Influence of Copper on Oleidesulfovibrio alaskensis G20 Biofilm Formation
Abstract
1. Introduction
2. Materials and Methods
2.1. Bacterial Strain, Culture, and Growth
2.2. Experimental Setup for Planktonic Cells
2.3. Biofilm Formation in 96-Well Plates
2.4. Biofilm Growth on Glass Coupons
2.5. Estimation of Protein and Carbohydrate Content
2.6. RNA Isolation and cDNA Synthesis
2.7. RT-qPCR Gene Expression
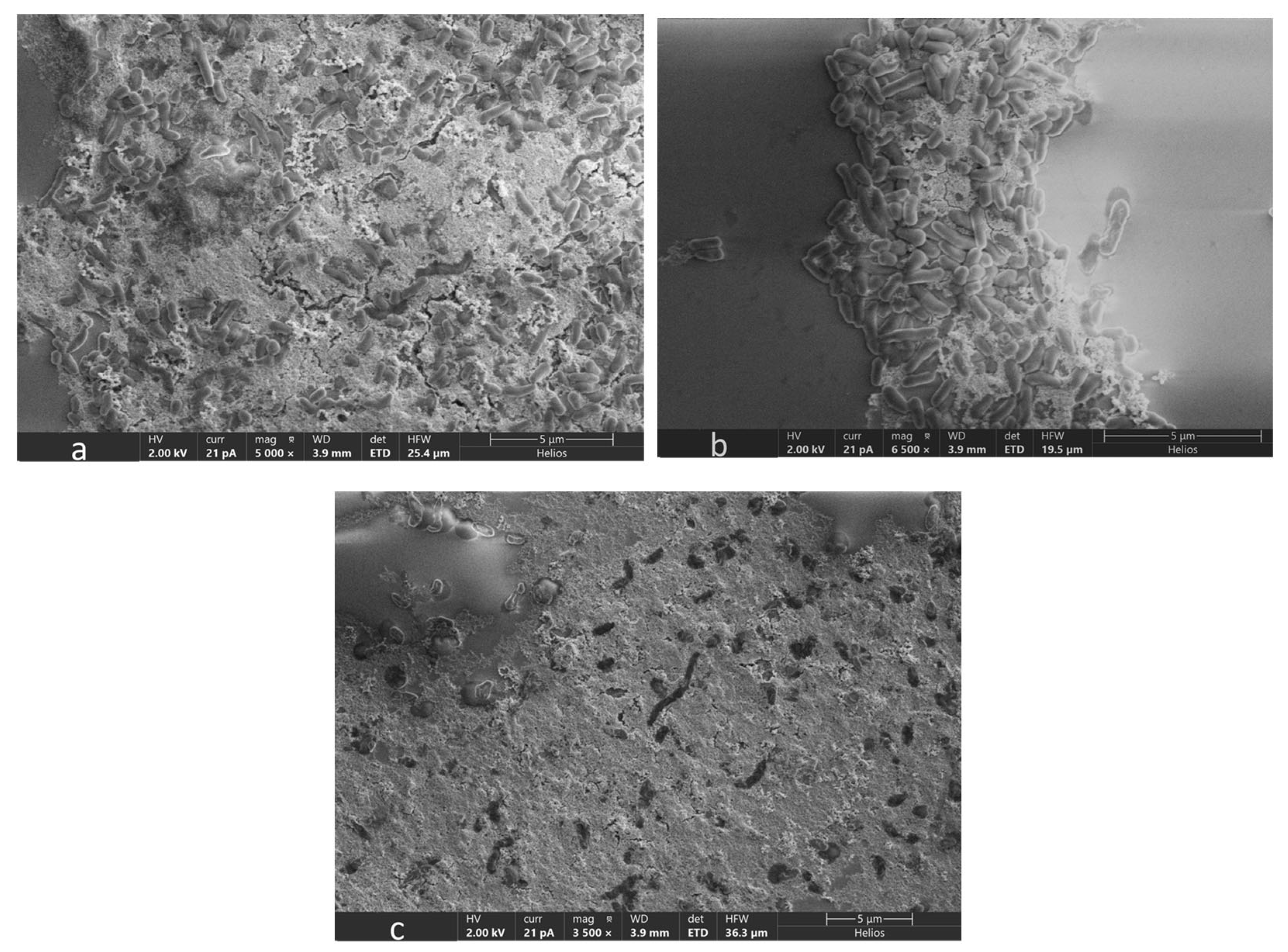
2.8. Scanning Electron Microscopy (SEM)
2.9. Confocal Microscopy
3. Results and Discussions
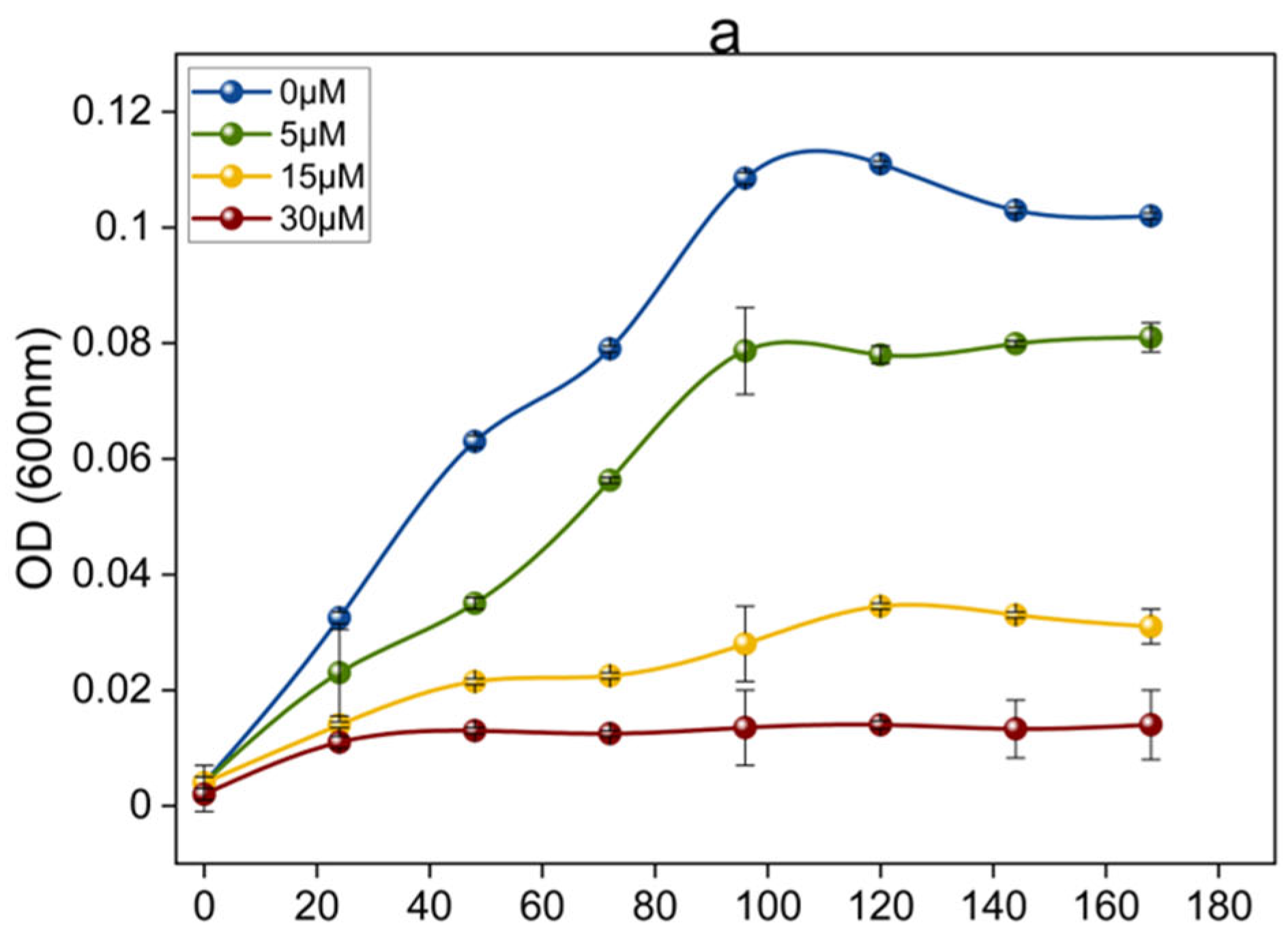
3.1. Growth of OA G20 Under Variable Cu Concentrations
3.2. Biofilm Formation Under Copper Stress
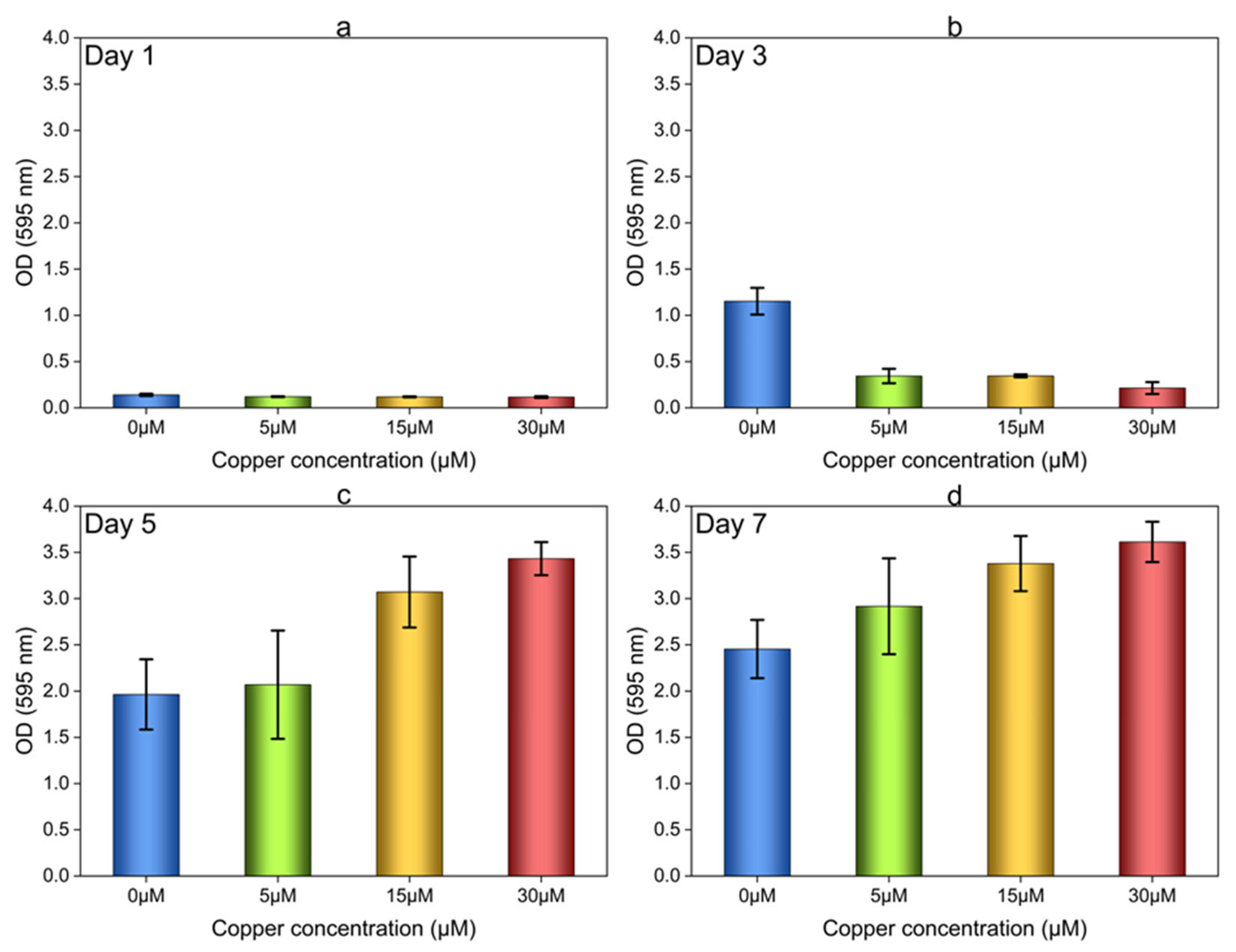
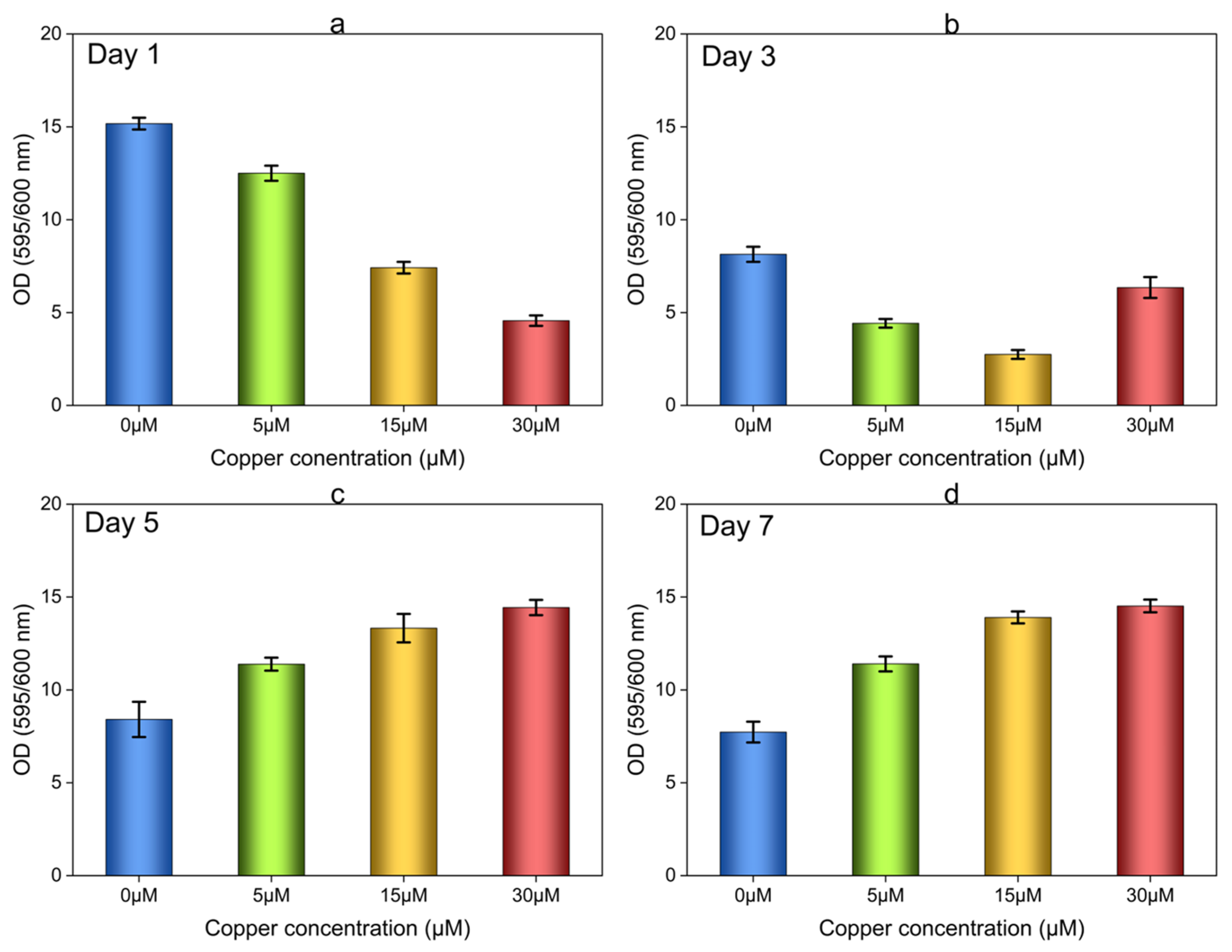
3.2.1. Biofilm Growth in 96-Well Plates
3.2.2. Biofilm Formation on Glass Coupons
3.3. Copper Resistance Level of OA- G20 Planktonic and Biofilm Cells
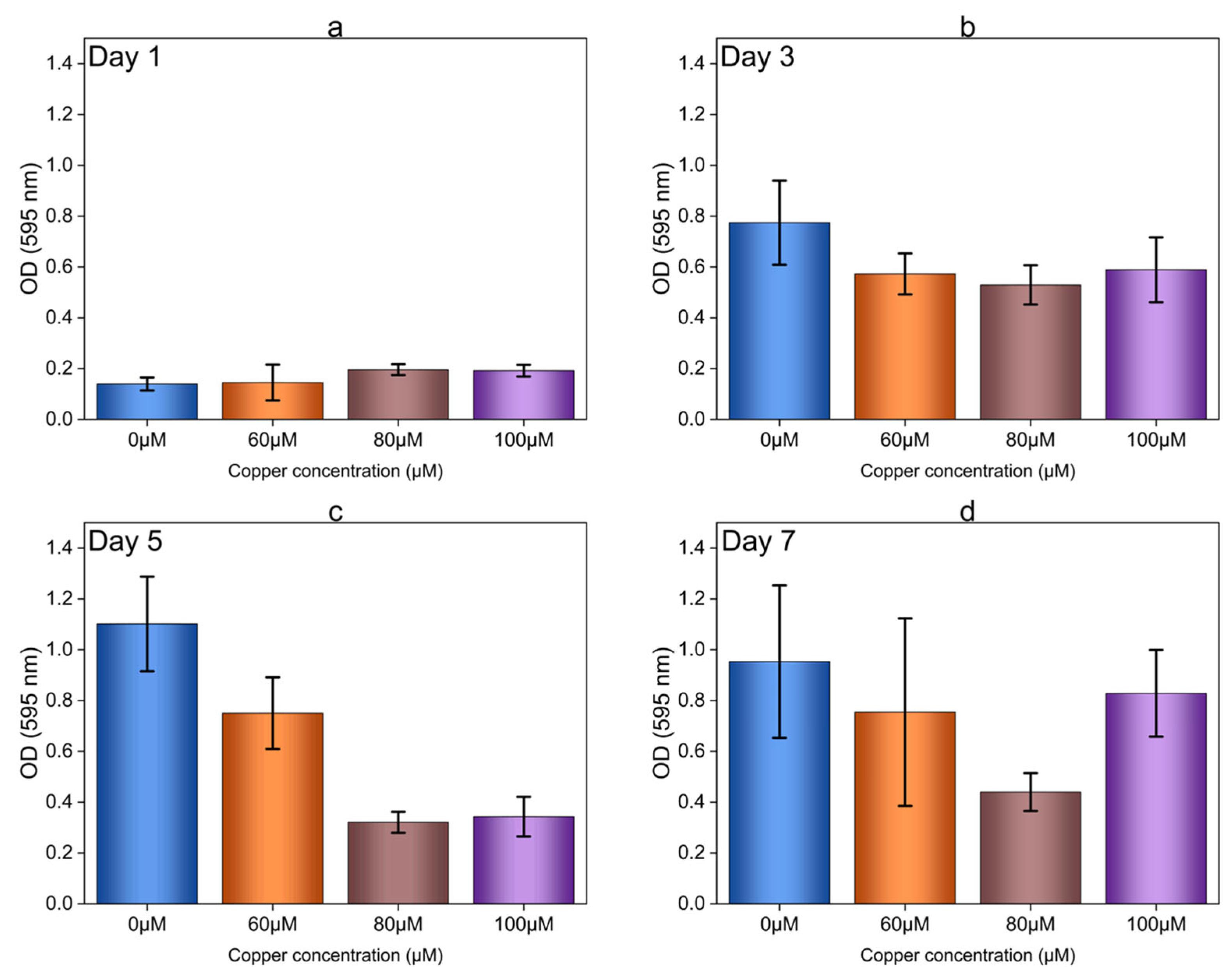
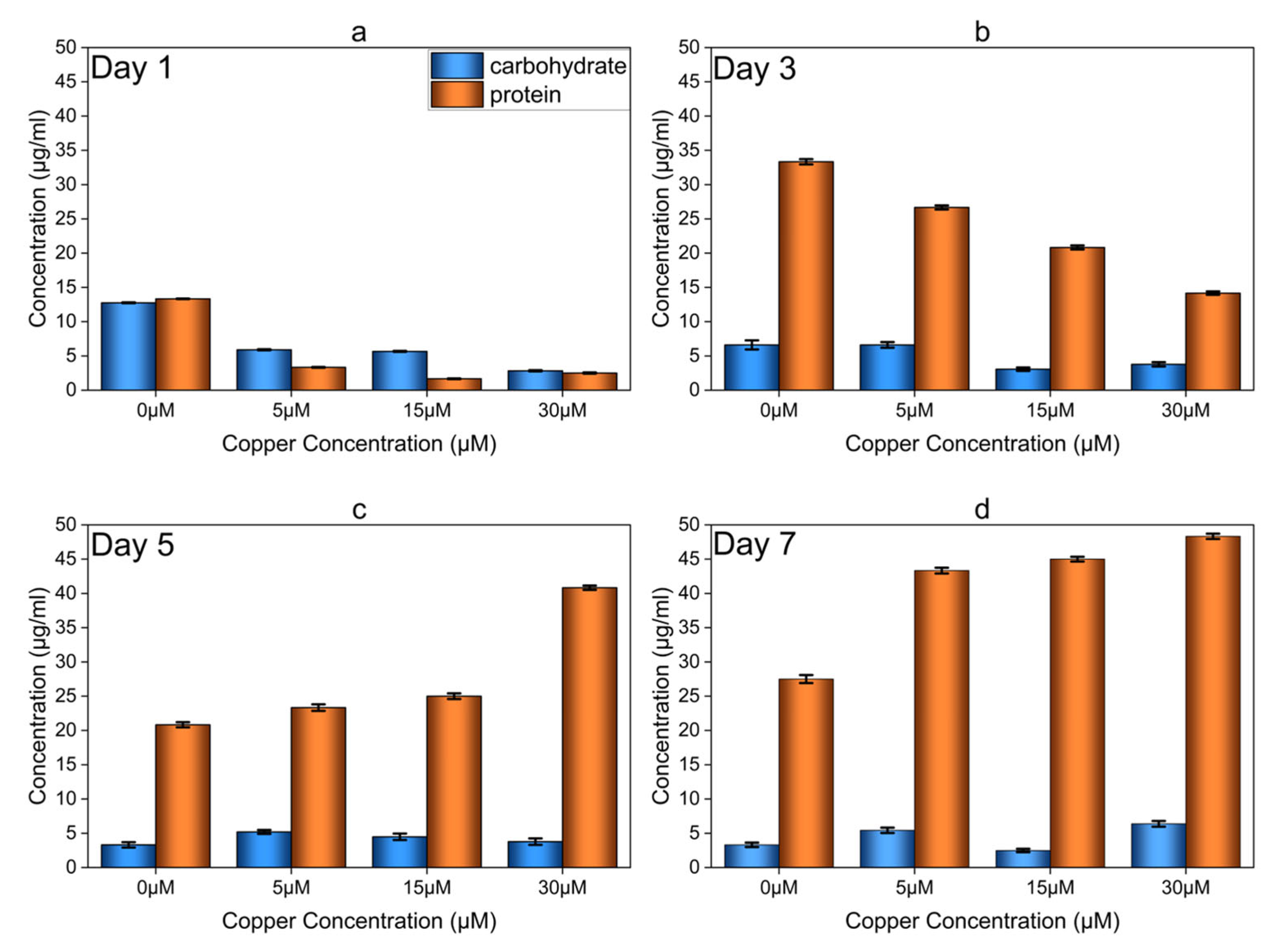
3.4. Total Protein and Carbohydrate Analysis of Copper-Induced Biofilms
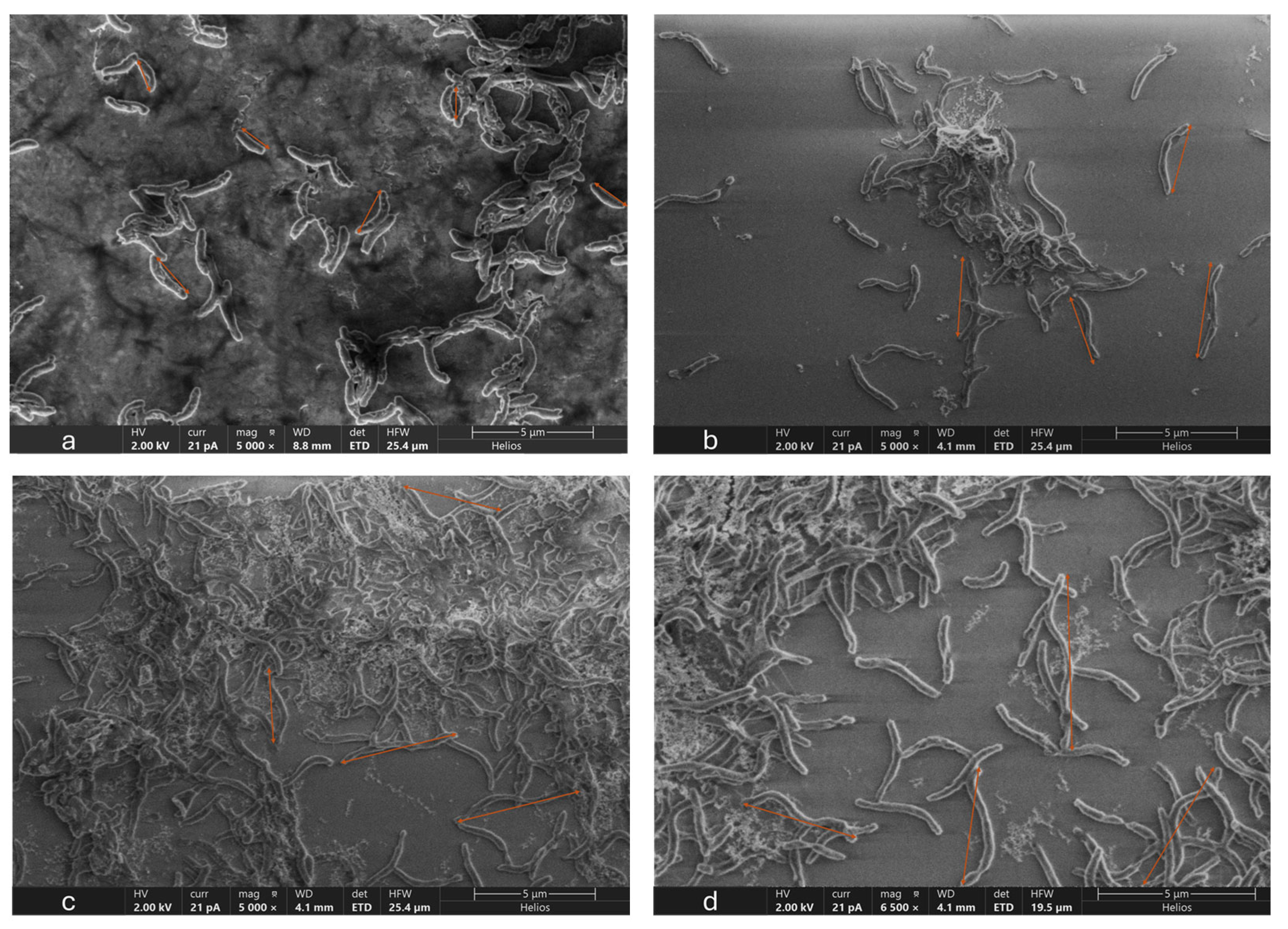
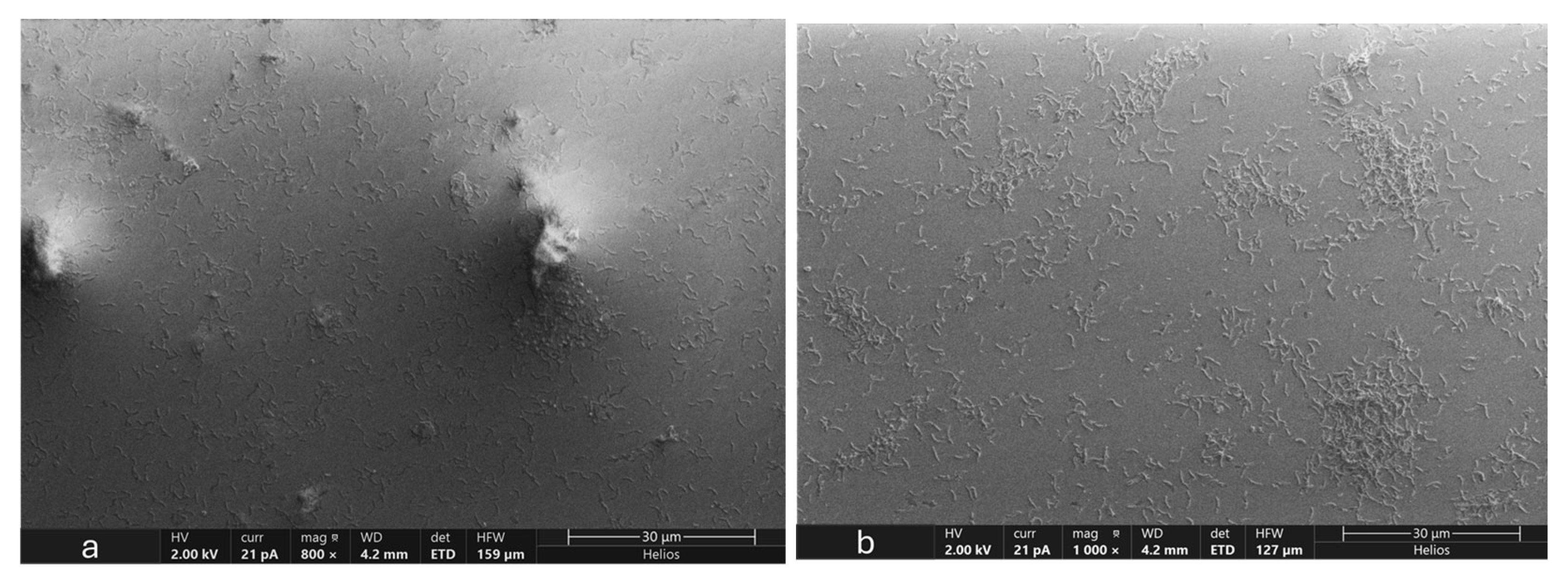
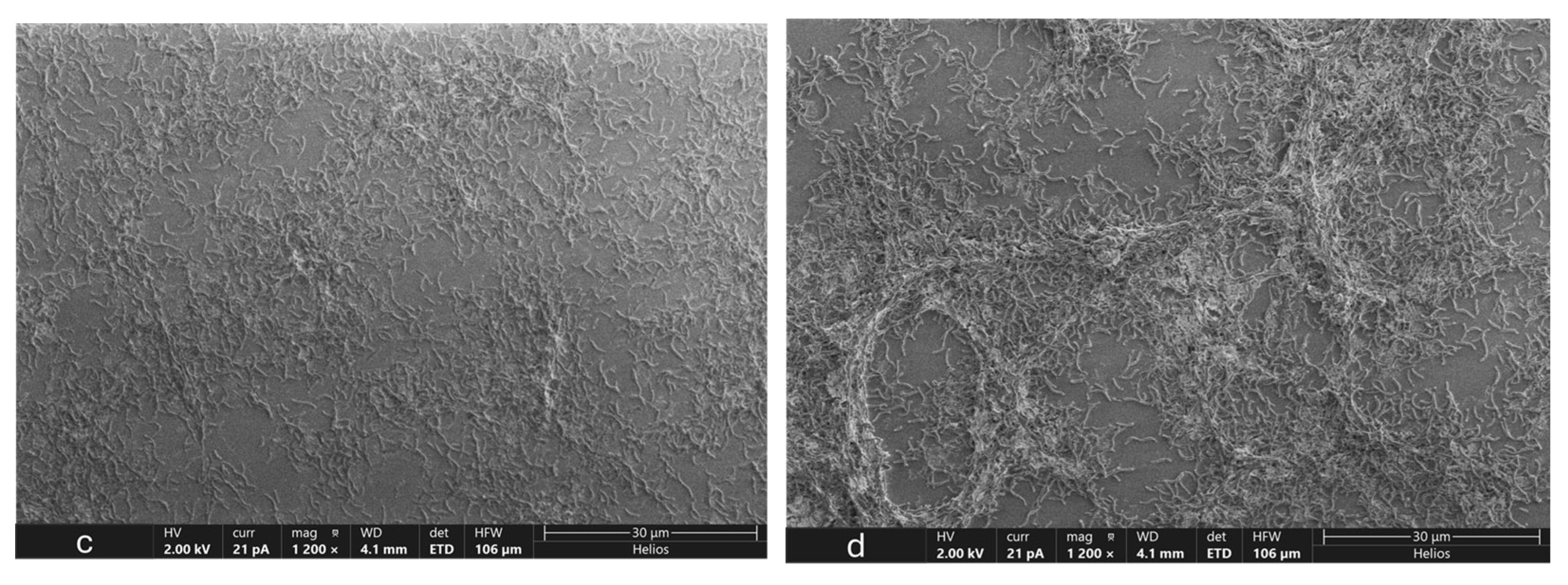
3.5. Microscopic Analysis of OA G20 Biofilm
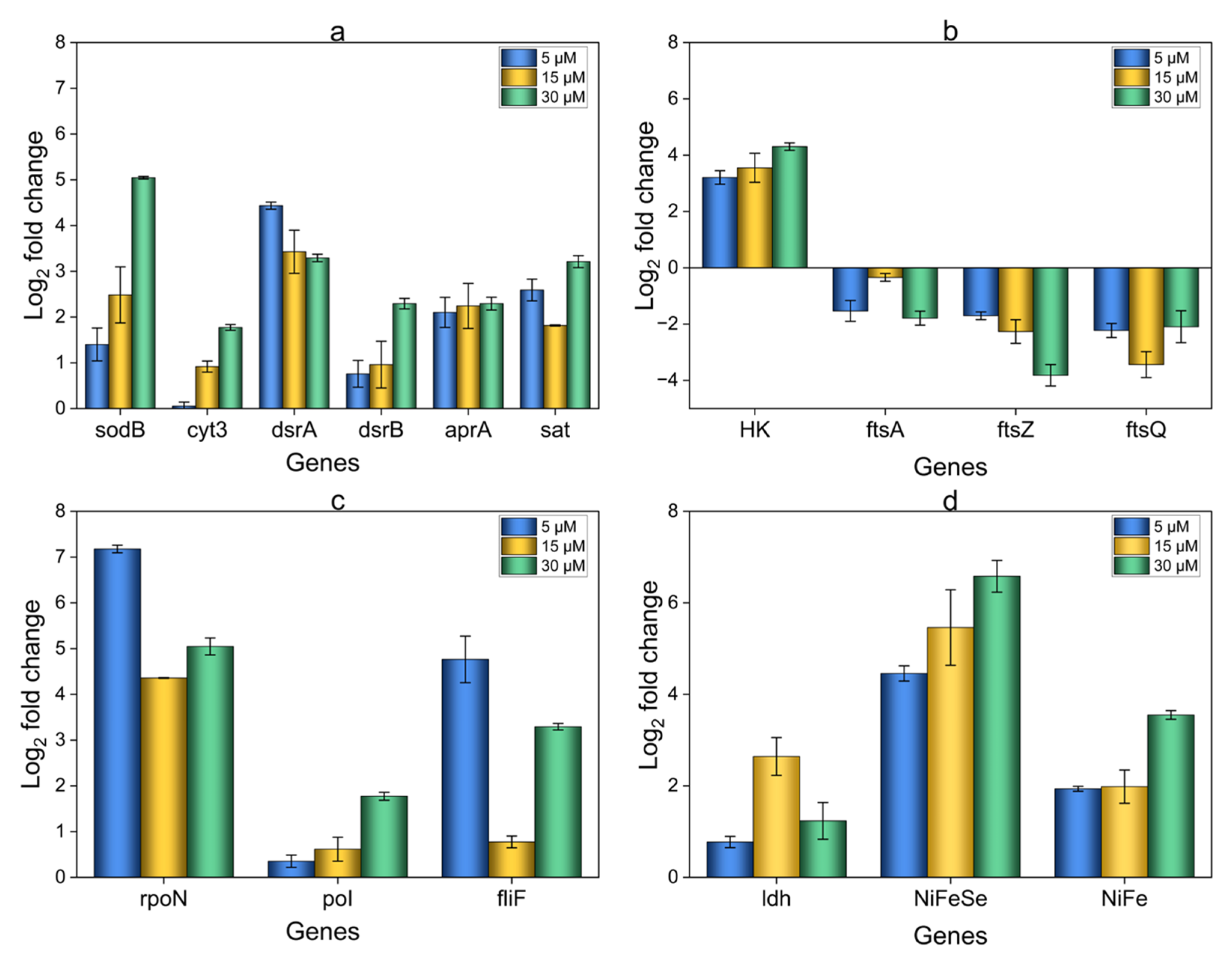
3.6. Relative Expression Analysis of OA G20 Biofilms
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Crispim, J.S.; Dias, R.S.; Laguardia, C.N.; Araújo, L.C.; da Silva, J.D.; Vidigal, P.M.P.; de Sousa, M.P.; da Silva, C.C.; Santana, M.F.; de Paula, S.O. Desulfovibrio alaskensis prophages and their possible involvement in the horizontal transfer of genes by outer membrane vesicles. Gene 2019, 703, 50–57. [Google Scholar] [CrossRef] [PubMed]
- Beech, I.B.; Sunner, J.A. Sulphate-reducing bacteria and their role in. In Sulphate-Reducing Bacteria: Environmental and Engineered Systems; Cambridge University Press: Cambridge, UK, 2007. [Google Scholar]
- Tripathi, A.K.; Thakur, P.; Saxena, P.; Rauniyar, S.; Gopalakrishnan, V.; Singh, R.N.; Gadhamshetty, V.; Gnimpieba, E.Z.; Jasthi, B.K.; Sani, R.K. Gene Sets and Mechanisms of Sulfate-Reducing Bacteria Biofilm Formation and Quorum Sensing With Impact on Corrosion. Front. Microbiol. 2021, 12, 754140. [Google Scholar] [CrossRef]
