The Effect of Intercropping with Different Leguminous Green Manures on the Soil Environment and Tea Quality in Tea Plantations
Abstract
:1. Introduction
2. Materials and Methods
2.1. Site Selection, Soil Sampling, and Basic Soil Conditions
2.2. Experimental Treatments
2.3. Determination of Tea Biochemical Quality
2.4. Determination of Soil Chemical Properties
2.5. Soil Enzyme Activity Determination
2.6. DNA Sequencing Analysis of Soil Bacteria
2.7. Bioinformatics and Statistical Analysis
3. Results
3.1. Effects of Intercropping with Leguminous Green Manure on Tea Quality Indicators, Soil Organic Carbon, and Enzyme Activity in Tea Plantations
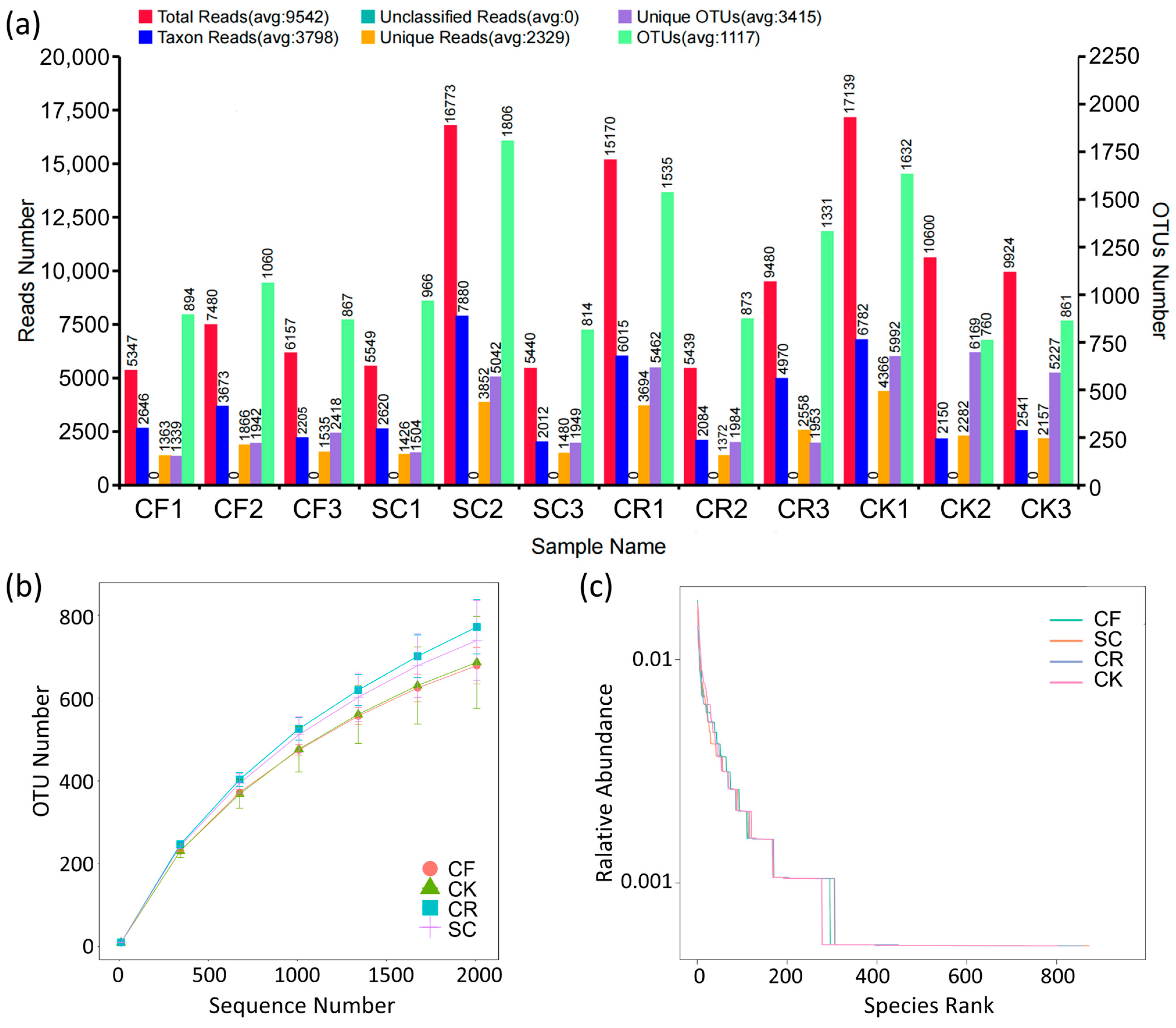
3.2. Effect of Leguminous Green Manure on Taxa and Microbial Community Structure
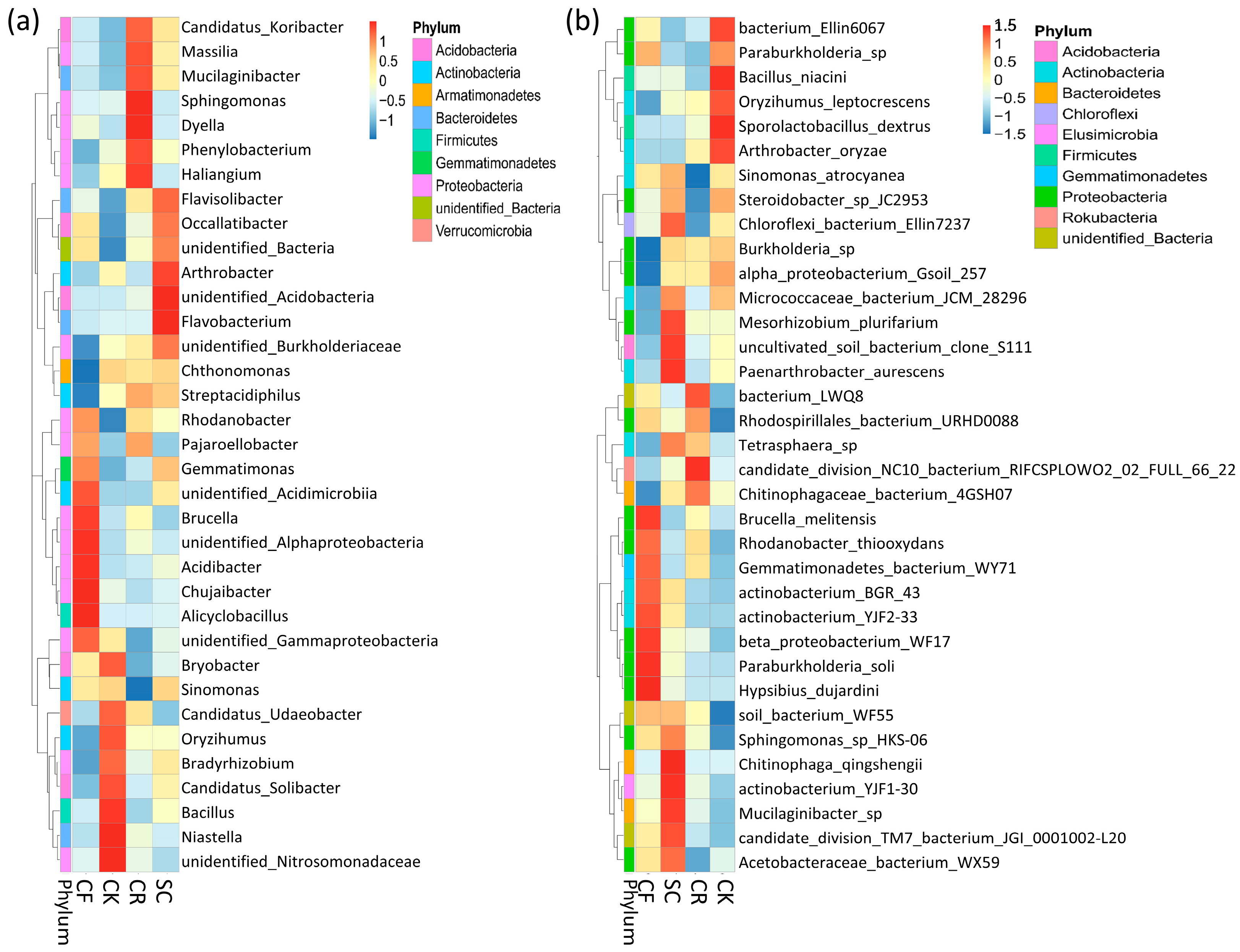
3.3. Composition of Bacterial Community
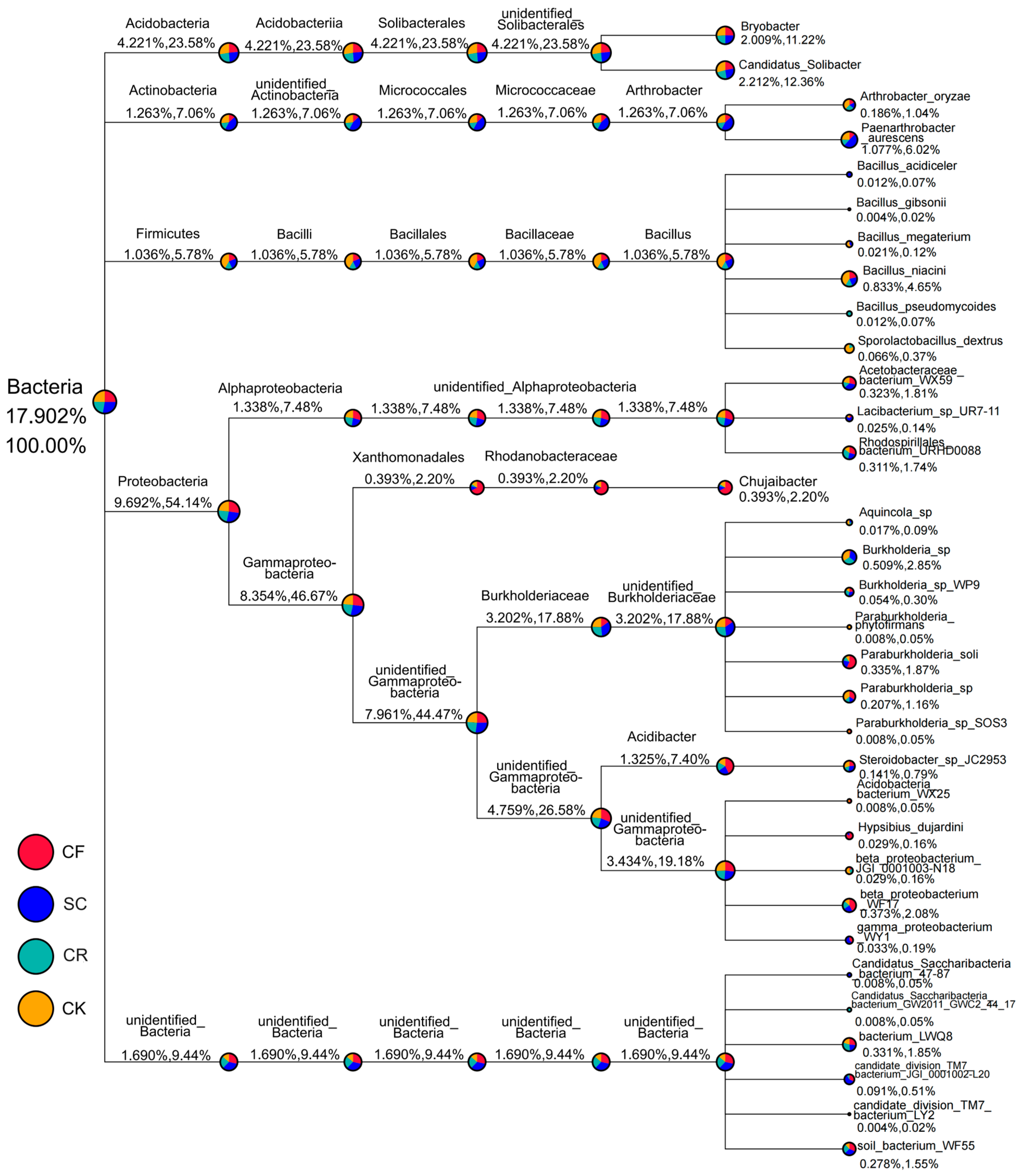
3.4. Phylogenetic Tree
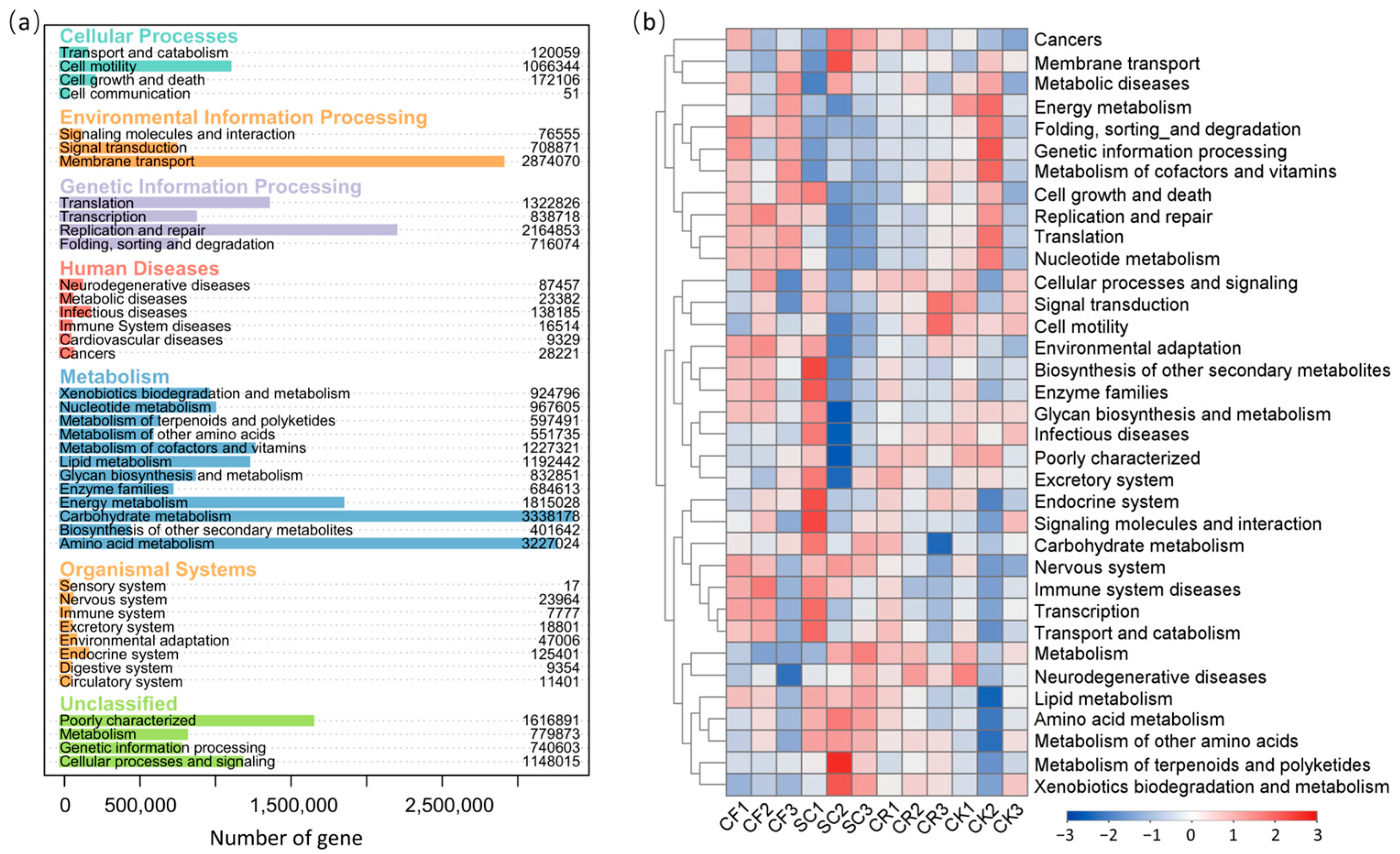
3.5. Functional Prediction of Soil Bacterial Communities
3.6. Correlation Analysis of Intercropping with Leguminous Green Manure in Tea Garden on Tea Quality Indicators, Soil Properties, and Soil Bacterial
4. Discussion
4.1. Effects on the Formation of Tea Key Quality Components in Tea Leaves
4.2. Effect of Intercropping Leguminous Green Manure on Soil Bacterial Community
4.3. Mutual Influence of Soil Properties, Soil Bacterial Communities, and Tea Quality Indicators
