Laundry Isolate Delftia sp. UBM14 Capable of Biodegrading Industrially Relevant Aminophosphonates
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Enrichment Procedure and the Purification of Phosphonate Degrading Isolates
2.3. Nucleic Acid Extraction and Sequencing of the 16S rRNA Gene
2.4. Antimicrobial Susceptibility Testing
2.5. Physiological and Biochemical Characteristics
2.6. Amplification of PhnJ and PhnX Genes
2.7. Biodegradation Batch Test with Delftia sp. UMB14
2.8. Biodegradation Batch Test with Different Phosphorus Concentrations
2.9. Adaptation Test with ATMP or EDTMP Preconditioned Bacteria
2.10. Analytical Methods
3. Results
3.1. Identification and Characterization
3.2. Substrate Utilization Pattern
3.3. Sequencing and Phylogenetic Analysis
3.4. Antimicrobial Susceptibility Testing
3.5. Detection of phnJ and phnX Genes
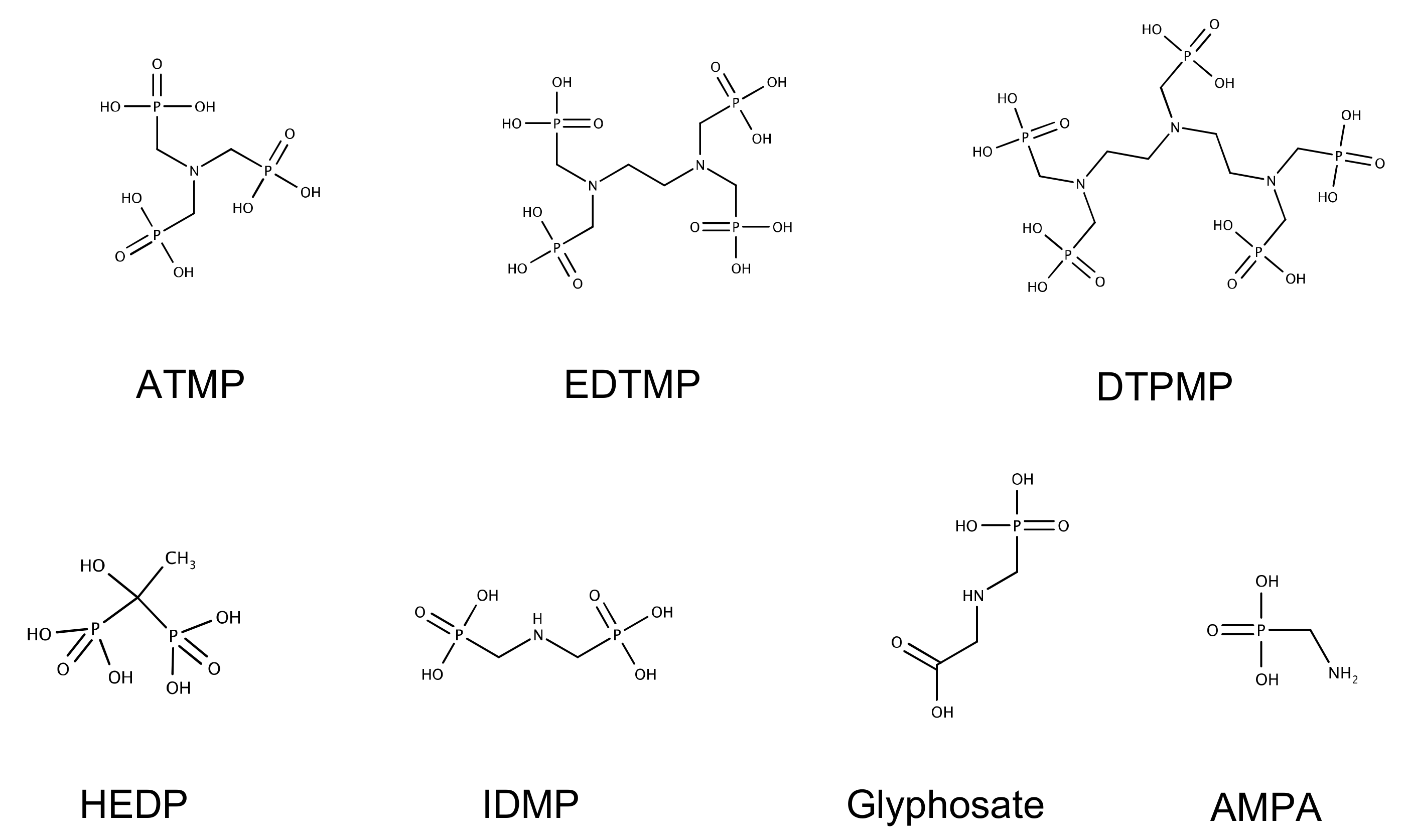
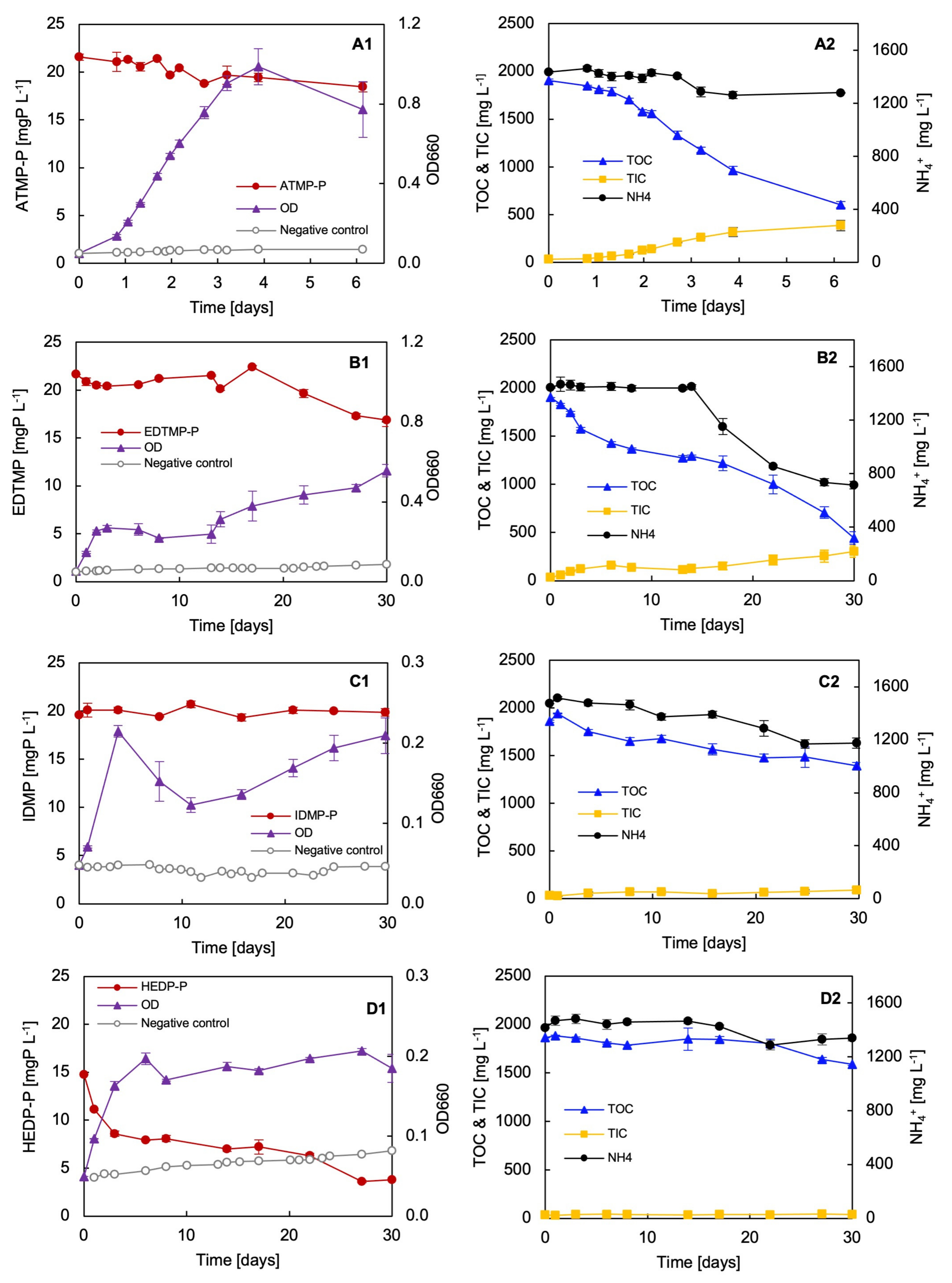
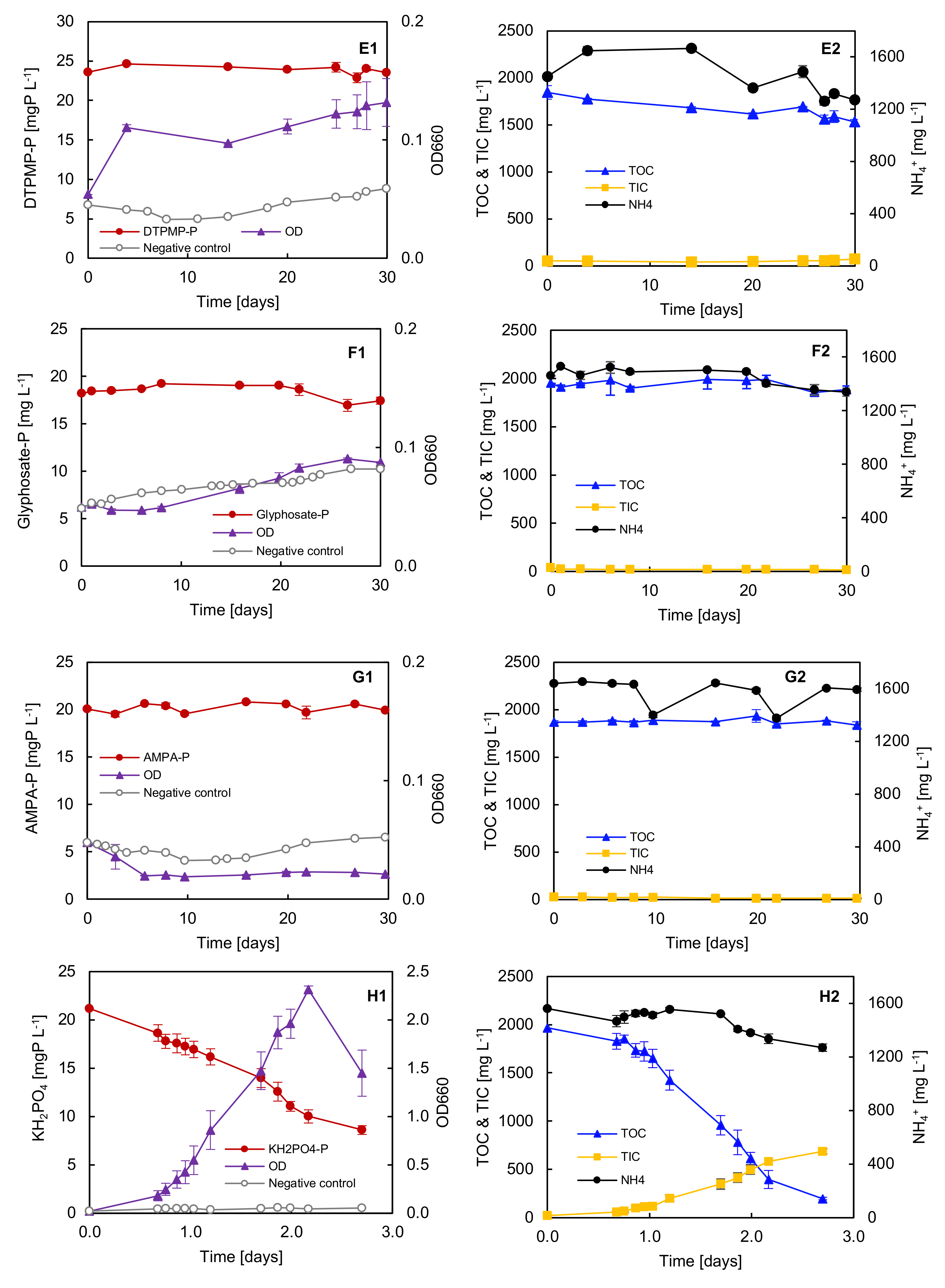
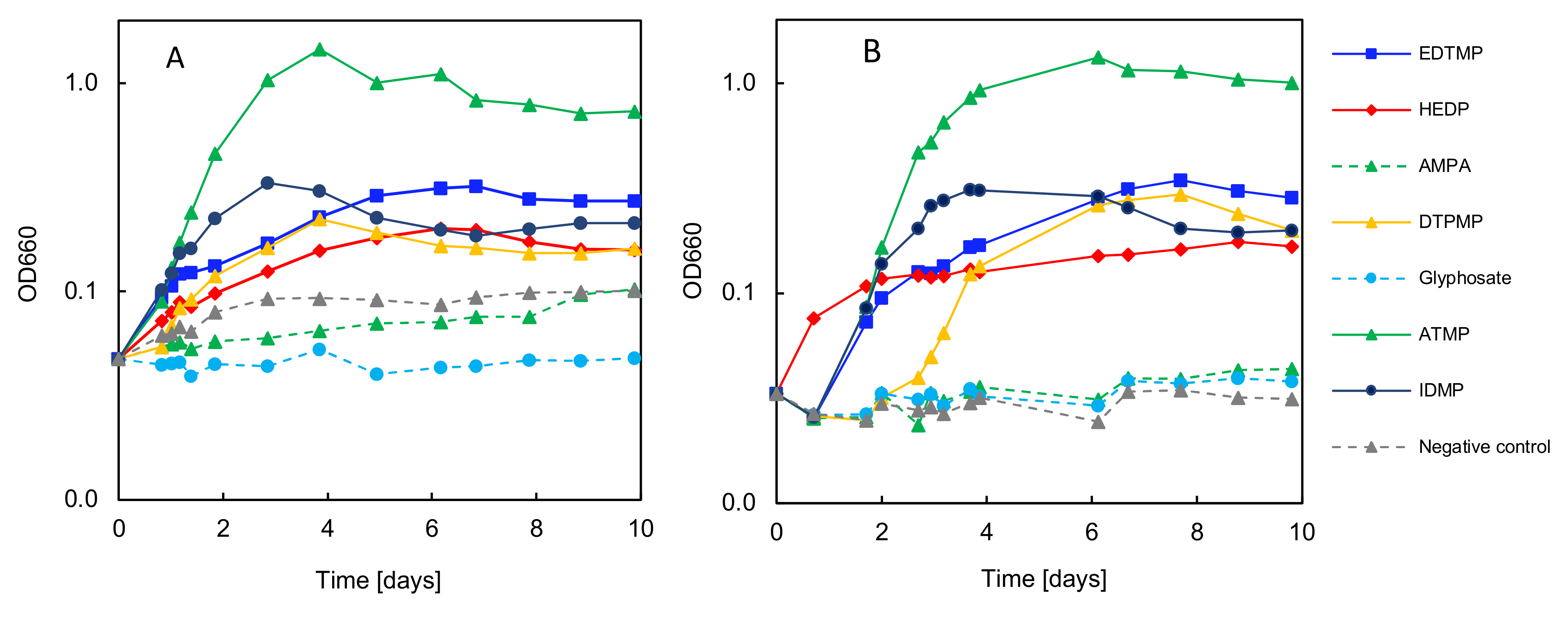
3.6. Biodegradation Test with Selected Synthetic Phosphonates
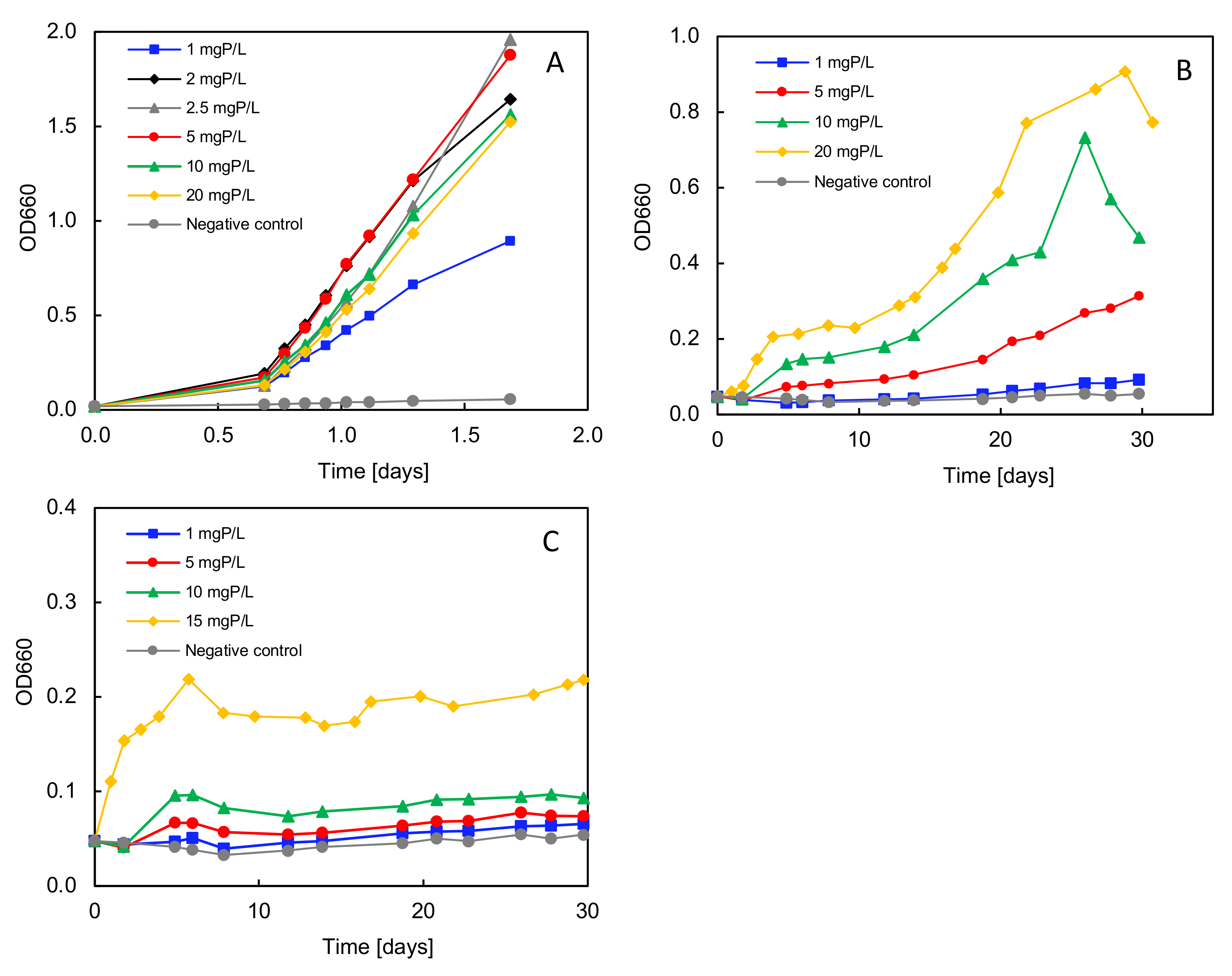
3.7. Phosphorus Requirements for Growth with Different P Sole Sources
3.8. Adaptation Test with ATMP and EDTMP
4. Discussion
4.1. Biodegradation of Phosphonates by Delftia sp. UMB14
4.2. Potential Enzymatic Pathway Responsible for the Biodegrading of Phosphonates
4.3. Factors Influencing the Degradation Efficiency during Phosphonate Degradation
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mazuryk, J.; Klepacka, K.; Kutner, W.; Sharma, P.S. Glyphosate: Impact on the microbiota-gut-brain axis and the immune-nervous system, and clinical cases of multiorgan toxicity. Ecotoxicol. Environ. Saf. 2024, 271, 115965. [Google Scholar] [CrossRef]
- López, A.; Ruiz, P.; Fuentes, E.; Yusà, V.; Dualde, P.; Miralles, P.; Coscollà, C. Simultaneous direct determination of Glyphosate and AMPA in the ambient air and inhalation risk assessment in a Mediterranean Region (Spain). Atmos. Environ. 2024, 317, 120204. [Google Scholar] [CrossRef]