- Rodrigues, C.M.; Takita, M.A.; Coletta-Filho, H.D.; Olivato, J.C.; Caserta, R.; Machado, M.A.; De Souza, A.A. Copper resistance of biofilm cells of the plant pathogen Xylella fastidiosa. Appl. Microbiol. Biotechnol. 2008, 77, 1145–1157. [Google Scholar] [CrossRef] [PubMed]
- Roy, P.K.; Ha, A.J.-W.; Mizan, M.F.R.; Hossain, M.I.; Ashrafudoulla, M.; Toushik, S.H.; Nahar, S.; Kim, Y.K.; Ha, S.-D. Effects of environmental conditions (temperature, pH, and glucose) on biofilm formation of Salmonella enterica serotype Kentucky and virulence gene expression. Poult. Sci. 2021, 100, 101209. [Google Scholar] [CrossRef] [PubMed]
- Clark, M.E.; Edelmann, R.E.; Duley, M.L.; Wall, J.D.; Fields, M.W. Biofilm formation in Desulfovibrio vulgaris Hildenborough is dependent upon protein filaments. Environ. Microbiol. 2007, 9, 2844–2854. [Google Scholar] [CrossRef]
- Lopez, P.P.; Neria, G.M.; Flores, C.L.; Aguilar, L.R. A mathematical model for cadmium removal using a sulfate reducing bacterium: Desulfovibrio alaskensis 6SR. Int. J. Environ. Res. 2013, 7, 501–512. [Google Scholar]
- White, C.; Gadd, G.M. Copper accumulation by sulfate-reducing bacterial biofilms. FEMS Microbiol. Lett. 2000, 183, 313–318. [Google Scholar] [CrossRef] [PubMed]
- White, C.; Gadd, G.M. Accumulation and effects of cadmium on sulphate-reducing bacterial biofilms. Microbiology 1998, 144, 1407–1415. [Google Scholar] [CrossRef]
- Bautista, B.E.T.; Wikieł, A.J.; Datsenko, I.; Vera, M.; Sand, W.; Seyeux, A.; Zanna, S.; Frateur, I.; Marcus, P. Influence of extracellular polymeric substances (EPS) from Pseudomonas NCIMB 2021 on the corrosion behaviour of 70Cu–30Ni alloy in seawater. J. Electroanal. Chem. 2015, 737, 184–197. [Google Scholar] [CrossRef]
- Wikieł, A.J.; Datsenko, I.; Vera, M.; Sand, W. Impact of Desulfovibrio alaskensis biofilms on corrosion behaviour of carbon steel in marine environment. Bioelectrochemistry 2014, 97, 52–60. [Google Scholar] [CrossRef]
- Elmouaden, K.; Jodeh, S.; Chaouay, A.; Oukhrib, R.; Salghi, R.; Bazzi, L.; Hilali, M. Sulfate-reducing bacteria impact on copper corrosion behavior in natural seawater environment. J. Surf. Eng. Mater. Adv. Technol. 2016, 6, 36–46. [Google Scholar] [CrossRef]
