Response of Escherichia coli to Acid Stress: Mechanisms and Applications—A Narrative Review
Abstract
:1. Introduction
2. Methods
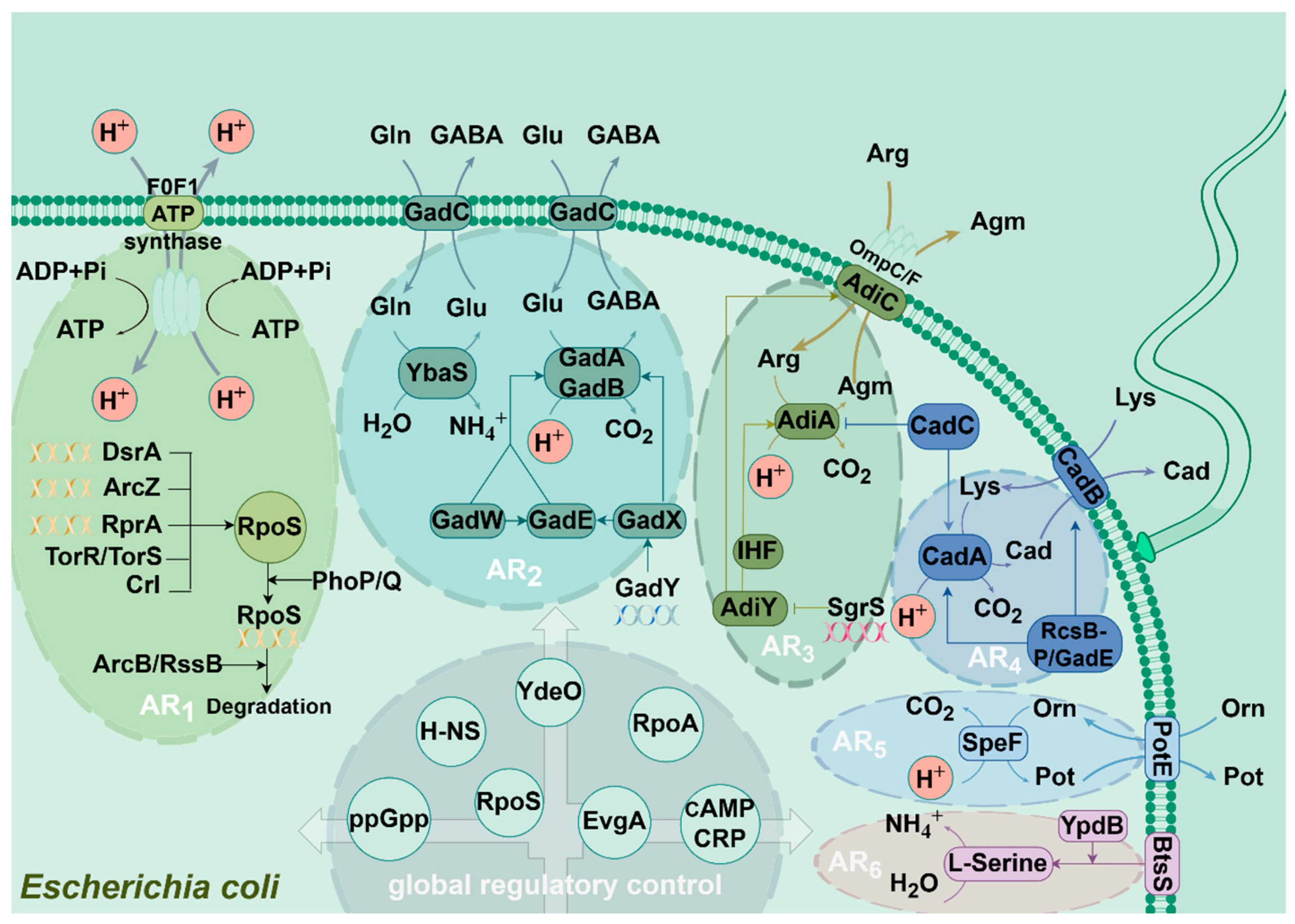
3. Mechanism of Different Acid-Resistance Systems in E. coli
3.1. AR1
3.2. AR2
3.3. AR3
3.4. AR4
3.5. AR5
3.6. AR6
4. Cell Membrane Protection
5. Macromolecular Repair
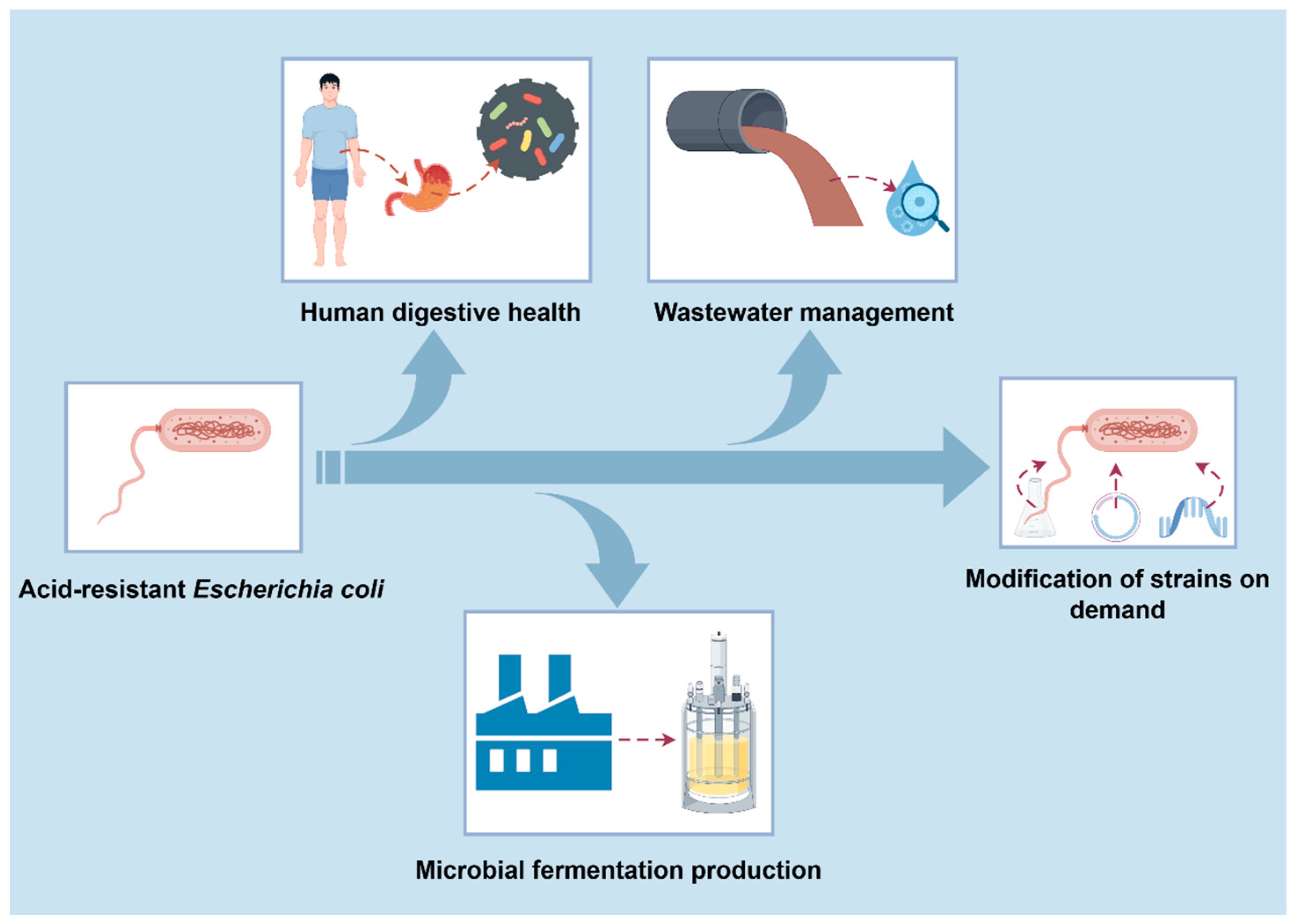
6. Potential Application and Development of Acid-Resistant E. coli in Industry
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Bao, Z.; Gao, Y.; Song, Y.; Ding, N.; Li, W.; Wu, Q.; Zhang, X.; Zheng, Y.; Li, J.; Hu, X. Construction of an Escherichia coli chassis for efficient biosynthesis of human-like N-linked glycoproteins. Front. Bioeng. Biotechnol. 2024, 12, 1370685. [Google Scholar] [CrossRef]
- Zhang, Z.; Chu, R.; Wei, W.; Song, W.; Ye, C.; Chen, X.; Wu, J.; Liu, L.; Gao, C. Systems engineering of Escherichia coli for high-level glutarate production from glucose. Nat. Commun. 2024, 15, 1032. [Google Scholar] [CrossRef]
- Luqman, A.; Hassan, A.; Ullah, M.; Naseem, S.; Ullah, M.; Zhang, L.; Din, A.U.; Ullah, K.; Ahmad, W.; Wang, G. Role of the intestinal microbiome and its therapeutic intervention in cardiovascular disorder. Front. Immunol. 2024, 15, 1321395. [Google Scholar] [CrossRef]
- Ma, G.; Shi, Y.; Meng, L.; Fan, H.; Tang, X.; Luo, H.; Wang, D.; Zhou, J.; Xiao, X. Factors affecting the early establishment of neonatal intestinal flora and its intervention measures. Front. Cell. Infect. Microbiol. 2023, 13, 1295111. [Google Scholar] [CrossRef]
- Mitra, S.D.; Shome, R.; Bandopadhyay, S.; Geddam, S.; Kumar, A.P.; Murugesan, D.; Shome, A.; Shome, B.R. Genetic insights of antibiotic resistance, pathogenicity (virulence) and phylogenetic relationship of Escherichia coli strains isolated from livestock, poultry and their handlers-a one health snapshot. Mol. Biol. Rep. 2024, 51, 404. [Google Scholar] [CrossRef]
- Yim, J.; Seo, K.; Chon, J.; Jeong, D.; Song, K. Status and prospects of PCR detection methods for diagnosing pathogenic Escherichia coli: A review. J. Dairy Sci. Biotechnol. 2021, 39, 51–62. [Google Scholar] [CrossRef]
- Park, N.; Hur, J.I.; Lee, S.; Ryu, S. Prevalence of CTX-M types among ESBL-producing pathogenic Escherichia coli isolates from foodborne diarrheal patients in Gyeonggi-do, South Korea. Food Sci. Biotechnol. 2024, 33, 2825–2833. [Google Scholar] [CrossRef]
