Genomic Functional Analysis of Novel Radiation-Resistant Species of Knollia sp. nov. S7-12T from the North Slope of Mount Everest
Abstract
1. Introduction
2. Materials and Methods
2.1. Bacterial Isolation and Culture
2.2. Morphological, Physiological, and Biochemical Analysis
2.3. Chemotaxonomic Analysis
2.4. Phylogenetic Analysis
2.5. Genomic Analysis and Prediction
2.6. Radiation-Resistance Analysis
3. Results and Discussion
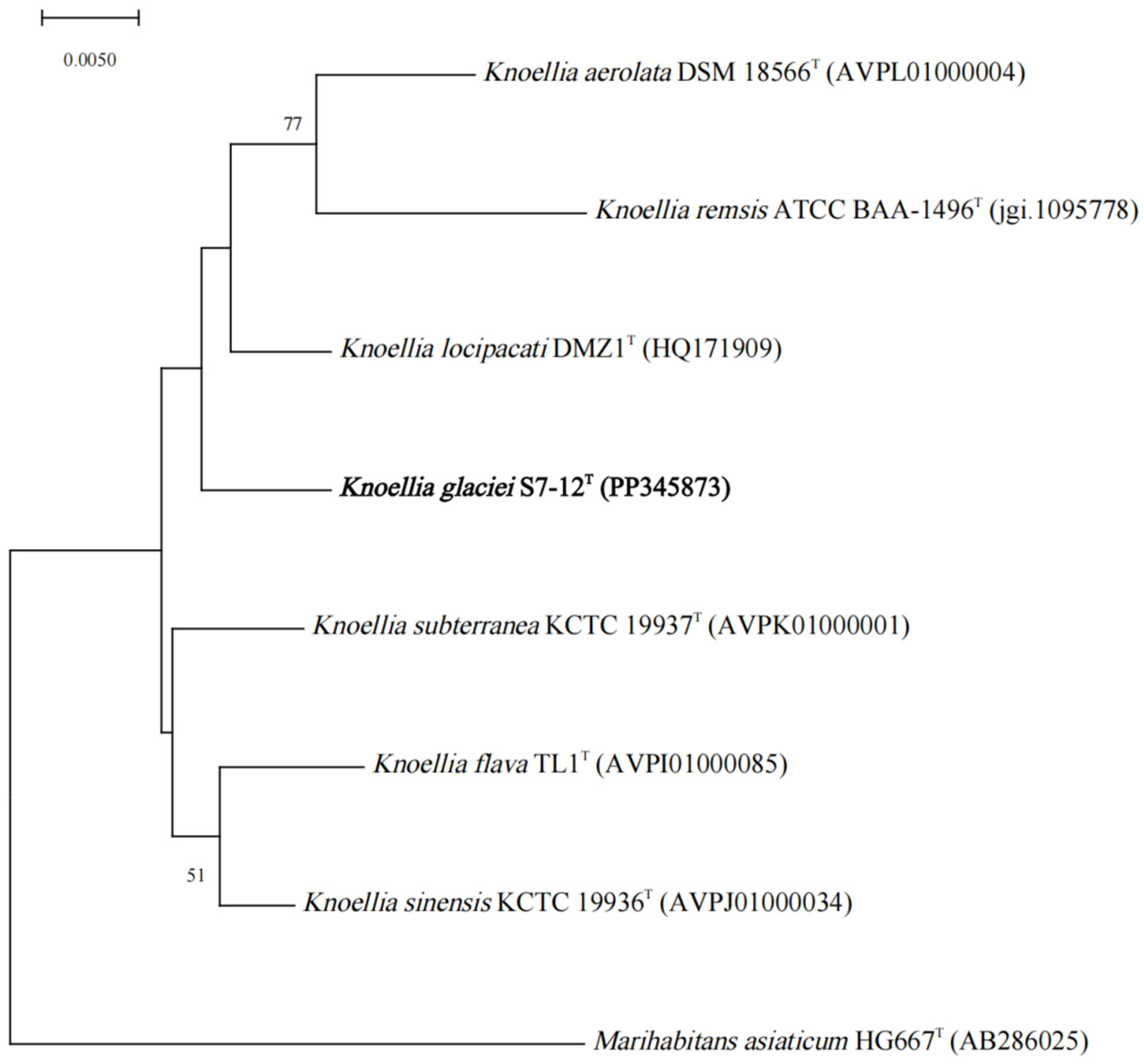
3.1. Phylogenetic Characterization Based on 16S rRNA Gene Sequencing
3.2. Phenotypic Characterization
3.3. Chemotaxonomic Characteristics
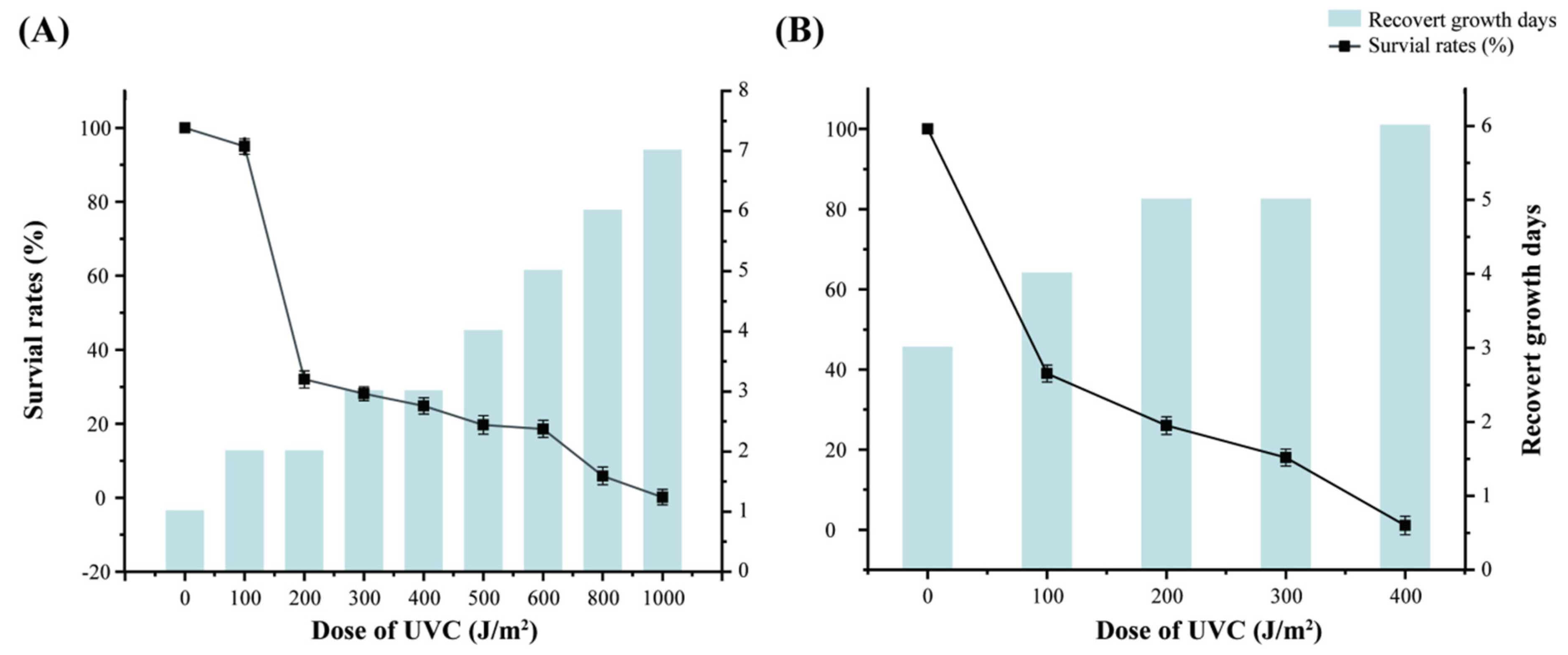
3.4. Radiation Resistance
3.5. Genomic Analysis
3.5.1. General Genome Features
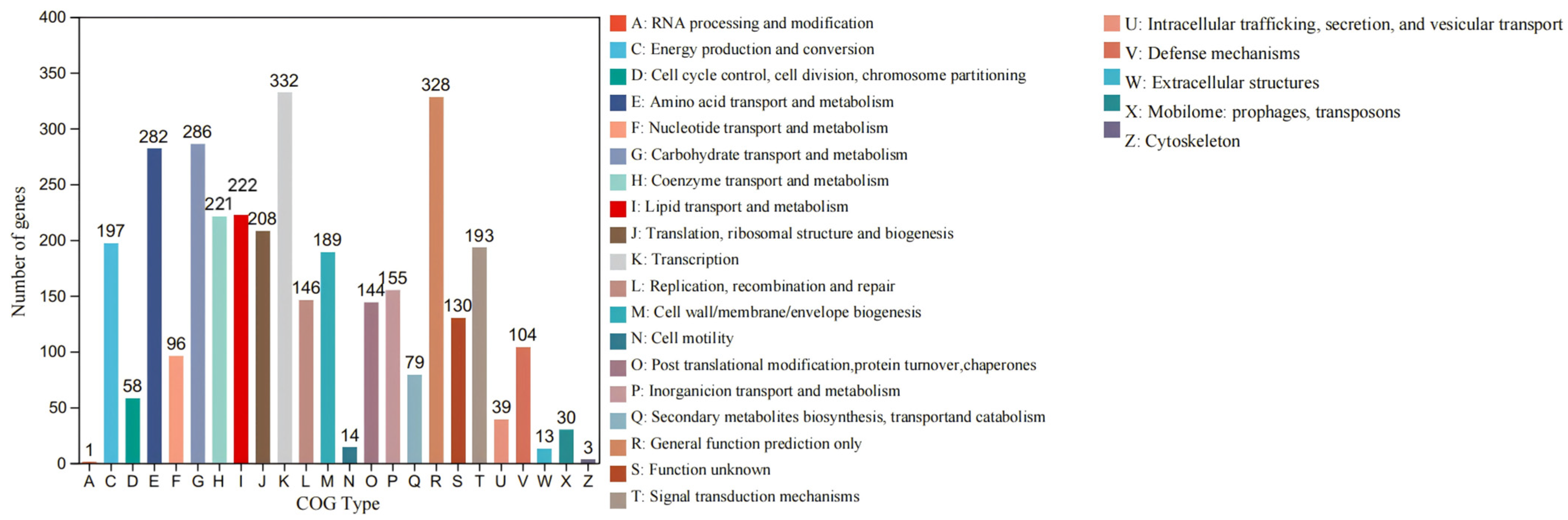
3.5.2. COG Analysis
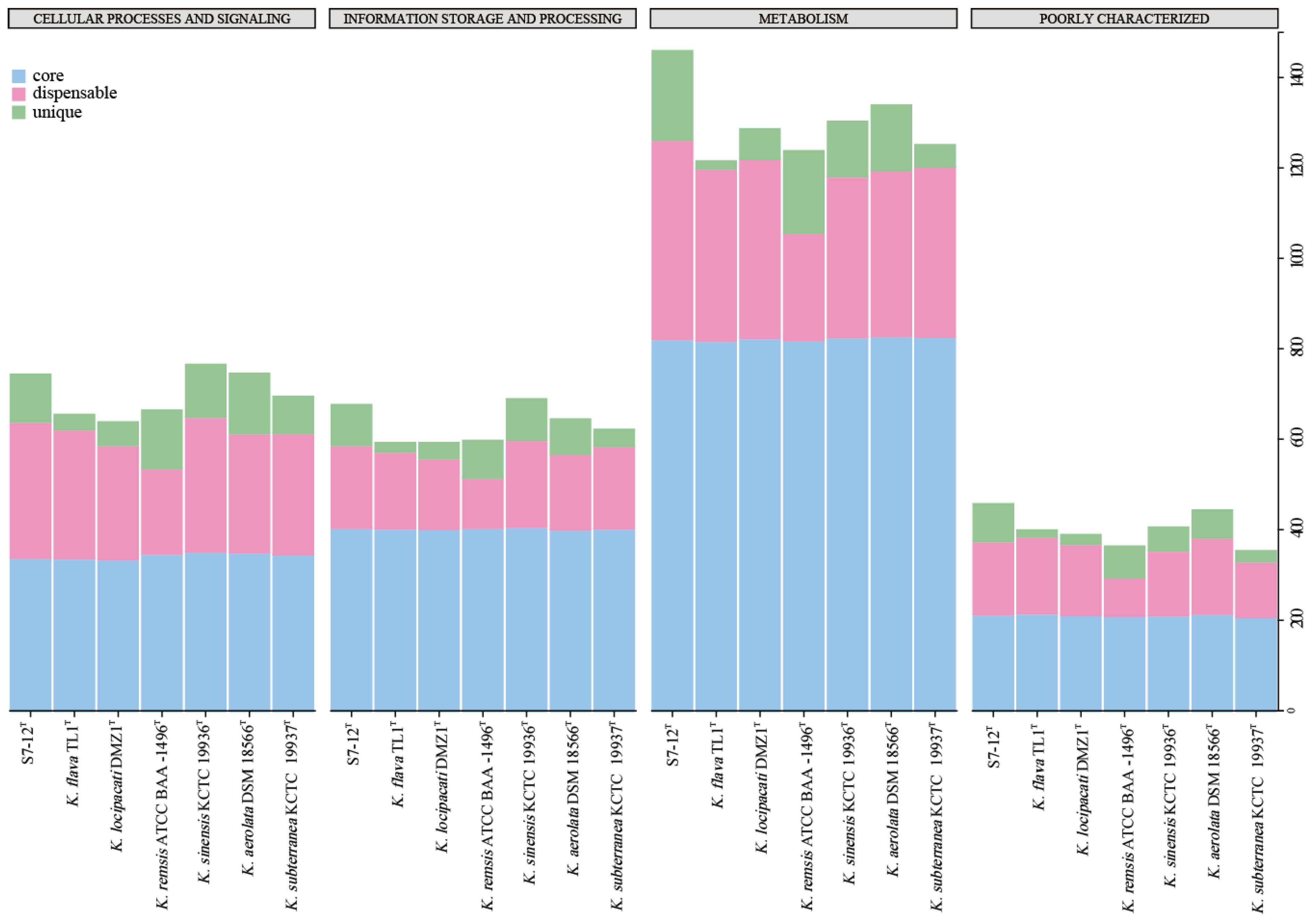
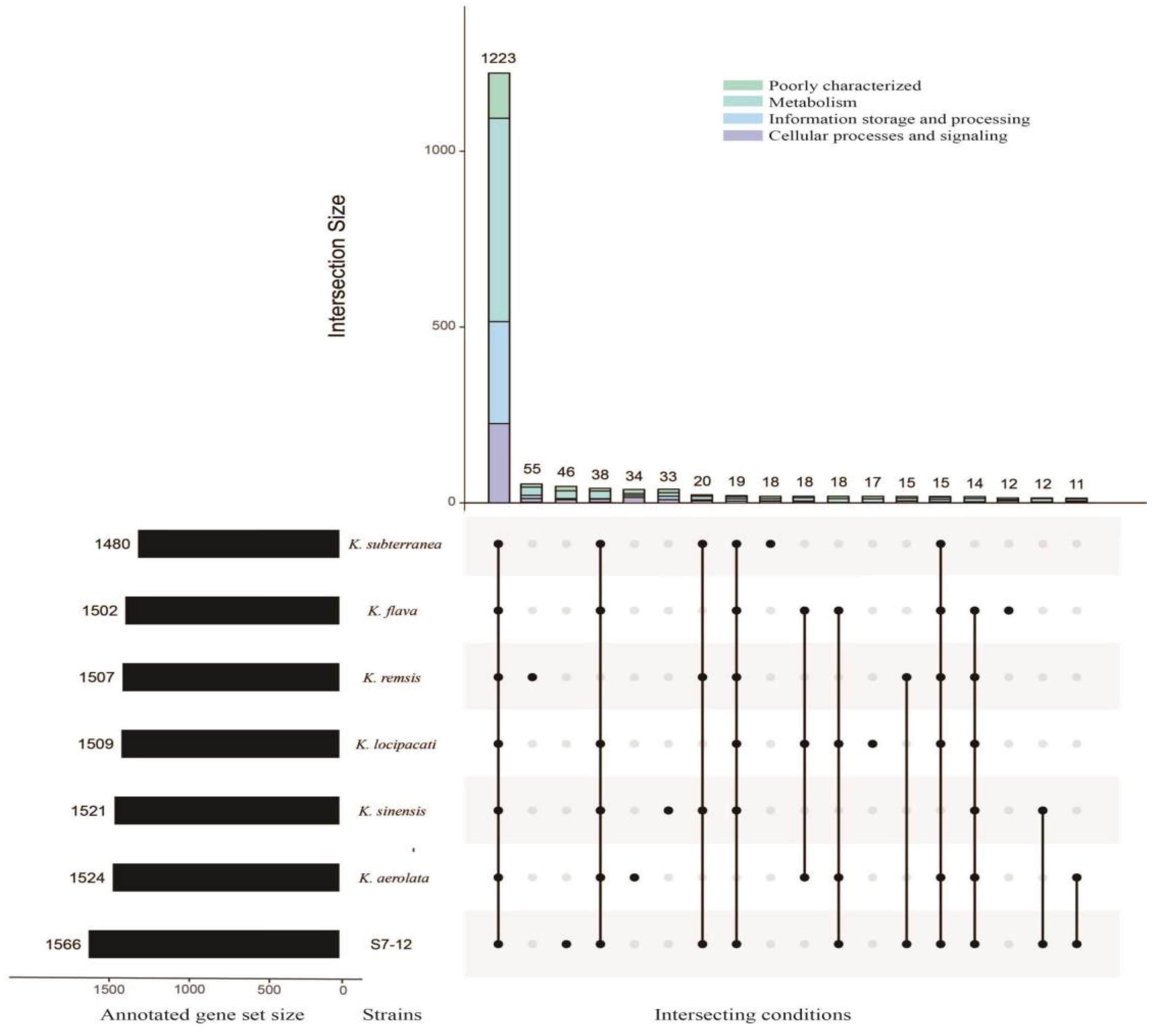
3.5.3. Pan-Genome Analysis
3.5.4. Horizontal Gene Transfer Analysis
4. Conclusions
Description of Knoellia glaciei sp. nov.
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Matthews, T.; Perry, L.B.; Lane, T.P.; Elmore, A.C.; Khadka, A.; Aryal, D.; Shrestha, D.; Tuladhar, S.; Baidya, S.K.; Gajurel, A.; et al. Into Thick(er) Air? Oxygen Availability at Humans’ Physiological Frontier on Mount Everest. iScience 2020, 23, 101718. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Cui, X.; Yang, R.; Zhang, Y.; Xu, Y.; Liu, G.; Zhang, B.; Wang, J.; Wang, X.; Zhang, W.; et al. Genomic Insights into the Radiation-Resistant Capability of Sphingomonas qomolangmaensis S5-59T and Sphingomonas glaciei S8-45T, Two Novel Bacteria from the North Slope of Mount Everest. Microorganisms 2022, 10, 2037. [Google Scholar] [CrossRef] [PubMed]
- Cui, X.; Liu, Y.; Xu, Y.; Chen, T.; Zhang, S.; Wang, J.; Yang, R.; Liu, G.; Zhang, W.; Zhang, G. Paracoccus everestensis sp. nov., a novel bacterium with great antioxidant capacity isolated from the north slope of Mount Everest. Int. J. Syst. Evol. Microbiol. 2022, 72, 005562. [Google Scholar] [CrossRef]
- Sood, U.; Dhingra, G.G.; Anand, S.; Hira, P.; Kumar, R.; Kaur, J.; Verma, M.; Singhvi, N.; Lal, S.; Rawat, C.D.; et al. Microbial Journey: Mount Everest to Mars. Indian. J. Microbiol. 2022, 62, 323–337. [Google Scholar] [CrossRef] [PubMed]
- Ji, M.; Kong, W.; Jia, H.; Delgado-Baquerizo, M.; Zhou, T.; Liu, X.; Ferrari, B.C.; Malard, L.; Liang, C.; Xue, K.; et al. Polar soils exhibit distinct patterns in microbial diversity and dominant phylotypes. Soil. Biol. Biochem. 2022, 166, 108550. [Google Scholar] [CrossRef]
- Zhang, G.-Q.; Liu, Q.; Liu, H.-C.; Zhou, Y.-G.; Xin, Y.-H. Flavobacterium ranwuense sp. nov., isolated from glacier. Int. J. Syst. Evol. Microbiol. 2019, 69, 3812–3817. [Google Scholar] [CrossRef]
- Xie, J.; Ren, L.; Wei, Z.; Peng, X.; Qin, K.; Peng, F. Pengzhenrongella phosphoraccumulans sp. nov., isolated from high Arctic glacial till, and emended description of the genus Pengzhenrongella. Int. J. Syst. Evol. Microbiol. 2024, 74, 006368. [Google Scholar] [CrossRef] [PubMed]
- Valenzuela-Ibaceta, F.; Carrasco, V.; Lagos-Moraga, S.; Dietz-Vargas, C.; Navarro, C.A.; Pérez-Donoso, J.M. Arthrobacter vasquezii sp. nov., isolated from a soil sample from Union Glacier, Antarctica. Int. J. Syst. Evol. Microbiol. 2023, 73, 006095. [Google Scholar] [CrossRef]
- Weon, H.-Y.; Kim, B.-Y.; Schumann, P.; Kroppenstedt, R.M.; Noh, H.-J.; Park, C.-W.; Kwon, S.-W. Knoellia aerolata sp nov., isolated from an air sample in Korea. Int. J. Syst. Evol. Microbiol. 2007, 57, 2861–2864. [Google Scholar] [CrossRef][Green Version]