- Acharya, C. Uranium bioremediation: Approaches and challenges. In Environmental Microbial Biotechnology; Springer: Cham, Switzerland, 2015; pp. 119–132. [Google Scholar]
- Joo, J.O.; Choi, J.-H.; Kim, I.H.; Kim, Y.-K.; Oh, B.-K. Effective bioremediation of Cadmium (II), nickel (II), and chromium (VI) in a marine environment by using Desulfovibrio desulfuricans. Biotechnol. Bioprocess Eng. 2015, 20, 937–941. [Google Scholar] [CrossRef]
- Li, Y.; Yang, X.; Geng, B. Preparation of immobilized sulfate-reducing bacteria-microalgae beads for effective bioremediation of copper-containing wastewater. Water Air Soil Pollut. 2018, 229, 54. [Google Scholar] [CrossRef]
- Raya, D.; Shreya, A.; Kumar, A.; Giri, S.K.; Salem, D.R.; Gnimpieba, E.Z.; Gadhamshetty, V.; Dhiman, S.S. Molecular regulation of conditioning film formation and quorum quenching in sulfate reducing bacteria. Front. Microbiol. 2022, 13, 1008536. [Google Scholar] [CrossRef]
- Huang, N.; Mao, J.; Zhao, Y.; Hu, M.; Wang, X. Multiple transcriptional mechanisms collectively mediate copper resistance in Cupriavidus gilardii CR3. Environ. Sci. Technol. 2019, 53, 4609–4618. [Google Scholar] [CrossRef] [PubMed]
- Huang, N.; Mao, J.; Hu, M.; Wang, X.; Huo, M. Responses to copper stress in the metal-resistant bacterium Cupriavidus gilardii CR3: A whole-transcriptome analysis. J. Basic Microbiol. 2019, 59, 446–457. [Google Scholar] [CrossRef] [PubMed]
- Utgikar, V.P.; Tabak, H.H.; Haines, J.R.; Govind, R. Quantification of toxic and inhibitory impact of copper and zinc on mixed cultures of sulfate-reducing bacteria. Biotechnol. Bioeng. 2003, 82, 306–312. [Google Scholar] [CrossRef] [PubMed]
- Sani, R.K.; Peyton, B.M.; Brown, L.T. Copper-induced inhibition of growth of Desulfovibrio desulfuricans G20: Assessment of its toxicity and correlation with those of zinc and lead. Appl. Environ. Microbiol. 2001, 67, 4765–4772. [Google Scholar] [CrossRef]
- Jin, S.; Drever, J.I.; Colberg, P.J.S. Effects of copper on sulfate reduction in bacterial consortia enriched from metal-contaminated and uncontaminated sediments. Environ. Toxicol. Chem. Int. J. 2007, 26, 225–230. [Google Scholar] [CrossRef]