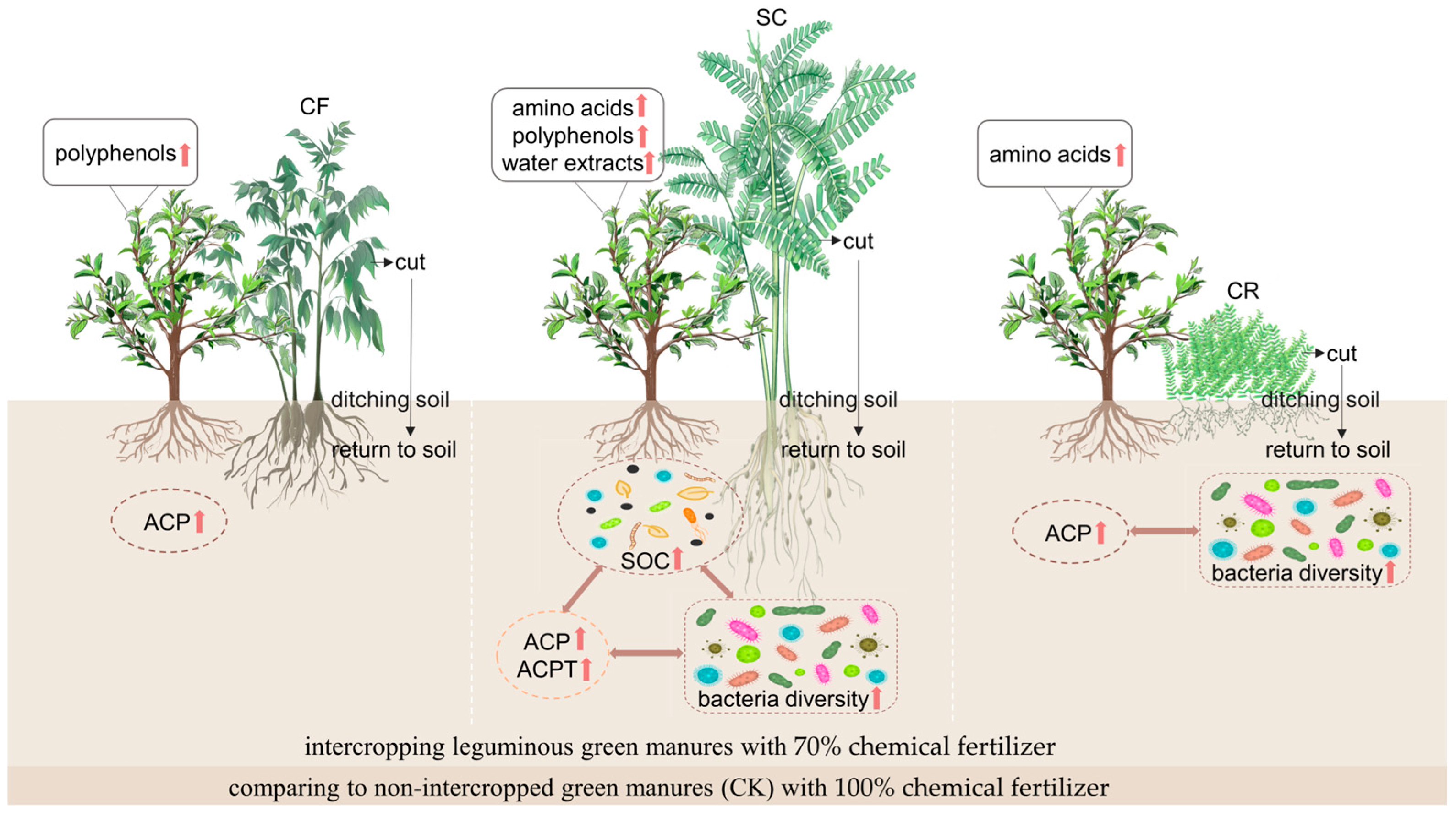
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Zhang, W.; Zhang, Y.; Qiu, H.; Guo, Y.; Wan, H.; Zhang, X.; Scossa, F.; Alseekh, S.; Zhang, Q.; Wang, P.; et al. Genome assembly of wild tea tree DASZ reveals pedigree and selection history of tea varieties. Nat. Commun. 2020, 11, 3719. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Xiao, J.; Chen, S.; Yu, Y.; Ma, J.; Lin, Y.; Li, R.; Lin, J.; Fu, Z.; Zhou, Q.; et al. Metabolite signatures of diverse Camellia sinensis tea populations. Nat. Commun. 2020, 11, 5586. [Google Scholar] [CrossRef] [PubMed]
- Vuong, Q.V. Epidemiological evidence linking tea consumption to human health: A review. Crit. Rev. Food Sci. Nutr. 2014, 54, 523–536. [Google Scholar] [CrossRef]
- Yue, C.; Peng, H.; Li, W.; Tong, Z.; Wang, Z.; Yang, P. Untargeted metabolomics and transcriptomics reveal the mechanism of metabolite differences in spring tender shoots of tea plants of different ages. Foods 2022, 11, 2303. [Google Scholar] [CrossRef]
- Jiang, Y.; Arafat, Y.; Letuma, P.; Ali, L.; Tayyab, M.; Waqas, M.; Li, Y.; Lin, W.; Lin, S.; Lin, W. Restoration of Long-term monoculture degraded tea orchard by green and goat manures applications system. Sustainability 2019, 11, 1011. [Google Scholar] [CrossRef]
- Fu, H.; Li, H.; Yin, P.; Mei, H.; Li, J.; Zhou, P.; Wang, Y.; Ma, Q.; Jeyaraj, A.; Thangaraj, K.; et al. Integrated application of rapeseed cake and green manure enhances soil nutrients and microbial communities in tea garden soil. Sustainability 2021, 13, 2967. [Google Scholar] [CrossRef]