- Shin, N.-R.; Roh, S.W.; Kim, M.-S.; Jung, M.-J.; Whon, T.W.; Bae, J.-W. Knoellia locipacati sp. nov., from soil of the Demilitarized Zone in South Korea. Int. J. Syst. Evol. Microbiol. 2012, 62, 342–346. [Google Scholar] [CrossRef]
- Osman, S.; Moissl, C.; Hosoya, N.; Briegel, A.; Mayilraj, S.; Satomi, M.; Venkateswaran, K. Tetrasphaera remsis sp. nov., isolated from the Regenerative Enclosed Life Support Module Simulator (REMS) air system. Int. J. Syst. Evol. Microbiol. 2007, 57, 2749–2753. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Du, Y.; Wang, G. Knoellia flava sp. nov., isolated from pig manure. Int. J. Syst. Evol. Microbiol. 2012, 62, 384–389. [Google Scholar] [CrossRef] [PubMed]
- Groth, I.; Schumann, P.; Schütze, B.; Augsten, K.; Stackebrandt, E. Knoellia sinensis gen. nov., sp. nov. and Knoellia subterranea sp. nov., two novel actinobacteria isolated from a cave. Int. J. Syst. Evol. Microbiol. 2002, 52, 77–84. [Google Scholar] [CrossRef]
- Battista, J.R. Against all odds: The survival strategies of Deinococcus radiodurans. Annu. Rev. Microbiol. 1997, 51, 203–224. [Google Scholar] [CrossRef]
- Liu, F.; Li, N.; Zhang, Y. The radioresistant and survival mechanisms of Deinococcus radiodurans. Radiat. Med. Prot. 2023, 4, 70–79. [Google Scholar] [CrossRef]
- Lieberman, P.; Morey, A.; Hochstadt, J.; Larson, M.; Mather, S. Mount Everest: A space analogue for speech monitoring of cognitive deficits and stress. Aviat. Space Env. Med. 2005, 76, B198–B207. [Google Scholar]
- Reasoner, D.J.; Geldreich, E.E. A new medium for the enumeration and subculture of bacteria from potable water. Appl. Env. Microbiol. 1985, 49, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Shirling, E.B.; Gottlieb, D. Methods for characterization of Streptomyces species1. Int. J. Syst. Evol. Microbiol. 1966, 16, 313–340. [Google Scholar] [CrossRef]
- Williams, S.T.; Goodfellow, M.; Alderson, G.; Wellington, E.M.H.; Sneath, P.H.A.; Sackin, M.J. Numerical Classification of Streptomyces and Related Genera. Microbiology 1983, 129, 1743–1813. [Google Scholar] [CrossRef]
- Kurup, P.V.; Schmitt, J.A. Numerical taxonomy of Nocardia. Can. J. Microbiol. 1973, 19, 1035–1048. [Google Scholar] [CrossRef] [PubMed]
- Collins, M.D.; Pirouz, T.; Goodfellow, M.; Minnikin, D.E. Distribution of menaquinones in actinomycetes and corynebacteria. J. Gen. Microbiol. 1977, 100, 221–230. [Google Scholar] [CrossRef] [PubMed]
- Lechevalier, H.A.; Lechevalier, M.P.; Gerber, N.N. Chemical Composition as a Criterion in the Classification of Actinomycetes. In Advances in Applied Microbiology; Perlman, D., Ed.; Academic Press: Cambridge, MA, USA, 1971; Volume 14, pp. 47–72. [Google Scholar]
- Staneck, J.L.; Roberts, G.D. Simplified approach to identification of aerobic actinomycetes by thin-layer chromatography. Appl. Microbiol. 1974, 28, 226–231. [Google Scholar] [CrossRef]
- Luo, R.; Liu, B.; Xie, Y.; Li, Z.; Huang, W.; Yuan, J.; He, G.; Chen, Y.; Pan, Q.; Liu, Y.; et al. SOAPdenovo2: An empirically improved memory-efficient short-read de novo assembler. Gigascience 2012, 1, 18. [Google Scholar] [CrossRef] [PubMed]
- Wick, R.R.; Judd, L.M.; Gorrie, C.L.; Holt, K.E. Unicycler: Resolving bacterial genome assemblies from short and long sequencing reads. PLoS Comput. Biol. 2017, 13, e1005595. [Google Scholar] [CrossRef]
- Lee, I.; Chalita, M.; Ha, S.M.; Na, S.I.; Yoon, S.H.; Chun, J. ContEst16S: An algorithm that identifies contaminated prokaryotic genomes using 16S RNA gene sequences. Int. J. Syst. Evol. Microbiol. 2017, 67, 2053–2057. [Google Scholar] [CrossRef]
