Enzymatic Stoichiometry Reveals the Metabolic Limitations of Soil Microbes under Nitrogen and Phosphorus Addition in Chinese Fir Plantations
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Sites and Experimental Design
2.2. Soil Sampling and Preparation
2.3. Analysis of the Soil’s Physicochemical Properties
2.4. Measurements of Phospholipid Fatty Acids (PLFAs) in the Soil
2.5. Soil Enzymatic Activity Analyses
2.6. Vector Analysis of Resource Limitations of Soil Microbes
2.7. Statistical Analysis
3. Results
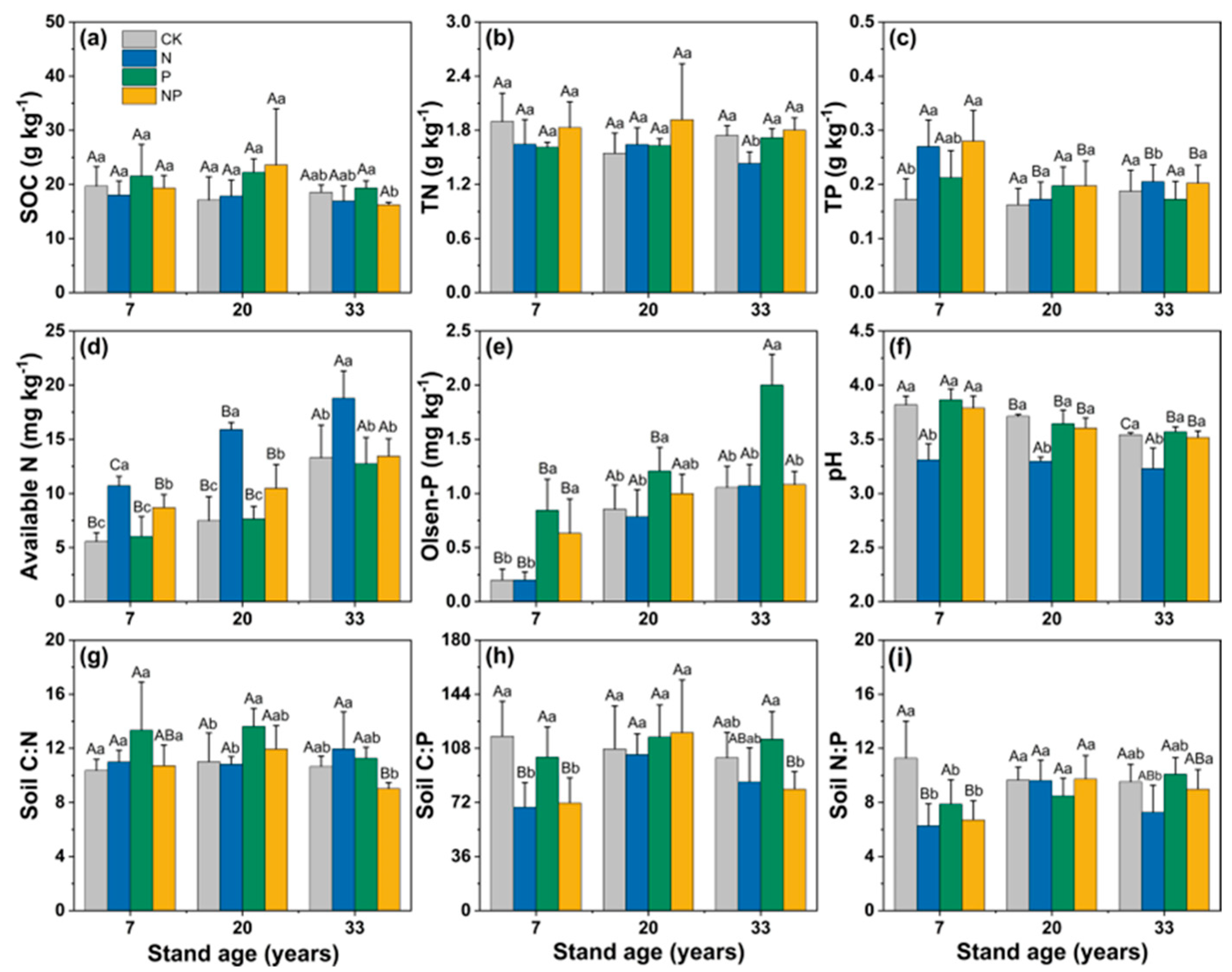
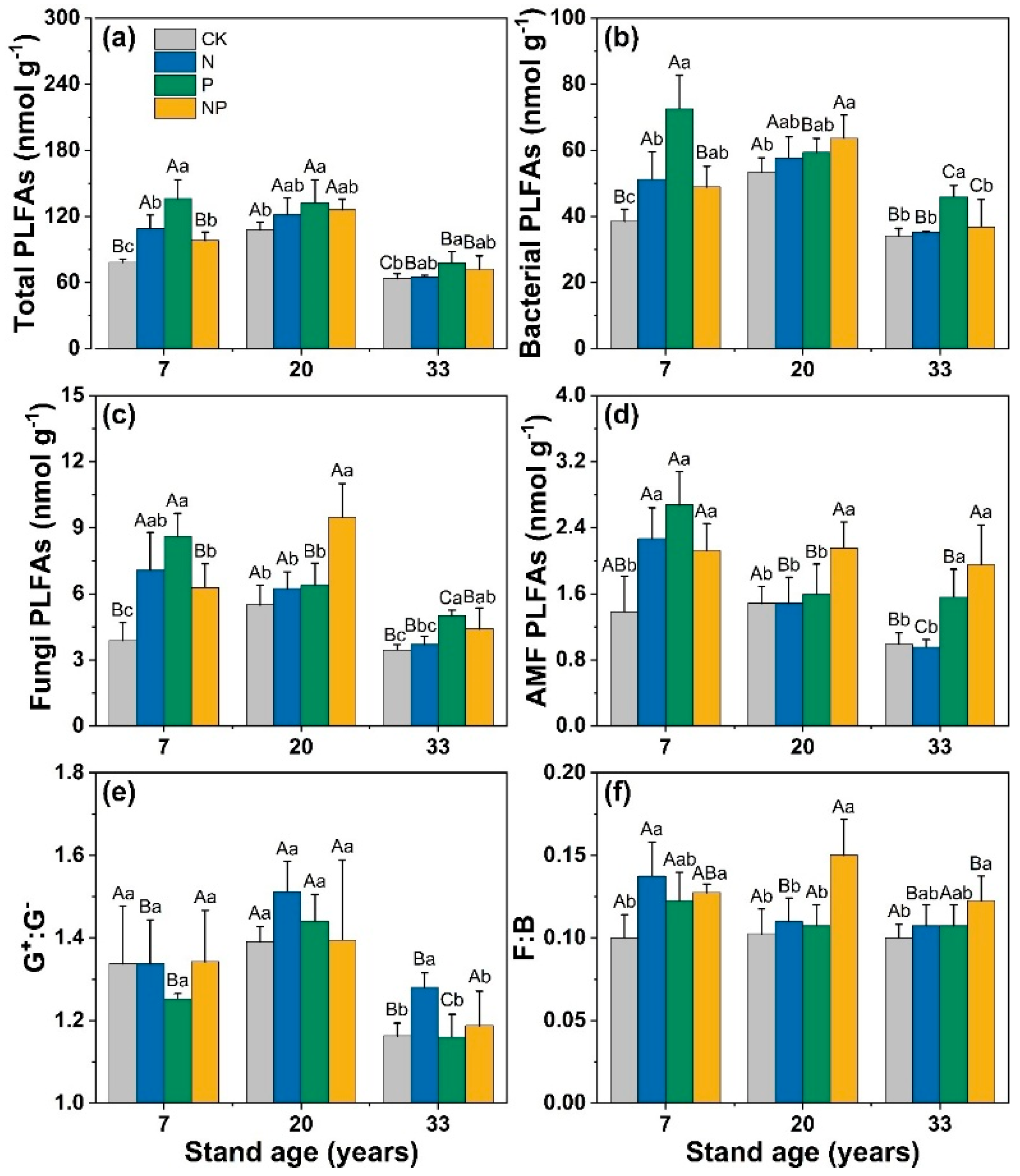
3.1. Changes in Soil Properties and Microbial Structure
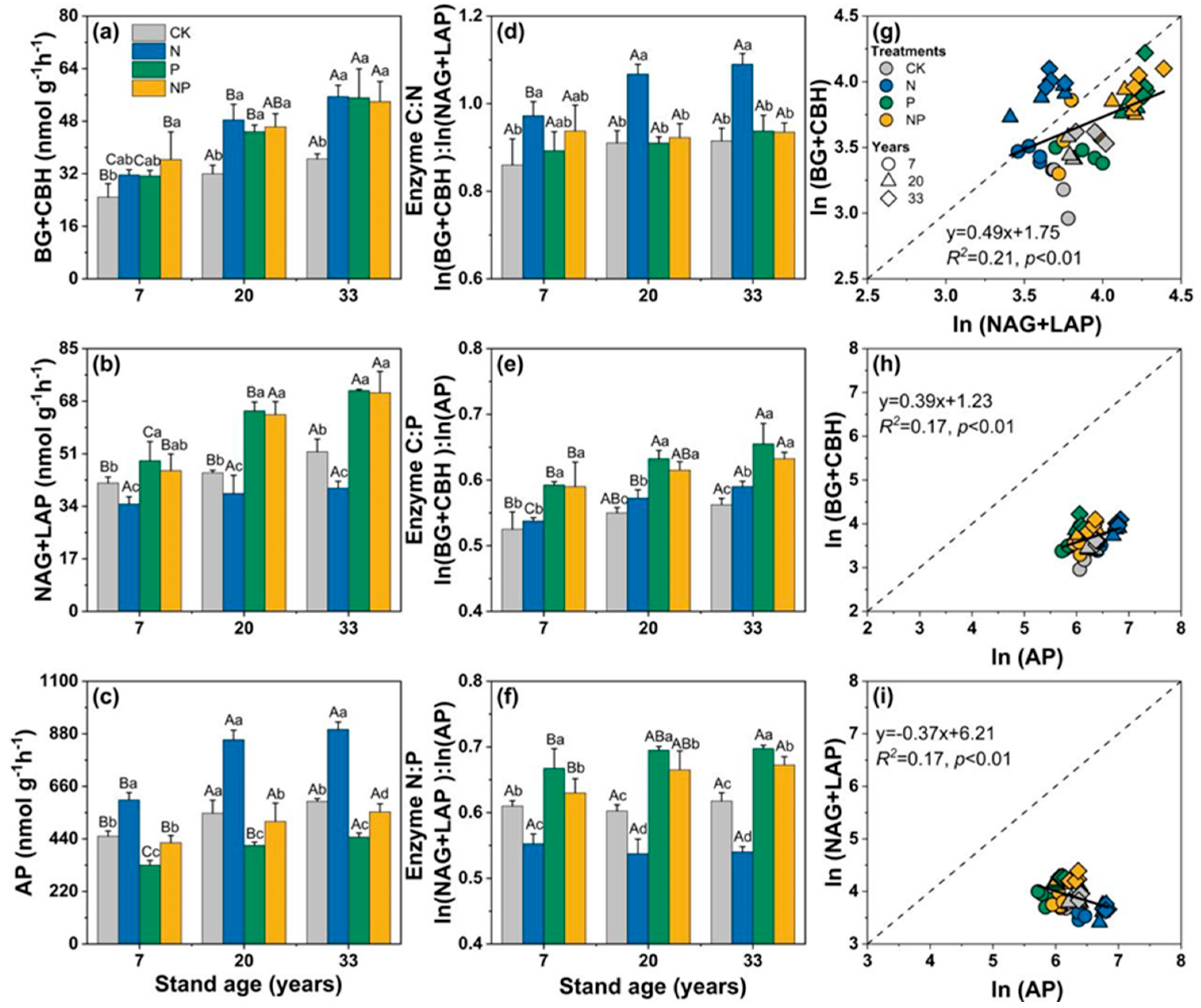
3.2. Changes in the Activity of Soil Extracellular Enzymes
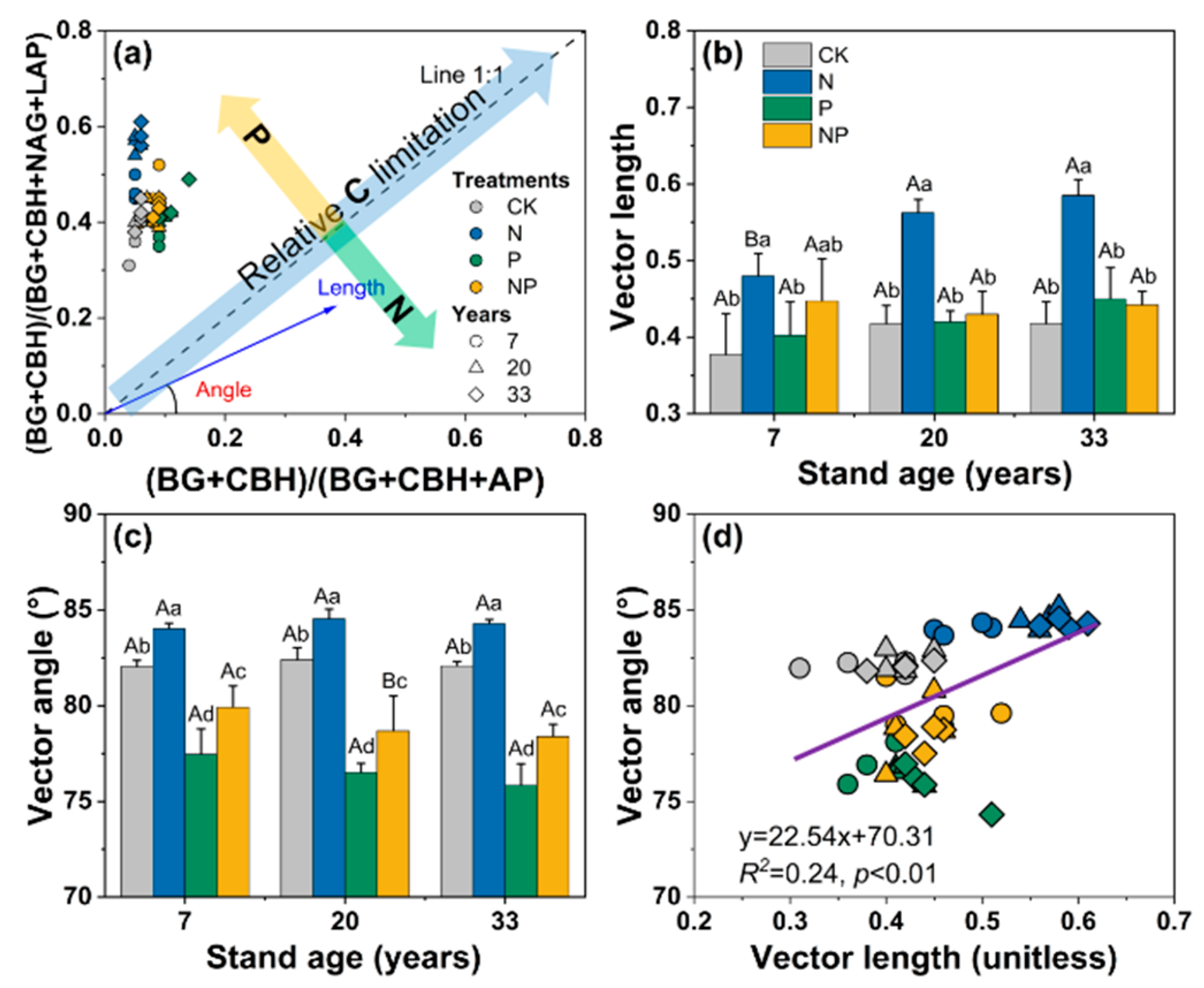
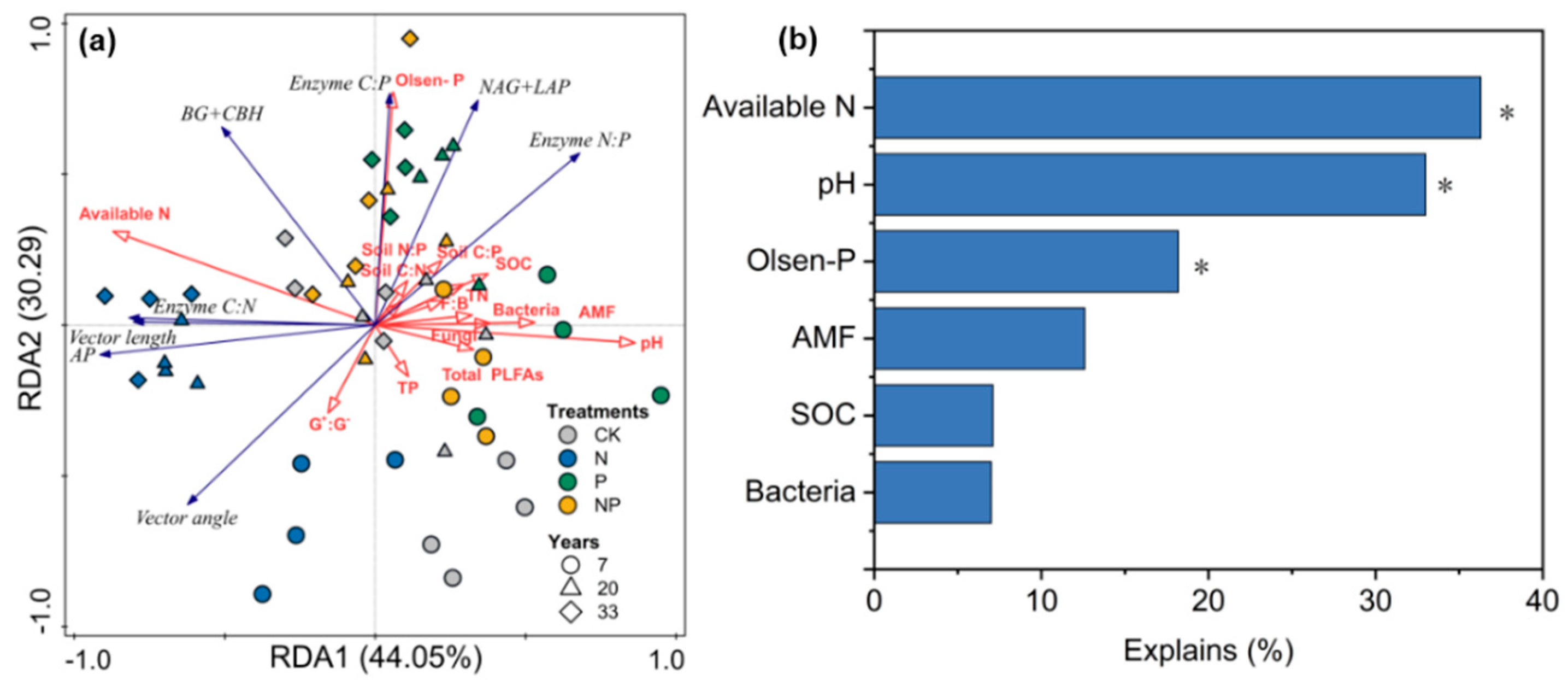
3.3. Vector-Based Characteristics of Extracellular Enzyme Stoichiometry
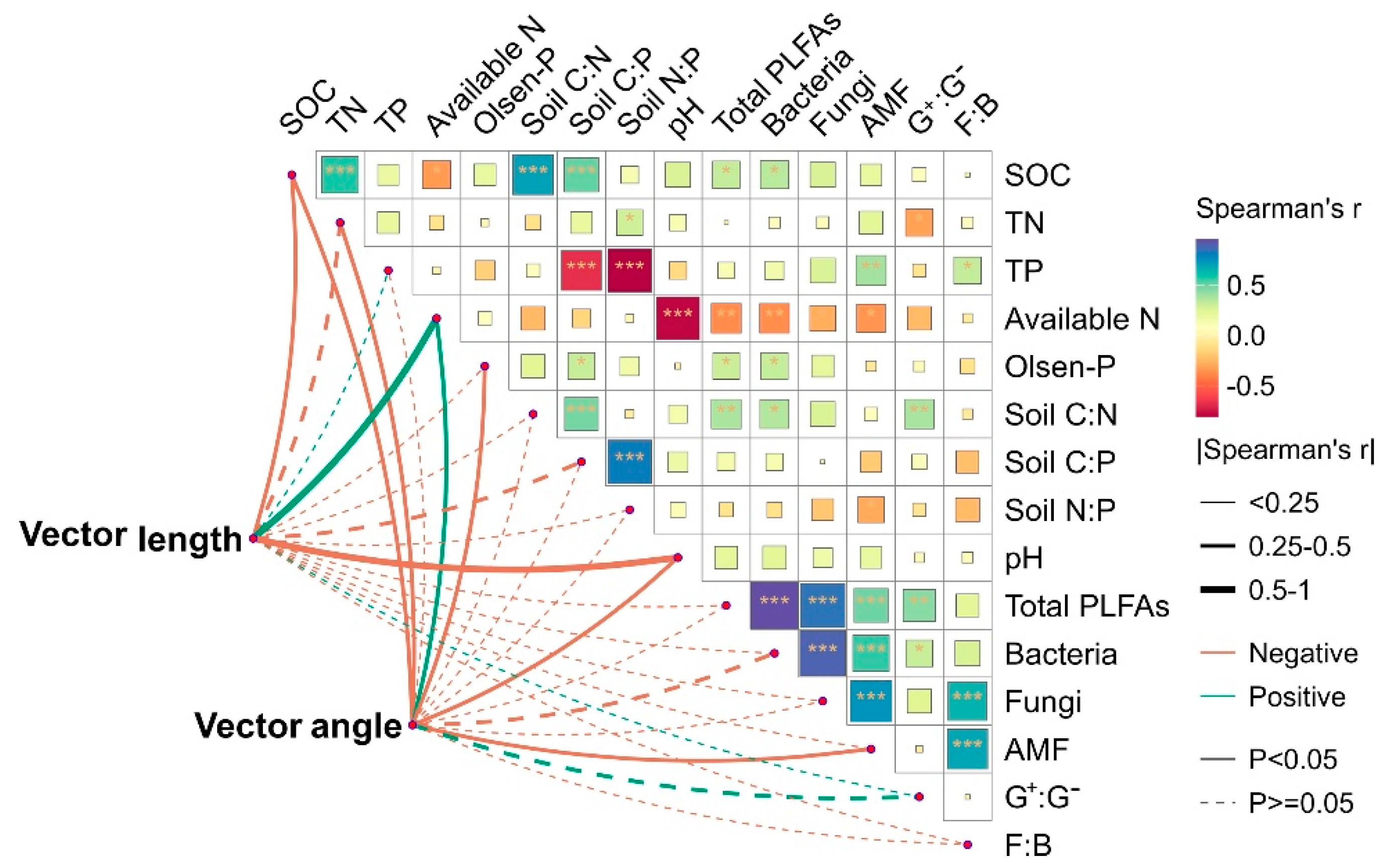
3.4. Correlation of Enzymatic Activity and Stoichiometry with the Soil’s Properties and Microbial Structure
4. Discussion
4.1. Effect of N Addition on Soil Enzyme Activity and Stoichiometry
4.2. Effect of P Addition on Soil Enzyme Activity and Stoichiometry
4.3. Implications for Plantation Management
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wieder, W.R.; Cleveland, C.C.; Smith, W.K.; Todd-Brown, K. Future productivity and carbon storage limited by terrestrial nutrient availability. Nat. Geosci. 2015, 8, 441–444. [Google Scholar] [CrossRef]
- Jiang, M.K.; Crous, K.Y.; Carrillo, Y.; Macdonald, C.A.; Anderson, I.C.; Boer, M.M.; Farrell, M.; Gherlenda, A.N.; Castañeda-Gómez, L.; Hasegawa, S.; et al. Microbial competition for phosphorus limits the CO2 response of a mature forest. Nature 2024, 630, 660–665. [Google Scholar] [CrossRef] [PubMed]
- Galloway, J.N.; Townsend, A.R.; Erisman, J.W.; Bekunda, M.; Cai, Z.; Freney, J.R.; Martinelli, L.A.; Seitzinger, S.P.; Sutton, M.A. Transformation of the nitrogen cycle: Recent trends, questions, and potential solutions. Science 2008, 320, 889–892. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Y.; Li, Y.; Mou, Z.J.; Kuang, L.H.; Wu, W.J.; Zhang, J.; Wang, F.M.; Hui, D.F.; Penuelas, J.; Sardans, J.; et al. Phosphorus addition decreases microbial residual contribution to soil organic carbon pool in a tropical coastal forest. Global Chang. Biol. 2021, 27, 454–466. [Google Scholar] [CrossRef]
