Strain-Dependent Adhesion Variations of Shouchella clausii Isolated from Healthy Human Volunteers: A Study on Cell Surface Properties and Potential Probiotic Benefits
Abstract
1. Introduction
2. Materials and Methods
2.1. Bacterial Strains and Culture Conditions
2.2. Characterization of S. clausii Strains
2.3. Adhesion of S. clausii Strains to HT-29 and Caco-2 Cell Lines
2.3.1. Cell Adhesion Assay
2.3.2. Competitive Exclusion of Salmonella Typhimurium Adhesion from HT-29 Cell Line Using the S. clausii Strains
2.3.3. Adhesion to ECM Components
2.3.4. Determination of Cell Surface Characteristics
Cell Surface Hydrophobicity
Auto-Aggregation and Co-Aggregation Assay
2.4. Genome Sequencing and Annotation
2.5. In Silico Characterization of Cell Surface Protein
2.6. Molecular Docking Studies
2.7. Statistical Analysis
3. Results and Discussion
3.1. In Vitro Resistance of S. clausii Strains to Simulated Gastric and Intestinal Fluids
3.2. Safety Assessment of S. clausii Strains
3.3. Disease-Preventing Traits of S. clausii Strains
3.4. Health-Promoting Effects of S. clausii Strains
| Properties | S. clausii Strains | |||
|---|---|---|---|---|
| B619/R | B603/Nb | B106 | B637/Nm | |
| Identification | ||||
| % 16S rRNA gene identity with S. clausii DSM 8716 | 99.85 | 99.85 | 99.85 | 99.87 |
| Average Nucleotide Identity (ANI) (%) | 95.45 | 95.42 | 95.44 | 95.44 |
| Digital DNA-DNA Hybridization (DDH) (%) | 62.5 | |||
| Physiological properties a | ||||
| Optimum Temperature (°C) | 30 (22–45) | 30 (22–45) | 30 (22–45) | 30 (22–45) |
| Optimum pH | 10 (7–10) | 10 (7–10) | 8 (7–10) | 10 (7–10) |
| Growth in presence of NaCl (%) | 4 (0–10%) | 2 (0–10%) | 4 (0–10%) | 4 (0–10%) |
| Survival in Gastrointestinal fluids b | ||||
| Survival (%) in SGF (120 min) | 88 ± 8 | 57 ± 3 | 94 ± 19 | 88 ± 4 |
| Survival (%) in SIF (180 min) | 70 ± 1 | 36 ± 0 | 66 ± 2 | 71 ± 2 |
| Antibiotic resistance profile c (*strains were resistant to the listed antibiotics) | Streptomycin, Neomycin, Clarithromycin, Metronidazole, Chloramphenicol | Novobiocin, Rifampicin, Clarithromycin, Metronidazole, Amoxicillin, Chloramphenicol | Chloramphenicol, Clarithromycin, Metronidazole | Tetracycline, Chloramphenicol Clarithromycin, Metronidazole, Amoxicillin |
| Disease Preventing traits | ||||
| Antimicrobial activity against pathogens d | P. aeruginosa (14.5 ± 0.7 mm) | P. aeruginosa (27 ± 1.4 mm), S. aureus subsp. aureus (20 ± 2.1 mm) | P. aeruginosa (11 ± 0.8 mm), E. faecalis (24.5 ± 0.7 mm), and S. aureus subsp. aureus (23 ± 2.6 mm) | P. aeruginosa (15.6 ± 1.1 mm), E. faecalis (10.6 ± 1.1 mm), and S. aureus subsp. aureus (18 ± 2.8 mm) |
| Health Promoting traits | ||||
| Protease activity e | + | + | + | + |
| Lipase activity f | + | + | + | - |
| β-galactosidase activity g | + | + | + | + |
| Antioxidant activity (%) h | 80 ± 2 | 95 ± 1 | 73 ± 3 | 68 ± 1 |
| Cholesterol-reducing ability (%) in SIF i | 46 ± 7 | 55 ± 1 | 52 ± 1 | 60 ± 2 |
| Exo-polysaccharide production (mg/L) j | 467 ± 10 | 463 ± 9 | 625 ± 11 | 532 ± 5 |
3.5. Adhesion of S. clausii Strains to Human Colonic Epithelial Cells
3.6. Competitive Exclusion of S. Typhimurium Adhesion by S. clausii to HT-29 Cells
3.7. In Vitro Binding of S. clausii Strains to ECM Components
3.8. Cell Surface Properties of S. clausii Strains
3.9. Genomic Insights into the Adhesion Potential of S. clausii Strains
3.10. Comparative Analysis of Adhesion Proteins in S. clausii Strains
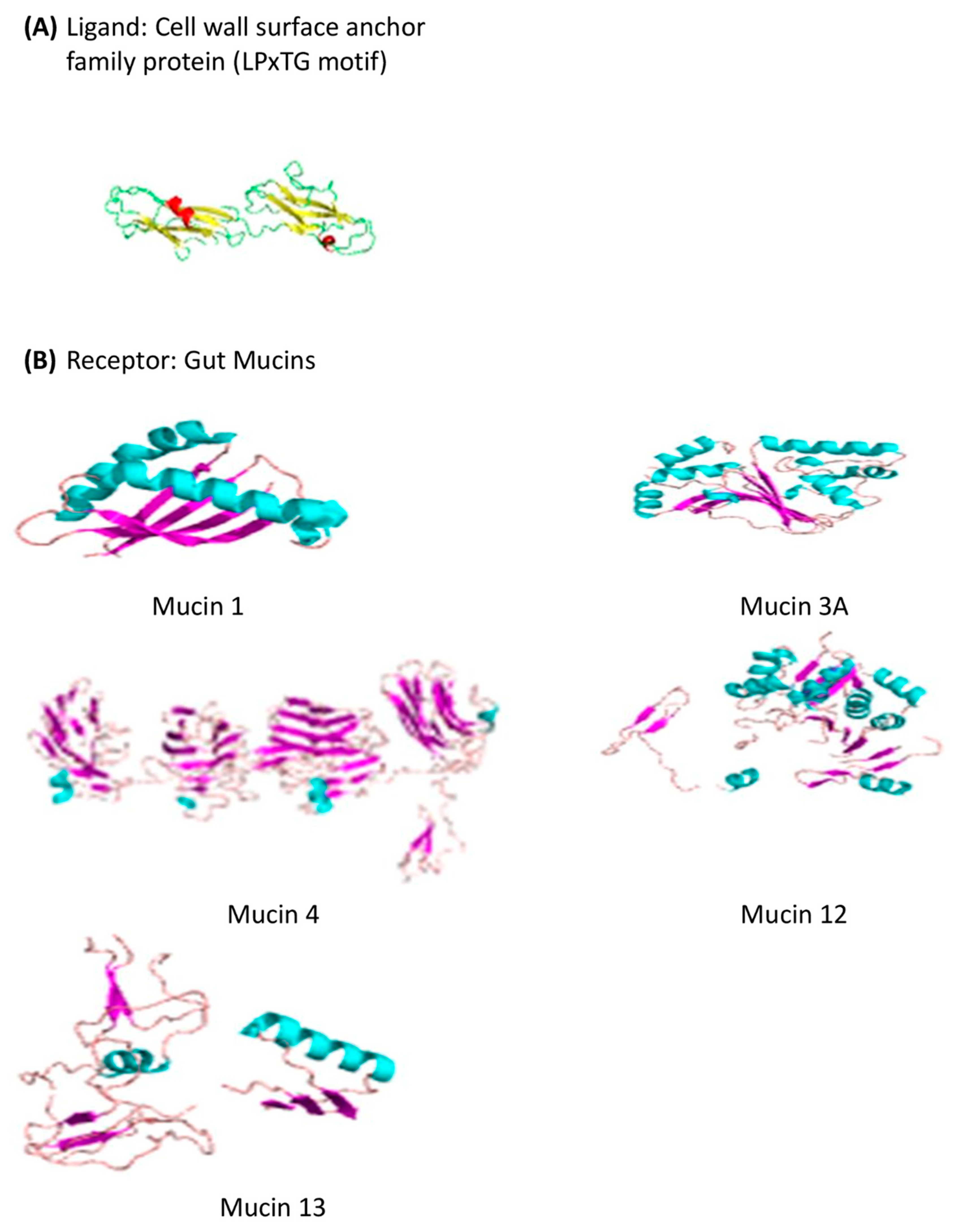
3.11. In Silico Characterization of the Cell Surface Protein of S. clausii Strains
3.11.1. Predicted 3D-Structure of the Cell Surface Protein
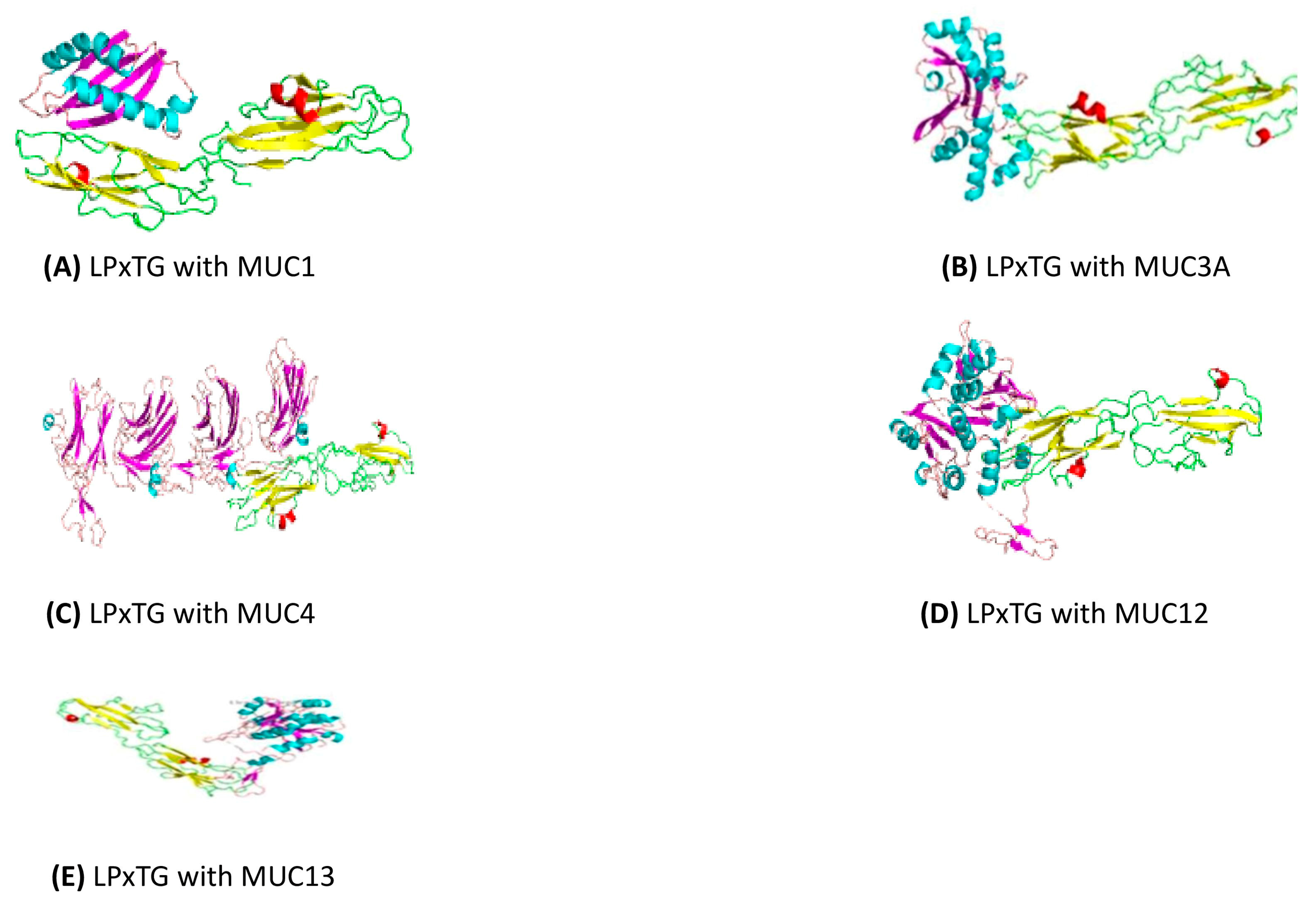
3.11.2. Docking of the Cell Wall Surface Anchor Family Protein (LPxTG Motif) with Gut Mucins
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hill, C.; Guarner, F.; Reid, G.; Gibson, G.R.; Merenstein, D.J.; Pot, B.; Morelli, L.; Canani, R.B.; Flint, H.J.; Salminen, S.; et al. The International Scientific Association for Probiotics and Prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat. Rev. Gastroenterol. Hepatol. 2014, 11, 506–514. [Google Scholar] [CrossRef] [PubMed]