- Richter, M.; Rosselló-Móra, R.; Oliver Glöckner, F.; Peplies, J. JSpeciesWS: A web server for prokaryotic species circumscription based on pairwise genome comparison. Bioinformatics 2016, 32, 929–931. [Google Scholar] [CrossRef] [PubMed]
- Chun, J.; Oren, A.; Ventosa, A.; Christensen, H.; Arahal, D.R.; da Costa, M.S.; Rooney, A.P.; Yi, H.; Xu, X.W.; De Meyer, S.; et al. Proposed minimal standards for the use of genome data for the taxonomy of prokaryotes. Int. J. Syst. Evol. Microbiol. 2018, 68, 461–466. [Google Scholar] [CrossRef]
- Yoon, S.H.; Ha, S.M.; Kwon, S.; Lim, J.; Kim, Y.; Seo, H.; Chun, J. Introducing EzBioCloud: A taxonomically united database of 16S rRNA gene sequences and whole-genome assemblies. Int. J. Syst. Evol. Microbiol. 2017, 67, 1613–1617. [Google Scholar] [CrossRef]
- Rodriguez-R, L.M.; Konstantinidis, K.T. Bypassing Cultivation To Identify Bacterial Species: Culture-independent genomic approaches identify credibly distinct clusters, avoid cultivation bias, and provide true insights into microbial species. Microbe Mag. 2014, 9, 111–118. [Google Scholar] [CrossRef]
- Meier-Kolthoff, J.P.; Auch, A.F.; Klenk, H.P.; Göker, M. Genome sequence-based species delimitation with confidence intervals and improved distance functions. BMC Bioinform. 2013, 14, 60. [Google Scholar] [CrossRef]
- Zhang, S.; Gan, Y.; Xu, B. Application of Plant-Growth-Promoting Fungi Trichoderma longibrachiatum T6 Enhances Tolerance of Wheat to Salt Stress through Improvement of Antioxidative Defense System and Gene Expression. Front. Plant Sci. 2016, 7, 1405. [Google Scholar] [CrossRef] [PubMed]
- Larkin, M.A.; Blackshields, G.; Brown, N.P.; Chenna, R.; McGettigan, P.A.; McWilliam, H.; Valentin, F.; Wallace, I.M.; Wilm, A.; Lopez, R.; et al. Clustal W and Clustal X version 2.0. Bioinformatics 2007, 23, 2947–2948. [Google Scholar] [CrossRef] [PubMed]
- Saitou, N.; Nei, M. The neighbor-joining method: A new method for reconstructing phylogenetic trees. Mol. Biol. Evol. 1987, 4, 406–425. [Google Scholar] [CrossRef]
- Felsenstein, J. Evolutionary trees from DNA sequences: A maximum likelihood approach. J. Mol. Evol. 1981, 17, 368–376. [Google Scholar] [CrossRef]
- Tamura, K.; Stecher, G.; Kumar, S. MEGA11: Molecular Evolutionary Genetics Analysis Version 11. Mol. Biol. Evol. 2021, 38, 3022–3027. [Google Scholar] [CrossRef]
- Nishimaki, T.; Sato, K. An Extension of the Kimura Two-Parameter Model to the Natural Evolutionary Process. J. Mol. Evol. 2019, 87, 60–67. [Google Scholar] [CrossRef]
- Na, S.I.; Kim, Y.O.; Yoon, S.H.; Ha, S.M.; Baek, I.; Chun, J. UBCG: Up-to-date bacterial core gene set and pipeline for phylogenomic tree reconstruction. J. Microbiol. 2018, 56, 280–285. [Google Scholar] [CrossRef]
- Tatusova, T.; DiCuccio, M.; Badretdin, A.; Chetvernin, V.; Nawrocki, E.P.; Zaslavsky, L.; Lomsadze, A.; Pruitt, K.D.; Borodovsky, M.; Ostell, J. NCBI prokaryotic genome annotation pipeline. Nucleic Acids Res. 2016, 44, 6614–6624. [Google Scholar] [CrossRef] [PubMed]
- Overbeek, R.; Olson, R.; Pusch, G.D.; Olsen, G.J.; Davis, J.J.; Disz, T.; Edwards, R.A.; Gerdes, S.; Parrello, B.; Shukla, M.; et al. The SEED and the Rapid Annotation of microbial genomes using Subsystems Technology (RAST). Nucleic Acids Res. 2014, 42, D206–D214. [Google Scholar] [CrossRef]
- Kanehisa, M.; Goto, S.; Kawashima, S.; Okuno, Y.; Hattori, M. The KEGG resource for deciphering the genome. Nucleic Acids Res. 2004, 32, D277–D280. [Google Scholar] [CrossRef]
- Cantalapiedra, C.P.; Hernández-Plaza, A.; Letunic, I.; Bork, P.; Huerta-Cepas, J. eggNOG-mapper v2: Functional Annotation, Orthology Assignments, and Domain Prediction at the Metagenomic Scale. Mol. Biol. Evol. 2021, 38, 5825–5829. [Google Scholar] [CrossRef] [PubMed]