- Qiu, L.; Chirman, D.; Clark, J.R.; Xing, Y.; Hernandez Santos, H.; Vaughan, E.E.; Maresso, A.W. Vaccines against extraintestinal pathogenic Escherichia coli (ExPEC): Progress and challenges. Gut Microbes 2024, 16, 2359691. [Google Scholar] [CrossRef]
- Guan, N.; Liu, L. Microbial response to acid stress: Mechanisms and applications. Appl. Microbiol. Biotechnol. 2020, 104, 51–65. [Google Scholar] [CrossRef]
- Hao, X.; Chen, B.; An, T. Pathway modification of industrial microorganisms to improve acid-stress tolerance. Sheng Wu Gong Cheng Xue Bao 2015, 31, 1151–1161. [Google Scholar]
- Li, Z.; Jiang, B.; Zhang, X.; Yang, Y.; Zhu, G. The role of bacterial cell envelope structures in acid stress resistance in E. coli. Appl. Microbiol. Biotechnol. 2020, 104, 2911–2921. [Google Scholar] [CrossRef] [PubMed]
- De Biase, D.; Lund, P.A. The Escherichia coli acid stress response and its significance for pathogenesis. Adv. Appl. Microbiol. 2015, 92, 49–88. [Google Scholar] [PubMed]
- Evans, D.F.; Pye, G.; Bramley, R.; Clark, A.G.; Dyson, T.J.; Hardcastle, J.D. Measurement of gastrointestinal pH profiles in normal ambulant human subjects. Gut 1988, 29, 1035–1041. [Google Scholar] [CrossRef] [PubMed]
- Ibekwe, V.C.; Fadda, H.M.; Mcconnell, E.L.; Khela, M.K.; Evans, D.F.; Basit, A.W. Interplay between intestinal pH, transit time and feed status on the in vivo performance of pH responsive ileo-colonic release systems. Pharm. Res. 2008, 25, 1828–1835. [Google Scholar] [CrossRef]
- Slonczewski, J.L.; Rosen, B.P.; Alger, J.R.; Macnab, R.M. pH homeostasis in Escherichia coli: Measurement by 31P nuclear magnetic resonance of methylphosphonate and phosphate. Proc. Natl. Acad. Sci. USA 1981, 78, 6271–6275. [Google Scholar] [CrossRef]
- Wilks, J.C.; Slonczewski, J.L. pH of the cytoplasm and periplasm of Escherichia coli: Rapid measurement by green fluorescent protein fluorimetry. J. Bacteriol. 2007, 189, 5601–5607. [Google Scholar] [CrossRef]
- Zilberstein, D.; Agmon, V.; Schuldiner, S.; Padan, E. Escherichia coli intracellular pH, membrane potential, and cell growth. J. Bacteriol. 1984, 158, 246–252. [Google Scholar] [CrossRef]
- Yang, J.; Zhang, J.; Zhu, Z.; Du, G. The challenges and prospects of Escherichia coli as an organic acid production host under acid stress. Appl. Microbiol. Biotechnol. 2021, 105, 8091–8107. [Google Scholar] [CrossRef]
- Lins, M.; Puppin Zandonadi, R.; Raposo, A.; Ginani, V.C. Food waste on foodservice: An overview through the perspective of sustainable dimensions. Foods 2021, 10, 1175. [Google Scholar] [CrossRef]