- Costas-Ferreira, C.; Durán, R.; Faro, L.R.F. Toxic Effects of Glyphosate on the Nervous System: A Systematic Review. Int. J. Mol. Sci. 2022, 23, 4605. [Google Scholar] [CrossRef] [PubMed]
- Nowack, B. Environmental chemistry of phosphonates. Water Res. 2003, 37, 2533–2546. [Google Scholar] [CrossRef] [PubMed]
- Yawen, H.; Yu, Y.; Jiajun, M.; Junxiao, Y. Preparation of ferric phosphonate/phosphonate and their special action on flame retardancy of epoxy resin. J. Appl. Polym. Sci. 2017, 135, 46206–46216. [Google Scholar] [CrossRef]
- Khormali, A.; Sharifov, A.R.; Torba, D.I. Increasing efficiency of calcium sulphate prevention using a new mixture of phosphonate scale inhibitors during waterflooding. J. Petrol. Sci. Eng. 2018, 164, 245–258. [Google Scholar] [CrossRef]
- Kong, G.; Kashyap, R.; Hicks, R.J. Adverse effects of radionuclide therapy. In Radiotheranostics—A Primer for Medical Physicists IN: Physics, Chemistry, Biology and Clinical Application, 1st ed.; Borrás, C., Stabin, M.G., Eds.; CRC Press: Boca Raton, FL, USA, 2024; Volume 1. [Google Scholar]
- Lange, R.; Heine, R.; Knapp, R.; de Klerk, J.M.H.; Bloemendal, H.J.; Hendrikse, H. Pharmaceuticial and clinical development of phosphonate-based radiopharmaceuticals for the targeted treatment of bone metastases. Bone 2016, 91, 159–179. [Google Scholar] [CrossRef] [PubMed]
- Taheri, M.; Azizmohammadi, Z.; Ansari, M.; Dadkhah, P.; Dehghan, K.; Valizadeh, R.; Assadi, M. 153Sm-EDTMP and 177Lu-EDTMP are equally safe and effective in pain palliation from skeletal metastases. Nuklearmedizin 2018, 57, 174–180. [Google Scholar] [CrossRef] [PubMed]
- Armbruster, D.; Rott, E.; Minke, R.; Happel, O. Trace-level determination of phosphonates in liquid and solid phase of wastewater and environmental samples by IC-ESI MS/MS. Anal. Bioanal. Chem. 2020, 412, 4807–4825. [Google Scholar] [CrossRef]