- O’Leary, B.; Finn, R.S.; Turner, N.C. Treating cancer with selective CDK4/6 inhibitors. Nat. Rev. Clin. Oncol. 2016, 13, 417–430. [Google Scholar] [CrossRef] [PubMed]
- Finn, R.S.; Martin, M.; Rugo, H.S.; Jones, S.; Im, S.A.; Gelmon, K.; Harbeck, N.; Lipatov, O.N.; Walshe, J.M.; Moulder, S.; et al. Palbociclib and Letrozole in Advanced Breast Cancer. N. Engl. J. Med. 2016, 375, 1925–1936. [Google Scholar] [CrossRef]
- Bairoch, A. The ENZYME database in 2000. Nucleic Acids Res 2000, 28, 304–305. [Google Scholar] [CrossRef]
- Cantarel, B.L.; Coutinho, P.M.; Rancurel, C.; Bernard, T.; Lombard, V.; Henrissat, B. The Carbohydrate-Active EnZymes database (CAZy): An expert resource for Glycogenomics. Nucleic Acids Res. 2009, 37, D233–D238. [Google Scholar] [CrossRef] [PubMed]
- Blin, K.; Shaw, S.; Kloosterman, A.M.; Charlop-Powers, Z.; van Wezel, G.P.; Medema, M.H.; Weber, T. antiSMASH 6.0: Improving cluster detection and comparison capabilities. Nucleic Acids Res. 2021, 49, W29–W35. [Google Scholar] [CrossRef]
- Yang, T.; Gao, F. High-quality pan-genome of Escherichia coli generated by excluding confounding and highly similar strains reveals an association between unique gene clusters and genomic islands. Brief. Bioinform. 2022, 23, bbac283. [Google Scholar] [CrossRef]
- Chaudhari, N.M.; Gupta, V.K.; Dutta, C. BPGA- an ultra-fast pan-genome analysis pipeline. Sci. Rep. 2016, 6, 24373. [Google Scholar] [CrossRef]
- Yang, X.; Garuglieri, E.; Van Goethem, M.W.; Marasco, R.; Fusi, M.; Daffonchio, D. Mangrovimonas cancribranchiae sp. nov., a novel bacterial species associated with the gills of the fiddler crab Cranuca inversa (Brachyura, Ocypodidae) from Red Sea mangroves. Int. J. Syst. Evol. Microbiol. 2024, 74, 006415. [Google Scholar] [CrossRef]
- Molina-Menor, E.; Gimeno-Valero, H.; Pascual, J.; Peretó, J.; Porcar, M. High Culturable Bacterial Diversity from a European Desert: The Tabernas Desert. Front. Microbiol. 2020, 11, 583120. [Google Scholar] [CrossRef]
- Kim, M.; Oh, H.S.; Park, S.C.; Chun, J. Towards a taxonomic coherence between average nucleotide identity and 16S rRNA gene sequence similarity for species demarcation of prokaryotes. Int. J. Syst. Evol. Microbiol. 2014, 64, 346–351. [Google Scholar] [CrossRef]
- International Committee on Systematic Bacteriology announcement of the report of the ad hoc Committee on Reconciliation of Approaches to Bacterial Systematics. J. Appl. Bacteriol. 1988, 64, 283–284. [CrossRef]
- Costa, S.S.; Guimaraes, L.C.; Silva, A.; Soares, S.C.; Barauna, R.A. First Steps in the Analysis of Prokaryotic Pan-Genomes. Bioinform. Biol. Insights 2020, 14, 1177932220938064. [Google Scholar] [CrossRef] [PubMed]
- Page, A.J.; Cummins, C.A.; Hunt, M.; Wong, V.K.; Reuter, S.; Holden, M.T.G.; Fookes, M.; Falush, D.; Keane, J.A.; Parkhill, J. Roary: Rapid large-scale prokaryote pan genome analysis. Bioinformatics 2015, 31, 3691–3693. [Google Scholar] [CrossRef] [PubMed]
- Luo, H.; Lin, Y.; Gao, F.; Zhang, C.T.; Zhang, R. DEG 10, an update of the database of essential genes that includes both protein-coding genes and noncoding genomic elements. Nucleic Acids Res. 2014, 42, D574–D580. [Google Scholar] [CrossRef]
- Wu, H.; Wang, D.; Gao, F. Toward a high-quality pan-genome landscape of Bacillus subtilis by removal of confounding strains. Brief. Bioinform. 2021, 22, 1951–1971. [Google Scholar] [CrossRef]
- Tettelin, H.; Riley, D.; Cattuto, C.; Medini, D. Comparative genomics: The bacterial pan-genome. Curr. Opin. Microbiol. 2008, 11, 472–477. [Google Scholar] [CrossRef]
