Evaluation of the Antifungal and Biochemical Activities of Fungicides and Biological Agents against Ginseng Sclerotinia Root Rot Caused by Sclerotinia nivalis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Determination of the Indoor Toxicity of the Chemicals
2.3. Determination of the Indoor Toxicity of the Biocontrol Agents
2.4. Effects of the Different Treatments on the Formation of Sclerotia by Sclerotinia nivalis
2.5. Protective and Curative Efficacy In Vitro
(total root number × maximum grade)
disease index in control
2.6. Control Efficacy of the Agents on Ginseng Sclerotinia Root Rot in a Field Trial
2.7. Biochemical Action of the Agents on S. nivalis YC5
2.8. Data Analysis
3. Results
3.1. Indoor Sensitivity of the Chemical Fungicides
3.2. Screening Results of the Biocontrol Agents (Strains)
3.3. Effects of Different Fungicides on the Number of Sclerotia
3.4. Protective and Curative Efficacy
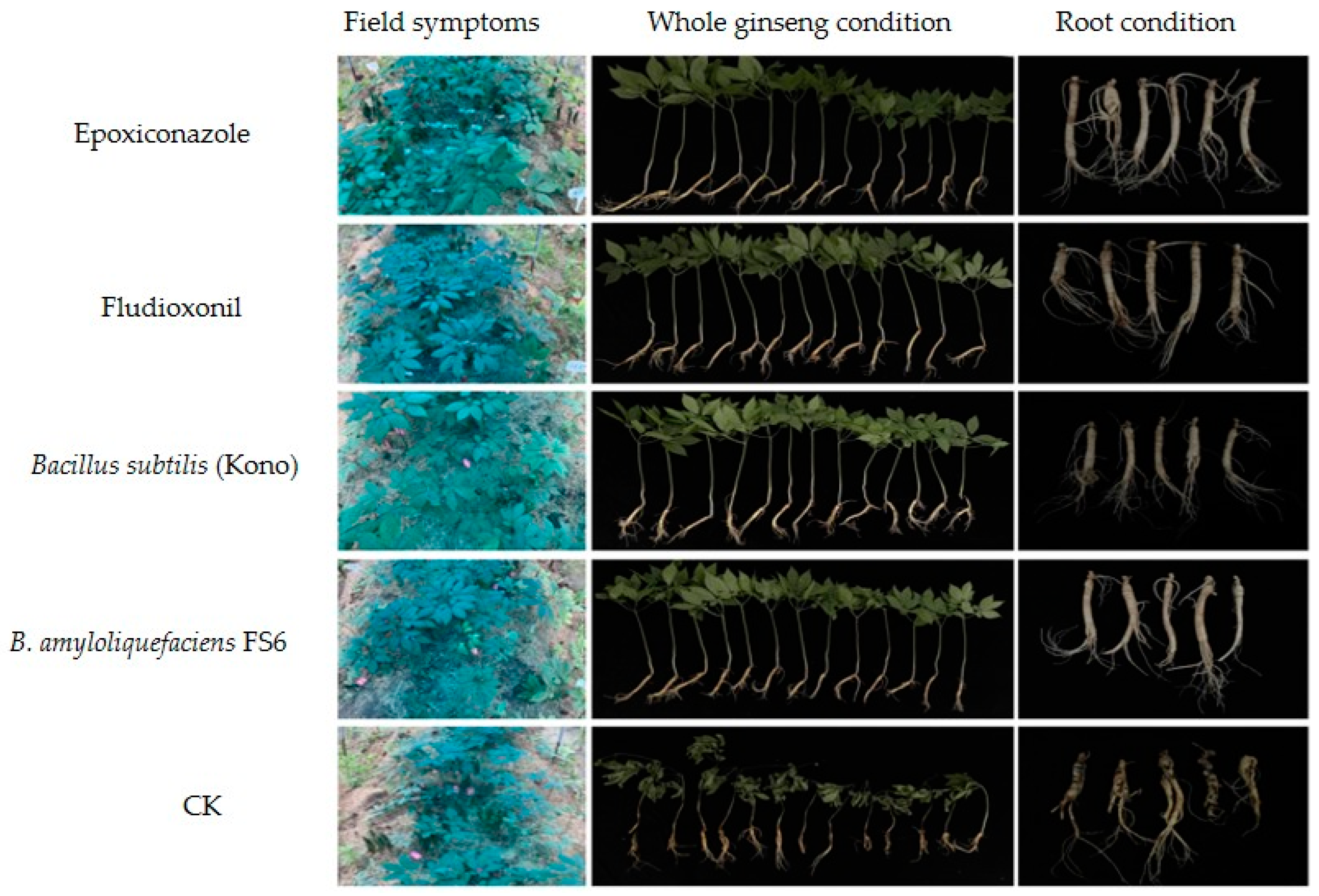
3.5. Effects of Different Agents on the Field Control of Sclerotia nivalis
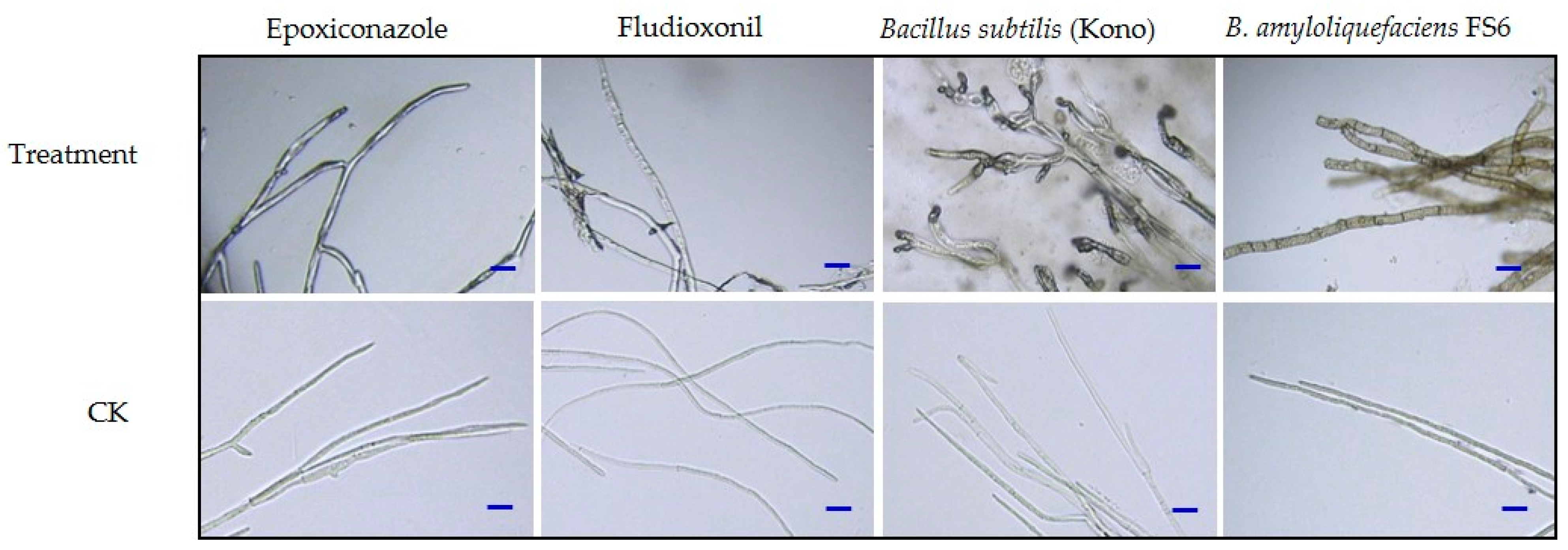
3.6. Effects of the Different Agents on Mycelial Morphology
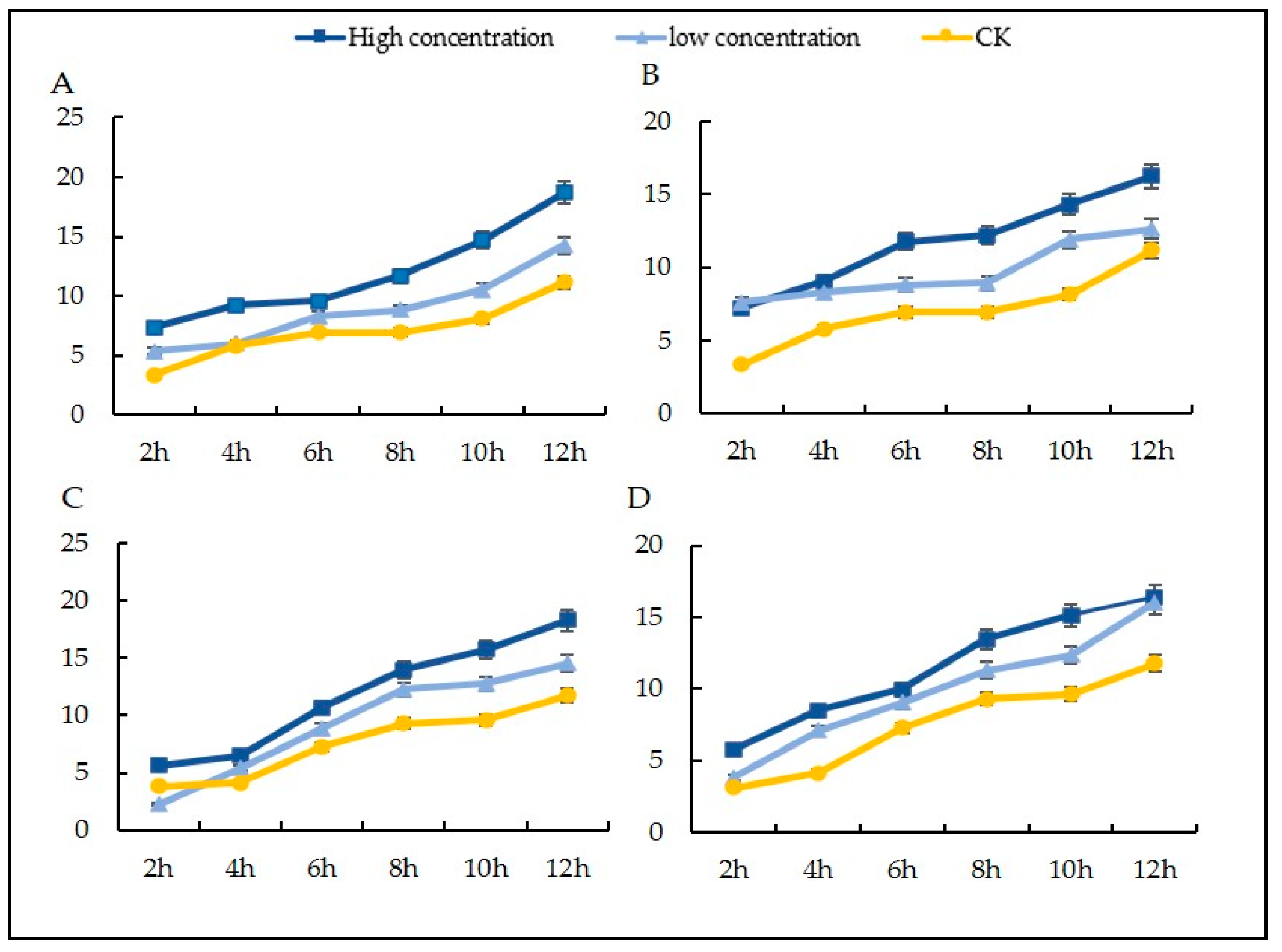
3.7. Effects of Different Agents on the Mycelial Membrane Permeability of Sclerotinia nivalis
3.8. Leakage of Nucleic Acids and Proteins in Mycelia after Treatment with Different Agents
3.9. Effects of the Different Agents on Ergosterol
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Yun, T.K. Brief introduction of Panax ginseng C.A. Meyer. J. Kor. Med. Sci. 2001, 16, S3–S5. [Google Scholar] [CrossRef] [PubMed]
- Editorial Board of the Chinese Materia Medica, State Administration of Traditional Chinese Medicine. Chinese Materia Medica; Shanghai Science and Technology Press: Beijing, China, 1999; Volume 5, p. 867. [Google Scholar]
- Wang, D.; Wang, S.R.; Yang, M.J.; Lu, B.H.; Liu, L.P.; Gao, J. Detection of the resistance of Alternaria panax and cross-resistance on ginseng in Jilin Province. Agrochemicals 2018, 57, 603–605. [Google Scholar]
- Li, G.; Wang, H.; Dong, Z.Q.; Wang, Y.P.; Wang, T.; Yao, X.; Chen, Y.; Jia, H.B.; Sun, X.B. Analysis of the Current Status and Trends of the Chinese Ginseng Industry Development. J. Tradit. Chin. Med. 2024, in press. [Google Scholar]
- Wang, C.R.; Chen, C.F.; Chen, J.; Fu, J.F. Sclerotinia ginseng, a new species of Sclerotinia. Acta Mycol. Sin. 1995, 14, 187–188. [Google Scholar]
- Boland, G.J.; Hall, R. Index of plant hosts of Sclerotinia sclerotiorum. Can. J. Plant Pathol. 1994, 16, 93–108. [Google Scholar] [CrossRef]
- Liang, X.; Rollins, J.A. Mechanisms of Broad Host Range Necrotrophic Pathogenesis in Sclerotinia sclerotiorum. Phytopathology 2018, 108, 1128–1140. [Google Scholar] [CrossRef]
