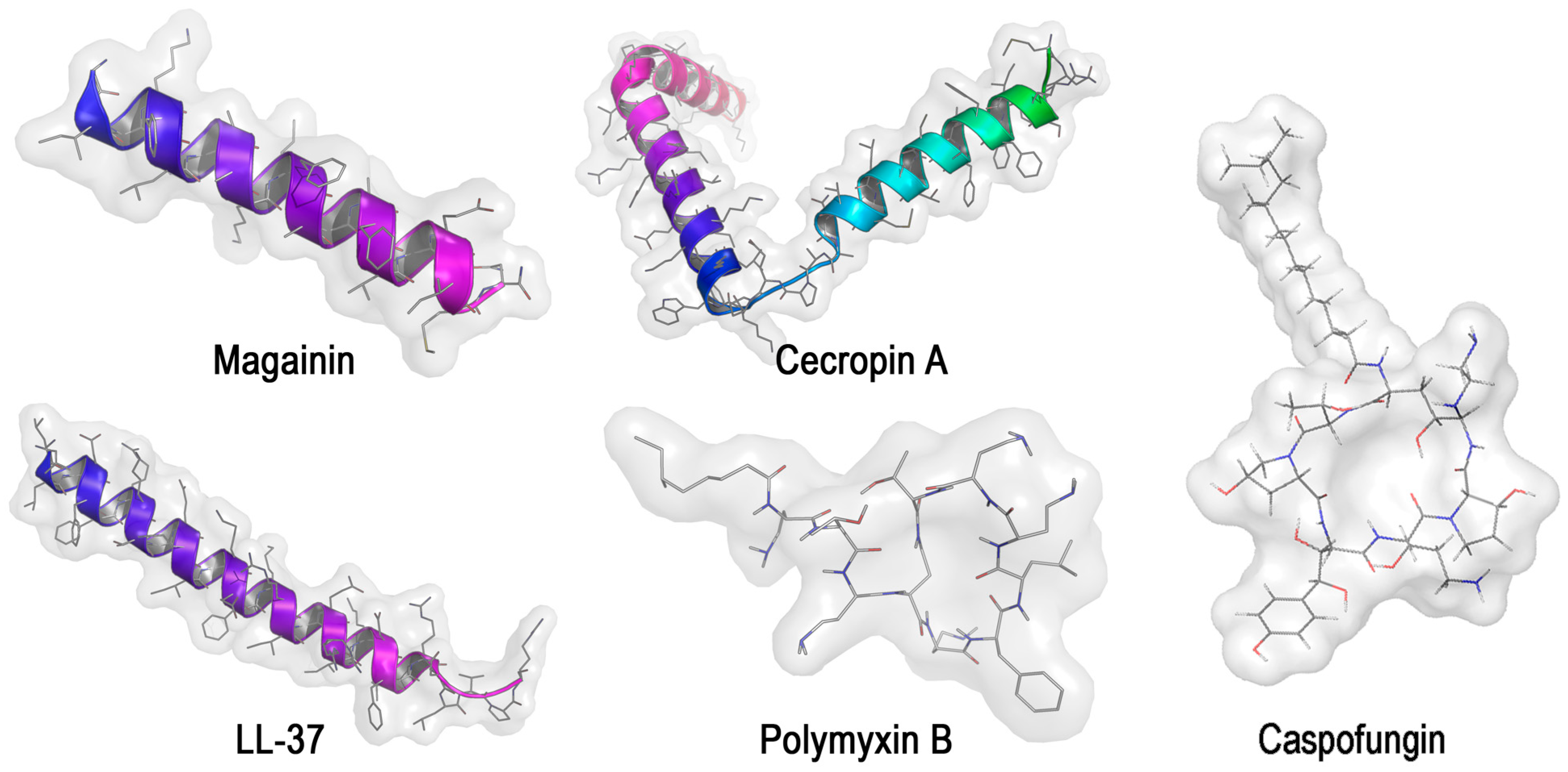
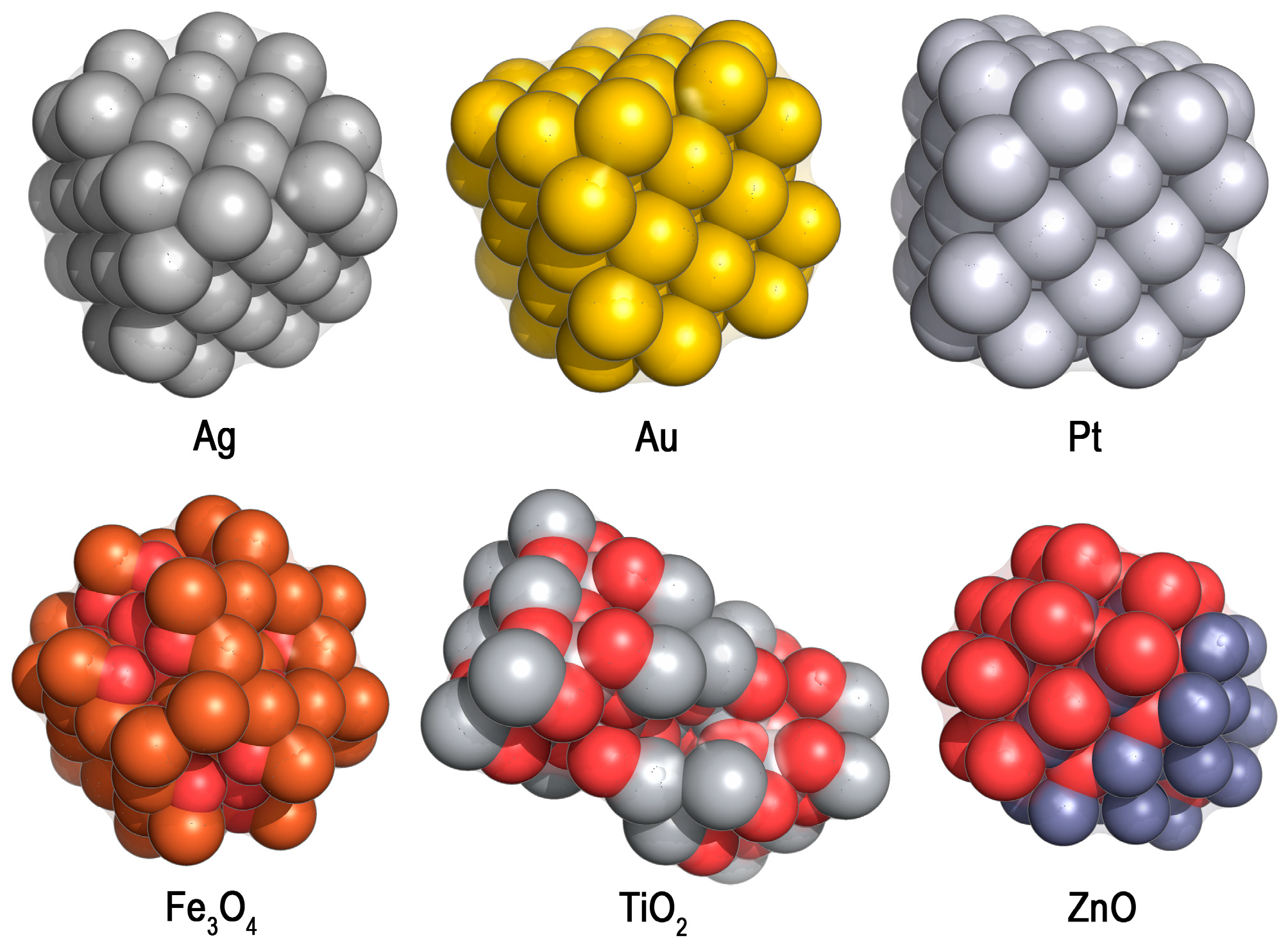
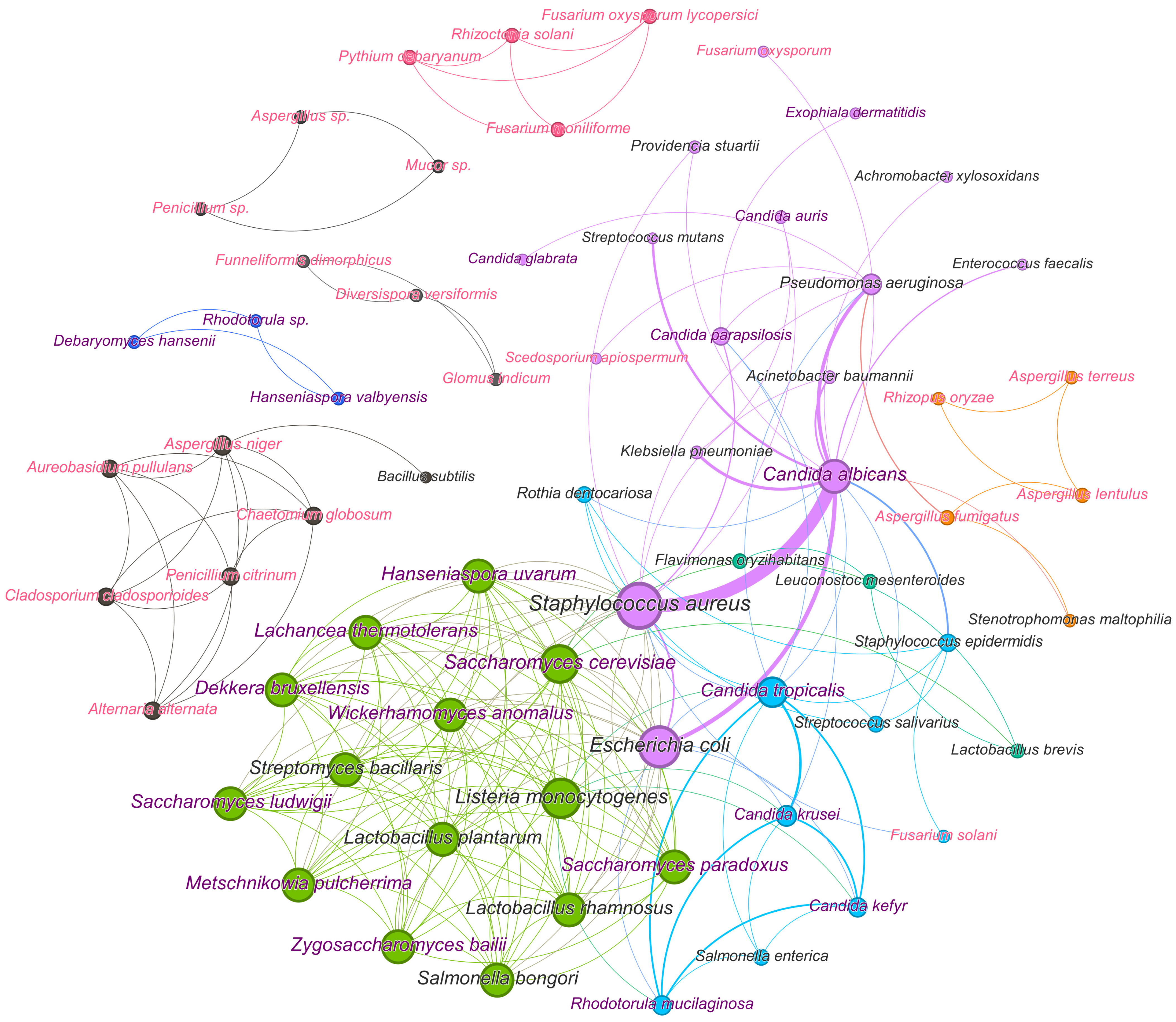

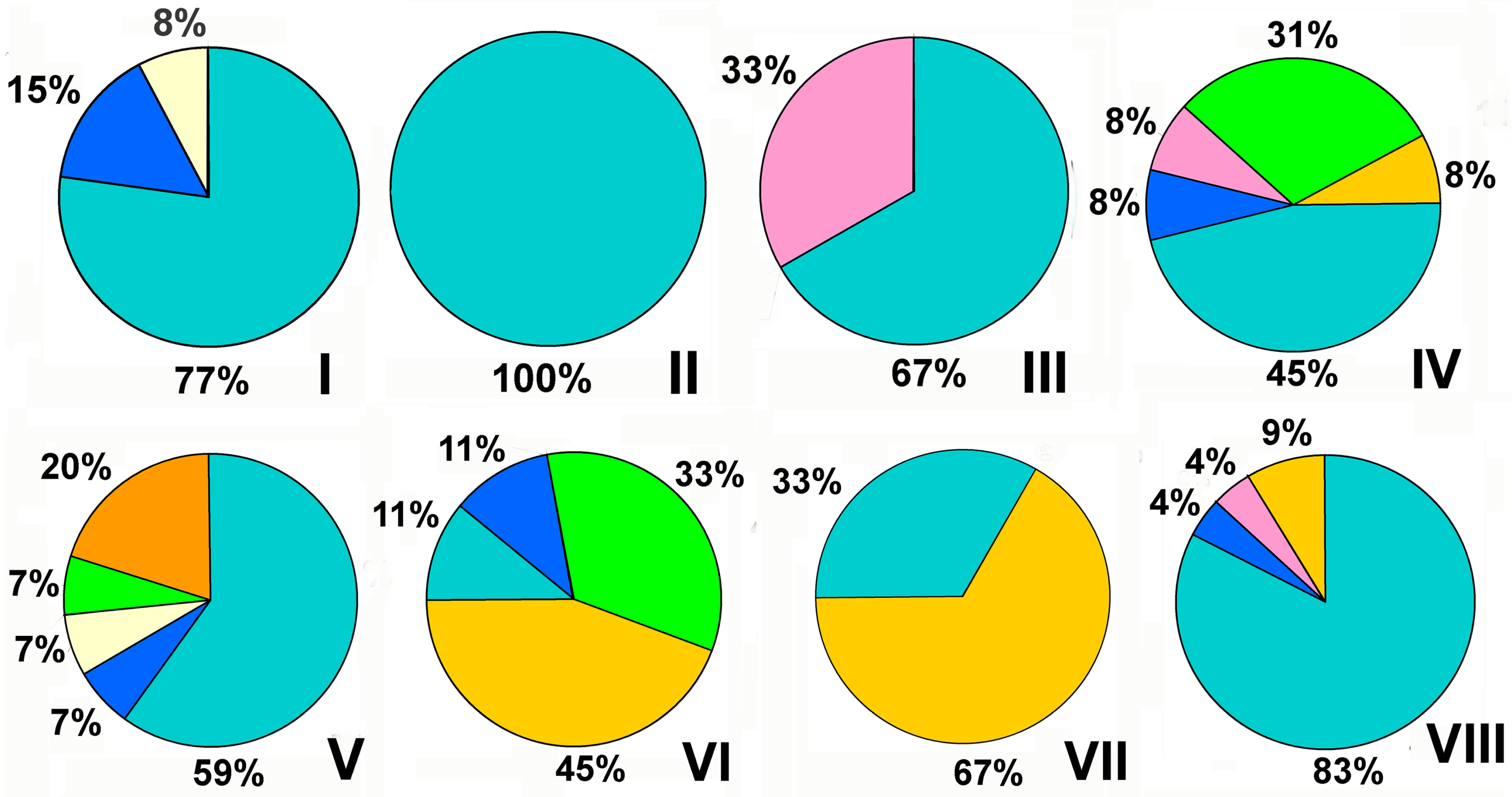
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Efremenko, E.; Stepanov, N.; Senko, O.; Maslova, O.; Lyagin, I.; Domnin, M.; Aslanli, A. “Stop, Little Pot” as the Motto of Suppressive Management of Various Microbial Consortia. Microorganisms 2024, 12, 1650. https://doi.org/10.3390/microorganisms12081650
Efremenko E, Stepanov N, Senko O, Maslova O, Lyagin I, Domnin M, Aslanli A. “Stop, Little Pot” as the Motto of Suppressive Management of Various Microbial Consortia. Microorganisms. 2024; 12(8):1650. https://doi.org/10.3390/microorganisms12081650
Chicago/Turabian StyleEfremenko, Elena, Nikolay Stepanov, Olga Senko, Olga Maslova, Ilya Lyagin, Maksim Domnin, and Aysel Aslanli. 2024. "“Stop, Little Pot” as the Motto of Suppressive Management of Various Microbial Consortia" Microorganisms 12, no. 8: 1650. https://doi.org/10.3390/microorganisms12081650