- Tripathi, A.K.; Saxena, P.; Thakur, P.; Rauniyar, S.; Samanta, D.; Gopalakrishnan, V.; Singh, R.N.; Sani, R.K. Transcriptomics and Functional Analysis of Copper Stress Response in the Sulfate-Reducing Bacterium Desulfovibrio alaskensis G20. Int. J. Mol. Sci. 2022, 23, 1396. [Google Scholar] [CrossRef] [PubMed]
- Lovley, D.R.; Phillips, E.J.P. Reduction of chromate by Desulfovibrio vulgaris and its c 3 cytochrome. Appl. Environ. Microbiol. 1994, 60, 726–728. [Google Scholar] [CrossRef] [PubMed]
- Payne, R.B.; Gentry, D.M.; Rapp-Giles, B.J.; Casalot, L.; Wall, J.D. Uranium reduction by Desulfovibrio desulfuricans strain G20 and a cytochrome c 3 mutant. Appl. Environ. Microbiol. 2002, 68, 3129–3132. [Google Scholar] [CrossRef]
- Pal, V.K.; Bandyopadhyay, P.; Singh, A. Hydrogen sulfide in physiology and pathogenesis of bacteria and viruses. IUBMB Life 2018, 70, 393–410. [Google Scholar] [CrossRef] [PubMed]
- Chien, C.-C.; Lin, B.-C.; Wu, C.-H. Biofilm formation and heavy metal resistance by an environmental Pseudomonas sp. Biochem. Eng. J. 2013, 78, 132–137. [Google Scholar] [CrossRef]
- Sani, R.K.; Geesey, G.; Peyton, B.M. Assessment of lead toxicity to Desulfovibrio desulfuricans G20: Influence of components of lactate C medium. Adv. Environ. Res. 2001, 5, 269–276. [Google Scholar] [CrossRef]
- Haney, E.F.; Trimble, M.J.; Cheng, J.T.; Vallé, Q.; Hancock, R.E. Critical assessment of methods to quantify biofilm growth and evaluate antibiofilm activity of host defence peptides. Biomolecules 2018, 8, 29. [Google Scholar] [CrossRef]
- Agency, U.E.P.; Programs, O.o.P. Proposed Procedure: EPA MLB SOP MB-19: Growing a Biofilm Using the CDC Biofilm Reactor. 07/20/16. Available online: https://www3.epa.gov/pesticides/chem_search/reg_actions/mlb-sop-mb19-EPA-HQ-OPP-2016-0357-0003.pdf (accessed on 10 July 2024).
- Chiba, A.; Sugimoto, S.; Sato, F.; Hori, S.; Mizunoe, Y. A refined technique for extraction of extracellular matrices from bacterial biofilms and its applicability. Microb. Biotechnol. 2015, 8, 392–403. [Google Scholar] [CrossRef]