- Binda, S.; Hill, C.; Johansen, E.; Obis, D.; Pot, B.; Sanders, M.E.; Tremblay, A.; Ouwehand, A.C. Criteria to qualify microorganisms as “probiotic” in foods and dietary supplements. Front. Microbiol. 2020, 11, 1662. [Google Scholar] [CrossRef]
- Fijan, S.; Frauwallner, A.; Langerholc, T.; Krebs, B.; ter Haar, J.A.; Heschl, A.; Mičetić Turk, D.; Rogelj, I. Efficacy of using probiotics with antagonistic activity against pathogens of wound infections: An integrative review of literature. Biomed. Res. Int. 2019, 2019, 7585486. [Google Scholar] [CrossRef]
- Anselmo, A.C.; McHugh, K.J.; Webster, J.; Langer, R.; Jaklenec, A. Layer by layer encapsulation of probiotics for delivery to the microbiome. Adv. Mater. 2016, 28, 9486–9490. [Google Scholar] [CrossRef] [PubMed]
- Bernardeau, M.; Lehtinen, M.J.; Forssten, S.D.; Nurminen, P. Importance of the gastrointestinal life cycle of Bacillus for probiotic functionality. J. Food Sci. Technol. 2017, 54, 2570–2584. [Google Scholar] [CrossRef] [PubMed]
- Łubkowska, B.; Jeżewska-Frąckowiak, J.; Sroczyński, M.; Dzitkowska-Zabielska, M.; Bojarczuk, A.; Skowron, P.M.; Cięszczyk, P. Analysis of industrial Bacillus species as potential probiotics for dietary supplements. Microorganisms 2023, 11, 488. [Google Scholar] [CrossRef]
- Joshi, A.; Thite, S.; Karodi, P.; Joseph, N.; Lodha, T. Corrigendum: Alkalihalobacterium elongatum gen. nov. sp. nov.: An antibiotic-producing bacterium isolated from Lonar Lake and reclassification of the genus Alkalihalobacillus into seven novel genera. Front. Microbiol. 2022, 13, 871596. [Google Scholar] [CrossRef]
- Ghelardi, E.; Mazzantini, D.; Celandroni, F.; Calvigioni, M.; Panattoni, A.; Lupetti, A.; de Fer, B.B.; Perez, M., III. Analysis of the microbial content of probiotic products commercialized worldwide and survivability in conditions mimicking the human gut environment. Front. Microbiol. 2023, 14, 1127321. [Google Scholar] [CrossRef]
- Nista, E.C.; Candelli, M.; Cremonini, F.; Cazzato, I.A.; Zocco, M.A.; Franceschi, F.; Cammarota, G.; Gasbarrini, G.; Gasbarrini, A. Bacillus clausii therapy to reduce side-effects of anti-Helicobacter pylori treatment: Randomized, double-blind, placebo controlled trial. Aliment. Pharmacol. Ther. 2004, 20, 1181–1188. [Google Scholar] [CrossRef] [PubMed]
- Acosta-Rodríguez-Bueno, C.P.; Abreu y Abreu, A.T.; Guarner, F.; Guno, M.J.V.; Pehlivanoğlu, E.; Perez, M., III. Bacillus clausii for gastrointestinal disorders: A narrative literature review. Adv. Ther. 2022, 39, 4854–4874. [Google Scholar] [CrossRef]
- Ianiro, G.; Rizzatti, G.; Plomer, M.; Lopetuso, L.; Scaldaferri, F.; Franceschi, F.; Cammarota, G.; Gasbarrini, A. Bacillus clausii for the treatment of acute diarrhea in children: A systematic review and meta-analysis of randomized controlled trials. Nutrients 2018, 10, 1074. [Google Scholar] [CrossRef] [PubMed]
- Khokhlova, E.; Colom, J.; Simon, A.; Mazhar, S.; García-Lainez, G.; Llopis, S.; Gonzalez, N.; Enrique-López, M.; Álvarez, B.; Martorell, P.; et al. Immunomodulatory and antioxidant properties of a novel potential probiotic Bacillus clausii CSI08. Microorganisms 2023, 11, 240. [Google Scholar] [CrossRef] [PubMed]
- Tuomola, E.M.; Salminen, S.J. Adhesion of some probiotic and dairy Lactobacillus strains to Caco-2 cell cultures. Int. J. Food Microbiol. 1998, 41, 45–51. [Google Scholar] [CrossRef] [PubMed]
- Ouwehand, A.C.; Tuomola, E.M.; Tölkkö, S.; Salminen, S. Assessment of adhesion properties of novel probiotic strains to human intestinal mucus. Int. J. Food Microbiol. 2001, 64, 119–126. [Google Scholar] [CrossRef] [PubMed]
- Štyriak, I.; Nemcova, R.; Chang, Y.H.; Ljungh, Å. Binding of extracellular matrix molecules by probiotic bacteria. Lett. Appl. Microbiol. 2003, 37, 329–333. [Google Scholar] [CrossRef] [PubMed]
- Ahire, J.J.; Kashikar, M.S.; Madempudi, R.S. Comparative accounts of probiotic properties of spore and vegetative cells of Bacillus clausii UBBC07 and in silico analysis of probiotic function. 3 Biotech 2021, 11, 116. [Google Scholar] [CrossRef] [PubMed]