- Cho, H.S.; Shin, J.S.; Kim, J.H.; Hong, T.K.; Kang, J.Y. First Report of Sclerotinia White Rot Caused by Sclerotinia nivalis on Panax ginseng in Korea. Res. Plant Dis. 2013, 19, 49–54. [Google Scholar] [CrossRef]
- Wang, C.; Shang, W.; Wang, Q.; Fan, S.; Hu, X. White rot of Panax quinquefolius caused by Sclerotinia nivalis. Plant Pathol. 2021, 70, 2034–2045. [Google Scholar] [CrossRef]
- Liu, X.; Yin, Y.; Yan, L.; Michailides, T.J.; Ma, Z. Sensitivity to iprodione and boscalid of Sclerotinia sclerotiorum isolates collected from rapeseed in China. Pestic. Biochem. Phys. 2009, 95, 106–112. [Google Scholar] [CrossRef]
- Sasirekhamani, M.; Ebenezer, P.; Nirmal Nevedhana, K.B.; Vijayan, V. The consequences of inhibition of ergosterol biosynthesis in Sclerotinia sclerotiorum (Lib) de Bary by Hexaconazole. Int. J. Pharm. Life Sci. 2013, 4, 2595–2604. [Google Scholar]
- Zhang, R.; Xu, Q.; Zhang, Y.; Zhu, F. Baseline Sensitivity and Toxic Actions of Prochloraz to Sclerotinia sclerotiorum. Plant Dis. 2018, 102, 2149–2157. [Google Scholar] [CrossRef] [PubMed]
- Hu, S.; Zhang, J.; Zhang, Y.; He, S.; Zhu, F. Baseline sensitivity and toxic actions of boscalid against Sclerotinia sclerotiorum. Crop Prot. 2018, 110, 83–90. [Google Scholar] [CrossRef]
- Di, Y.L.; Zhu, Z.Q.; Lu, X.M.; Zhu, F.X. Baseline sensitivity and efficacy of trifloxystrobin against Sclerotinia sclerotiorum. Crop Prot. 2016, 87, 31–36. [Google Scholar] [CrossRef]
- Zhang, J.; Hu, S.; Xu, Q.; You, H.; Zhu, F. Baseline sensitivity and control efficacy of propiconazole against Sclerotinia sclerotiorum. Crop Prot. 2018, 114, 208–214. [Google Scholar] [CrossRef]
- Lu, X.M.; Zhu, Z.Q.; Di, Y.L.; Zhu, F.X. Baseline sensitivity and toxic action of flusilazole to Sclerotinia sclerotiorum. Crop Prot. 2015, 78, 92–98. [Google Scholar] [CrossRef]
- Zhang, F.; Ge, H.; Zhang, F.; Guo, N.; Wang, Y.; Chen, L.; Li, C. Biocontrol potential of Trichoderma harzianum isolate T-aloe against Sclerotinia sclerotiorum in soybean. Plant Physiol. Bioch. 2016, 100, 64–74. [Google Scholar] [CrossRef] [PubMed]
- Erickson, R.S.; Chatterton, S.; Huang, H.C. Sensitivity of Sclerotinia sclerotiorum and Its Two Biocontrol Agents, Ulocladium atrum and Coniothyrium minitans, to Boscalid and Benomyl. Plant Pathol. Bull. 2013, 22, 57–66. [Google Scholar]
- Ribeiro, I.; Bach, E.; Moreira, F.; Müller, A.R.; Passaglia, L. Antifungal potential against Sclerotinia sclerotiorum (Lib.) de Bary and plant growth promoting abilities of Bacillus isolates from canola (Brassica napus L.) roots. Microbiol. Res. 2021, 248, 126754. [Google Scholar] [CrossRef] [PubMed]