- Jiao, Y.; Cody, G.D.; Harding, A.K.; Wilmes, P.; Schrenk, M.; Wheeler, K.E.; Banfield, J.F.; Thelen, M.P. Characterization of extracellular polymeric substances from acidophilic microbial biofilms. Appl. Environ. Microbiol. 2010, 76, 2916–2922. [Google Scholar] [CrossRef]
- Samanta, D.; Govil, T.; Saxena, P.; Krumholz, L.; Gadhamshetty, V.; Goh, K.M.; Sani, R.K. Genetical and Biochemical Basis of Methane Monooxygenases of Methylosinus trichosporium OB3b in Response to Copper. Methane 2024, 3, 103–121. [Google Scholar] [CrossRef]
- Gonzalez-Machado, C.; Capita, R.; Riesco-Pelaez, F.; Alonso-Calleja, C. Visualization and quantification of the cellular and extracellular components of Salmonella Agona biofilms at different stages of development. PLoS ONE 2018, 13, e0200011. [Google Scholar] [CrossRef]
- Heggendorn, F.L.; Fraga, A.G.M.; Ferreira, D.d.C.; Gonçalves, L.S.; Lione, V.d.O.F.; Lutterbach, M.T.S. Sulfate-reducing bacteria: Biofilm formation and corrosive activity in endodontic files. Int. J. Dent. 2018. [Google Scholar] [CrossRef] [PubMed]
- Soler-Arango, J.; Figoli, C.; Muraca, G.; Bosch, A.; Brelles-Marino, G. The Pseudomonas aeruginosa biofilm matrix and cells are drastically impacted by gas discharge plasma treatment: A comprehensive model explaining plasma-mediated biofilm eradication. PLoS ONE 2019, 14, e0216817. [Google Scholar] [CrossRef] [PubMed]
- Dong, Y.; Gao, Z.; Di, J.; Wang, D.; Yang, Z.; Wang, Y.; Xie, Z. Study on the effectiveness of sulfate reducing bacteria to remove heavy metals (Fe, Mn, Cu, Cr) in acid mine drainage. Sustainability 2023, 15, 5486. [Google Scholar] [CrossRef]
- Moll, L.; Badosa, E.; Planas, M.; Feliu, L.; Montesinos, E.; Bonaterra, A. Antimicrobial peptides with antibiofilm activity against Xylella fastidiosa. Front. Microbiol. 2021, 12, 753874. [Google Scholar] [CrossRef]
- Zhu, Y.; Miao, L. Effects of Specific Surface Area of Artificial Carriers on Carbon Metabolism Activity of Biofilm. Water 2022, 14, 2735. [Google Scholar] [CrossRef]
- Tran, T.T.T.; Kannoorpatti, K.; Padovan, A.; Thennadil, S. A study of bacteria adhesion and microbial corrosion on different stainless steels in environment containing Desulfovibrio vulgaris. R. Soc. Open Sci. 2021, 8, 201577. [Google Scholar] [CrossRef]