- Jeon, H.L.; Lee, N.K.; Yang, S.J.; Kim, W.S.; Paik, H.D. Probiotic characterization of Bacillus subtilis P223 isolated from kimchi. Food Sci. Biotechnol. 2017, 26, 1641–1648. [Google Scholar] [CrossRef]
- Mazzantini, D.; Calvigioni, M.; Celandroni, F.; Lupetti, A.; Ghelardi, E. In vitro assessment of probiotic attributes for strains contained in commercial formulations. Sci. Rep. 2022, 12, 21640. [Google Scholar] [CrossRef] [PubMed]
- Bubnov, R.V.; Babenko, L.P.; Lazarenko, L.M.; Mokrozub, V.V.; Spivak, M.Y. Specific properties of probiotic strains: Relevance and benefits for the host. EPMA J. 2018, 9, 205–223. [Google Scholar] [CrossRef] [PubMed]
- Vecchione, A.; Celandroni, F.; Mazzantini, D.; Senesi, S.; Lupetti, A.; Ghelardi, E. Compositional quality and potential gastrointestinal behavior of probiotic products commercialized in Italy. Front. Med. 2018, 5, 59. [Google Scholar] [CrossRef]
- Jacobsen, C.N.; Rosenfeldt Nielsen, V.; Hayford, A.E.; Møller, P.L.; Michaelsen, K.F.; Paerregaard, A.; Sandstrom, B.; Tvede, M.; Jakobsen, M. Screening of probiotic activities of forty-seven strains of Lactobacillus spp. by in vitro techniques and evaluation of the colonization ability of five selected strains in humans. Appl. Environ. Microbiol. 1999, 65, 4949–4956. [Google Scholar] [CrossRef] [PubMed]
- Lim, Y.H.; Foo, H.L.; Loh, T.C.; Mohamad, R.; Abdullah, N. Comparative studies of versatile extracellular proteolytic activities of lactic acid bacteria and their potential for extracellular amino acid productions as feed supplements. J. Anim. Sci. Biotechnol. 2019, 10, 15. [Google Scholar] [CrossRef] [PubMed]
- Vijayakumari Nadaraja, A.; Jayakumaran Nair, A.; Prameela, M.; Hari, N.; Balakrishnan, N. Evaluation of currently employed food preservation conditions to tackle biofilm forming food pathogens. J. Food Saf. 2018, 38, e12407. [Google Scholar] [CrossRef]
- Mu, G.; Gao, Y.; Tuo, Y.; Li, H.; Zhang, Y.; Qian, F.; Jiang, S. Assessing and comparing antioxidant activities of lactobacilli strains by using different chemical and cellular antioxidant methods. J. Dairy Sci. 2018, 101, 10792–10806. [Google Scholar] [CrossRef] [PubMed]
- Tarrah, A.; dos Santos Cruz, B.C.; Sousa Dias, R.; da Silva Duarte, V.; Pakroo, S.; de Oliveira, L.L.; Gouveia Peluzio, M.C.; Corich, V.; Giacomini, A.; Oliveira de Paula, S. Lactobacillus paracasei DTA81, a cholesterol-lowering strain having immunomodulatory activity, reveals gut microbiota regulation capability in BALB/c mice receiving high-fat diet. J. Appl. Microbiol. 2021, 131, 1942–1957. [Google Scholar] [CrossRef] [PubMed]
- Mıdık, F.; Tokatlı, M.; Bağder Elmacı, S.; Özçelik, F. Influence of different culture conditions on exopolysaccharide production by indigenous lactic acid bacteria isolated from pickles. Arch. Microbiol. 2020, 202, 875–885. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Kanwar, S.S. Adherence potential of indigenous lactic acid bacterial isolates obtained from fermented foods of Western Himalayas to intestinal epithelial Caco-2 and HT-29 cell lines. J. Food Sci. Technol. 2017, 54, 3504–3511. [Google Scholar] [CrossRef] [PubMed]
- Lebeer, S.; Claes, I.; Tytgat, H.L.; Verhoeven, T.L.; Marien, E.; von Ossowski, I.; Reunanen, J.; Palva, A.; de Vos, W.M.; de Keersmaecker, S.C.; et al. Functional analysis of Lactobacillus rhamnosus GG pili in relation to adhesion and immunomodulatory interactions with intestinal epithelial cells. Appl. Environ. Microbiol. 2012, 78, 185–193. [Google Scholar] [CrossRef] [PubMed]
- Inturri, R.; Stivala, A.; Sinatra, F.; Morrone, R.; Blandino, G. Scanning electron microscopy observation of adhesion properties of Bifidobacterium longum W11 and chromatographic analysis of its exopolysaccaride. Food Nutr. Sci. 2014, 5, 1787. [Google Scholar]
- Choi, A.R.; Patra, J.K.; Kim, W.J.; Kang, S.S. Antagonistic activities and probiotic potential of lactic acid bacteria derived from a plant-based fermented food. Front. Microbiol. 2018, 9, 1963. [Google Scholar] [CrossRef] [PubMed]