- Feng, S.; Sun, G.G.; Feng, Z.W.; Bai, J.; Song, M.H.; Yang, L.N.; Wang, X.; Lu, B.H.; Gao, J. Screening of fungicides with high efficiency and low toxicity to control Sclerotinia root rot of ginseng. J. Jilin Agric. Univ. 2021, 45, 747–755. [Google Scholar]
- Da Silva, L.M.; Alves, K.S.; Del Ponte, E.M.; Pethybridge, S.J. Comparing the Fungicide Sensitivity of Sclerotinia sclerotiorum Using Mycelial Growth and Ascospore Germination Assays. Plant Dis. 2022, 106, 360–363. [Google Scholar] [CrossRef]
- Kim, J.O.; Shin, J.H.; Gumilang, A.; Chung, K.; Choi, K.Y.; Kim, K.S. Effectiveness of Different Classes of Fungicides on Botrytis cinerea Causing Gray Mold on Fruit and Vegetables. Plant Pathol. J. 2016, 32, 70–574. [Google Scholar] [CrossRef]
- Kamal, M.M.; Lindbeck, K.D.; Savocchia, S.; Ash, G.J. Biological control of sclerotinia stem rot of canola using antagonistic bacteria. Plant Pathol. 2015, 64, 1375–1384. [Google Scholar] [CrossRef]
- Wang, Y.; Sun, Y.; Zhang, Y.; Zhang, Y.; Feng, J. Sensitivity and biochemical characteristics of Sclerotinia sclerotiorum to propamidine. Pestic. Biochem. Phys. 2017, 135, 82–88. [Google Scholar] [CrossRef]
- Veloukas, T.; Karaoglanidis, G.S. Biological activity of the succinate dehydrogenase inhibitor fluopyram against Botrytis cinerea and fungal baseline sensitivity. Pest Manag. Sci. 2012, 68, 858–864. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.Y.; Zhang, Z.Q.; Chen, L.L.; He, L.M.; Lu, H.B.; Ren, Y.P.; Mu, W.; Liu, F. Baseline sensitivity of Botrytis cinerea to the succinate dehydrogenase inhibitor isopyrazam and efficacy of this fungicide. Plant Dis. 2016, 100, 1314–1320. [Google Scholar] [CrossRef] [PubMed]
- Que, H.; Jiang, J.; Deng, G.; Zhou, S.; Liu, Q. The Toxicity Tests and Field Control Effects of Five Fungicides against Sclerotinia sclerotiorum. Acta Agric. Univ. Jiangxiensis 2009, 31, 82–84. [Google Scholar]
- Wang, R.; Wang, C.; Zuo, B.; Liang, X.; Zhang, D.N.; Liu, R.; Yang, L.N.; Lu, B.H.; Wang, X.; Gao, J. A novel biocontrol strain Bacillus amyloliquefaciens FS6 for excellent control of gray mold and seedling diseases of ginseng. Plant Dis. 2021, 105, 1926–1935. [Google Scholar] [CrossRef]
- Mao, X.W.; Li, J.S.; Chen, Y.L.; Song, X.S.; Duan, Y.B.; Wang, J.X.; Chen, C.J.; Zhou, M.G.; Hou, Y.P. Resistance risk assessment for fluazinam in Sclerotinia sclerotiorum. Pestic. Biochem. Physiol. 2018, 144, 27–35. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.C.; Li, D.C.; Kong, F.Y. Various methodsused to extract the cell wall proteins from fungi mycelia. ShandongScience 2004, 1, 534–556. [Google Scholar]