- Peñuelas, J.; Poulter, B.; Sardans, J.; Ciais, P.; Van Der Velde, M.; Bopp, L.; Boucher, O.; Godderis, Y.; Hinsinger, P.; Llusia, J.; et al. Human-induced nitrogen-phosphorus imbalances alter natural and managed ecosystems across the globe. Nat. Commun. 2013, 4, 2934. [Google Scholar] [CrossRef]
- Ma, S.H.; Chen, G.P.; Tang, W.G.; Xing, A.J.; Chen, X.; Xiao, W.; Zhou, L.H.; Zhu, J.L.; Li, Y.D.; Zhu, B.; et al. Inconsistent responses of soil microbial community structure and enzyme activity to nitrogen and phosphorus additions in two tropical forests. Plant Soil 2021, 460, 453–468. [Google Scholar] [CrossRef]
- Crowther, T.W.; van den Hoogen, J.; Wan, J.; Mayes, M.A.; Keiser, A.D.; Mo, L.; Averill, C.; Maynard, D.S. The global soil community and its influence on biogeochemistry. Science 2019, 365, eaav0550. [Google Scholar] [CrossRef]
- Chen, H.; Chen, M.; Li, D.; Mao, Q.; Zhang, W.; Mo, J. Responses of soil phosphorus availability to nitrogen addition in a legume and a non-legume plantation. Geoderma 2018, 322, 12–18. [Google Scholar] [CrossRef]
- Cui, Y.; Bing, H.; Moorhead, D.L.; Delgado-Baquerizo, M.; Ye, L.; Yu, J.; Zhang, S.; Wang, X.; Peng, S.; Guo, X.; et al. Ecoenzymatic stoichiometry reveals widespread soil phosphorus limitation to microbial metabolism across Chinese forests. Commun. Earth Environ. 2022, 3, 184. [Google Scholar] [CrossRef]
- Waring, B.G.; Weintraub, S.R.; Sinsabaugh, R.L. Ecoenzymatic stoichiometry of microbial nutrient acquisition in tropical soils. Biogeochemistry 2014, 117, 101–113. [Google Scholar] [CrossRef]
- Sinsabaugh, R.L.; Moorhead, D.L. Resource allocation to extracellular enzyme production: A model for nitrogen and phosphorus control of litter decomposition. Soil Biol. Biochem. 1994, 26, 1305–1311. [Google Scholar] [CrossRef]
- Sinsabaugh, R.L.; Hill, B.H.; Follstad Shah, J.J. Ecoenzymatic stoichiometry of microbial organic nutrient acquisition in soil and sediment. Nature 2009, 462, 795–798. [Google Scholar] [CrossRef]
- Moorhead, D.L.; Sinsabaugh, R.L.; Hill, B.H.; Weintraub, M.N. Vector analysis of ecoenzyme activities reveal constraints on coupled C, N and P dynamics. Soil Biol. Biochem. 2016, 93, 1–7. [Google Scholar] [CrossRef]