- Muñoz-Provencio, D.; Llopis, M.; Antolín, M.; De Torres, I.; Guarner, F.; Pérez-Martínez, G.; Monedero, V. Adhesion properties of Lactobacillus casei strains to resected intestinal fragments and components of the extracellular matrix. Arch. Microbiol. 2009, 191, 153–161. [Google Scholar] [CrossRef] [PubMed]
- Rokana, N.; Singh, B.P.; Thakur, N.; Sharma, C.; Gulhane, R.D.; Panwar, H. Screening of cell surface properties of potential probiotic lactobacilli isolated from human milk. J. Dairy Res. 2018, 85, 347–354. [Google Scholar] [CrossRef] [PubMed]
- Han, Q.; Kong, B.; Chen, Q.; Sun, F.; Zhang, H. In vitro comparison of probiotic properties of lactic acid bacteria isolated from Harbin dry sausages and selected probiotics. J. Funct. Foods 2017, 32, 391–400. [Google Scholar] [CrossRef]
- Collado, M.C.; Isolauri, E.; Salminen, S. Specific probiotic strains and their combinations counteract adhesion of Enterobacter sakazakii to intestinal mucus. FEMS Microbiol. Lett. 2008, 285, 58–64. [Google Scholar] [CrossRef] [PubMed]
- Aziz, R.K.; Bartels, D.; Best, A.A.; DeJongh, M.; Disz, T.; Edwards, R.A.; Formsma, K.; Gerdes, S.; Glass, E.M.; Kubal, M.; et al. The RAST Server: Rapid annotations using subsystems technology. BMC Genom. 2008, 9, 75. [Google Scholar] [CrossRef] [PubMed]
- Carattoli, A.; Zankari, E.; García-Fernández, A.; Voldby Larsen, M.; Lund, O.; Villa, L.; Møller Aarestrup, F.; Hasman, H. In silico detection and typing of plasmids using PlasmidFinder and plasmid multilocus sequence typing. Antimicrob. Agents Chemother. 2014, 58, 3895–3903. [Google Scholar] [CrossRef]
- Malberg Tetzschner, A.M.; Johnson, J.R.; Johnston, B.D.; Lund, O.; Scheutz, F. In silico genotyping of Escherichia coli isolates for extraintestinal virulence genes by use of whole-genome sequencing data. J. Clin. Microbiol. 2020, 58, 10–1128. [Google Scholar] [CrossRef] [PubMed]
- Van Heel, A.J.; de Jong, A.; Song, C.; Viel, J.H.; Kok, J.; Kuipers, O.P. BAGEL4: A user-friendly web server to thoroughly mine RiPPs and bacteriocins. Nucleic Acids Res. 2018, 46, W278–W281. [Google Scholar] [CrossRef]
- Yang, J.; Zhang, Y. I-TASSER server: New development for protein structure and function predictions. Nucleic Acids Res. 2015, 43, W174–W181. [Google Scholar] [CrossRef] [PubMed]
- Pieper, U.; Webb, B.M.; Barkan, D.T.; Schneidman-Duhovny, D.; Schlessinger, A.; Braberg, H.; Yang, Z.; Meng, E.C.; Pettersen, E.F.; Huang, C.C.; et al. ModBase, a database of annotated comparative protein structure models, and associated resources. Nucleic Acids Res. 2010, 39 (Suppl. S1), D465–D474. [Google Scholar] [CrossRef]
- Kozakov, D.; Hall, D.R.; Xia, B.; Porter, K.A.; Padhorny, D.; Yueh, C.; Beglov, D.; Vajda, S. The ClusPro web server for protein–protein docking. Nat. Protoc. 2017, 12, 255–278. [Google Scholar] [CrossRef]
- McFarland, L.V.; Evans, C.T.; Goldstein, E.J. Strain-specificity and disease-specificity of probiotic efficacy: A systematic review and meta-analysis. Front. Med. 2018, 5, 124. [Google Scholar] [CrossRef] [PubMed]
- Malki, A.A.; Yoon, S.H.; Firoz, A.; Ali, H.M.; Park, Y.H.; Hor, Y.Y.; Rather, I.A. Characterization of new probiotic isolates from fermented ajwa dates of madinah and their anti-inflammatory potential. Appl. Sci. 2022, 12, 5082. [Google Scholar] [CrossRef]
- Aleman, R.S.; Yadav, A. Systematic Review of Probiotics and Their Potential for Developing Functional Nondairy Foods. Appl. Microbiol. 2023, 4, 47–69. [Google Scholar] [CrossRef]
- Cenci, G.; Trotta, F.; Caldini, G. Tolerance to challenges miming gastrointestinal transit by spores and vegetative cells of Bacillus clausii. J. Appl. Microbiol. 2006, 101, 1208–1215. [Google Scholar] [CrossRef]
- Goderska, K.; Agudo Pena, S.; Alarcon, T. Helicobacter pylori treatment: Antibiotics or probiotics. Appl. Microbiol. Biotechnol. 2018, 102, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Abbrescia, A.; Palese, L.L.; Papa, S.; Gaballo, A.; Alifano, P.; Sardanelli, A.M. Antibiotic sensitivity of Bacillus clausii strains in commercial preparation. Clin. Immunol. Endocr. Metab. Drugs 2014, 1, 102–110. [Google Scholar] [CrossRef]