- Rott, E.; Happel, O.; Armbruster, D.; Minke, R. Influence of wastewater discharge on the occurrence of PBTC, HEDP, and aminophosphonates in sediment, suspended matter, and the aqueous phase of rivers. Water 2020, 12, 803. [Google Scholar] [CrossRef]
- Rott, E.; Happel, O.; Armbruster, D.; Minke, R. Behavior of PBTC, HEDP, and aminophosphonates in the process of wastewater treatment. Water 2020, 12, 53. [Google Scholar] [CrossRef]
- Wang, S.; Zhang, B.; Shan, C.; Yan, X.; Chen, H.; Pan, B. Occurrence and transformation of phosphonates in textile dyeing wastewater along full-scale combined treatment process. Water Res. 2020, 184, 116173. [Google Scholar] [CrossRef] [PubMed]
- Schowanek, D.; Verstraete, W. Phosphonate utilization by bacterial cultures and enrichments from environmental samples. Appl. Environ. Microbiol. 1990, 56, 895–903. [Google Scholar] [CrossRef] [PubMed]
- Rott, E.; Steinmetz, H.; Metzger, J.W. Organophosphonates: A review on environmental relevance, biodegradability and removal in wastewater treatment plants. Sci. Total Environ. 2018, 615, 1176–1191. [Google Scholar] [CrossRef] [PubMed]
- Riedel, R.; Krahl, K.; Buder, K.; Böllmann, J.; Braun, B.; Martienssen, M. Novel standard biodegradation test for synthetic phosphonates. J. Microbiol. Method. 2023, 212, 106793. [Google Scholar] [CrossRef] [PubMed]
- Riedel, R.; Commichau, F.M.; Benndorf, D.; Hertel, R.; Holzer, K.; Hoelzle, L.E.; Mardoukhi, M.S.Y.; Noack, L.E.; Martienssen, M. Biodegradation of selected aminophosphonates by the novel bacterial isolate Ochrobactrum sp. BTU1. Microbiol. Res. 2024, 280, 127800. [Google Scholar] [CrossRef]
- Chen, C.M.; Ye, Q.Z.; Zhu, Z.M.; Wanner, B.L.; Walsh, C.T. Molecular biology of carbon-phosphorus bond cleavage. Cloning and sequencing of the phn (psiD) genes involved in alkylphosphonate uptake and C-P lyase activity in Escherichia coli K-12. J. Biol. Chem. 1990, 265, 4461–4471. [Google Scholar] [CrossRef] [PubMed]
- Hove-Jensen, B.; Zechel, D.; Jochimsen, B. Utilization of glyphosate as phosphate source: Biochemistry and genetics of bacterial carbon-phosphorus lyase. Microbiol. Mol. Biol. Rev. 2014, 78, 176–197. [Google Scholar] [CrossRef]