- Huang, Y.; Chakraborty, S.; Liang, H. Methods to probe the formation of biofilms: Applications in foods and related surfaces. Anal. Methods 2020, 12, 416–432. [Google Scholar] [CrossRef]
- Qi, Z.; Chen, L.; Zhang, W. Comparison of transcriptional heterogeneity of eight genes between batch Desulfovibrio vulgaris biofilm and planktonic culture at a single-cell level. Front. Microbiol. 2016, 7, 597. [Google Scholar] [CrossRef]
- Koechler, S.; Farasin, J.; Cleiss-Arnold, J.; Arsène-Ploetze, F. Toxic metal resistance in biofilms: Diversity of microbial responses and their evolution. Res. Microbiol. 2015, 166, 764–773. [Google Scholar] [CrossRef]
- Thakur, P.; Alaba, M.O.; Rauniyar, S.; Singh, R.N.; Saxena, P.; Bomgni, A.; Gnimpieba, E.Z.; Lushbough, C.; Goh, K.M.; Sani, R.K. Text-Mining to Identify Gene Sets Involved in Biocorrosion by Sulfate-Reducing Bacteria: A Semi-Automated Workflow. Microorganisms 2023, 11, 119. [Google Scholar] [CrossRef]
- van Hullebusch, E.D.; Zandvoort, M.H.; Lens, P.N.L. Metal immobilisation by biofilms: Mechanisms and analytical tools. Rev. Environ. Sci. Biotechnol. 2003, 2, 9–33. [Google Scholar] [CrossRef]
- Navarrete, F.; De La Fuente, L. Response of Xylella fastidiosa to zinc: Decreased culturability, increased exopolysaccharide production, and formation of resilient biofilms under flow conditions. Appl. Environ. Microbiol. 2014, 80, 1097–1107. [Google Scholar] [CrossRef]
- Jang, A.; Kim, S.M.; Kim, S.Y.; Lee, S.G.; Kim, I.S. Effect of heavy metals (Cu, Pb, and Ni) on the compositions of EPS in biofilms. Water Sci. Technol. 2001, 43, 41–48. [Google Scholar] [CrossRef] [PubMed]
- Telegdi, J.; Shaban, A.; Vastag, G. Biocorrosion–Steel; Elsevier: Amsterdam, The Netherlands, 2018. [Google Scholar]
- Carvalho, F.G.d.; Puppin-Rontani, R.M.; Fúcio, S.B.P.d.; Negrini, T.d.C.; Carlo, H.L.; Garcia-Godoy, F. Analysis by confocal laser scanning microscopy of the MDPB bactericidal effect on S. mutans biofilm CLSM analysis of MDPB bactericidal effect on biofilm. J. Appl. Oral Sci. 2012, 20, 568–575. [Google Scholar] [CrossRef]
- Hou, J.; Veeregowda, D.H.; van de Belt-Gritter, B.; Busscher, H.J.; van der Mei, H.C. Extracellular polymeric matrix production and relaxation under fluid shear and mechanical pressure in Staphylococcus aureus biofilms. Appl. Environ. Microbiol. 2018, 84, e01516–e01517. [Google Scholar] [CrossRef]