- Lakshmi, S.G.; Jayanthi, N.; Saravanan, M.; Ratna, M.S. Safety assesment of Bacillus clausii UBBC07, a spore forming probiotic. Toxicol. Rep. 2017, 4, 62–71. [Google Scholar] [CrossRef] [PubMed]
- Khatri, I.; Sharma, G.; Subramanian, S. Composite genome sequence of Bacillus clausii, a probiotic commercially available as Enterogermina®, and insights into its probiotic properties. BMC Microbiol. 2019, 19, 307. [Google Scholar] [CrossRef] [PubMed]
- Urdaci, M.C.; Bressollier, P.; Pinchuk, I. Bacillus clausii probiotic strains: Antimicrobial and immunomodulatory activities. J. Clin. Gastroenterol. 2004, 38, S86–S90. [Google Scholar] [CrossRef] [PubMed]
- Sharma, J.; Upadhya, S. Determination of antimicrobial potential of Saccharomyces boulardii and Bacillus clausii against some community acquired pathogens in vitro study. Int. J. Pharm. Sci. Res. 2015, 6, 1023–1026. [Google Scholar]
- Grove, T.L.; Himes, P.M.; Hwang, S.; Yumerefendi, H.; Bonanno, J.B.; Kuhlman, B.; Almo, S.C.; Bowers, A.A. Structural insights into thioether bond formation in the biosynthesis of sactipeptides. J. Am. Chem. Soc. 2017, 139, 11734–11744. [Google Scholar] [CrossRef] [PubMed]
- Cotter, P.D.; Ross, R.P.; Hill, C. Bacteriocins—A viable alternative to antibiotics? Nat. Rev. Microbiol. 2013, 11, 95–105. [Google Scholar] [CrossRef] [PubMed]
- Rahmdel, S.; Shekarforoush, S.S.; Hosseinzadeh, S.; Torriani, S.; Gatto, V. Antimicrobial spectrum activity of bacteriocinogenic Staphylococcus strains isolated from goat and sheep milk. J. Dairy Sci. 2019, 102, 2928–2940. [Google Scholar] [CrossRef] [PubMed]
- Ahire, J.J.; Kashikar, M.S.; Madempudi, R.S. Survival and germination of Bacillus clausii UBBC07 spores in in vitro human gastrointestinal tract simulation model and evaluation of clausin production. Front. Microbiol. 2020, 11, 1010. [Google Scholar] [CrossRef] [PubMed]
- Rochín-Medina, J.J.; Ramírez-Medina, H.K.; Rangel-Peraza, J.G.; Pineda-Hidalgo, K.V.; Iribe-Arellano, P. Use of whey as a culture medium for Bacillus clausii for the production of protein hydrolysates with antimicrobial and antioxidant activity. Food Sci. Technol. Int. 2018, 24, 35–42. [Google Scholar] [CrossRef] [PubMed]
- Ripert, G.; Racedo, S.M.; Elie, A.M.; Jacquot, C.; Bressollier, P.; Urdaci, M.C. Secreted compounds of the probiotic Bacillus clausii strain O/C inhibit the cytotoxic effects induced by Clostridium difficile and Bacillus cereus toxins. Antimicrob. Agents Chemother. 2016, 60, 3445–3454. [Google Scholar] [CrossRef] [PubMed]
- Patel, C.; Patel, P.; Acharya, S. Therapeutic prospective of a spore-forming probiotic---Bacillus clausii UBBC07 against acetaminophen-induced uremia in rats. Probiotics Antimicrob. Proteins 2020, 12, 253–258. [Google Scholar]
- Paparo, L.; Tripodi, L.; Luccioni, C.; Bruno, C.; Pisapia, L.; Damiano, C.; Pastore, L.; Berni Canani, R. Protective action of Bacillus clausii probiotic strains in an in vitro model of Rotavirus infection. Sci. Rep. 2020, 10, 12636. [Google Scholar]
- Ooi, L.G.; Liong, M.T. Cholesterol-lowering effects of probiotics and prebiotics: A review of in vivo and in vitro findings. Int. J. Mol. Sci. 2010, 11, 2499–2522. [Google Scholar] [CrossRef] [PubMed]
- Gorreja, F.; Walker, W.A. The potential role of adherence factors in probiotic function in the gastrointestinal tract of adults and pediatrics: A narrative review of experimental and human studies. Gut Microbes 2022, 14, 2149214. [Google Scholar] [CrossRef]
- Lau, L.Y.J.; Quek, S.Y. Probiotics: Health benefits, food application, and colonization in the human gastrointestinal tract. Food Bioeng. 2024, 3, 41–64. [Google Scholar] [CrossRef]
- Monteagudo-Mera, A.; Rastall, R.A.; Gibson, G.R.; Charalampopoulos, D.; Chatzifragkou, A. Adhesion mechanisms mediated by probiotics and prebiotics and their potential impact on human health. Appl. Microbiol. Biotechnol. 2019, 103, 6463–6472. [Google Scholar] [CrossRef] [PubMed]