- Bai, X.; Dippold, M.A.; An, S.; Wang, B.; Zhang, H.; Loeppmann, S. Extracellular enzyme activity and stoichiometry: The effect of soil microbial element limitation during leaf litter decomposition. Ecol. Indic. 2021, 121, 107200. [Google Scholar] [CrossRef]
- Wang, C.Y.; Han, G.M.; Jia, Y.; Feng, X.G.; Guo, P.; Tian, X.J. Response of litter decomposition and related soil enzyme activities to different forms of nitrogen fertilization in a subtropical forest. Ecol. Res. 2011, 26, 505–513. [Google Scholar] [CrossRef]
- Lazcano, C.; Gomez-Brandon, M.; Revilla, P.; Dominguez, J. Short-term effects of organic and inorganic fertilizers on soil microbial community structure and function. Biol. Fert. Soils 2013, 49, 723–733. [Google Scholar] [CrossRef]
- Dong, W.Y.; Zhang, X.Y.; Liu, X.Y.; Fu, X.L.; Chen, F.S.; Wang, H.M.; Sun, X.M.; Wen, X.F. Responses of soil microbial communities and enzyme activities to nitrogen and phosphorus additions in Chinese fir plantations of subtropical China. Biogeosciences 2015, 12, 5537–5546. [Google Scholar] [CrossRef]
- Marklein, A.R.; Houlton, B.Z. Nitrogen inputs accelerate phosphorus cycling rates across a wide variety of terrestrial ecosystems. New Phytol. 2012, 193, 696–704. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Gurmesa, G.A.; Zhang, W.; Zhu, X.M.; Zheng, M.H.; Mao, Q.G.; Zhang, T.; Mo, J.G. Nitrogen saturation in humid tropical forests after 6 years of nitrogen and phosphorus addition: Hypothesis testing. Funct. Ecol. 2016, 30, 305–313. [Google Scholar] [CrossRef]
- Jian, S.Y.; Li, J.W.; Chen, J.; Wang, G.S.; Mayes, M.A.; Dzantor, K.E.; Hui, D.F.; Luo, Y.Q. Soil extracellular enzyme activities, soil carbon and nitrogen storage under nitrogen fertilization: A meta–analysis. Soil Biol. Biochem. 2016, 101, 32–43. [Google Scholar] [CrossRef]
- Wang, R.; Cao, Y.; Wang, H.; Dijkstra, F.A.; Jiang, J.; Zhao, R.; Ma, W.; Li, T.; Dorodnikov, M.; Wang, Z. Exogenous P compounds differentially interacted with N availability to regulate enzymatic activities in a meadow steppe. Eur. J. Soil Sci. 2020, 71, 667–680. [Google Scholar] [CrossRef]
- Hu, Z.; Xiang, W. Inconsistent Responses of Rhizosphere Microbial Community Structure and Extracellular Enzyme Activity to Short-Term Nitrogen and Phosphorus Additions in Chinese Fir (Cunninghamia lanceolata) Plantations. Forests 2023, 14, 1532. [Google Scholar] [CrossRef]