- Hertel, R.; Gibhardt, J.; Martienssen, M.; Kuhn, R.; Commichau, F.M. Molecular mechanisms underlying glyphosate resistance in bacteria. Environ. Microbiol. 2012, 23, 2891–2905. [Google Scholar] [CrossRef]
- Kamat, S.S.; Wiliams, H.J.; Rauschel, F.M. Intermediates in the transformation of phosphonates to phosphate by bacteria. Nature 2011, 480, 570–573. [Google Scholar] [CrossRef]
- Lockwood, S.; Greening, C.; Baltar, F.; Morales, S.E. Global and seasonal variation of marine phosphonate metabolism. ISME 2022, 16, 2198–2212. [Google Scholar] [CrossRef]
- Ternan, N.G.; Quinn, J.P. In vitro cleavage of the carbon-phosphorus bons of phosphonopyruvate by cell extracts of an environmental Burkholdria cepacian isolate. Biochem. Biophys. Res. Commun. 1998, 248, 378–381. [Google Scholar] [CrossRef] [PubMed]
- McMullan, G.; Harrington, F.; Quinn, J.P. Metabolism of phosphonoacetate as the sole carbon and phosphorus source by an environmental bacterial isolate. Appl. Environ. Microbiol. 1992, 58, 1364–1366. [Google Scholar] [CrossRef] [PubMed]
- LaNauze, J.M.; Coggins, J.R.; Dixon, H.B.F. Aldolase-like imine formation in mechanism of action of phosphonoacetaldehyde hydrolase. Biochem. J. 1977, 165, 409–411. [Google Scholar] [CrossRef] [PubMed]
- Dumora, C.; Lacoste, A.M.; Cassaigne, A. Phosphonoacetaldehyde hydrolase from Pseudomonas aeruginosa—Purification, properties and comparison with Bacillus cereus enzyme. Biochem. Biophys. Acta 1989, 997, 193–198. [Google Scholar] [CrossRef] [PubMed]
- Martinez, A.; Tyson, G.W.; DeLong, E.F. Widespread known and novel phosphonate utilization pathways in marine bacteria revealed by functional screening and metagenomic analyses. Environ. Microbiol. 2010, 12, 222–238. [Google Scholar] [CrossRef] [PubMed]
- McScorley, A.; Wyatt, P.B.; Martinez, A.; DeLong, E.F.; Hove-Jeansen, B.; Zechel, D.L. PhnY and PhnZ comprise a new oxidative pathway for enzymatic cleavage of a carbon-phosphorus bond. J. Am. Chem. Soc. 2012, 134, 8364–8367. [Google Scholar] [CrossRef] [PubMed]