- Zhao, N.; Zhang, J.; Li, X.; Ma, J.; Cao, J.; Liu, H.; Wang, X.; Bai, L.; Wang, Z. Limited advantages of green manure planting on soil nutrients and productivity in intensive agriculture: A case study of wheat–maize–sunflower rotation in Hetao irrigation dDistrict. Agronomy 2023, 14, 100. [Google Scholar] [CrossRef]
- Liang, Q.; Zhang, T.; Liu, Z.; Gao, W.; Cheng, Y.; Feng, H. Effects of Different green manure crops on soil water, nitrogen, and yield: Preliminary results in an apple orchard on the Loess Plateau, China. Agronomy 2023, 13, 2009. [Google Scholar] [CrossRef]
- Asghar, W.; Kataoka, R. Green manure incorporation accelerates enzyme activity, plant growth, and changes in the fungal community of soil. Arch. Microbiol. 2021, 204, 7. [Google Scholar] [CrossRef]
- Dong, N.; Hu, G.; Zhang, Y.; Qi, J.; Chen, Y.; Hao, Y. Effects of green-manure and tillage management on soil microbial community composition, nutrients and tree growth in a walnut orchard. Sci. Rep. 2021, 11, 16882. [Google Scholar] [CrossRef]
- Li, D.; Yin, N.; Xu, R.; Wang, L.; Zhang, Z.; Li, K. Sludge amendment accelerating reclamation process of reconstructed mining substrates. Sci. Rep. 2021, 11, 2905. [Google Scholar] [CrossRef]