- Li, T.; Li, H.; Liu, T.; Zhu, J.; Zhang, L.; Mu, W.; Liu, F. Evaluation of the antifungal and biochemical activities of mefentrifluconazole against Botrytis cinerea. Pestic. Biochem. Phys. 2021, 173, 104784. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, C.W.; Gao, J.; Ma, J.J. Toxicity Test of Different Fungicides and Their Ratios against Sclerotinia sclerotiorum of Ginseng. North. Hortic. 2014, 7, 115–119. [Google Scholar]
- Wang, Y.; Lu, N.; Wang, K.; Li, Y.; Zhang, M.; Liu, S.; Li, Y.; Zhou, F. Fluxapyroxad Resistance Mechanisms in Sclerotinia sclerotiorum. Plant Dis. 2023, 107, 1035–1043. [Google Scholar] [CrossRef] [PubMed]
- Feng, S.; Zhang, C.; Wang, X.; Chen, C.; Lu, B.; Gao, J. Measuring Pathogenic Soil Fungi That Cause Sclerotinia Rot of Panax ginseng Using Real-Time Fluorescence Quantitative PCR. Agriculture 2023, 13, 1452. [Google Scholar] [CrossRef]
- Duan, Y.B.; Xiu, Q.; Li, H.R.; Tao, J.X.; Wang, M.G. Pharmacological characteristics and control efficacy of a novel SDHI fungicide pydiflumetofen against Sclerotinia sclerotiorum. Plant Dis. 2019, 103, 77–82. [Google Scholar] [CrossRef] [PubMed]




| Name | Active Ingredient Content (%) | Manufacturer |
|---|---|---|
| Difenoconazole | 96 | Hubei Jiufenglong Chemical Co., Ltd., Wuhan, China |
| Pyraclostrobine | 96 | Hebei Ruisheng Pharmaceutical Technology Co., Ltd., Shijiazhuang, China |
| Badistan | 98 | Jiangsu Rotam Chemistry Co., Ltd., Suzhou, China |
| Fluazinam | 95 | Hubei Jiufenglong Chemical Co., Ltd., Wuhan, China |
| Procymidone | 96 | Yibin Bei Chuan’an Chemical Co., Ltd., Yibin, China |
| Thiram | 95 | Hebei Guanlong Agrochemical Co., Ltd., Hengshui, China |
| Fluocycloprazole | 97 | Jiangsu Lanfeng Bio-chemical Co., Ltd., Xuzhou, China |
| Fluxapyroxad | 98 | BASF SE, Port Ludwig, Germany |
| Fludioxonil | 95 | Nantong Jiahe Chemicals Co., Ltd., Nantong, China |
| Thiophanate-methyl | 95 | Jiangsu Lanfeng Bio-chemical Co., Ltd., Xuzhou, China |
| Metalaxyl-M | 95 | Zibo Dezun Chemical Co., Ltd., Zibo, China |
| Myclobutanil | 95 | Zhejiang Heben Pesticide&Chemicals Co., Ltd., Wenzhou, China |
| Dimethachlon | 96 | Jiangxi Heyi Chemicals Co., Ltd., Jiujiang, China |
| Azoxystrobin | 95 | Zhejiang Heben Pesticide&Chemicals Co., Ltd., Wenzhou, China |
| Trifloxystrobin | 97 | Hubei Wanye Pharmaceutical Chemical Co., Ltd., Wuhan, China |
| Tebuconazole | 98 | Hailir Pesticides&Chemicals Co., Ltd., Weifang, China |
| Biological Agent (Strain) | Content 108 CFU/g (mL) | Manufacturer (Abbreviation) |
|---|---|---|
| Trichoderma harzianum T-22 | 3 | Bioworks Co., Ltd., NY, America |
| Bacillus subtilis (Deqiang) | 100 | Deqiang Biotechnology Co., Ltd., Haerbin, China |
| B. subtilis (Kono) | 1000 | Wuhan Kono Bio-tech Co., Ltd., Wuhan, China |