- Epiktetov, D.O.; Sviridov, A.V.; Tarlachkov, S.V.; Shushkova, T.V.; Leontievsky, A.A. Phosphonatase operons of organophosphonates-degrading soil bacteria of the genus Achromobacter. Microbiology 2023, 92, 45–49. [Google Scholar] [CrossRef]
- Zangelmi, E.; Ruffolo, F.; Dinhof, T.; Secchi, A.; Pallitsch, K.; Peracchi, A. Deciphering the role of recurrent FAD-dependent enzyme in bacterial phosphonate catabolism. Iscience 2023, 26, 108108. [Google Scholar] [CrossRef]
- Quinn, J.P.; Kulakova, A.N.; Cooley, N.A.; McGrath, J.W. New ways to break an old bond: The bacterial carbon-phosphorus hydrolases and their role in biogeochemical phosphorus cycling. Environ. Microbiol. 2007, 9, 2392–2400. [Google Scholar] [CrossRef]
- Zangelmi, E.; Stankovic, T.; Malatesta, M.; Acquotti, D.; Pallitsch, K.; Peracchi, A. Discovery of a new, recent enzyme in bacterial phosphonate degradation: (R)-1-hydroxy-2-aminoethylphosphonate ammonia-lyase. Biochemistry 2021, 60, 1214–1225. [Google Scholar] [CrossRef]
- Kim, A.D.; Baker, S.; Dunaway-Mariano, D.; Metcalf, W.W.; Wanner, B.L.; Martin, B.M. The 2-aminoethylphosphonate-specific transaminase of the 2-aminoethylphosphonate degradation pathway. J. Bacteriol. 2002, 184, 4134–4140. [Google Scholar] [CrossRef] [PubMed]
- Zangelmi, E. Identification and Characterization of New Enzymes Involved in Bacterial Phosphonate Catabolism. Ph.D. Thesis, University of Parma, Parma, Italy, 2021. [Google Scholar]
- Epiktetov, D.O.; Sviridov, A.V.; Tarlachkov, S.V.; Shushkova, T.V.; Toropygin, I.Y.; Leontievsky, A.A. Glyphosate-induced phosphonatase operons in soil bacteria of the genus Achromobacter. Int. J. Mol. Sci. 2024, 25, 6409. [Google Scholar] [CrossRef] [PubMed]
- Pfennig, N.; Trüper, H.G. The Prokaryotes, A Handbook on the Biology of Bacteria, Vol. 5 Proteobacteria: Alpha and Beta Subclasses, 3rd ed.Starr, M.P., Stolp, H., Trüper, H.G., Balows, A., Schegel, H.G., Eds.; Springer: Berlin, Germany, 1981; Volume 5, pp. 279–289. [Google Scholar] [CrossRef]