- Fang, X.; Qin, K.; Li, S.; Han, S.; Zhu, T.; Fang, X.; Qin, K. Whole genome sequence of Diaporthe capsici, a new pathogen of walnut blight. Genomics 2020, 112, 3751–3761. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.-J.; Lim, J.-M.; Hamada, M.; Ahn, J.-H.; Weon, H.-Y.; Suzuki, K.-i.; Ahn, T.-Y.; Kwon, S.-W. Oryzobacter terrae gen. nov., sp nov., isolated from paddy soil. Int. J. Syst. Evol. Microbiol. 2015, 65, 3190–3195. [Google Scholar] [CrossRef]
- Lamilla, C.; Pavez, M.; Santos, A.; Hermosilla, A.; Llanquinao, V.; Barrientos, L. Bioprospecting for extracellular enzymes from culturable Actinobacteria from the South Shetland Islands, Antarctica. Polar Biol. 2017, 40, 719–726. [Google Scholar] [CrossRef]
- Veech, R.L.; Todd King, M.; Pawlosky, R.; Kashiwaya, Y.; Bradshaw, P.C.; Curtis, W. The “great” controlling nucleotide coenzymes. IUBMB Life 2019, 71, 565–579. [Google Scholar] [CrossRef] [PubMed]
- Nirwal, S.; Czarnocki-Cieciura, M.; Chaudhary, A.; Zajko, W.; Skowronek, K.; Chamera, S.; Figiel, M.; Nowotny, M. Mechanism of RecF-RecO-RecR cooperation in bacterial homologous recombination. Nat. Struct. Mol. Biol. 2023, 30, 650–660. [Google Scholar] [CrossRef] [PubMed]
- Del Val, E.; Nasser, W.; Abaibou, H.; Reverchon, S. RecA and DNA recombination: A review of molecular mechanisms. Biochem. Soc. Trans. 2019, 47, 1511–1531. [Google Scholar] [CrossRef]
- Sedgwick, B.; Bates, P.A.; Paik, J.; Jacobs, S.C.; Lindahl, T. Repair of alkylated DNA: Recent advances. DNA Repair. 2007, 6, 429–442. [Google Scholar] [CrossRef]
- Xiumin, L.I.U.; Jing, W.U.; Wei, Z.; Wei, L.U.; Shuzhen, P.; Min, L.I.N.; Ming, C. Disruption and characterization of the excision repair pathway in the extremely radioresistant bacterium Deinococcus SP. BR501. Acta Agric. Nucleatae Sin. 2007, 21, 357–361. [Google Scholar]
- Mennecier, S.; Coste, G.; Servant, P.; Bailone, A.; Sommer, S. Mismatch repair ensures fidelity of replication and recombination in the radioresistant organism Deinococcus radiodurans. Mol. Genet. Genom. 2004, 272, 460–469. [Google Scholar] [CrossRef]
- Piróg, A.; Cantini, F.; Nierzwicki, Ł.; Obuchowski, I.; Tomiczek, B.; Czub, J.; Liberek, K. Two Bacterial Small Heat Shock Proteins, IbpA and IbpB, Form a Functional Heterodimer. J. Mol. Biol. 2021, 433, 167054. [Google Scholar] [CrossRef] [PubMed]
- Turan, M. Genome-wide analysis and characterization of HSP gene families (HSP20, HSP40, HSP60, HSP70, HSP90) in the yellow fever mosquito (Aedes aegypti) (Diptera: Culicidae). J. Insect Sci. 2023, 23, 27. [Google Scholar] [CrossRef]
- Yu, E.-m.; Yoshinaga, T.; Jalufka, F.L.; Ehsan, H.; Mark Welch, D.B.; Kaneko, G. The complex evolution of the metazoan HSP70 gene family. Sci. Rep. 2021, 11, 17794. [Google Scholar] [CrossRef] [PubMed]
- Genest, O.; Wickner, S.; Doyle, S.M. Hsp90 and Hsp70 chaperones: Collaborators in protein remodeling. J. Biol. Chem. 2019, 294, 2109–2120. [Google Scholar] [CrossRef]
- Yamanaka, K.; Fang, L.; Inouye, M. The CspA family in Escherichia coli: Multiple gene duplication for stress adaptation. Mol. Microbiol. 1998, 27, 247–255. [Google Scholar] [CrossRef] [PubMed]
- Bisht, S.C.; Joshi, G.K.; Mishra, P.K. CspA encodes a major cold shock protein in Himalayan psychrotolerant Pseudomonas strains. Interdiscip. Sci. 2014, 6, 140–148. [Google Scholar] [CrossRef] [PubMed]
- Sützl, L.; Foley, G.; Gillam, E.M.J.; Bodén, M.; Haltrich, D. The GMC superfamily of oxidoreductases revisited: Analysis and evolution of fungal GMC oxidoreductases. Biotechnol. Biofuels 2019, 12, 118. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, T.; Sugano, Y. A structural and functional perspective of DyP-type peroxidase family. Arch. Biochem. Biophys. 2015, 574, 49–55. [Google Scholar] [CrossRef]