- Foster, J.W. Acid stress responses of Salmonella and E. coli: Survival mechanisms, regulation, and implications for pathogenesis. J. Microbiol. 2001, 39, 89–94. [Google Scholar]
- Peter, L.; Angela, T.; Daniela, D.B. Coping with low pH: Molecular strategies in neutralophilic bacteria. FEMS Microbiol. Rev. 2015, 38, 1091–1125. [Google Scholar]
- Huang, D.H.; Wang, K.; Chiu, C.P.; Pan, T.M.; Tsai, T.Y. Effects of chemical and low-temperature treatments and adaption on the responses of virulence factor genes and outer membrane proteins in Escherichia coli O157:H7. J. Microbiol. Immunol. 2015, 48, 604–612. [Google Scholar] [CrossRef]
- Lin, J.; Lee, I.S.; Frey, J.; Slonczewski, J.L.; Foster, J.W. Comparative analysis of extreme acid survival in Salmonella typhimurium, Shigella flexneri, and Escherichia coli. J. Bacteriol. 1995, 177, 4097–4104. [Google Scholar] [CrossRef]
- Li, G.; Morigen; Yao, Y. TorR/TorS Two-Component system resists extreme acid environment by regulating the key response factor RpoS in Escherichia coli. Gene 2022, 821, 146295. [Google Scholar] [CrossRef]
- Dong, T.; Kirchhof, M.G.; Schellhorn, H.E. RpoS regulation of gene expression during exponential growth of Escherichia coli K12. Mol. Genet. Genom. 2008, 279, 267–277. [Google Scholar] [CrossRef]
- Gama-Castro, S.; Salgado, H.; Peralta-Gil, M.; Santos-Zavaleta, A.; Muniz-Rascado, L.; Solano-Lira, H.; Jimenez-Jacinto, V.; Weiss, V.; Garcia-Sotelo, J.S.; Lopez-Fuentes, A.; et al. RegulonDB version 7.0: Transcriptional regulation of Escherichia coli K-12 integrated within genetic sensory response units (Gensor Units). Nucleic Acids. Res. 2010, 39, D98–D105. [Google Scholar] [CrossRef]
- Geng, H.; Jiang, R. CAMP receptor protein (CRP)-mediated resistance/tolerance in bacteria: Mechanism and utilization in biotechnology. Appl. Microbiol. Biot. 2015, 99, 4533–4543. [Google Scholar] [CrossRef]
- Chakraborti, S.; Dhalla, N.S. Regulation of Ca2+-ATPases, V-ATPases and F-ATPases, 1st ed.; Springer International Publishing: Cham, Switzerland, 2016; pp. 459–469. [Google Scholar]
- Richard, H.; Foster, J.W. Escherichia coli glutamate- and arginine-dependent acid resistance systems increase internal pH and reverse transmembrane potential. J. Bacteriol. 2004, 186, 6032–6041. [Google Scholar] [CrossRef]
- Biase, D.D.; Pennacchietti, E. Glutamate decarboxylase-dependent acid resistance in orally acquired bacteria: Function, distribution and biomedical implications of the gadBC operon. Mol. Microbiol. 2012, 86, 770–786. [Google Scholar] [CrossRef]