- Marchesi, J.R.; Sato, T.; Weightman, A.J.; Martin, T.A.; Fry, J.C.; Hiom, S.J.; Wade, W.G. Design and evaluation of useful bacterium-specific PCR primers that amplify genes coding for bacterial 16S rRNA. Appl. Environ. Microbiol. 1998, 64, 795–799. [Google Scholar] [CrossRef]
- Engelen, B.; Meinken, K.; von Wintzingerode, F.; Heuer, H.; Malkomes, H.P.; Backhaus, H. Monitoring impact of a pesticide treatment on bacterial soil communities by metabolic and genetic fingerprinting in addition to conventional testing procedures. Appl. Environ. Microbiol. 1998, 64, 2814–2821. [Google Scholar] [CrossRef] [PubMed]
- Morales, M.E.; Allegrini, M.; Basualdo, J.; Villamil, M.B.; Zabaloy, M.C. Primer design to assess bacterial degradation of glyphosate and other phosphonates. J. Microbiol. Methods. 2020, 169, 105814. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, Y.J.; Wanner, B.L. Global regulation by the seven-component Pi signaling system. Curr. Opin. Microbiol. 2010, 13, 198–203. [Google Scholar] [CrossRef] [PubMed]
- Bergkemper, F.; Kublik, S.; Lang, F.; Krüger, J.; Vestergaard, G.; Schloter, M.; Schulz, S. Novel oligonucleotide primers reveal a high diversity of microbes which drive phosphorous turnover in soil. J. Microbiol. Methods 2016, 125, 91–97. [Google Scholar] [CrossRef]
- DIN 38406-5:1983-10; German Standard Methods for the Examination of Water, Waste Water and Sludge; Cations (Group E); Determination of Ammonia-Nitrogen (E 5). DIN Media: Berlin, Germany, 1983. [CrossRef]
- DIN EN ISO 6878:2004-09; Water Quality-Determination of Phosphorus—Ammonium Molybdate Spectrometric Method (ISO 6878:2004); German Version EN ISO 6878:2004. DIN Media: Berlin, Germany, 2004. [CrossRef]
- DIN EN 12260:2003-12; Water Quality—Determination of Nitrogen—Determination of Bound Nitrogen (TN), Following Oxidation to Nitrogen Oxides. German Institute for Standardisation (Deutsches Institut für Normung): Berlin, Germany, 2003.