- Zawistowska-Rojek, A.; Kośmider, A.; Stępień, K.; Tyski, S. Adhesion and aggregation properties of Lactobacillaceae strains as protection ways against enteropathogenic bacteria. Arch. Microbiol. 2022, 204, 285. [Google Scholar]
- Lea, T. Caco-2 cell line. In The Impact of Food Bioactives on Health: In Vitro and Ex Vivo Models; Springer: Cham, Switzerland, 2015; pp. 103–111. [Google Scholar]
- Haeri, A.; Khodaii, Z.; Ghaderian, S.M.H.; Tabatabaei Panah, A.S.; Akbarzadeh Najar, R. Comparison of adherence patterns of a selection of probiotic bacteria to Caco-2, HEp-2, and T84 cell lines. Ann. Microbiol. 2012, 62, 339–344. [Google Scholar] [CrossRef]
- Sambuy, Y.; De Angelis, I.; Ranaldi, G.; Scarino, M.L.; Stammati, A.; Zucco, F. The Caco-2 cell line as a model of the intestinal barrier: Influence of cell and culture-related factors on Caco-2 cell functional characteristics. Cell Biol. Toxicol. 2005, 21, 1–26. [Google Scholar] [CrossRef]
- Kleiveland, C.R. Co-cultivation of Caco-2 and HT-29MTX. In The Impact of Food Bioactives on Health: In Vitro and Ex Vivo Models; Springer: Cham, Switzerland, 2015; pp. 135–140. [Google Scholar]
- De Vecchi, E.; Nicola, L.; Zanini, S.; Drago, L. In vitro screening of probiotic characteristics of some Italian products. J. Chemother. 2008, 20, 341–347. [Google Scholar]
- Haddaji, N.; Mahdhi, A.K.; Krifi, B.; Ismail, M.B.; Bakhrouf, A. Change in cell surface properties of Lactobacillus casei under heat shock treatment. FEMS Microbiol. Lett. 2015, 362, fnv047. [Google Scholar]
- Lee, Y.K.; Puong, K.Y.; Ouwehand, A.C.; Salminen, S. Displacement of bacterial pathogens from mucus and Caco-2 cell surface by lactobacilli. J. Appl. Microbiol. 2003, 52, 925–930. [Google Scholar] [CrossRef]
- Vélez, M.P.; Hermans, K.; Verhoeven, T.L.A.; Lebeer, S.E.; Vanderleyden, J.; De Keersmaecker, S.C.J. Identification and characterization of starter lactic acid bacteria and probiotics from Columbian dairy products. J. Appl. Microbiol. 2007, 103, 666–674. [Google Scholar] [CrossRef]
- Andriantsoanirina, V.; Teolis, A.C.; Xin, L.X.; Butel, M.J.; Aires, J. Bifidobacterium longum and Bifidobacterium breve isolates from preterm and full term neonates: Comparison of cell surface properties. Anaerobe 2014, 28, 212–215. [Google Scholar] [CrossRef]
- Krausova, G.; Hyrslova, I.; Hynstova, I. In vitro evaluation of adhesion capacity, hydrophobicity, and auto-aggregation of newly isolated potential probiotic strains. Fermentation 2019, 5, 100. [Google Scholar] [CrossRef]
- Ehrmann, M.A.; Kurzak, P.; Bauer, J.; Vogel, R.F. Characterization of lactobacilli towards their use as probiotic adjuncts in poultry. J. Appl. Microbiol. 2002, 92, 966–975. [Google Scholar] [CrossRef] [PubMed]
- Vinderola, C.G.; Medici, M.; Perdigon, G. Relationship between interaction sites in the gut, hydrophobicity, mucosal immunomodulating capacities and cell wall protein profiles in indigenous and exogenous bacteria. J. Appl. Microbiol. 2004, 96, 230–243. [Google Scholar] [CrossRef]
- García-Cayuela, T.; Korany, A.M.; Bustos, I.; de Cadiñanos, L.P.G.; Requena, T.; Peláez, C.; Martínez-Cuesta, M.C. Adhesion abilities of dairy Lactobacillus plantarum strains showing an aggregation phenotype. Food Res. Int. 2014, 57, 44–50. [Google Scholar] [CrossRef]
- Campana, R.; van Hemert, S.; Baffone, W. Strain-specific probiotic properties of lactic acid bacteria and their interference with human intestinal pathogens invasion. Gut Pathog. 2017, 9, 28286570. [Google Scholar]
- Chaffanel, F.; Charron-Bourgoin, F.; Soligot, C.; Kebouchi, M.; Bertin, S.; Payot, S.; Le Roux, Y.; Leblond-Bourget, N. Surface proteins involved in the adhesion of Streptococcus salivarius to human intestinal epithelial cells. Appl. Microbiol. Biotechnol. 2018, 102, 2851–2865. [Google Scholar] [CrossRef] [PubMed]
- Kapczynski, D.R.; Meinersmann, R.J.; Lee, M.D. Adherence of Lactobacillus to intestinal 407 cells in culture correlates with fibronectin binding. Curr. Microbiol. 2000, 41, 136–141. [Google Scholar] [CrossRef]