- Castanie-Cornet, M.; Penfound, T.A.; Smith, D.; Elliott, J.F.; Foster, J.W. Control of acid resistance in Escherichia coli. J. Bacteriol. 1999, 181, 3525–3535. [Google Scholar] [CrossRef]
- Patten, C.L.; Kirchhof, M.G.; Schertzberg, M.R.; Morton, R.A.; Schellhorn, H.E. Microarray analysis of RpoS-mediated gene expression in Escherichia coli K-12. Mol. Genet. Genom. 2004, 272, 580–591. [Google Scholar] [CrossRef] [PubMed]
- Thompson, K.M.; Gottesman, S. The MiaA tRNA modification enzyme is necessary for robust RpoS expression in Escherichia coli. J. Bacteriol. 2014, 196, 754–761. [Google Scholar] [CrossRef] [PubMed]
- Weber, H.; Polen, T.; Heuveling, J.; Wendisch, V.F.; Hengge, R. Genome-wide analysis of the general stress response network in Escherichia coli: σS-dependent genes, promoters, and sigma factor selectivity. J. Bacteriol. 2005, 187, 1591–1603. [Google Scholar] [CrossRef] [PubMed]
- Kowalczyk, L.; Ratera, M.; Paladino, A.; Bartoccioni, P.; Errasti-Murugarren, E.; Valencia, E.; Portella, G.; Bial, S.; Zorzano, A.; Fita, I.; et al. Molecular basis of substrate-induced permeation by an amino acid antiporter. Proc. Natl. Acad. Sci. USA 2011, 108, 3935–3940. [Google Scholar] [CrossRef] [PubMed]
- Lu, P.; Ma, D.; Chen, Y.; Guo, Y.; Chen, G.Q.; Deng, H.; Shi, Y. L-glutamine provides acid resistance for Escherichia coli through enzymatic release of ammonia. Cell Res. 2013, 23, 635–644. [Google Scholar] [CrossRef]
- Ma, Z.; Gong, S.; Richard, H.; Tucker, D.L.; Conway, T.; Foster, J.W. GadE (YhiE) activates glutamate decarboxylase-dependent acid resistance in Escherichia coli K-12. Mol. Microbiol. 2010, 49, 1309–1320. [Google Scholar] [CrossRef]
- Castaniecornet, M.P.; Foster, J.W. Escherichia coli acid resistance: cAMP receptor protein and a 20 bp cis-acting sequence control pH and stationary phase expression of the gadA and gadBC glutamate decarboxylase genes. Microbiology 2001, 147, 709–715. [Google Scholar] [CrossRef]
- Fontenot, E.M.; Ezelle, K.E.; Gabreski, L.N.; Giglio, E.R.; Mcafee, J.M.; Mills, A.C.; Qureshi, M.N.; Salmon, K.M.; Toyota, C.G. YfdW and YfdU are required for oxalate-induced acid tolerance in Escherichia coli K-12. J. Bacteriol. 2013, 195, 1446–1455. [Google Scholar] [CrossRef]
- Krin, E.; Danchin, A.; Soutourina, O. Decrypting the H-NS-dependent regulatory cascade of acid stress resistance in Escherichia coli. BMC Microbiol. 2010, 10, 273. [Google Scholar] [CrossRef]
- Ma, X.; Zhang, S.; Xu, Z.; Li, H.; Lu, Y. SdiA Improves the Acid Tolerance of E. coli by Regulating GadW and GadY Expression. Front. Microbiol. 2020, 11, 1078. [Google Scholar] [CrossRef]