- Mathivanan, K.; Chandirika, J.U.; Vinothkanna, A.; Yin, H.; Liu, X.; Meng, D. Bacterial adaptive strategies to cope with metal toxicity in the contaminated environment–A review. Ecotoxicol. Environ. Saf. 2021, 226, 112863. [Google Scholar] [CrossRef]
- Mohite, B.V.; Koli, S.H.; Patil, S.V. Heavy metal stress and its consequences on exopolysaccharide (EPS)-producing Pantoea agglomerans. Appl. Biochem. Biotechnol. 2018, 186, 199–216. [Google Scholar] [CrossRef]
- Mathivanan, K.; Chandirika, J.U.; Mathimani, T.; Rajaram, R.; Annadurai, G.; Yin, H. Production and functionality of exopolysaccharides in bacteria exposed to a toxic metal environment. Ecotoxicol. Environ. Saf. 2021, 208, 111567. [Google Scholar] [CrossRef]
- Jawaharraj, K.; Peta, V.; Dhiman, S.S.; Gnimpieba, E.Z.; Gadhamshetty, V. Transcriptome-wide marker gene expression analysis of stress-responsive sulfate-reducing bacteria. Sci. Rep. 2023, 13, 16181. [Google Scholar] [CrossRef]
- Mussmann, M.; Richter, M.; Lombardot, T.; Meyerdierks, A.; Kuever, J.; Kube, M.; Glöckner, F.O.; Amann, R. Clustered genes related to sulfate respiration in uncultured prokaryotes support the theory of their concomitant horizontal transfer. J. Bacteriol. 2005, 187, 7126–7137. [Google Scholar] [CrossRef]
- Scarascia, G.; Lehmann, R.; Machuca, L.L.; Morris, C.; Cheng, K.Y.; Kaksonen, A.; Hong, P.-Y. Effect of quorum sensing on the ability of Desulfovibrio vulgaris to form biofilms and to biocorrode carbon steel in saline conditions. Appl. Environ. Microbiol. 2019, 86, e01664-19. [Google Scholar] [CrossRef] [PubMed]
- Leavitt, W.D.; Venceslau, S.S.; Waldbauer, J.; Smith, D.A.; Pereira, I.A.C.; Bradley, A.S. Proteomic and isotopic response of Desulfovibrio vulgaris to DsrC perturbation. Front. Microbiol. 2019, 10, 658. [Google Scholar] [CrossRef]
- Database, K.M. Sulfur Metabolism. Available online: https://www.genome.jp/dbget-bin/www_bget?path:map00920 (accessed on 10 July 2024).
- KEGG. Two-Component System. Available online: https://www.genome.jp/dbget-bin/www_bget?path:map02020 (accessed on 10 July 2024).