- Claesson, M.J.; Li, Y.; Leahy, S.; Canchaya, C.; van Pijkeren, J.P.; Cerdeño-Tárraga, A.M.; Parkhill, J.; Flynn, S.; O’Sullivan, G.C.; Collins, J.K.; et al. Multireplicon genome architecture of Lactobacillus salivarius. Proc. Natl. Acad. Sci. USA 2006, 103, 6718–6723. [Google Scholar] [CrossRef] [PubMed]
- Kebouchi, M.; Galia, W.; Genay, M.; Soligot, C.; Lecomte, X.; Awussi, A.A.; Perrin, C.; Roux, E.; Dary-Mourot, A.; Le Roux, Y. Implication of sortase-dependent proteins of Streptococcus thermophilus in adhesion to human intestinal epithelial cell lines and bile salt tolerance. Appl. Microbiol. Biotechnol. 2016, 100, 3667–3679. [Google Scholar] [CrossRef] [PubMed]
- Kainulainen, V.; Korhonen, T.K. Dancing to another tune—Adhesive moonlighting proteins in bacteria. Biology 2014, 3, 178–204. [Google Scholar] [CrossRef] [PubMed]
- Nagarajan, R.; Sankar, S.; Ponnuraj, K. Crystal structure of GAPDH of Streptococcus agalactiae and characterization of its interaction with extracellular matrix molecules. Microb. Pathog. 2019, 127, 359–367. [Google Scholar] [CrossRef] [PubMed]
- Nelson, D.; Goldstein, J.M.; Boatright, K.; Harty, D.W.S.; Cook, S.L.; Hickman, P.J.; Potempa, J.; Travis, J.; Mayo, J.A. pH-regulated secretion of a glyceraldehyde-3-phosphate dehydrogenase from Streptococcus gordonii FSS2: Purification, characterization, and cloning of the gene encoding this enzyme. J. Dent. Res. 2001, 80, 371–377. [Google Scholar] [CrossRef] [PubMed]
- Kinoshita, H.; Uchida, H.; Kawai, Y.; Kawasaki, T.; Wakahara, N.; Matsuo, H.; Watanabe, M.; Kitazawa, H.; Ohnuma, S.; Miura, K.; et al. Cell surface Lactobacillus plantarum LA 318 glyceraldehyde-3-phosphate dehydrogenase (GAPDH) adheres to human colonic mucin. J. Appl. Microbiol. 2008, 104, 1667–1674. [Google Scholar] [CrossRef] [PubMed]
- Antikainen, J.; Kuparinen, V.; Lähteenmäki, K.; Korhonen, T.K. Enolases from Gram-positive bacterial pathogens and commensal lactobacilli share functional similarity in virulence-associated traits. FEMS Immunol. Med. Microbiol. 2007, 51, 526–534. [Google Scholar] [CrossRef] [PubMed]
- Sanchez, B.; Bressollier, P.; Urdaci, M.C. Exported proteins in probiotic bacteria: Adhesion to intestinal surfaces, host immunomodulation and molecular cross-talking with the host. FEMS Immunol. Med. Microbiol. 2008, 54, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Granato, D.; Bergonzelli, G.E.; Pridmore, R.D.; Marvin, L.; Rouvet, M.; Corthésy-Theulaz, I.E. Cell surface-associated elongation factor Tu mediates the attachment of Lactobacillus johnsonii NCC533 (La1) to human intestinal cells and mucins. Infect. Immun. 2004, 72, 2160–2169. [Google Scholar] [CrossRef] [PubMed]
- Bergonzelli, G.E.; Granato, D.; Pridmore, R.D.; Marvin-Guy, L.F.; Donnicola, D.; Corthésy-Theulaz, I.E. GroEL of Lactobacillus johnsonii La1 (NCC 533) is cell surface associated: Potential role in interactions with the host and the gastric pathogen Helicobacter pylori. Infect. Immun. 2006, 74, 425–434. [Google Scholar] [CrossRef] [PubMed]
- Marraffini, L.A.; DeDent, A.C.; Schneewind, O. Sortases and the art of anchoring proteins to the envelopes of gram-positive bacteria. Microbiol. Mol. Biol. Rev. 2006, 70, 192–221. [Google Scholar]
- Van Pijkeren, J.P.; Canchaya, C.; Ryan, K.A.; Li, Y.; Claesson, M.J.; Sheil, B.; Steidler, L.; O’Mahony, L.; Fitzgerald, G.F.; van Sinderen, D.; et al. Comparative and functional analysis of sortase-dependent proteins in the predicted secretome of Lactobacillus salivarius UCC118. Appl. Environ. Microbiol. 2006, 72, 4143–4153. [Google Scholar] [CrossRef]
- Kumar, R.; Bansal, P.; Singh, J.; Dhanda, S.; Bhardwaj, J.K. Aggregation, adhesion and efficacy studies of probiotic candidate Pediococcus acidilactici NCDC 252: A strain of dairy origin. World J. Microbiol. Biotechnol. 2020, 36, 10. [Google Scholar] [CrossRef] [PubMed]
- Das, J.K.; Mahapatra, R.K.; Patro, S.; Goswami, C.; Suar, M. Lactobacillus acidophilus binds to MUC3 component of cultured intestinal epithelial cells with highest affinity. FEMS Microbiol. Lett. 2016, 363, fnw050. [Google Scholar] [CrossRef] [PubMed][Green Version]