- Kaurin, A.; Gluhar, S.; Tilikj, N.; Lestan, D. Soil washing with biodegradable chelating agents and EDTA: Effect on soil properties and plant growth. Chemosphere 2020, 260, 127673. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Zhang, J.; Shi, W.; Sun, J.; Xia, T.; Huang, F.; Liu, Y.; Li, H.; Teng, K.; Zhong, J. Dynamic changes in fermentation quality and structure and function of the microbiome during mixed silage of Sesbania cannabina and Sweet Sorghum grown on Saline-Alkaline land. Microbiol. Spectr. 2022, 10, e0248322. [Google Scholar] [CrossRef]
- Duan, Y.; Wang, T.; Lei, X.; Cao, Y.; Liu, L.; Zou, Z.; Ma, Y.; Zhu, X.; Fang, W. Leguminous green manure intercropping changes the soil microbial community and increases soil nutrients and key quality components of tea leaves. Hortic. Res. 2024, 11, uhae018. [Google Scholar] [CrossRef]
- Balachandar, R.; Baskaran, L.; Yuvaraj, A.; Thangaraj, R.; Subbaiya, R.; Ravindran, B.; Chang, S.W.; Karmegam, N. Enriched pressmud vermicompost production with green manure plants using Eudrilus eugeniae. Bioresour. Technol. 2020, 299, 122578. [Google Scholar] [CrossRef]
- Ruan, L.; Wei, K.; Li, J.; He, M.; Wu, L.; Aktar, S.; Wang, L.; Cheng, H. Responses of tea plants (Camellia sinensis) with different low-nitrogen tolerances during recovery from nitrogen deficiency. J. Sci. Food Agric. 2022, 102, 1405–1414. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.; Cui, C.; Cao, Y.; Dai, J.; Cheng, X.; Hua, S.; Wang, W.; Duan, Y.; Petropoulos, E.; Wang, H.; et al. Tea plant–legume intercropping simultaneously improves soil fertility and tea quality by changing Bacillus species composition. Hortic. Res. 2022, 9, uhac046. [Google Scholar] [CrossRef]