- Geisseler, D.; Scow, K.M. Long-term effects of mineral fertilizers on soil microorganisms—A review. Soil Biol. Biochem. 2014, 75, 54–63. [Google Scholar] [CrossRef]
- Tong, R.; Zhou, B.; Jiang, L.; Ge, X.; Cao, Y.; Shi, J. Leaf litter carbon, nitrogen and phosphorus stoichiometry of Chinese fir (Cunninghamia lanceolata) across China. Global Ecol. Conserv. 2021, 27, e01542. [Google Scholar] [CrossRef]
- National Forestry and Grassland Administration. China Forest Resource Report (2014–2019); China Forestry Publishing House: Beijing, China, 2019. [Google Scholar]
- Selvaraj, S.; Duraisamy, V.; Huang, Z.J.; Guo, F.T.; Ma, X.Q. Influence of long-term successive rotations and stand age of Chinese fir (Cunninghamia lanceolata) plantations on soil properties. Geoderma 2017, 306, 127–134. [Google Scholar] [CrossRef]
- Dong, C.; Wang, W.; Liu, H.; Xu, X.; Zeng, H. Temperate grassland shifted from nitrogen to phosphorus limitation induced by degradation and nitrogen deposition: Evidence from soil extracellular enzyme stoichiometry. Ecol. Indic. 2019, 101, 453–464. [Google Scholar] [CrossRef]
- Wu, H.L.; Xiang, W.H.; Ouyang, S.; Xiao, W.F.; Li, S.G.; Chen, L.; Lei, P.F.; Deng, X.W.; Zeng, Y.L.; Zeng, L.X. Tree growth rate and soil nutrient status determine the shift in nutrient-use strategy of Chinese fi plantations along a chronosequence. For. Ecol. Manag. 2020, 460, 117896. [Google Scholar] [CrossRef]
- Ma, Y.; Zhu, B.; Sun, Z.; Zhao, C.; Yang, Y.; Piao, S. The effects of simulated nitrogen deposition on extracellular enzyme activities of litter and soil among different-aged stands of larch. J. Plant Ecol. 2014, 7, 240–249. [Google Scholar] [CrossRef]
- Chen, L.; Xiang, W.H.; Ouyang, S.; Wu, H.L.; Xia, Q.; Ma, J.N.; Zeng, Y.L.; Lei, P.F.; Xiao, W.F.; Li, S.G.; et al. Tight coupling of fungal community composition with soil quality in a Chinese fir plantation chronosequence. Land Degrad. Dev. 2021, 32, 1164–1178. [Google Scholar] [CrossRef]
- Walkley, A. A critical examination of a rapid method for determining organic carbon in soils—Effect of variations in digestion conditions and of inorganic soil constituents. Soil Sci. 1947, 63, 251–264. [Google Scholar] [CrossRef]