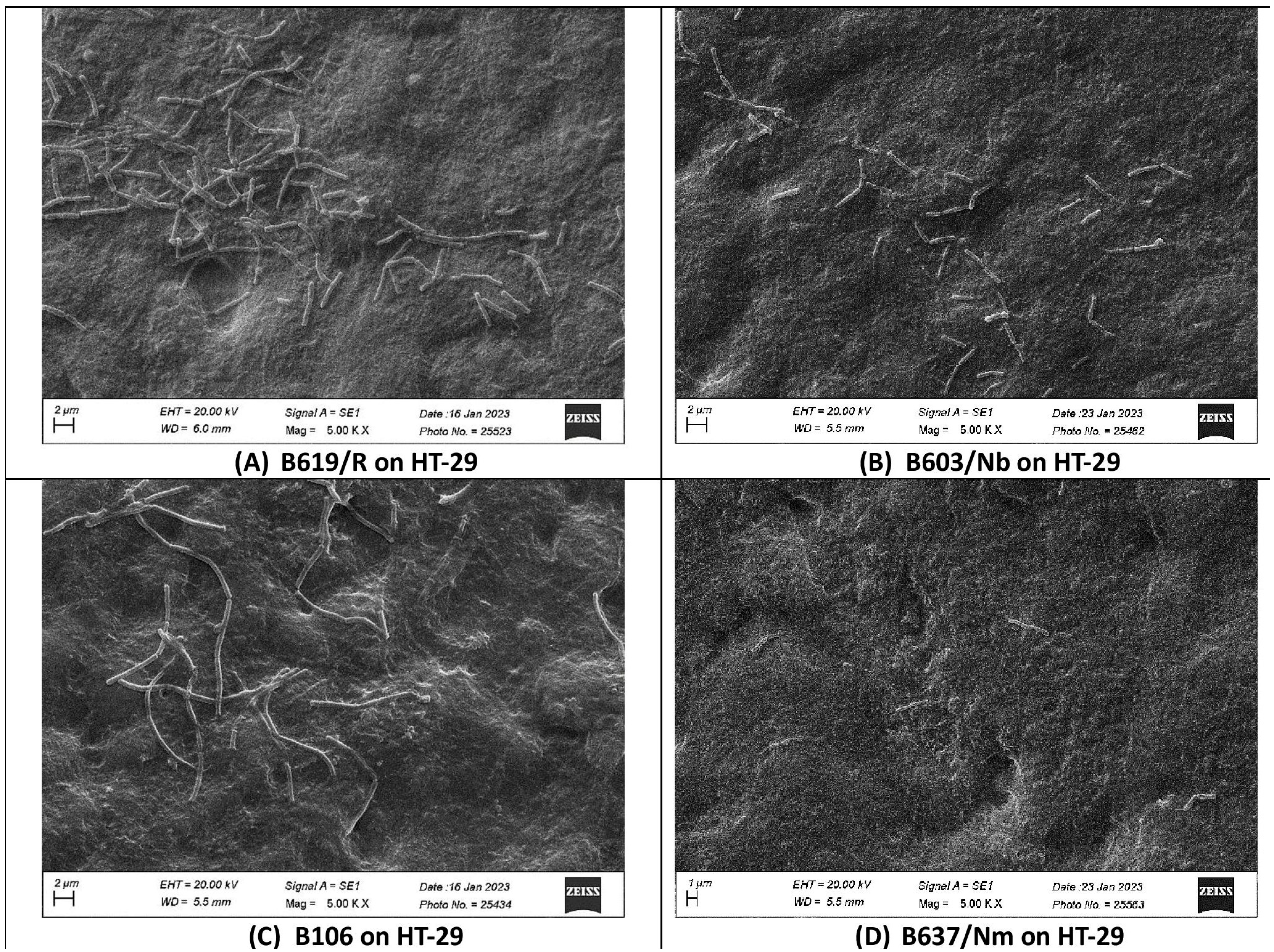
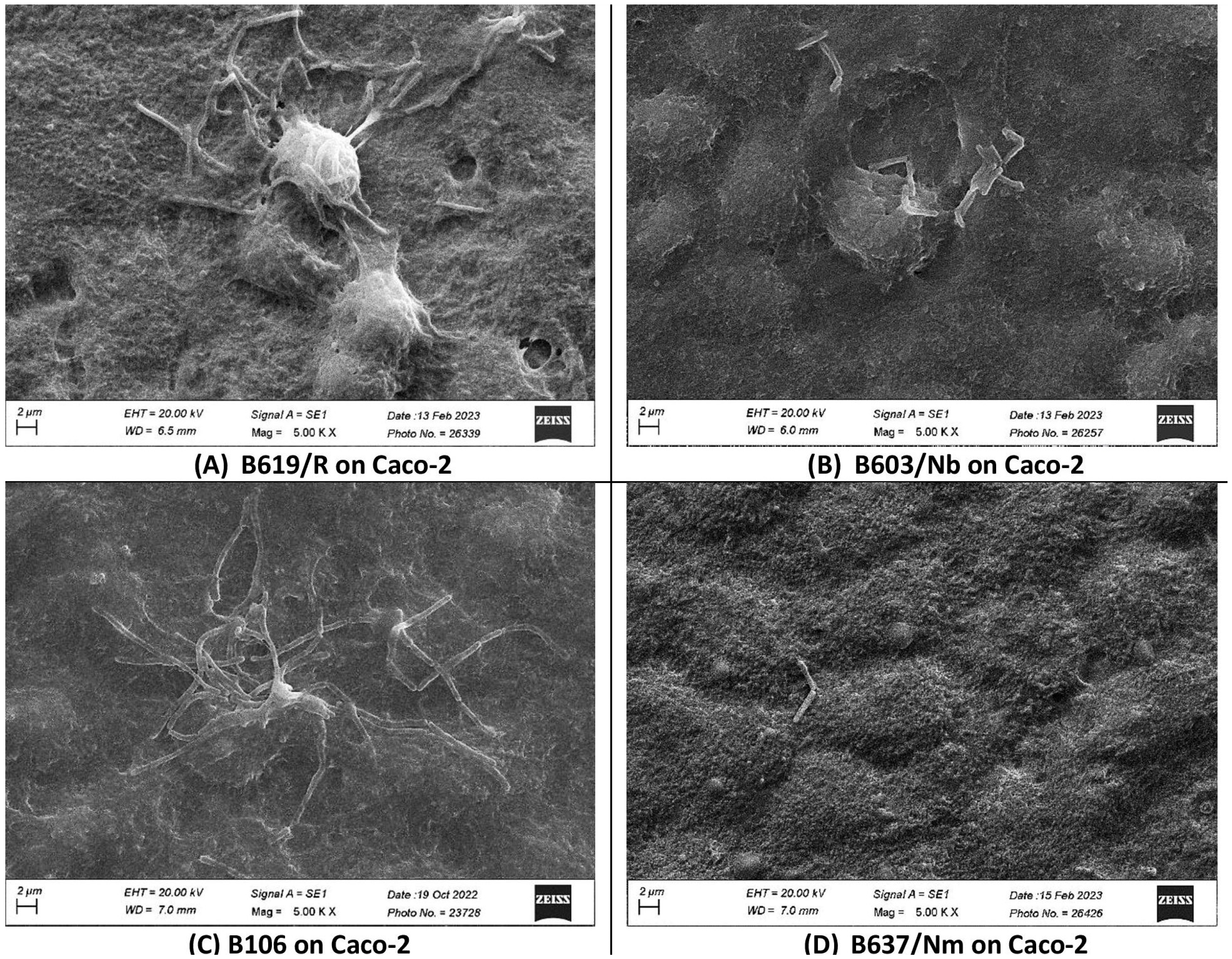

| S. clausii Strain | Number of S. clausii Cells Adhering/100 HT-29 Cells | Number of S. clausii Cells Adhering/100 Caco-2 Cells |
|---|---|---|
| B619/R | 360 ± 14 | 182 ± 10 |
| B603/Nb | 114 ± 6 | 106 ± 9 |
| B106 | 211 ± 23 | 210 ± 30 |
| B637/Nm | 11 ± 3 | 19 ± 1 |
| S. clausii Strain | % Exclusion of S. Typhimurium from HT-29 Cell Line by S. clausii Strain |
|---|---|
| B619/R | 37 ± 3 |
| B603/Nb | 19 ± 1 |
| B106 | 7 ± 1 |
| B637/Nm | 18 ± 1 |
| S. clausii Strain | Binding to ECM Components (O.D. 600 nm) | ||
|---|---|---|---|
| Mucin | Fibrinogen | Collagen | |
| B619/R | 0.250 ± 0.008 | 0.203 ± 0.008 | 0.175 ± 0.004 |
| B603/Nb | 0.206 ± 0.039 | 0.105 ± 0.002 | 0.202 ± 0.003 |
| B106 | 0.241 ± 0.032 | 0.154 ± 0.005 | 0.163 ± 0.003 |
| B637/Nm | 0.247 ± 0.009 | 0.204 ± 0.002 | 0.048 ± 0.002 |
| Strains of S. clausii | Hydrophobicity (%) | ||||
|---|---|---|---|---|---|
| Chloroform | Ethyl Acetate | Toluene | Xylene | n-Hexane | |
| B619/R | 19.1 ± 7.3 | 19.3 ± 0.5 | 32.7 ± 1.8 | 12.1 ± 0.1 | 3.1 ± 1.2 |
| B603/Nb | 17.6 ± 0.2 | 20.4 ± 0.3 | 44.8 ± 0.5 | 33.1 ± 1.3 | 19.7 ± 0.7 |
| B106 | 11.0 ± 0 | 4.4 ± 1.5 | 11.2 ± 0.2 | 4.2 ± 0.1 | 4.6 ± 1.2 |
| B637/Nm | 27.3 ± 3.6 | 57.4 ± 0.5 | 67.4 ± 1.1 | 61.7 ± 0.4 | 60.4 ± 0 |
| Strains of S. clausii | Auto-aggregation (%) | % Co-Aggregation of S. clausii Strains with Pathogens after 4 h | |||||||
|---|---|---|---|---|---|---|---|---|---|
| E. coli | S. aureus subsp. aureus | E. faecalis | S. dysenteriae | K. pneumoniae | P. aeruginosa | E. aerogenes | S. Typhimurium | ||
| B619/R | 43.1 ± 4.4 | 14.8 ± 1.7 | 38.1 ± 3.6 | 16.2 ± 1.0 | 42.1 ± 1.3 | 10.4 ± 1.1 | 12.4 ± 1.5 | 37.3 ± 4.4 | 24.3 ± 3.6 |
| B603/Nb | 35.8 ± 2.4 | 45.5 ± 0.2 | 39 ±0.5 | 28 ± 0.01 | 28.5 ± 5.4 | 28.4 ± 0.8 | 41.2 ± 9.3 | 27.1 ± 2.1 | 32.1 ± 0.3 |
| B106 | 45.7 ± 0.4 | 30 ± 1.2 | 42.9 ± 0.8 | 28 ± 3.4 | 26.3 ± 3.3 | 21.6 ± 2.1 | 42.5 ± 3.4 | 26.9 ± 3.1 | 24.5 ± 0.6 |
| B637/Nm | 75.9 ± 0.9 | 33 ± 0 | 43.9 ± 0.1 | 34.1 ± 0.08 | 41.6 ± 2.2 | 30.3 ± 1.2 | 35.8 ± 0.08 | 40.0 ± 0.07 | 29.3 ± 1.4 |
| Docking Complex | Energy (kcal/mol) |
|---|---|
| LPxTG with MUC1 | −635.4 |
| LPxTG with MUC3A | −938.5 |
| LPxTG with MUC4 | −1317.1 |
| LPxTG with MUC12 | −1122.9 |
| LPxTG with MUC13 | −952.1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dhakephalkar, T.; Pisu, V.; Margale, P.; Chandras, S.; Shetty, D.; Wagh, S.; Dagar, S.S.; Kapse, N.; Dhakephalkar, P.K. Strain-Dependent Adhesion Variations of Shouchella clausii Isolated from Healthy Human Volunteers: A Study on Cell Surface Properties and Potential Probiotic Benefits. Microorganisms 2024, 12, 1771. https://doi.org/10.3390/microorganisms12091771
Dhakephalkar T, Pisu V, Margale P, Chandras S, Shetty D, Wagh S, Dagar SS, Kapse N, Dhakephalkar PK. Strain-Dependent Adhesion Variations of Shouchella clausii Isolated from Healthy Human Volunteers: A Study on Cell Surface Properties and Potential Probiotic Benefits. Microorganisms. 2024; 12(9):1771. https://doi.org/10.3390/microorganisms12091771
Chicago/Turabian StyleDhakephalkar, Tanisha, Vaidehi Pisu, Prajakta Margale, Siddhi Chandras, Deepa Shetty, Shilpa Wagh, Sumit Singh Dagar, Neelam Kapse, and Prashant K. Dhakephalkar. 2024. "Strain-Dependent Adhesion Variations of Shouchella clausii Isolated from Healthy Human Volunteers: A Study on Cell Surface Properties and Potential Probiotic Benefits" Microorganisms 12, no. 9: 1771. https://doi.org/10.3390/microorganisms12091771
APA StyleDhakephalkar, T., Pisu, V., Margale, P., Chandras, S., Shetty, D., Wagh, S., Dagar, S. S., Kapse, N., & Dhakephalkar, P. K. (2024). Strain-Dependent Adhesion Variations of Shouchella clausii Isolated from Healthy Human Volunteers: A Study on Cell Surface Properties and Potential Probiotic Benefits. Microorganisms, 12(9), 1771. https://doi.org/10.3390/microorganisms12091771