- Borisov, V.B.; Siletsky, S.A.; Paiardini, A.; Hoogewijs, D.; Forte, E.; Giuffrè, A.; Poole, R.K. Bacterial Oxidases of the Cytochrome bd Family: Redox Enzymes of Unique Structure, Function, and Utility as Drug Targets. Antioxid. Redox Signal 2021, 34, 1280–1318. [Google Scholar] [CrossRef]
- Yin, V.; Shaw, G.S.; Konermann, L. Cytochrome c as a Peroxidase: Activation of the Precatalytic Native State by H2O2-Induced Covalent Modifications. J. Am. Chem. Soc. 2017, 139, 15701–15709. [Google Scholar] [CrossRef]
- Kehl-Fie, T.E.; Zhang, Y.; Moore, J.L.; Farrand, A.J.; Hood, M.I.; Rathi, S.; Chazin, W.J.; Caprioli, R.M.; Skaar, E.P. MntABC and MntH contribute to systemic Staphylococcus aureus infection by competing with calprotectin for nutrient manganese. Infect. Immun. 2013, 81, 3395–3405. [Google Scholar] [CrossRef]
- Sikora, A.E.; Gomez, C.; Le Van, A.; Baarda, B.I.; Darnell, S.; Martinez, F.G.; Zielke, R.A.; Bonventre, J.A.; Jerse, A.E. A novel gonorrhea vaccine composed of MetQ lipoprotein formulated with CpG shortens experimental murine infection. Vaccine 2020, 38, 8175–8184. [Google Scholar] [CrossRef] [PubMed]
- Kohm, K.; Floccari, V.A.; Lutz, V.T.; Nordmann, B.; Mittelstädt, C.; Poehlein, A.; Dragoš, A.; Commichau, F.M.; Hertel, R. The Bacillus phage SPβ and its relatives: A temperate phage model system reveals new strains, species, prophage integration loci, conserved proteins and lysogeny management components. bioRxiv 2021. [Google Scholar] [CrossRef]
- Bay, D.C.; Rommens, K.L.; Turner, R.J. Small multidrug resistance proteins: A multidrug transporter family that continues to grow. Biochim. Biophys. Acta 2008, 1778, 1814–1838. [Google Scholar] [CrossRef]
- Cruz, A.; Micaelo, N.; Félix, V.; Song, J.Y.; Kitamura, S.; Suzuki, S.; Mendo, S. sugE: A gene involved in tributyltin (TBT) resistance of Aeromonas molluscorum Av27. J. Gen. Appl. Microbiol. 2013, 59, 39–47. [Google Scholar] [CrossRef]
- Verma, D.; Gupta, V. New insights into the structure and function of an emerging drug target CysE. 3 Biotech 2021, 11, 373. [Google Scholar] [CrossRef]
- Siegers, K.; Heinzmann, S.; Entian, K.D. Biosynthesis of lantibiotic nisin—Posttranslational modification of its prepeptide occurs at a multimeric membrane-associated lanthionine synthetase complex. J. Biol. Chem. 1996, 271, 12294–12301. [Google Scholar] [CrossRef] [PubMed]
- Fahey, D.; O’Brien, J.; Pagnon, J.; Page, S.; Wilson, R.; Slamen, N.; Roddam, L.; Ambrose, M. DinB (DNA polymerase IV), ImuBC and RpoS contribute to the generation of ciprofloxacin-resistance mutations in Pseudomonas aeruginosa. Mutat. Res. 2023, 827, 111836. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Zhao, H.; Yang, H.; He, C.; Shu, W.; Cui, Z.; Liu, Q. Insights Into the Impact of Small RNA SprC on the Metabolism and Virulence of Staphylococcus aureus. Front. Cell Infect. Microbiol. 2022, 12, 746746. [Google Scholar] [CrossRef] [PubMed]
- Marcus, S.A.; Sidiropoulos, S.W.; Steinberg, H.; Talaat, A.M. CsoR Is Essential for Maintaining Copper Homeostasis in Mycobacterium tuberculosis. PLoS ONE 2016, 11, e0151816. [Google Scholar] [CrossRef]
- Hirose, M.; Kuroda, Y.; Murata, E. NGF/TrkA Signaling as a Therapeutic Target for Pain. Pain. Pr. 2016, 16, 175–182. [Google Scholar] [CrossRef] [PubMed]
- Schnell, R.; Sriram, D.; Schneider, G. Pyridoxal-phosphate dependent mycobacterial cysteine synthases: Structure, mechanism and potential as drug targets. Biochim. Biophys. Acta 2015, 1854, 1175–1183. [Google Scholar] [CrossRef]