- Schwarz, J.; Schumacher, K.; Brameyer, S.; Jung, K. Bacterial battle against acidity. FEMS Microbiol. Rev. 2022, 46, fuac037. [Google Scholar] [CrossRef] [PubMed]
- Tramonti, A.; Santis, F.D.; Pennacchietti, E.; Biase, D.D. The yhiM gene codes for an inner membrane protein involved in GABA export in Escherichia coli. AIMS Microbiol. 2017, 3, 71. [Google Scholar] [CrossRef] [PubMed]
- Iyer, R.; Williams, C.; Miller, C. Arginine-agmatine antiporter in extreme acid resistance in Escherichia coli. J. Bacteriol. 2003, 185, 6556–6561. [Google Scholar] [CrossRef] [PubMed]
- Richard, H.T.; Foster, J.W. Acid resistance in Escherichia coli. Adv. Appl. Microbiol. 2003, 52, 167–186. [Google Scholar]
- Fang, Y.; Kolmakova-Partensky, L.; Miller, C. A bacterial arginine-agmatine exchange transporter involved in extreme acid resistance. J. Biol. Chem. 2007, 282, 176–182. [Google Scholar] [CrossRef]
- Gong, S.; Ma, Z.; Foster, J.W. The Era-like GTPase TrmE conditionally activates gadE and glutamate-dependent acid resistance in Escherichia coli. Mol. Microbiol. 2004, 54, 948–961. [Google Scholar] [CrossRef] [PubMed]
- Bekhit, A.; Fukamachi, T.; Saito, H.; Kobayashi, H. The role of OmpC and OmpF in acidic resistance in Escherichia coli. Biol. Pharm. Bull. 2011, 34, 330–334. [Google Scholar] [CrossRef]
- Tsai, M.F.; Miller, C. An arginine-agmatine antiporter optimized for extreme acid resistance in enteric bacteria. Biophys. J. 2013, 104, 300a. [Google Scholar] [CrossRef]
- Moreau, P.L. The lysine decarboxylase CadA protects Escherichia coli starved of phosphate against fermentation acids. J. Bacteriol. 2007, 189, 2249–2261. [Google Scholar] [CrossRef]
- Neely, M.N.; Dell, C.L.; Olson, E.R. Roles of LysP and CadC in mediating the lysine requirement for acid induction of the Escherichia coli cad operon. J. Bacteriol. 1994, 176, 3278–3285. [Google Scholar] [CrossRef]
- Rauschmeier, M.; Schüppel, V.; Tetsch, L.; Jung, K. New insights into the interplay between the lysine transporter LysP and the pH sensor CadC in Escherichia coli. J. Mol. Biol. 2014, 426, 215–229. [Google Scholar] [CrossRef]
- Torres, A.G. The cad locus of Enterobacteriaceae: More than just lysine decarboxylation. Anaerobe 2009, 15, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Guerra, P.R.; Herrero-Fresno, A.; Ladero, V.; Redruello, B.; Dos Santos, T.P.; Spiegelhauer, M.R.; Jelsbak, L.; Olsen, J.E. Putrescine biosynthesis and export genes are essential for normal growth of avian pathogenic Escherichia coli. BMC Microbiol. 2018, 18, 226. [Google Scholar] [CrossRef]