- Madrid, F. Methods in Agricultural Chemical Analysis. J. Environ. Qual. 2004, 33, 1580–1581. [Google Scholar] [CrossRef]
- Frostegård, Å.; Bååth, E.; Tunlio, A. Shifts in the structure of soil microbial communities in limed forests as revealed by phospholipid fatty acid analysis. Soil Biol. Biochem. 1993, 25, 723–730. [Google Scholar] [CrossRef]
- Saiya-Cork, K.; Sinsabaugh, R.; Zak, D. The effects of long term nitrogen deposition on extracellular enzyme activity in an Acer saccharum forest soil. Soil Biol. Biochem. 2002, 34, 1309–1315. [Google Scholar] [CrossRef]
- Cui, Y.; Zhang, Y.; Duan, C.; Wang, X.; Zhang, X.; Ju, W.; Chen, H.; Yue, S.; Wang, Y.; Li, S.; et al. Ecoenzymatic stoichiometry reveals microbial phosphorus limitation decreases the nitrogen cycling potential of soils in semi-arid agricultural ecosystems. Soil Till. Res. 2020, 197, 104463. [Google Scholar] [CrossRef]
- Moorhead, D.L.; Rinkes, Z.L.; Sinsabaugh, R.L.; Weintraub, M.N. Dynamic relationships between microbial biomass, respiration, inorganic nutrients and enzyme activities: Informing enzyme-based decomposition models. Front. Microbiol. 2013, 4, 223. [Google Scholar] [CrossRef]
- Cui, Y.X.; Fang, L.C.; Guo, X.B.; Han, F.; Ju, W.L.; Ye, L.P.; Wang, X.; Tan, W.F.; Zhang, X.C. Natural grassland as the optimal pattern of vegetation restoration in arid and semi-arid regions: Evidence from nutrient limitation of soil microbes. Sci. Total Environ. 2019, 648, 388–397. [Google Scholar] [CrossRef] [PubMed]
- Sinsabaugh, R.L.; Lauber, C.L.; Weintraub, M.N.; Ahmed, B.; Zeglin, L.H. Stoichiometry of soil enzyme activity at global scale. Ecol. Lett. 2008, 11, 1252–1264. [Google Scholar] [PubMed]
- Keeler, B.L.; Hobbie, S.E.; Kellogg, L.E. Effects of long-term nitrogen addition on microbial enzyme activity in eight forested and grassland sites: Implications for litter and soil organic matter decomposition. Ecosystems 2009, 12, 1–15. [Google Scholar] [CrossRef]
- Dong, H.; Ge, J.; Sun, K.; Wang, B.; Xu, J. Change in root-associated fungal communities affects soil enzymatic activities during Pinus massoniana forest development in subtropical China. Forest Ecol. Manag. 2021, 482, 118817. [Google Scholar] [CrossRef]