| Paenibacillus polymyxa | 50 | Shanxi Linyi Zhongjin Chemical Co., Ltd., Yuncheng, China |
| P. polymyxa KN-03 | 5 | Wuhan Kono Bio-tech Co., Ltd., Wuhan, China |
| B. amyloliquefaciens B7900 | 10 | Shanxi Xiannong Biotechnology Co., Ltd.,Xian, China |
| B. amyloliquefaciens FS6 | 10 | Laboratory of Comprehensive Management of Plant Pathology, Jilin Agricultural University (JLAU), Changchun, China. |
| Bacillus velezensis 2-8 | 5 | Comprehensive Management of Plant Pathology, JLAU., Changchun, China. |
| B. subtilis Y4 | 5 | Comprehensive Management of Plant Pathology, JLAU., Changchun, China. |
| B. amyloliquefaciens XH1 | 5 | Comprehensive Management of Plant Pathology, JLAU., Changchun, China. |
| Fungicides | Mass Concentration (mg·L−1) | |||||
|---|---|---|---|---|---|---|
| Epoxiconazole | 5 × 10−2 | 1 × 10−2 | 8 × 10−3 | 5 × 10−3 | 1 × 10−3 | 5 × 10−4 |
| Fludioxonil | 5 × 10−2 | 1 × 10−2 | 5 × 10−3 | 1 × 10−3 | 5 × 10−4 | 1 × 10−4 |
| Tebuconazole | 1 × 100 | 1 × 10−1 | 5 × 10−2 | 1 × 10−2 | 5 × 10−3 | 1 × 10−3 |
| Fluazinam | 1 × 101 | 1 × 100 | 1 × 10−1 | 1 × 10−2 | 5 × 10−3 | 1 × 10−3 |
| Dimethachlon | 1 × 100 | 5 × 10−1 | 1 × 10−1 | 5 × 10−2 | 1 × 10−2 | 5 × 10−3 |
| Fluxapyroxad | 1 × 100 | 1 × 10−1 | 5 × 10−2 | 1 × 10−2 | 8 × 10−3 | 5 × 10−3 |
| Difenoconazole | 5 × 100 | 5 × 10−1 | 1 × 10−1 | 5 × 10−2 | 1 × 10−2 | 5 × 10−3 |
| Azoxystrobin | 5 × 100 | 1 × 100 | 5 × 10−1 | 1 × 10−1 | 5 × 10−2 | 1 × 10−2 |
| Pyraclostrobine | 1 × 100 | 5 × 10−1 | 1 × 10−1 | 5 × 10−2 | 1 × 10−2 | 5 × 10−2 |
| Thiram | 1 × 101 | 5 × 100 | 1 × 100 | 1 × 10−1 | 5 × 10−2 | 5 × 10−2 |
| Myclobutanil | 1 × 101 | 1 × 100 | 5 × 10−1 | 1 × 10−1 | 5 × 10−2 | 1 × 10−2 |
| Procymidone | 1 × 102 | 1 × 101 | 1 × 100 | 1 × 10−1 | 5 × 10−2 | 1 × 10−2 |
| Thiophanate-methyl | 1 × 102 | 1 × 101 | 1 × 100 | 5 × 10−1 | 1 × 10−2 | 1 × 10−2 |
| Trifloxystrobin | 1 × 102 | 1 × 101 | 1 × 100 | 1 × 10−1 | 5 × 10−2 | 1 × 10−2 |
| Badistan | 5 × 102 | 1 × 102 | 1 × 101 | 1 × 100 | 1 × 10−1 | 5 × 10−2 |
| Metalaxyl-M | 5 × 102 | 5 × 101 | 1 × 101 | 1 × 100 | 5 × 10−1 | 1 × 10−1 |
| Fungicide Names | Regression Equation | Correlation/r | EC50/(mg·L−1) | EC90/(mg·L−1) | 95% Confidence Interval (mg·L−1) |
|---|---|---|---|---|---|
| Epoxiconazole | y = 15.6571x + 0.4920 | 0.9445 | 0.0004 | 0.0052 | 0.0000~0.0020 |
| Fluazinam | y = 11.5506x + 0.3127 | 0.8887 | 0.0008 | 0.0478 | 0.0003~0.0050 |
| Dimethachlon | y = 9.4442x + 0.2167 | 0.9713 | 0.0012 | 0.4560 | 0.0011~0.1370 |
| Fludioxonil | y = 11.3572x + 0.3516 | 0.9817 | 0.0140 | 0.5353 | 0.0002~1.1370 |
| Difenoconazole | y = 11.5989x + 0.3767 | 0.9801 | 0.0246 | 0.7364 | 0.0100~0.1740 |