- Shin, S.Y.; Choi, J.Y.; Ko, K.S. Four cases of possible human infections with Delftia lacustris. Infection 2012, 40, 709–712. [Google Scholar] [CrossRef]
- Sohn, K.M.; Baek, J.Y.; Cheon, S.; Kim, Y.S.; Koo, S.H. Ocular infection associated with Delftia lacustris: First report. Braz. J. Infect. Dis. 2015, 19, 449–450. [Google Scholar] [CrossRef]
- Cheng, C.; Zhou, W.; Dong, X.; Zhang, P.; Zhou, K.; Zhou, D.; Qian, C.; Lin, X.; Li, P.; Li, K.; et al. Genomic analysis of Delftia tsuruhatensis strain TR1180 isolated from a patient from China with in4-like integron-associated antimicrobial resistance. Front. Cell Infect. Microbiol. 2021, 11, 663933. [Google Scholar] [CrossRef] [PubMed]
- Preiswerk, B.; Ullrich, S.; Speich, R.; Bloemberg, G.V.; Hombach, M. Human Infection with Delftia tsuruhatensis isolated from a central venous catheter. J. Med. Microbiol. 2011, 60, 246–248. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Zhang, X.; Bai, H.; Li, T.; Zhang, Z.; Zong, X.; Shang, X.; Lui, Z.; Fan, L. Characterization and genome analysis of the Delftia lacustris strain LzhVag01 isolated from vaginal discharge. Curr. Microbiol. 2024, 81, 232. [Google Scholar] [CrossRef] [PubMed]
- Jørgensen, N.O.; Brandt, K.K.; Nybroe, O.; Hansen, M. Delftia lacustris sp. nov., a peptidoglycan-degrading bacterium from fresh water, and emended description of Delftia tsuruhatensis as a peptidoglycan-degrading bacterium. Int. J. Syst. Evol. Microbiol. 2009, 59, 2195–2199. [Google Scholar] [CrossRef] [PubMed]
- Wen, A.; Fegan, M.; Hayward, C.; Chakraborty, S.; Sly, L.I. Phylogenetic Relationships Among Members of the Comamonadaceae, and Description of Delftia Acidovorans (Den Dooren De Jong 1926 and Tamaoka Et Al. 1987) Gen. Nov., Comb. Nov. Int. J. Syst. Bacteriol. 1999, 49, 567–576. [Google Scholar] [CrossRef] [PubMed]
- Shigematsu, T.; Yumihara, K.; Ueda, Y.; Numaguchi, M.; Morimura, S.; Kida, K. Delftia tsuruhatensis sp. nov., a terephthalate-assimilating bacterium isolated from activated sludge. Inter. J. Syst. Evol. Microbiol. 2003, 53, 1479–1483. [Google Scholar] [CrossRef] [PubMed]
- Ren, J.; Wang, Z.; Deng, L.; Niu, D.; Fan, B.; Huhe, T.; Li, Z.; Zhang, J.; Li, C. Biodegradation of erythromycin by Delftia lacustris RJJ-61 and characterization of its erythromycin esterase. J. Basic. Microbiol. 2021, 61, 55–62. [Google Scholar] [CrossRef] [PubMed]
- Cai, X.; Li, J.; Guan, F.; Luo, F.; Yu, Z.; Yuan, Y. Complete pentachlorophenol biodegradation in a dual-working electrode bioelectrochemical system: Performance and functional microorganism identification. Water Res. 2023, 230, 119529. [Google Scholar] [CrossRef] [PubMed]
- Cattò, C.; Sanmartín, P.; Gulotta, D.; Troiano, F.; Cappitelli, F. Bioremoval of graffiti using novel commercial strains of bacteria. Sci. Total Environ. 2021, 756, 144075. [Google Scholar] [CrossRef]
- Ahmed, M.M.A.; Boudreau, P.D. LCMS-Metabolomic profiling and genome mining of Delftia lacustris DSM 21246 revealing lipophilic delftibactin metallophores. J. Nat. Prod. 2024, 87, 1384–1393. [Google Scholar] [CrossRef]
- Wadgaonkar, S.L.; Nancharaiah, Y.V.; Jacob, C.; Esposito, G.; Lens, P.N.L. Microbial transformation of Se oxyanions in cultures of Delftia lacustris grown under aerobic conditions. J. Microbiol. 2019, 57, 362–371. [Google Scholar] [CrossRef] [PubMed]
- US Food and Drug Administration. Delftia Lacustris Genome Assembly ASM1602781v1 [WWW Document]. NCBI. 2020. Available online: https://www.ncbi.nlm.nih.gov/data-hub/assembly/GCF_016027815.1/ (accessed on 7 July 2024).