- Hedo, J.; Lucas-Borja, M.E.; Vinegla, B.; Cerda, A.; Candel-Perez, D. Unravelling the importance of forest age stand and forest structure driving microbiological soil properties, enzymatic activities and soil nutrients content in Mediterranean Spanish black pine(Pinus nigra Ar. ssp. salzmannii). Forest. Sci. Total Environ. 2016, 562, 145–154. [Google Scholar]
- Kara, Ö.; Bolat, İ.; Çakıroğlu, K.; Öztürk, M. Plant canopy effects on litter accumulation and soil microbial biomass in two temperate forests. Biol. Fertil. Soils 2008, 45, 193–198. [Google Scholar] [CrossRef]
- Wei, C.C.; Liu, X.F.; Lin, C.F.; Li, X.F.; Li, Y.; Zheng, Y.X. Response of soil enzyme activities to litter input changes in two secondary Castanopsis carlessii forests in subtropical China. Chin. J. Plant Ecol. 2018, 42, 692. [Google Scholar]
- Zhong, Y.; Yan, W.; Canisares, L.P.; Wang, S.; Brodie, E.L. Alterations in soil pH emerge as a key driver of the impact of global change on soil microbial nitrogen cycling: Evidence from a global meta-analysis. Glob. Ecol. Biogeogr. 2023, 32, 145–165. [Google Scholar] [CrossRef]
- Sinsabaugh, R.L.; Shah, J.J.F. Integrating resource utilization and temperature in metabolic scaling of riverine bacterial production. Ecology 2010, 91, 1455–1465. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Tang, Y.; Shi, Y.; He, N.; Wen, X.; Yu, Q.; Zheng, C.; Sun, X.; Qiu, W. Responses of soil hydrolytic enzymes, ammonia-oxidizing bacteria and archaea to nitrogen applications in a temperate grassland in Inner Mongolia. Sci. Rep. 2016, 6, 32791. [Google Scholar] [CrossRef] [PubMed]
- Skeffington, R.A. Accelerated nitrogen inputs—A new problem or a new perspective? Plant Soil 1990, 128, 1–11. [Google Scholar] [CrossRef]
- Ning, Q.S.; Hattenschwiler, S.; Lu, X.T.; Kardol, P.; Zhang, Y.H.; Wei, C.Z.; Xu, C.Y.; Huang, J.H.; Li, A.; Yang, J.; et al. Carbon limitation overrides acidification in mediating soil microbial activity to nitrogen enrichment in a temperate grassland. Glob. Chang. Biol. 2021, 27, 5976–5988. [Google Scholar] [CrossRef]
- Allison, S.D.; Vitousek, P.M. Responses of extracellular enzymes to simple and complex nutrient inputs. Soil Biol. Biochem. 2005, 37, 937–944. [Google Scholar] [CrossRef]