- Feng, Y.; Sunderland, T. Feasibility of tea/tree intercropping plantations on soil ecological service function in China. Agronomy 2023, 13, 1548. [Google Scholar] [CrossRef]
- Wang, L.; Huang, D.; Wang, F.; Li, Q.; He, C.; Liu, C.; Huang, Y. Bacterial communities in acid tea soils treated for 10 years with chemical vs. integrated fertilizers. Commun. Soil Sci. Plan 2019, 50, 307–320. [Google Scholar] [CrossRef]
- Duan, Y.; Wang, T.; Zhang, P.; Zhao, X.; Jiang, J.; Ma, Y.; Zhu, X.; Fang, W. The effect of intercropping leguminous green manure on theanine accumulation in the tea plant: A metagenomic analysis. Plant Cell Environ. 2024, 47, 1141–1159. [Google Scholar] [CrossRef]
- Wang, T.; Duan, Y.; Liu, G.; Shang, X.; Liu, L.; Zhang, K.; Li, J.; Zou, Z.; Zhu, X.; Fang, W. Tea plantation intercropping green manure enhances soil functional microbial abundance and multifunctionality resistance to drying-rewetting cycles. Sci. Total Environ. 2022, 810, 151282. [Google Scholar] [CrossRef]
- Ma, Q.; Song, L.; Niu, Z.; Qiu, Z.; Sun, H.; Ren, Z.; Wu, H.; Wang, Y.; Mei, H.; Li, X.; et al. Pea-Tea intercropping improves tea quality through regulating amino acid metabolism and flavonoid biosynthesis. Foods 2022, 11, 3746. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Dong, X.; Wang, Y.; Maker, G.; Agarwal, M.; Ding, Z. Tea-Soybean intercropping improves tea quality and nutrition uptake by inducing changes of Rhizosphere bacterial communities. Microorganisms 2022, 10, 2149. [Google Scholar] [CrossRef]
- Shen, F.; Lin, S. Shifts in bacterial community associated with green manure Soybean intercropping and edaphic properties in a tea plantation. Sustainability 2021, 13, 11478. [Google Scholar] [CrossRef]
- Xie, S.; Yang, F.; Feng, H.; Yu, Z.; Wei, X.; Liu, C.; Wei, C. Potential to reduce chemical fertilizer application in tea plantations at various spatial scales. Int. J. Environ. Res. Public Health 2022, 19, 5243. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Dong, C.; Yang, T.; Bao, S.; Fang, W.; Lucas, W.J.; Zhang, Z. The tea plant CsLHT1 and CsLHT6 transporters take up amino acids, as a nitrogen source, from the soil of organic tea plantations. Hortic. Res. 2021, 8, 178. [Google Scholar] [CrossRef]
- GB/T 8314-2013; Tea-Determination of Free Amino Acids Content. General Administration of Quality Supervision, Inspection and Quarantine of the People’s Republic of China, and Standardization Administration of the People’s Republic of China: Beijing, China, 2013.
- GB/T 8313-2018; Determination of Total Polyphenols and Catechins Content in Tea. State Administration for Market Regulation of China and Standardization Administration of the People’s Republic of China: Beijing, China, 2018.