- Kashiwagi, K.; Suzuki, T.; Suzuki, F.; Furuchi, T.; Igarashi, K. Coexistence of the genes for putrescine transport protein and ornithine decarboxylase at 16 min on Escherichia coli chromosome. J. Biol. Chem. 1991, 266, 20922–20927. [Google Scholar] [CrossRef] [PubMed]
- Wiebe, M.A.; Brannon, J.R.; Steiner, B.D.; Bamidele, A.; Schrimpe-Rutledge, A.C.; Codreanu, S.G.; Sherrod, S.D.; Mclean, J.A.; Hadjifrangiskou, M. Serine deamination is a new acid tolerance mechanism observed in uropathogenic Escherichia coli. mBio 2022, 13, e02922–e02963. [Google Scholar] [CrossRef]
- Vilhena, C.; Kaganovitch, E.; Shin, J.Y.; Grünberger, A.; Behr, S.; Kristoficova, I.; Brameyer, S.; Kohlheyer, D.; Jung, K. A single-cell view of the BtsSR/YpdAB pyruvate sensing network in Escherichia coli and its biological relevance. J. Bacteriol. 2018, 200, 10–1128. [Google Scholar] [CrossRef]
- Xu, W.; Mukherjee, S.; Ning, Y.; Hsu, F.F.; Zhang, K. Cyclopropane fatty acid synthesis affects cell shape and acid resistance in Leishmania mexicana. Int. J. Parasitol. 2018, 48, 245–256. [Google Scholar] [CrossRef]
- Morè, N.; Martorana, A.M.; Biboy, J.; Otten, C.; Winkle, M.; Serrano, C.K.G.; Montón Silva, A.; Atkinson, L.; Yau, H.; Breukink, E. Peptidoglycan remodeling enables Escherichia coli to survive severe outer membrane assembly defect. mBio 2019, 10, e02729-18. [Google Scholar] [CrossRef] [PubMed]
- Yuan, J.; Jin, F.; Glatter, T.; Sourjik, V. Osmosensing by the bacterial PhoQ/PhoP two-component system. Proc. Natl. Acad. Sci. USA 2017, 114, E10792–E10798. [Google Scholar] [CrossRef]
- Chakraborty, S.; Winardhi, R.S.; Morgan, L.K.; Yan, J.; Kenney, L.J. Non-canonical activation of OmpR drives acid and osmotic stress responses in single bacterial cells. Nat. Commun. 2017, 8, 1587. [Google Scholar] [CrossRef]
- Sperandeo, P.; Martorana, A.M.; Polissi, A. Lipopolysaccharide biosynthesis and transport to the outer membrane of Gram-negative bacteria. In Bacterial Cell Walls and Membranes; Subcellular Biochemistry; Springer: Berlin/Heidelberg, Germany, 2019; pp. 9–37. [Google Scholar]
- Dahl, J.U.; Koldewey, P.; Salmon, L.C.; Horowitz, S.; Bardwell, J.C.A.; Jakob, U. HdeB functions as an acid-protective chaperone in bacteria. J. Biol. Chem. 2015, 290, 65–75. [Google Scholar] [CrossRef]
- Foit, L.; George, J.S.; Zhang, B.W.; Brooks, C.L.; Bardwell, J.C.A. Chaperone activation by unfolding. Proc. Natl. Acad. Sci. USA 2013, 110, E1254–E1262. [Google Scholar] [CrossRef] [PubMed]