- Fan, Y.; Bai, J.; Xi, D.; Yang, B. RpoE Facilitates Stress-Resistance, Invasion, and Pathogenicity of Escherichia coli K1. Microorganisms 2022, 10, 879. [Google Scholar] [CrossRef]
- Tran, H.T.; Bonilla, C.Y. SigB-regulated antioxidant functions in gram-positive bacteria. World J. Microbiol. Biotechnol. 2021, 37, 38. [Google Scholar] [CrossRef]
- Cabrejos, M.E.; Zhao, H.L.; Guacucano, M.; Bueno, S.; Levican, G.; Garcia, E.; Jedlicki, E.; Holmes, D.S. IST1 insertional inactivation of the resB gene: Implications for phenotypic switching in Thiobacillus ferrooxidans. FEMS Microbiol. Lett. 1999, 175, 223–229. [Google Scholar] [CrossRef]
- Hübschmann, T.; Jorissen, H.; Börner, T.; Gärtner, W.; de Marsac, N.T. Phosphorylation of proteins in the light-dependent signalling pathway of a filamentous cyanobacterium. Eur. J. Biochem. 2001, 268, 3383–3389. [Google Scholar] [CrossRef] [PubMed]
- Whipp, M.J.; Pittard, A.J. A reassessment of the relationship between arok-encoded and arol-encoded shikimate kinase enzymes of Escherichia-coli. J. Bacteriol. 1995, 177, 1627–1629. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Munavar, M.H.; Madhavi, K.; Jayaraman, R. Aberrant transcription in fit mutants of Escherichia-coli and its alleviation by suppressor mutations. J. Biosci. 1993, 18, 37–45. [Google Scholar] [CrossRef]
- Skotnicová, P.; Srivastava, A.; Aggarwal, D.; Talbot, J.; Karlínová, I.; Moos, M.; Mares, J.; Bucinská, L.; Koník, P.; Simek, P.; et al. A thylakoid biogenesis BtpA protein is required for the initial step of tetrapyrrole biosynthesis in cyanobacteria. New Phytol. 2024, 241, 1236–1249. [Google Scholar] [CrossRef] [PubMed]
- Hou, J.; Gao, C.; Chen, X.; Liu, L. Using dynamic molecular switches for shikimic acid production in Escherichia coli. Sheng Wu Gong Cheng Xue Bao 2020, 36, 2104–2112. [Google Scholar] [CrossRef]
- He, Z.; Wang, H. Functions of bacterial Toxin-Antitoxin systems. Sheng Wu Gong Cheng Xue Bao 2018, 34, 1270–1278. [Google Scholar] [CrossRef]




| Characteristics | 1 | 2 | 3 | 4 | 5 | 6 | 7 |
|---|---|---|---|---|---|---|---|
| Isolation source | Moraine | Soil | Air | Soil | Pig manure | Air | Soil |
| Colony Color | White | White | White | White | White | White | White |
| Growth temperature (°C) range (optium) | 10–45 (30) | 28–37 (28) | 10–35 (30) | 10–35 (30) | 4–42 (28) | 15–45 (25) | 28–37 (28) |
| pH range (optium) | 6–8 (7) | (5–9) | 5–9 (6–7) | 6–9 (7–8) | 4–10 | 6–8 (7) | (5–9) |
| NaCl tolerance range (optium) (%, w/v) | 0–10 (1) | (4) | 0–2 (1) | 0–5 (1) | 0–5(0) | ND | (4) |
| Oxidase activity | − | − | − | − | − | − | − |
| Production of H2S | + | + | ND | ND | + | − | + |
| Reduction of nitrate | + | + | + | ND | + | − | + |
| Hydrolysis of: | |||||||
| Urea | − | w | − | ND | − | − | W |
| Tween 20 | + | ND | ND | ND | + | ND | ND |
| Tween 80 | + | + | + | ND | + | + | + |
| Gelatin | + | + | + | ND | + | + | + |
| Starch | + | + | + | + | + | ND | + |
| Utilization as carbon sources | |||||||
| L-Arabinose | + | ND | − | ND | − | − | ND |
| D-Fructose | + | + | + | + | ND | + | − |
| D-Glucose | + | ND | + | + | + | − | ND |
| D-Lactose | ND | ND | ND | ND | ND | − | ND |
| D-Galactose | + | + | w | + | ND | + | W |
| D-Mannitol | + | + | + | + | + | ND | − |
| Sucrose | + | w | + | + | + | + | − |
| D-xylose | W | − | w | w | ND | + | − |
| Enzymatic activity | |||||||
| Alkaline phosphatase | + | + | + | + | + | ND | + |
| Esterase (C4) | + | + | + | + | + | ND | + |
| Lip esterase (C8) | + | + | + | + | + | ND | + |
| Lipase (C14) | − | w | − | + | w | ND | W |