- GB/T 8312-2013; Tea-Determination of Caffeine Content. State Administration for Market Regulation of China and Standardization Administration of the People’s Republic of China: Beijing, China, 2013.
- GB/T 8305-2002; Tea-Determination of Water Extracts Content. General Administration of Quality Supervision, Inspection and Quarantine of the People’s Republic of China, and Standardization Administration of the People’s Republic of China: Beijing, China, 2002.
- Caporaso, J.G.; Lauber, C.L.; Walters, W.A.; Berg-Lyons, D.; Lozupone, C.A.; Turnbaugh, P.J.; Fierer, N.; Knight, R. Global patterns of 16S rRNA diversity at a depth of millions of sequences per sample. Proc. Natl. Acad. Sci. USA 2010, 108, 4516–4522. [Google Scholar] [CrossRef]
- Tian, C.F.; Zhou, Y.J.; Zhang, Y.M.; Li, Q.Q.; Zhang, Y.Z.; Li, D.F.; Wang, S.; Wang, J.; Gilbert, L.B.; Li, Y.R.; et al. Comparative genomics of rhizobia nodulating soybean suggests extensive recruitment of lineage-specific genes in adaptations. Proc. Natl. Acad. Sci. USA 2012, 109, 8629–8634. [Google Scholar] [CrossRef]
- Tejada, M.; Gonzalez, J.L.; Garcia-Martinez, A.M.; Parrado, J. Application of a green manure and green manure composted with beet vinasse on soil restoration: Effects on soil properties. Bioresour. Technol. 2008, 99, 4949–4957. [Google Scholar] [CrossRef] [PubMed]
- Tejada, M.; Gonzalez, J.L.; Garcia-Martinez, A.M.; Parrado, J. Effects of different green manures on soil biological properties and maize yield. Bioresour. Technol. 2008, 99, 1758–1767. [Google Scholar] [CrossRef]
- Srinivas, N.; Jetter, P.; Ueberbacher, B.J.; Werneburg, M.; Zerbe, K.; Steinmann, J.; Van der Meijden, B.; Bernardini, F.; Lederer, A.; Dias, R.L.; et al. Peptidomimetic antibiotics target outer-membrane biogenesis in Pseudomonas aeruginosa. Science 2010, 327, 1010–1013. [Google Scholar] [CrossRef] [PubMed]
- Xu, A.; Li, L.; Xie, J.; Zhang, R.; Luo, Z.; Cai, L.; Liu, C.; Wang, L.; Anwar, S.; Jiang, Y. Bacterial diversity and potential functions in response to long-term nitrogen fertilizer on the semiarid Loess Plateau. Microorganisms 2022, 10, 1579. [Google Scholar] [CrossRef] [PubMed]
- Yu, Z.; Yang, Z. Understanding different regulatory mechanisms of proteinaceous and non-proteinaceous amino acid formation in tea (Camellia sinensis) provides new insights into the safe and effective alteration of tea flavor and function. Crit Rev. Food Sci. Nutr. 2020, 60, 844–858. [Google Scholar] [CrossRef]
- Zhang, C.; Wang, M.; Gao, X.; Zhou, F.; Shen, C.; Liu, Z. Multi-omics research in albino tea plants: Past, present, and future. Sci. Hortic. 2020, 261, 108943. [Google Scholar] [CrossRef]
- Biteen, J.S.; Blainey, P.C.; Cardon, Z.G.; Chun, M.; Church, G.M.; Dorrestein, P.C.; Fraser, S.E.; Gilbert, J.A.; Jansson, J.K.; Knight, R.; et al. Tools for the microbiome: Nano and beyond. ACS Nano 2016, 10, 6–37. [Google Scholar] [CrossRef]
- Sun, L.; Wang, Y.; Ma, D.; Wang, L.; Zhang, X.; Ding, Y.; Fan, K.; Xu, Z.; Yuan, C.; Jia, H.; et al. Differential responses of the rhizosphere microbiome structure and soil metabolites in tea (Camellia sinensis) upon application of cow manure. BMC Microbiol. 2022, 22, 55. [Google Scholar] [CrossRef]
- Beckers, B.; Op De Beeck, M.; Weyens, N.; Boerjan, W.; Vangronsveld, J. Structural variability and niche differentiation in the rhizosphere and endosphere bacterial microbiome of field-grown poplar trees. Microbiome 2017, 5, 25. [Google Scholar] [CrossRef]
- Gottel, N.R.; Castro, H.F.; Kerley, M.; Yang, Z.; Pelletier, D.A.; Podar, M.; Karpinets, T.; Uberbacher, E.; Tuskan, G.A.; Vilgalys, R.; et al. Distinct microbial communities within the endosphere and rhizosphere of Populus deltoides roots across contrasting soil types. Appl. Environ. Microbiol. 2011, 77, 5934–5944. [Google Scholar] [CrossRef]