- Fu, X.; Wang, Y.; Shao, H.; Ma, J.; Song, X.; Zhang, M.; Chang, Z. DegP functions as a critical protease for bacterial acid resistance. FEBS J. 2018, 285, 3525–3538. [Google Scholar] [CrossRef]
- Huber, D.; Bukau, B. DegP: A Protein “Death Star”. Structure 2008, 16, 989–990. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Zhang, J.; Zhu, Z.; Jiang, X.; Zheng, T.; Du, G. Revealing novel synergistic defense and acid tolerant performance of Escherichia coli in response to organic acid stimulation. Appl. Microbiol. Biotechnol. 2022, 106, 7577–7594. [Google Scholar] [CrossRef] [PubMed]
- Peters, K.; Kannan, S.; Rao, V.A.; Biboy, J.; Vollmer, W. The redundancy of peptidoglycan carboxypeptidases ensures robust cell shape maintenance in Escherichia coli. mBio 2016, 7, e00816–e00819. [Google Scholar] [CrossRef] [PubMed]
- Shayanfar, S.; Broumand, A.; Pillai, D.S. Acid stress induces differential accumulation of metabolites in Escherichia coli O26:H11. J. Appl. Microbiol. 2018, 125, 1911–1919. [Google Scholar] [CrossRef]
- Xu, J.; Guo, L.; Zhao, N.; Meng, X.; Zhang, J.; Wang, T.; Wei, X.; Fan, M. Response mechanisms to acid stress of acid-resistant bacteria and biotechnological applications in the food industry. Crit. Rev. Biotechnol. 2023, 43, 258–274. [Google Scholar] [CrossRef]
- Nudler, E. Transcription-coupled global genomic repair in E. coli. Trends Biochem. Sci. 2023, 48, 873–882. [Google Scholar] [CrossRef]
- Duprie, M. Recruitment and Function of Mlh1-Pms1 in DNA Mismatch Repair; University of California: San Diego, CA, USA, 2020. [Google Scholar]
- Zhang, J.; Wang, S.; Abee, T.; Veen, S. Role of base excision repair in Listeria monocytogenes DNA stress survival during infections. J. Infect. Dis. 2021, 223, 721–734. [Google Scholar] [CrossRef]
- Payne-Dwyer, A.L.; Syeda, A.H.; Shepherd, J.W.; Frame, L.; Leake, M.C. RecA and RecB: Probing complexes of DNA repair proteins with mitomycin C in live Escherichia coli with single-molecule sensitivity. J. R. Soc. Interface 2022, 19, 20220437. [Google Scholar] [CrossRef] [PubMed]
- Mallick, S.; Das, S. Acid-tolerant bacteria and prospects in industrial and environmental applications. Appl. Microbiol. Biotechnol. 2023, 107, 3355–3374. [Google Scholar] [CrossRef] [PubMed]