- Jiang, W.; Metcalf, W.W.; Lee, K.S.; Wanner, B.L. Molecular cloning, mapping, and regulation of Pho regulon genes for phosphonate breakdown by the phosphonatase pathway of Salmonella typhimurium LT2. J. Bacteriol. 1995, 177, 6411–6421. [Google Scholar] [CrossRef] [PubMed]
- Villarreal-Chiu, J.F. The genes and enzymes of phosphonate metabolism by bacteria, and their distribution in the marine environment. Front. Microbiol. 2012, 3, 19. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.S.; Metcalf, W.W.; Wanner, B.L. Evidence for two phosphonate degradative pathways in Enterobacter aerogenes. J. Bacteriol. 1992, 174, 2501–2510. [Google Scholar] [CrossRef]
- Rizk, S.S.; Cuneo, M.J.; Hellinga, H.W. Identification of cognate ligands for the Escherichia coli phnD protein product and engineering of a reagentless fluorescent biosensor for phosphonates. Protein Sci. 2006, 15, 1745–1751. [Google Scholar] [CrossRef]
| (A) | |||||
|---|---|---|---|---|---|
| Parameter | Unit | KH2PO4 | ATMP | EDTMP | IDMP |
| Growth | µ [days−1] | 3.22 ± 0.2 | 1.30 ± 0.1 | 0.80 ± 0.0 | 0.44 ± 0.0 |
| td [h] | 5.2 ± 0.4 | 12.8 ± 0.7 | 20.8 ± 0.3 | 38.1 ± 0.6 | |
| P/S [-] | - | 0.41 ± 0.0 | 0.25 ± 0.0 | 0.14 ± 0.0 | |
| Consumption | C [mgCorg L−1] | 1770.1 ± 13.8 | 1301.5 ± 47.8 | 1459.9 ± 63.4 | 469.1 ± 49.4 |
| N [mgN L−1] | 227.0 ± 21.7 | 122.6 ± 9.6 | 567.8 ± 22.0 | 232.3 ± 31.3 | |
| P [mgP L−1] | 12.5 ± 2.1 | 3.9 ± 0.2 | 2.9 ± 0.6 | 1.7 ± 0.2 | |
| Efficiency | C [%] | 96.6 ± 3.8 | 72.7 ± 2.8 | 76.8 ± 3.4 | 26.8 ± 2.9 |
| N [%] | 18.8 ± 2.1 | 11.0 ± 0.8 | 50.2 ± 0.2 | 18.5 ± 2.6 | |
| P [%] | 56.5 ± 9.4 | 18.2 ± 0.8 | 13.6 ± 2.8 | 7.8 ± 0.9 | |
| (B) | |||||
| Parameter | Unit | HEDP | DTPMP | GS | AMPA |
| Growth | µ [days−1] | 0.50 ± 0.0 | 0.18 ± 0.0 | 0.06 ± 0.0 | - |
| td [h] | 33.4 ± 1.2 | 90.6 ± 2.6 | 301.5 ± 35.5 | - | |
| P/S [-] | 0.16 ± 0.0 | 0.06 ± 0.0 | 0.02 ± 0.0 | - | |
| Consumption | C [mgCorg L−1] | 276.5 ± 8.9 | 310.3 ± 76.3 | 130.9 ± 18.9 | 32.3 ± 26.3 |
| N [mgN L−1] | 58.8 ± 17.0 | 156.1 ± 31.0 | 95.1 ± 22.6 | 36.0 ± 9.0 | |
| P [mgP L−1] | 2.4 ± 0.5 | 0.7 ± 0.1 | 0.1 ± 0.3 | 0.2 ± 0.1 | |
| Efficiency | C [%] | 15.1 ± 0.5 | 17.8 ± 4.4 | 6.8 ± 1.0 | 1.7 ± 1.4 |
| N [%] | 5.8 ± 1.0 | 13.8 ± 2.5 | 7.2 ± 2.0 | 2.1 ± 1.0 | |
| P [%] | 16.1 ± 3.3 | 2.9 ± 0.3 | 5.0 ± 2.0 | 1.2 ± 0.7 | |
| P Source | Conc. [mg L−1] | µmax [days−1] | td [h] |
|---|---|---|---|
| KH2PO4 | 1 | 4.82 ± 0.4 | 3.5 ± 0.3 |
| 2 | 5.15 ± 0.7 | 3.3 ± 0.4 | |
| 2.5 | 5.60 ± 1.0 | 3.0 ± 0.5 | |
| 5 | 5.50 ± 1.0 | 3.1 ± 0.5 | |
| 10 | 4.75 ± 0.1 | 3.5 ± 0.1 | |
| 20 | 5.29 ± 0.6 | 3.2 ± 0.3 | |
| ATMP | 1 | 0.07 ± 0.01 | 243.5 ± 31.5 |
| 5 | 0.22 ± 0.01 | 75.9 ± 4.1 | |
| 10 | 0.38 ± 0.00 | 44.1 ± 0.4 | |
| 20 | 0.68 ± 0.05 | 24.7 ± 2.0 | |
| HEDP | 1 | 0.08 ± 0.01 | 222.6 ± 16.4 |
| 5 | 0.16 ± 0.01 | 101.6 ± 1.9 | |
| 10 | 0.27 ± 0.00 | 62.7 ± 0.8 | |
| 15 | 0.47 ± 0.10 | 35.1 ± 1.3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Riedel, R.; Meißner, K.; Kaschubowski, A.; Benndorf, D.; Martienssen, M.; Braun, B. Laundry Isolate Delftia sp. UBM14 Capable of Biodegrading Industrially Relevant Aminophosphonates. Microorganisms 2024, 12, 1664. https://doi.org/10.3390/microorganisms12081664
Riedel R, Meißner K, Kaschubowski A, Benndorf D, Martienssen M, Braun B. Laundry Isolate Delftia sp. UBM14 Capable of Biodegrading Industrially Relevant Aminophosphonates. Microorganisms. 2024; 12(8):1664. https://doi.org/10.3390/microorganisms12081664
Chicago/Turabian StyleRiedel, Ramona, Karsten Meißner, Arne Kaschubowski, Dirk Benndorf, Marion Martienssen, and Burga Braun. 2024. "Laundry Isolate Delftia sp. UBM14 Capable of Biodegrading Industrially Relevant Aminophosphonates" Microorganisms 12, no. 8: 1664. https://doi.org/10.3390/microorganisms12081664
APA StyleRiedel, R., Meißner, K., Kaschubowski, A., Benndorf, D., Martienssen, M., & Braun, B. (2024). Laundry Isolate Delftia sp. UBM14 Capable of Biodegrading Industrially Relevant Aminophosphonates. Microorganisms, 12(8), 1664. https://doi.org/10.3390/microorganisms12081664



.png)