- Deng, X.; Liu, Y.; Han, S. Carbon and nitrogen dynamics in early stages of forest litter decomposition as affected by nitrogen addition. J. Forestry Res. 2009, 20, 111–116. [Google Scholar] [CrossRef]
- Fan, Y.; Zhong, X.; Lin, T.-C.; Lyu, M.; Wang, M.; Hu, W.; Yang, Z.; Chen, G.; Guo, J.; Yang, Y. Effects of nitrogen addition on DOM-induced soil priming effects in a subtropical plantation forest and a natural forest. Biol. Fert. Soils 2020, 56, 205–216. [Google Scholar] [CrossRef]
- Gross, A.; Lin, Y.; Weber, P.K.; Pett-Ridge, J.; Silver, W.L. The role of soil redox conditions in microbial phosphorus cycling in humid tropical forests. Ecology 2020, 101, e02928. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.F.; Lin, W.S.; Chen, S.L.; Peng, J.Q.; Guo, J.F.; Yang, Y.S. Phosphorus addition accelerates fine root decomposition by stimulating extracellular enzyme activity in a subtropical natural evergreen broad-leaved forest. Eur. J. Forest Res. 2019, 138, 917–928. [Google Scholar] [CrossRef]
- Olander, L.P.; Vitousek, P.M. Regulation of soil phosphatase and chitinase activity by N and P availability. Biogeochemistry 2000, 49, 175–191. [Google Scholar] [CrossRef]
- Speir, T.W.; Cowling, J.C. Phosphatase activities of pasture plants and soils: Relationship with plant productivity and soil P fertility indices. Biol. Fert. Soils 1991, 12, 189–194. [Google Scholar] [CrossRef]
- Allison, S.D.; Weintraub, M.N.; Gartner, T.B.; Waldrop, M.P. Evolutionary-economic principles as regulators of soil enzyme production and ecosystem function. Soil Enzymol. 2011, 22, 229–243. [Google Scholar]
- Turner, B.L.; Wright, S.J. The response of microbial biomass and hydrolytic enzymes to a decade of nitrogen, phosphorus, and potassium addition in a lowland tropical rain forest. Biogeochemistry 2014, 117, 115–130. [Google Scholar] [CrossRef]
- Zheng, M.H.; Huang, J.; Chen, H.; Wang, H.; Mo, J.M. Responses of soil acid phosphatase and beta-glucosidase to nitrogen and phosphorus addition in two subtropical forests in southern China. Eur. J. Soil Biol. 2015, 68, 77–84. [Google Scholar] [CrossRef]
- Yokoyama, D.; Imai, N.; Kitayama, K. Effects of nitrogen and phosphorus fertilization on the activities of four different classes of fine-root and soil phosphatases in Bornean tropical rain forests. Plant Soil 2017, 416, 463–476. [Google Scholar] [CrossRef]