| Fluxapyroxad | y = 8.7231x + 0.1757 | 0.9946 | 0.0006 | 0.9139 | 0.001~0.0340 |
| Tebuconazole | y = 11.3066x + 0.3622 | 0.9806 | 0.0275 | 0.9407 | 0.0060~0.0860 |
| Myclobutanil | y = 10.8126x + 0.3505 | 0.9841 | 0.0628 | 2.4207 | 0.0600~0.8300 |
| Procymidone | y = 12.4986x + 0.5083 | 0.9409 | 0.3915 | 4.8580 | 0.0220~0.8240 |
| Pyraclostrobine | y = 11.7435x + 0.4578 | 0.9563 | 0.4012 | 6.5695 | 0.0130~10.2480 |
| Badistan | y = 9.2158x + 0.2725 | 0.9233 | 0.1912 | 20.9562 | 0.0010~0.9810 |
| Azoxystrobin | y = 9.4945x + 0.3009 | 0.9954 | 0.3264 | 22.9565 | 0.0040~0.0100 |
| Metalaxyl-M | y = 9.5073x + 0.3218 | 0.9675 | 0.8254 | 44.0798 | 0.2155~1.1092 |
| Thiophanate-methyl | y = 8.7453x + 0.2744 | 0.9426 | 1.17900 | 125.2117 | 0.1370~2.4810 |
| Thiram | y = 7.4638x + 0.1452 | 0.9816 | 0.0429 | 288.3154 | 0.0010~0.0620 |
| Trifloxystrobin | y = 7.3871x + 0.2243 | 0.9429 | 23.9115 | 7189.6160 | 3.5950~63.9240 |
| Fungicide Names | Regression Equation | Correlation/ r | EC50/ (CFU·mL−1) | EC90/ (CFU·mL−1) | 95% Confidence Interval (CFU·mL−1) |
|---|---|---|---|---|---|
| Bacillus subtilis (Kono) | y = 6.9784x + 0.0823 | 0.9767 | 3.6552 × 102 | 2.0676 × 108 | 0.0000~462.0690 |
| Paenibacillus polymyxa (Linyi) | y = 6.7956x + 0.0883 | 0.9370 | 1.5000 × 103 | 2.9025 × 109 | 5.4348~7.0373 × 103 |
| B. amyloliquefaciens FS6 | y = 7.2164x + 0.1179 | 0.9460 | 6.8000 × 103 | 3.5505 × 108 | 1.2740 × 100~2.6092 × 104 |
| B. subtilis (Deqiang) | y = 6.8627x + 0.1064 | 0.9229 | 2.5000 × 104 | 4.1835 × 109 | 4.4258 × 103~5.4075 × 104 |
| B. subtilis Y4 | y = 6.9229x + 0.1467 | 0.9414 | 2.0253 × 106 | 1.2484 × 1010 | 2.2271 × 106~6.1604 × 109 |
| B. amyloliquefaciens XH1 | y = 6.4724x + 0.1376 | 0.9420 | 2.2507 × 107 | 2.4700 × 1011 | 4.6127 × 106~1.8557 × 1013 |
| P. polymyxa KN-03 | y = 6.2071x + 0.1152 | 0.9734 | 2.8101 × 107 | 1.8824 × 1012 | 1.6513 × 106~2.5609 × 1015 |
| B. velezensis 2-8 | y = 8.0255x + 0.2888 | 0.9402 | 2.8216 × 107 | 2.3726 × 109 | 1.1886 × 106~8.0405 × 1010 |
| B. amyloliquefaciens B7900 | y = 7.0324x + 0.2365 | 0.9670 | 1.8541 × 108 | 4.1541 × 1010 | 8.3402 × 107~4.1027 × 1012 |
| Trichoderma harzianum T-22 | y = 6.3308x + 0.1921 | 0.9955 | 9.7956 × 108 | 7.6761 × 1011 | 1.7560 × 108~9.4916 × 1011 |
| Fungicide Names | Inhibition Rate of the Formation of Sclerotia at Different Concentrations of Fungicides (%) | ||||||
|---|---|---|---|---|---|---|---|
| Concentration 1 | Concentration 2 | Concentration 3 | Concentration 4 | Concentration 5 | Concentration 6 | The Average Inhibition Rate | |
| Epoxiconazole | 76.39 ± 3.20 | 70.33 ± 6.32 | 49.14 ± 4.84 | 47.13 ± 3.34 | 43.69 ± 1.82 | 34.01 ± 1.21 | 53.45 ± 3.46 |