| Acid phosphatase | − | w | − | + | + | ND | W |
| Naphthol-AS-BI-phosphate hydrolase | + | + | ND | + | + | ND | + |
| N-acetyl-β-glucosaminase | − | − | − | − | − | − | − |
| α-Mannosidase | ND | − | − | ND | ND | ND | − |
| Motility | − | − | − | − | − | ND | − |
| Spore formation | − | − | − | − | − | − | − |
| Major fatty acids | iso-C16:0H, isoC16:0 and C17:1ω8c | i-C15:0, i-C17:0, i-C16:0 and ai-C17:0 | iso-C16:0, C17:1ω8c and iso-C15:0 | iso-C16:0, iso-C15:0 and iso-C14:0 | iso-C16:0, iso-C15:0 and C17:1ω8c | iso-C16:0, C18:0 and C18:1 | i-C15:0, i-C17:0, i-C16:0 and ai-C17:0 |
| DNA G+C content (mol%) | 67.8 | 68.0–69.0 | 73.0 | 72.6 | 70.9 | 69.2 | 68.0–69.0 |
| Fatty Acids (%) | 1 | 2 | 3 | 4 | 5 | 6 | 7 |
|---|---|---|---|---|---|---|---|
| iso-C13:0 | ND | ND | 0.6 | ND | ND | ND | ND |
| C14:0 | 0.1 | ND | 0.6 | ND | 1.0 | 0.3 | 0.3 |
| iso-C14:0 | 3.2 | 8.3 | 2.5 | 10.6 | 0.8 | 7.3 | 5.1 |
| C15:0 | ND | 3.1 | ND | 3.9 | ND | ND | 1.7 |
| iso-C15:0 | 3.3 | 15.9 | 15.5 | 12.6 | 9.0 | 11.0 | 6.4 |
| anteiso-C15:0 | 0.3 | ND | 1.8 | ND | ND | 0.7 | 1.8 |
| C16:0 | 0.9 | 1.2 | 1.8 | 1.4 | 5.8 | 1.3 | 2.1 |
| iso-C16:0 | 26.3 | 33.6 | 20.7 | 32.6 | 13.7 | 46.6 | 37.2 |
| iso-C16:1H | 17.1 | ND | 0.5 | 1.1 | ND | 1.0 | 1.6 |
| C17:0 | 0.4 | 5.5 | 9.5 | 6.1 | 6.7 | 0.6 | 1.1 |
| C17:0 10-methyl | ND | 3.7 | 1.0 | 9.8 | ND | 4.8 | 13.0 |
| C17:1ω8c | 26.8 | 11.5 | 18.5 | 9.4 | ND | 0.6 | 6.6 |
| iso-C17:0 | 0.2 | 2.1 | 4.4 | ND | 6.1 | 8.1 | 2.9 |
| iso-C17:1ω9c | 0.5 | 1.7 | 2.5 | ND | ND | 11.4 | 6.2 |
| anteiso-C17:0 | 0.5 | ND | 6.7 | ND | 1.8 | 1.7 | 3.2 |
| anteiso-C17:1ω9c | 0.5 | ND | 1.9 | ND | ND | ND | ND |
| C18:0 | 0.6 | ND | 1.1 | ND | 12.9 | ND | 2.1 |
| C18:1ω5c | ND | ND | ND | ND | ND | ND | 0.8 |
| C18:1ω7c | ND | ND | ND | ND | ND | ND | 1.2 |
| C18:1ω9c | 9.6 | 2.1 | 5.2 | ND | ND | ND | 1.5 |
| iso-C18:0 | 0.3 | 1.2 | 1.1 | ND | 2.4 | 2.1 | 2.0 |
| C19:0 | ND | ND | ND | ND | ND | ND | ND |
| TBSA | ND | ND | ND | ND | ND | ND | 0.7 |
| C14:02-OH | ND | ND | ND | ND | ND | ND | ND |
| C15:02-OH | ND | ND | ND | ND | ND | ND | ND |
| C16:02-OH | ND | ND | ND | ND | ND | ND | 1.3 |
| C17:03-OH | ND | ND | ND | 4.1 | ND | ND | ND |
| iso-C17:03-OH | ND | ND | 3.4 | ND | ND | 1.9 | ND |
| Summed Feature 3 | 5.2 | 1.6 | 1.6 | ND | ND | 1.3 | 2.1 |
| Summed Feature 6 | 2.1 | 2.5 | 2.3 | ND | ND | ND | ND |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, X.; Liu, Y.; Chen, Z.; Wang, K.; Liu, G.; Chen, T.; Zhang, B. Genomic Functional Analysis of Novel Radiation-Resistant Species of Knollia sp. nov. S7-12T from the North Slope of Mount Everest. Microorganisms 2024, 12, 1748. https://doi.org/10.3390/microorganisms12091748
Wang X, Liu Y, Chen Z, Wang K, Liu G, Chen T, Zhang B. Genomic Functional Analysis of Novel Radiation-Resistant Species of Knollia sp. nov. S7-12T from the North Slope of Mount Everest. Microorganisms. 2024; 12(9):1748. https://doi.org/10.3390/microorganisms12091748
Chicago/Turabian StyleWang, Xinyue, Yang Liu, Zhiyuan Chen, Kexin Wang, Guangxiu Liu, Tuo Chen, and Binglin Zhang. 2024. "Genomic Functional Analysis of Novel Radiation-Resistant Species of Knollia sp. nov. S7-12T from the North Slope of Mount Everest" Microorganisms 12, no. 9: 1748. https://doi.org/10.3390/microorganisms12091748
APA StyleWang, X., Liu, Y., Chen, Z., Wang, K., Liu, G., Chen, T., & Zhang, B. (2024). Genomic Functional Analysis of Novel Radiation-Resistant Species of Knollia sp. nov. S7-12T from the North Slope of Mount Everest. Microorganisms, 12(9), 1748. https://doi.org/10.3390/microorganisms12091748