- Marques, M.d.V.; Gomes, S.L.; Gober, J.W. A gene coding for a putative sigma 54 activator is developmentally regulated in Caulobacter crescentus. J. Bacteriol. 1997, 179, 5502–5510. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; Li, J.; Ding, Y.; Shao, X.; Sun, Y.; Xie, F.; Liu, S.; Tang, S.; Deng, X. An atlas of bacterial two-component systems reveals function and plasticity in signal transduction. Cell Rep. 2022, 41, 111502. [Google Scholar] [CrossRef] [PubMed]
- Gudipaty, S.A.; McEvoy, M.M. The histidine kinase CusS senses silver ions through direct binding by its sensor domain. Biochim. Et Biophys. Acta (BBA)-Proteins Proteom. 2014, 1844, 1656–1661. [Google Scholar] [CrossRef]
- Rismondo, J.; Große, C.; Nies, D.H. The Sensory Histidine Kinase CusS of Escherichia coli Senses Periplasmic Copper Ions. Microbiol. Spectr. 2023, 11, e00291-23. [Google Scholar] [CrossRef]
- Chen, Z.; Srivastava, P.; Zarazúa-Osorio, B.; Marathe, A.; Fujita, M.; Igoshin, O.A. Bacillus subtilis histidine kinase KinC activates biofilm formation by controlling heterogeneity of single-cell responses. MBio 2022, 13, e01694-21. [Google Scholar] [CrossRef]
- Falke, J.J.; Bass, R.B.; Butler, S.L.; Chervitz, S.A.; Danielson, M.A. The two-component signaling pathway of bacterial chemotaxis: A molecular view of signal transduction by receptors, kinases, and adaptation enzymes. Annu. Rev. Cell Dev. Biol. 1997, 13, 457. [Google Scholar] [CrossRef]
- Francke, C.; Groot Kormelink, T.; Hagemeijer, Y.; Overmars, L.; Sluijter, V.; Moezelaar, R.; Siezen, R.J. Comparative analyses imply that the enigmatic Sigma factor 54 is a central controller of the bacterial exterior. BMC Genom. 2011, 12, 1–21. [Google Scholar] [CrossRef]
- Liu, X.; Ye, Y.; Zhu, Y.; Wang, L.; Yuan, L.; Zhu, J.; Sun, A. Involvement of RpoN in regulating motility, biofilm, resistance, and spoilage potential of Pseudomonas fluorescens. Front. Microbiol. 2021, 12, 641844. [Google Scholar] [CrossRef]
- Jiang, Z.; Zhang, Y.; Zhu, X.; Zhou, Y.; Qian, Q.; Gao, X.; Jiang, Q.; Zhang, X. Involvement of RpoN in regulating stress response, biofilm formation and virulence of Vibrio mimicus. Aquaculture 2024, 578, 740116. [Google Scholar] [CrossRef]
- Lloyd, M.G.; Lundgren, B.R.; Hall, C.W.; Gagnon, L.B.; Mah, T.F.; Moffat, J.F.; Nomura, C.T. Targeting the alternative sigma factor RpoN to combat virulence in Pseudomonas aeruginosa. Sci. Rep. 2017, 7, 12615. [Google Scholar] [CrossRef] [PubMed]
- Mahmud, A.K.M.F.; Nilsson, K.; Fahlgren, A.; Navais, R.; Choudhury, R.; Avican, K.; Fällman, M. Genome-scale mapping reveals complex regulatory activities of RpoN in Yersinia pseudotuberculosis. Msystems 2020, 5, 10–1128. [Google Scholar] [CrossRef]
- Zhang, W.; Culley, D.E.; Nie, L.; Scholten, J. Comparative transcriptome analysis of Desulfovibrio vulgaris grown in planktonic culture and mature biofilm on a steel surface. Appl. Microbiol. Biotechnol. 2007, 76, 447–457. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Chakraborty, S.; Hosseinzadeh, P.; Yu, Y.; Tian, S.; Petrik, I.; Bhagi, A.; Lu, Y. Metalloproteins containing cytochrome, iron–sulfur, or copper redox centers. Chem. Rev. 2014, 114, 4366–4469. [Google Scholar] [PubMed]
- Pereira, I.A.C.; Ramos, A.R.; Grein, F.; Marques, M.C.; Da Silva, S.M.; Venceslau, S.S. A comparative genomic analysis of energy metabolism in sulfate reducing bacteria and archaea. Front. Microbiol. 2011, 2, 69. [Google Scholar] [CrossRef]
- Beliaev, D.V.; Tereshonok, D.V.; Lunkova, N.F.; Baranova, E.N.; Osipova, E.S.; Lisovskii, S.V.; Raldugina, G.N.; Kuznetsov, V.V. Expression of Cytochrome c3 from Desulfovibrio vulgaris in Plant Leaves Enhances Uranium Uptake and Tolerance of Tobacco. Int. J. Mol. Sci. 2021, 22, 12622. [Google Scholar] [CrossRef] [PubMed]
- Haveman, S.A.; Greene, E.A.; Stilwell, C.P.; Voordouw, J.K.; Voordouw, G. Physiological and gene expression analysis of inhibition of Desulfovibrio vulgaris Hildenborough by nitrite. J. Bacteriol. 2004, 186, 7944–7950. [Google Scholar] [CrossRef]
- Larsson, J. Redox Regulation and Stress Responses-Studies in Bacillus subtilis. Ph.D. Thesis, Lund University, Lund, Sweden, 2005. [Google Scholar]
- Durand, A.; Azzouzi, A.; Bourbon, M.-L.; Steunou, A.-S.; Liotenberg, S.; Maeshima, A.; Astier, C.; Argentini, M.; Saito, S.; Ouchane, S. c-Type cytochrome assembly is a key target of copper toxicity within the bacterial periplasm. MBio 2015, 6, 10–1128. [Google Scholar] [CrossRef]
- Zhou, A.; Baidoo, E.; He, Z.; Mukhopadhyay, A.; Baumohl, J.K.; Benke, P.; Joachimiak, M.P.; Xie, M.; Song, R.; Arkin, A.P. Characterization of NaCl tolerance in Desulfovibrio vulgaris Hildenborough through experimental evolution. ISME J. 2013, 7, 1790–1802. [Google Scholar] [CrossRef]
- Pereira, P.M.; He, Q.; Xavier, A.V.; Zhou, J.; Pereira, I.A.C.; Louro, R.O. Transcriptional response of Desulfovibrio vulgaris Hildenborough to oxidative stress mimicking environmental conditions. Arch. Microbiol. 2008, 189, 451–461. [Google Scholar] [CrossRef][Green Version]
- Chien, A.-C.; Hill, N.S.; Levin, P.A. Cell size control in bacteria. Curr. Biol. 2012, 22, R340–R349. [Google Scholar] [CrossRef]
- Yoon, M.Y.; Lee, K.-M.; Park, Y.; Yoon, S.S. Contribution of cell elongation to the biofilm formation of Pseudomonas aeruginosa during anaerobic respiration. PLoS ONE 2011, 6, e16105. [Google Scholar] [CrossRef] [PubMed]
- Pratt, L.A.; Kolter, R. Genetic analysis of Escherichia coli biofilm formation: Roles of flagella, motility, chemotaxis and type I pili. Mol. Microbiol. 1998, 30, 285–293. [Google Scholar] [CrossRef] [PubMed]
- López-Sánchez, A.; Leal-Morales, A.; Jiménez-Díaz, L.; Platero, A.I.; Bardallo-Pérez, J.; Díaz-Romero, A.; Acemel, R.D.; Illán, J.M.; Jiménez-López, J.; Govantes, F. Biofilm formation-defective mutants in Pseudomonas putida. FEMS Microbiol. Lett. 2016, 363, fnw127. [Google Scholar] [CrossRef]
- Yang, S.; Ma, L.; Xu, X.; Peng, Q.; Zhong, H.; Gong, Y.; Shi, L.; He, M.; Shi, B.; Qiao, Y. Physiological and Transcriptomic Analyses of Escherichia coli Serotype O157: H7 in Response to Rhamnolipid Treatment. Microorganisms 2023, 11, 2112. [Google Scholar] [CrossRef]
- Zhou, A.; He, Z.; Redding-Johanson, A.M.; Mukhopadhyay, A.; Hemme, C.L.; Joachimiak, M.P.; Luo, F.; Deng, Y.; Bender, K.S.; He, Q. Hydrogen peroxide-induced oxidative stress responses in Desulfovibrio vulgaris Hildenborough. Environ. Microbiol. 2010, 12, 2645–2657. [Google Scholar] [CrossRef]
- Ghosh, P.; Mondal, J.; Ben-Jacob, E.; Levine, H. Mechanically-driven phase separation in a growing bacterial colony. Proc. Natl. Acad. Sci. USA 2015, 112, E2166–E2173. [Google Scholar] [CrossRef] [PubMed]
- Marvasi, M.; Visscher, P.T.; Casillas Martinez, L. Exopolymeric substances (EPS) from Bacillus subtilis: Polymers and genes encoding their synthesis. FEMS Microbiol. Lett. 2010, 313, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Gupta, P.; Diwan, B. Bacterial exopolysaccharide mediated heavy metal removal: A review on biosynthesis, mechanism and remediation strategies. Biotechnol. Rep. 2017, 13, 58–71. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Thakur, P.; Gopalakrishnan, V.; Saxena, P.; Subramaniam, M.; Goh, K.M.; Peyton, B.; Fields, M.; Sani, R.K. Influence of Copper on Oleidesulfovibrio alaskensis G20 Biofilm Formation. Microorganisms 2024, 12, 1747. https://doi.org/10.3390/microorganisms12091747
Thakur P, Gopalakrishnan V, Saxena P, Subramaniam M, Goh KM, Peyton B, Fields M, Sani RK. Influence of Copper on Oleidesulfovibrio alaskensis G20 Biofilm Formation. Microorganisms. 2024; 12(9):1747. https://doi.org/10.3390/microorganisms12091747
Chicago/Turabian StyleThakur, Payal, Vinoj Gopalakrishnan, Priya Saxena, Mahadevan Subramaniam, Kian Mau Goh, Brent Peyton, Matthew Fields, and Rajesh Kumar Sani. 2024. "Influence of Copper on Oleidesulfovibrio alaskensis G20 Biofilm Formation" Microorganisms 12, no. 9: 1747. https://doi.org/10.3390/microorganisms12091747
APA StyleThakur, P., Gopalakrishnan, V., Saxena, P., Subramaniam, M., Goh, K. M., Peyton, B., Fields, M., & Sani, R. K. (2024). Influence of Copper on Oleidesulfovibrio alaskensis G20 Biofilm Formation. Microorganisms, 12(9), 1747. https://doi.org/10.3390/microorganisms12091747