- Yao, X.; Liu, P.; Chen, B.; Wang, X.; Tao, F.; Lin, Z.; Yang, X. Synthetic acid stress-tolerance modules improve growth robustness and lysine productivity of industrial Escherichia coli in fermentation at low pH. Microb. Cell Fact. 2022, 21, 68. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Peng, Z.; Zhu, Q.; Zhang, J.; Du, G. [NiFe] Hydrogenase accessory proteins HypB–HypC accelerate proton conversion to enhance the acid resistance and D-lactic acid production of Escherichia coli. ACS Synth. Biol. 2022, 11, 1521–1530. [Google Scholar] [CrossRef]
- Tan, Z.; Yoon, J.M.; Nielsen, D.R.; Shanks, J.V.; Jarboe, L.R. Membrane engineering via trans unsaturated fatty acids production improves Escherichia coli robustness and production of biorenewables. Metab. Eng. 2016, 35, 105–113. [Google Scholar] [CrossRef]
- Hou, D.; O Connor, D.; Igalavithana, A.D.; Alessi, D.S.; Luo, J.; Tsang, D.C.W.; Sparks, D.L.; Yamauchi, Y.; Rinklebe, J.; Ok, Y.S. Metal contamination and bioremediation of agricultural soils for food safety and sustainability. Nat. Rev. Earth Environ. 2020, 1, 366–381. [Google Scholar] [CrossRef]
- Virpiranta, H.; Sotaniemi, V.; Leiviskä, T.; Taskila, S.; Rämö, J.; Johnson, D.B.; Tanskanen, J. Continuous removal of sulfate and metals from acidic mining-impacted waters at low temperature using a sulfate-reducing bacterial consortium. Chem. Eng. J. 2022, 427, 132050. [Google Scholar] [CrossRef]
- Diels, L.; De Smet, M.; Hooyberghs, L.; Corbisier, P. Heavy metals bioremediation of soil. Mol. Biotechnol. 1999, 12, 149–158. [Google Scholar] [CrossRef]
- Kang, S.H.; Singh, S.; Kim, J.; Lee, W.; Mulchandani, A.; Chen, W. Bacteria metabolically engineered for enhanced phytochelatin production and cadmium accumulation. Appl. Environ. Microbiol. 2007, 73, 6317–6320. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Z.; Huang, Z.; Gu, P. Response of Escherichia coli to Acid Stress: Mechanisms and Applications—A Narrative Review. Microorganisms 2024, 12, 1774. https://doi.org/10.3390/microorganisms12091774
Li Z, Huang Z, Gu P. Response of Escherichia coli to Acid Stress: Mechanisms and Applications—A Narrative Review. Microorganisms. 2024; 12(9):1774. https://doi.org/10.3390/microorganisms12091774
Chicago/Turabian StyleLi, Zepeng, Zhaosong Huang, and Pengfei Gu. 2024. "Response of Escherichia coli to Acid Stress: Mechanisms and Applications—A Narrative Review" Microorganisms 12, no. 9: 1774. https://doi.org/10.3390/microorganisms12091774
APA StyleLi, Z., Huang, Z., & Gu, P. (2024). Response of Escherichia coli to Acid Stress: Mechanisms and Applications—A Narrative Review. Microorganisms, 12(9), 1774. https://doi.org/10.3390/microorganisms12091774