- Treseder, K.K.; Vitousek, P.M. Effects of soil nutrient availability on investment in acquisition of N and P in Hawaiian rain forests. Ecology 2001, 82, 946–954. [Google Scholar] [CrossRef]
- Lu, X.; Mo, J.; Zhang, W.; Mao, Q.; Liu, R.; Wang, C.; Zheng, M.; Wang, S.; Mori, T.; Mao, J. Effects of simulated atmospheric nitrogen deposition on forest ecosystems in China: An overview. J. Trop. Subtr. Botany 2019, 27, 500–522. [Google Scholar]
- Pant, H.; Warman, P. Enzymatic hydrolysis of soil organic phosphorus by immobilized phosphatases. Biol. Fert. Soils 2000, 30, 306–311. [Google Scholar] [CrossRef]
- Liu, M.H.; Gan, B.P.; Li, Q.; Xiao, W.F.; Song, X.Z. Effects of nitrogen and phosphorus addition on soil extracellular enzyme activity and stoichiometry in Chinese fir (Cunninghamia lanceolata) Forests. Front. Plant Sci. 2022, 13, 834184. [Google Scholar] [CrossRef] [PubMed]
- Zheng, L.; Chen, H.; Wang, Y.; Mao, Q.; Zheng, M.; Su, Y.; Xiao, K.; Wang, K.; Li, D. Responses of soil microbial resource limitation to multiple fertilization strategies. Soil Till. Res. 2020, 196, 104474. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ren, Y.; Wang, Y.; Zhang, X.; Liu, X.; Liu, P.; Chen, L. Enzymatic Stoichiometry Reveals the Metabolic Limitations of Soil Microbes under Nitrogen and Phosphorus Addition in Chinese Fir Plantations. Microorganisms 2024, 12, 1716. https://doi.org/10.3390/microorganisms12081716
Ren Y, Wang Y, Zhang X, Liu X, Liu P, Chen L. Enzymatic Stoichiometry Reveals the Metabolic Limitations of Soil Microbes under Nitrogen and Phosphorus Addition in Chinese Fir Plantations. Microorganisms. 2024; 12(8):1716. https://doi.org/10.3390/microorganisms12081716
Chicago/Turabian StyleRen, Yan, Ying Wang, Xiulan Zhang, Xionghui Liu, Pei Liu, and Liang Chen. 2024. "Enzymatic Stoichiometry Reveals the Metabolic Limitations of Soil Microbes under Nitrogen and Phosphorus Addition in Chinese Fir Plantations" Microorganisms 12, no. 8: 1716. https://doi.org/10.3390/microorganisms12081716
APA StyleRen, Y., Wang, Y., Zhang, X., Liu, X., Liu, P., & Chen, L. (2024). Enzymatic Stoichiometry Reveals the Metabolic Limitations of Soil Microbes under Nitrogen and Phosphorus Addition in Chinese Fir Plantations. Microorganisms, 12(8), 1716. https://doi.org/10.3390/microorganisms12081716