| Fludioxonil | 97.38 ± 4.55 | 96.97 ± 3.37 | 95.56 ± 4.25 | 95.16 ± 4.57 | 80.43 ± 1.85 | 72.96 ± 3.47 | 89.74 ± 3.68 |
| Tebuconazole | 85.27 ± 4.12 | 72.56 ± 1.53 | 34.41 ± 8.82 | 27.35 ± 4.84 | 20.28 ± 5.76 | 7.97 ± 3.10 | 41.31 ± 4.70 |
| Fluazinam | 92.73 ± 1.60 | 71.95 ± 3.65 | 64.28 ± 0.61 | 56.41 ± 3.03 | 12.62 ± 0.93 | 8.84 ± 5.55 | 51.14 ± 2.56 |
| Dimethachlon | 80.83 ± 2.29 | 74.98 ± 2.13 | 48.13 ± 1.94 | 32.39 ± 2.29 | 31.18 ± 2.73 | 15.04 ± 3.45 | 47.09 ± 2.47 |
| Fluxapyroxad | 96.16 ± 1.93 | 95.82 ± 4.25 | 94.85 ± 4.38 | 92.43 ± 3.17 | 83.64 ± 1.85 | 69.98 ± 1.65 | 88.81 ± 2.87 |
| Difenoconazole | 74.57 ± 3.37 | 64.48 ± 2.45 | 58.03 ± 2.13 | 54.59 ± 3.68 | 44.75 ± 5.45 | 33.40 ± 6.16 | 54.97 ± 3.87 |
| Azoxystrobin | 65.29 ± 22.20 | 60.45 ± 10.08 | 34.61 ± 1.60 | 25.94 ± 1.94 | 24.52 ± 5.04 | 21.70 ± 5.49 | 38.75 ± 7.73 |
| Pyraclostrobine | 73.97 ± 2.47 | 64.68 ± 2.43 | 58.03 ± 2.73 | 55.40 ± 2.44 | 51.41 ± 3.23 | 45.01 ± 3.15 | 58.08 ± 2.74 |
| Thiram | 98.59 ± 2.44 | 95.96 ± 2.45 | 84.46 ± 2.80 | 50.55 ± 7.02 | 46.92 ± 1.53 | 31.18 ± 14.04 | 67.94 ± 5.05 |
| Myclobutanil | 87.09 ± 1.53 | 86.88 ± 3.78 | 76.79 ± 2.86 | 68.92 ± 2.45 | 57.01 ± 4.84 | 22.70 ± 9.99 | 66.57 ± 4.24 |
| Procymidone | 92.72 ± 3.51 | 90.72 ± 4.58 | 85.67 ± 1.26 | 70.13 ± 6.18 | 65.08 ± 8.82 | 26.54 ± 3.34 | 71.81 ± 4.62 |
| Thiophanate-methyl | 83.65 ± 3.78 | 79.61 ± 1.26 | 59.64 ± 9.43 | 58.63 ± 0.93 | 40.67 ± 7.44 | 17.25 ± 2.86 | 56.58 ± 4.28 |
| Trifloxystrobin | 72.35 ± 4.58 | 64.28 ± 2.77 | 61.45 ± 5.22 | 58.22 ± 6.98 | 34.61 ± 2.19 | 0.00 ± 0.00 | 48.49 ± 3.62 |
| Badistan | 75.98 ± 8.51 | 68.52 ± 3.78 | 59.44 ± 1.21 | 53.18 ± 3.51 | 41.80 ± 4.60 | 0.58 ± 4.86 | 49.92 ± 4.41 |
| Metalaxyl-M | 85.88 ± 2.29 | 74.37 ± 2.29 | 65.90 ± 3.65 | 42.48 ± 4.57 | 21.70 ± 7.18 | 2.52 ± 7.59 | 48.81 ± 4.60 |
| Biocontrol Agent (Strain) Names | Inhibition Rate of the Formation of Sclerotia at Different Concentrations of Biocontrol Agents (Strains) (%) | ||||||
|---|---|---|---|---|---|---|---|
| 106 CFU·mL−1 | 5 × 105 CFU·mL−1 | 105 CFU·mL−1 | 5 × 104 CFU·mL−1 | 104 CFU·mL−1 | 103 CFU·mL−1 | The Average Inhibition Rate | |
| Bacillus subtilis (Kono) | 100.00 ± 0.00 | 100.00 ± 0.00 | 100.00 ± 0.00 | 100.00 ± 0.00 | 100.00 ± 0.00 | 100.00 ± 0.00 | 100.00 ± 0.00 |
| B. subtilis (Deqiang) | 100.00 ± 0.00 | 100.00 ± 0.00 | 100.00 ± 0.00 | 100.00 ± 0.00 | 100.00 ± 0.00 | 100.00 ± 0.00 | 100.00 ± 0.00 |
| B. amyloliquefaciens FS6 | 100.00 ± 0.00 | 100.00 ± 0.00 | 100.00 ± 0.00 | 100.00 ± 0.00 | 100.00 ± 0.00 | 100.00 ± 0.00 | 100.00 ± 0.00 |
| P. polymyxa (Linyi) | 100.00 ± 0.00 | 100.00 ± 0.00 | 100.00 ± 0.00 | 100.00 ± 0.00 | 100.00 ± 0.00 | 100.00 ± 0.00 | 100.00 ± 0.00 |