- Zhou, X.; Zhang, Z.; Tian, L.; Li, X.; Tian, C. Microbial communities in peatlands along a chronosequence on the Sanjiang Plain, China. Sci. Rep. 2017, 7, 9567. [Google Scholar] [CrossRef]
- Pankratov, T.A.; Ivanova, A.O.; Dedysh, S.N.; Liesack, W. Bacterial populations and environmental factors controlling cellulose degradation in an acidic Sphagnum peat. Environ. Microbiol. 2011, 13, 1800–1814. [Google Scholar] [CrossRef]
- Wang, J.; Li, M.; Zhou, Q.; Zhang, T. Effects of continuous cropping Jiashi muskmelon on rhizosphere microbial community. Front. Microbiol. 2022, 13, 1086334. [Google Scholar] [CrossRef]
- Van der Meij, A.; Worsley, S.F.; Hutchings, M.I.; van Wezel, G.P. Chemical ecology of antibiotic production by actinomycetes. FEMS Microbiol. Rev. 2017, 41, 392–416. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Li, B.; Cai, S.; Zhang, Y.; Xu, M.; Zhang, C.; Yuan, B.; Xing, K.; Qin, S. Identification of Rhizospheric actinomycete streptomyces lavendulae SPS-33 and the inhibitory effect of its volatile organic compounds against ceratocystis fimbriata in postharvest sweet potato (Ipomoea batatas (L.) Lam.). Microorganisms 2020, 8, 319. [Google Scholar] [CrossRef] [PubMed]
- You, D.; Yin, B.C.; Li, Z.H.; Zhou, Y.; Yu, W.B.; Zuo, P.; Ye, B.C. Sirtuin-dependent reversible lysine acetylation of glutamine synthetases reveals an autofeedback loop in nitrogen metabolism. Proc. Natl. Acad. Sci. USA 2016, 113, 6653–6658. [Google Scholar] [CrossRef] [PubMed]
- Ji, T.; Guo, X.; Wu, F.; Wei, M.; Li, J.; Ji, P.; Wang, N.; Yang, F. Proper irrigation amount for eggplant cultivation in a solar greenhouse improved plant growth, fruit quality and yield by influencing the soil microbial community and rhizosphere environment. Front. Microbiol. 2022, 13, 981288. [Google Scholar] [CrossRef]
- Hou, E.; Chen, C.; Luo, Y.; Zhou, G.; Kuang, Y.; Zhang, Y.; Heenan, M.; Lu, X.; Wen, D. Effects of climate on soil phosphorus cycle and availability in natural terrestrial ecosystems. Glob. Change Biol. 2018, 24, 3344–3356. [Google Scholar] [CrossRef]
- Duan, Y.; Shen, J.; Zhang, X.; Wen, B.; Ma, Y.; Wang, Y.; Fang, W.; Zhu, X. Effects of soybean–tea intercropping on soil-available nutrients and tea quality. Acta Physiol. Plant. 2019, 41, 140. [Google Scholar] [CrossRef]
- Ning, C.; Gao, P.; Wang, B.; Lin, W.; Jiang, N.; Cai, K. Impacts of chemical fertilizer reduction and organic amendments supplementation on soil nutrient, enzyme activity and heavy metal content. J. Integr. Agric. 2017, 16, 1819–1831. [Google Scholar] [CrossRef]
- Yang, X.; Tsibart, A.; Nam, H.; Hur, J.; El-Naggar, A.; Tack, F.M.G.; Wang, C.H.; Lee, Y.H.; Tsang, D.C.W.; Ok, Y.S. Effect of gasification biochar application on soil quality: Trace metal behavior, microbial community, and soil dissolved organic matter. J. Hazard. Mater. 2019, 365, 684–694. [Google Scholar] [CrossRef]
- Sun, T.; Miao, J.; Saleem, M.; Zhang, H.; Yang, Y.; Zhang, Q. Bacterial compatibility and immobilization with biochar improved tebuconazole degradation, soil microbiome composition and functioning. J. Hazard. Mater. 2020, 398, 122941. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, P.; Chen, M.; Bao, Q.; Wang, H.; Wang, Y.; Fu, H. The Effect of Intercropping with Different Leguminous Green Manures on the Soil Environment and Tea Quality in Tea Plantations. Microorganisms 2024, 12, 1721. https://doi.org/10.3390/microorganisms12081721
Zhou P, Chen M, Bao Q, Wang H, Wang Y, Fu H. The Effect of Intercropping with Different Leguminous Green Manures on the Soil Environment and Tea Quality in Tea Plantations. Microorganisms. 2024; 12(8):1721. https://doi.org/10.3390/microorganisms12081721
Chicago/Turabian StyleZhou, Pinqian, Mengjuan Chen, Qiang Bao, Hua Wang, Yuanjiang Wang, and Haiping Fu. 2024. "The Effect of Intercropping with Different Leguminous Green Manures on the Soil Environment and Tea Quality in Tea Plantations" Microorganisms 12, no. 8: 1721. https://doi.org/10.3390/microorganisms12081721