| P. polymyxa KN-03 | 100.00 ± 0.00 | 100.00 ± 0.00 | 100.00 ± 0.00 | 100.00 ± 0.00 | 94.17 ± 2.35 | 81.21 ± 3.61 | 95.90 ± 0.99 |
| B. subtilis Y4 | 100.00 ± 0.00 | 98.90 ± 0.17 | 96.70 ± 1.83 | 96.33 ± 4.07 | 96.33 ± 4.13 | 94.91 ± 3.20 | 97.20 ± 2.23 |
| B. amyloliquefaciens XH1 | 73.47 ± 4.84 | 70.09 ± 2.66 | 64.59 ± 2.58 | 49.98 ± 3.46 | 44.59 ± 7.52 | 33.63 ± 4.73 | 56.06 ± 4.30 |
| Bacillus velezensis 2-8 | 98.35 ± 2.67 | 88.26 ± 4.13 | 81.47 ± 8.57 | 78.16 ± 2.11 | 69.36 ± 6.35 | 35.76 ± 2.14 | 75.23 ± 4.33 |
| Trichoderma harzianum T-22 | 100.00 ± 0.00 | 100.00 ± 0.00 | 100.00 ± 0.00 | 96.02 ± 2.52 | 70.64 ± 2.12 | 46.87 ± 6.30 | 85.59 ± 1.82 |
| B. amyloliquefaciens B7900 | 98.66 ± 2.29 | 94.86 ± 1.90 | 92.66 ± 4.22 | 90.46 ± 4.24 | 84.59 ± 6.73 | 75.15 ± 11.65 | 89.40 ± 5.17 |
| Agents | Concentration (mg·L−1 or CFU·mL−1) | Control Effect (%) | |
|---|---|---|---|
| 2022 | 2023 | ||
| Epoxiconazole | 0.052 | 98.16 ± 1.14 | 90.60 ± 2.61 |
| Fludioxonil | 5.353 | 98.16 ± 0.79 | 94.87 ± 3.49 |
| Bacillus subtilis (Kono) | 2.0676 × 108 | 97.04 ± 1.13 | 94.80 ± 2.32 |
| B. amyloliquefaciens FS6 | 3.5505 × 108 | 97.24 ± 3.13 | 95.73 ± 1.26 |
| CK | -- | -- | -- |
| Agents | Concentration (mg·L−1 or CFU·mL−1) | Ergosterol Content (μg·mL−1) |
|---|---|---|
| Epoxiconazole | 0.0520 | 8.63 ± 0.13 h |
| 0.0005 | 4.20 ± 0.04 i | |
| Fludioxonil | 5.3530 | 104.13 ± 2.27 b |
| 0.0535 | 30.68 ± 0.95 g | |
| Bacillus subtilis (Kono) | 2.0676 × 109 | 85.53 ± 1.32 c |
| 2.0676 × 107 | 37.66 ± 0.35 f | |
| B. amyloliquefaciens FS6 | 3.5505 × 109 | 77.05 ± 1.42 d |
| 3.5505 × 107 | 41.38 ± 0.24 e | |
| CK | -- | 197.87 ± 3.21 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Feng, S.; Wang, C.; Xu, Z.; Dou, B.; Wang, X.; Yang, L.; Lu, B.; Gao, J. Evaluation of the Antifungal and Biochemical Activities of Fungicides and Biological Agents against Ginseng Sclerotinia Root Rot Caused by Sclerotinia nivalis. Microorganisms 2024, 12, 1761. https://doi.org/10.3390/microorganisms12091761
Feng S, Wang C, Xu Z, Dou B, Wang X, Yang L, Lu B, Gao J. Evaluation of the Antifungal and Biochemical Activities of Fungicides and Biological Agents against Ginseng Sclerotinia Root Rot Caused by Sclerotinia nivalis. Microorganisms. 2024; 12(9):1761. https://doi.org/10.3390/microorganisms12091761
Chicago/Turabian StyleFeng, Shi, Chunlin Wang, Zhaoyang Xu, Baozhu Dou, Xue Wang, Lina Yang, Baohui Lu, and Jie Gao. 2024. "Evaluation of the Antifungal and Biochemical Activities of Fungicides and Biological Agents against Ginseng Sclerotinia Root Rot Caused by Sclerotinia nivalis" Microorganisms 12, no. 9: 1761. https://doi.org/10.3390/microorganisms12091761





