Shifts in Fusarium Communities and Mycotoxins in Maize Residues, Soils, and Wheat Grains throughout the Wheat Cycle: Implications for Fusarium Head Blight Epidemiology
Abstract
:1. Introduction
2. Materials and Methods
2.1. Field Sampling
2.2. Disease Evaluation at Flowering and Harvest
2.3. DNA Extraction
2.4. Illumina MiSeq Sequencing
2.5. Processing of Sequencing Data
2.6. qPCR Quantification of Dominant Fusarium Species
2.7. Mycotoxin Extraction and Analysis
2.8. Statistical Analyses
3. Results
3.1. FHB Development and Mycotoxin Contamination in Grains at Harvest
3.2. Fusarium Diversity in Grains by EF1α Metabarcoding
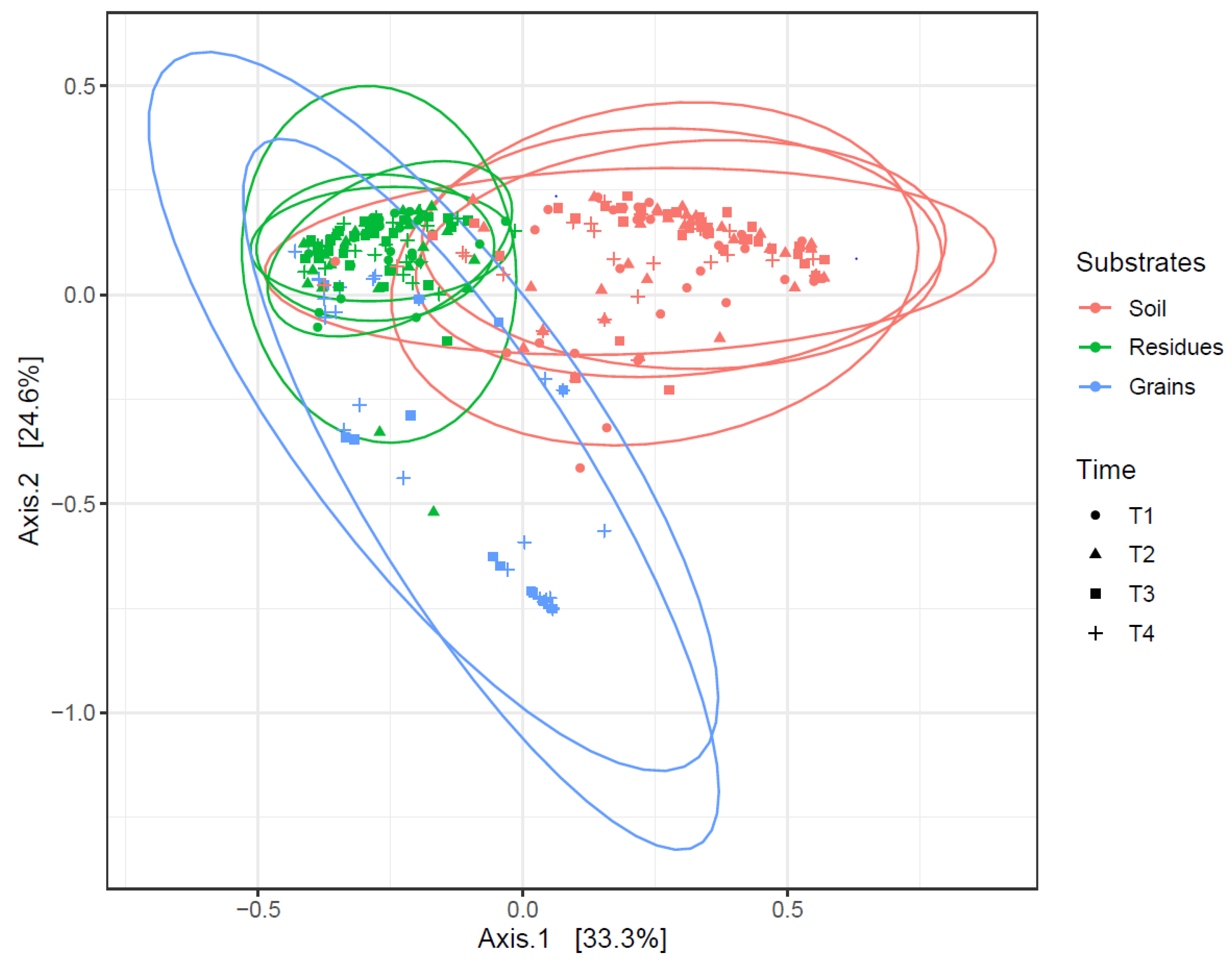
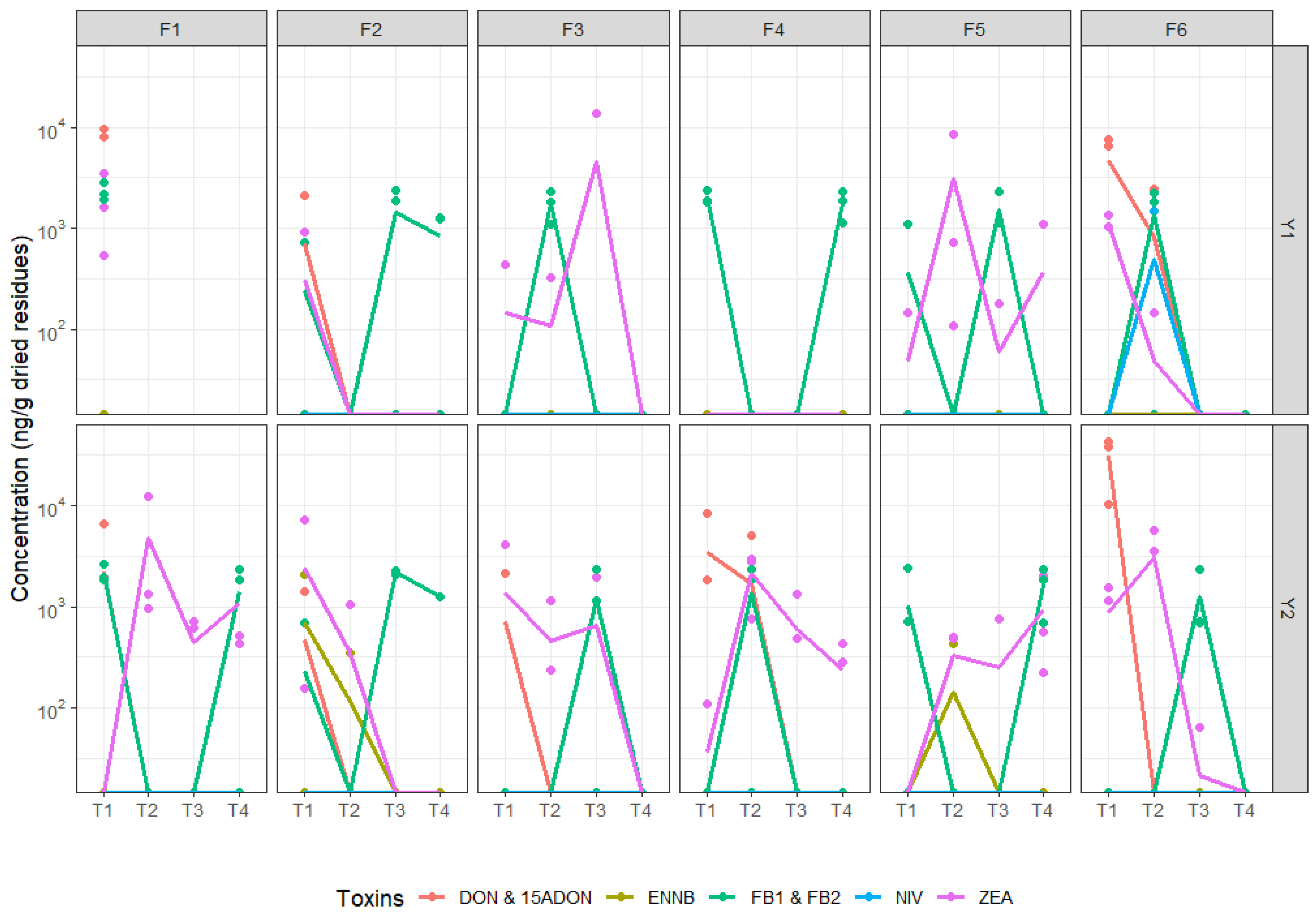
3.3. Fusarium Pathobiome Composition and Associated Toxins in Soils and Maize Residues
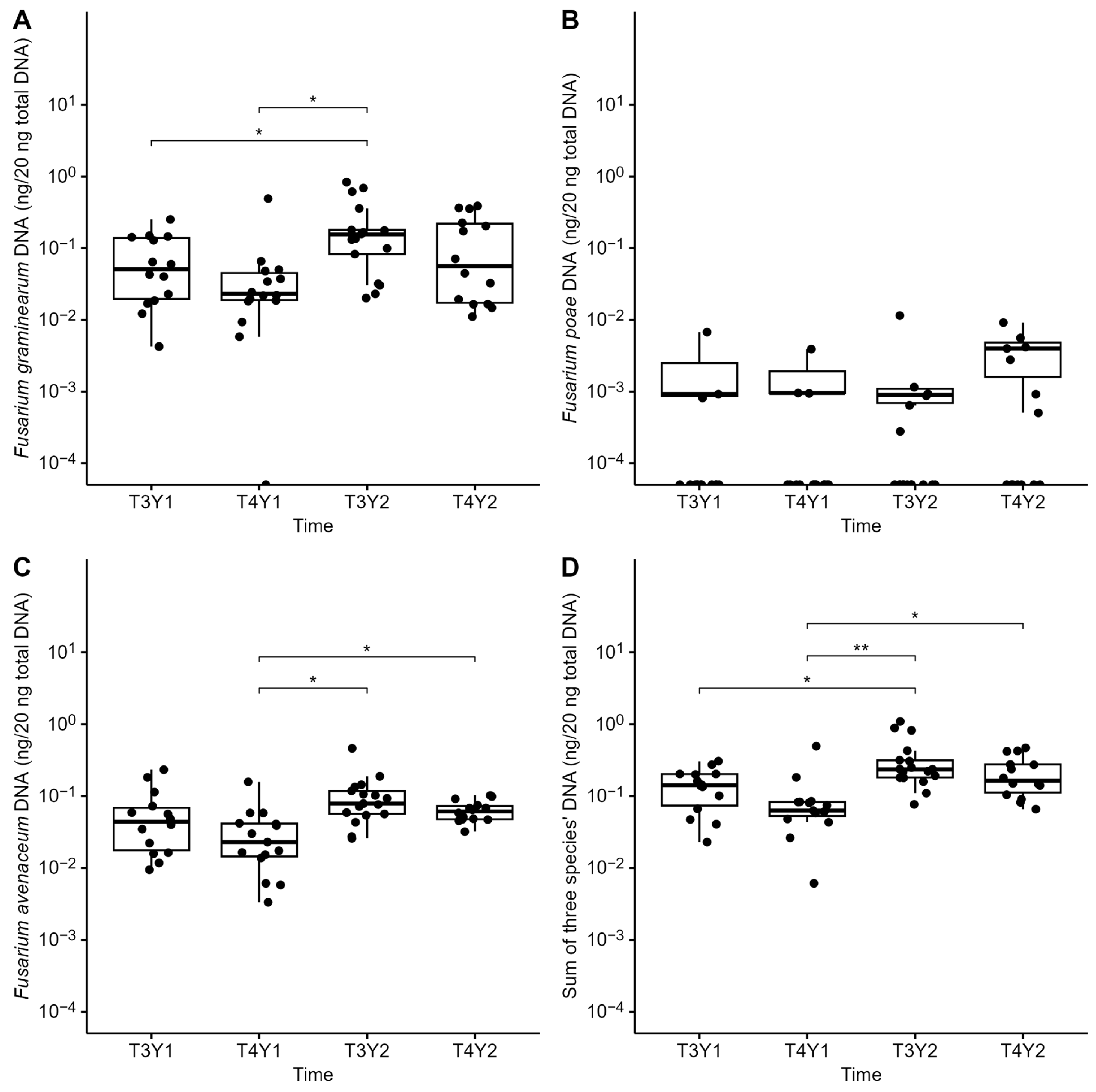
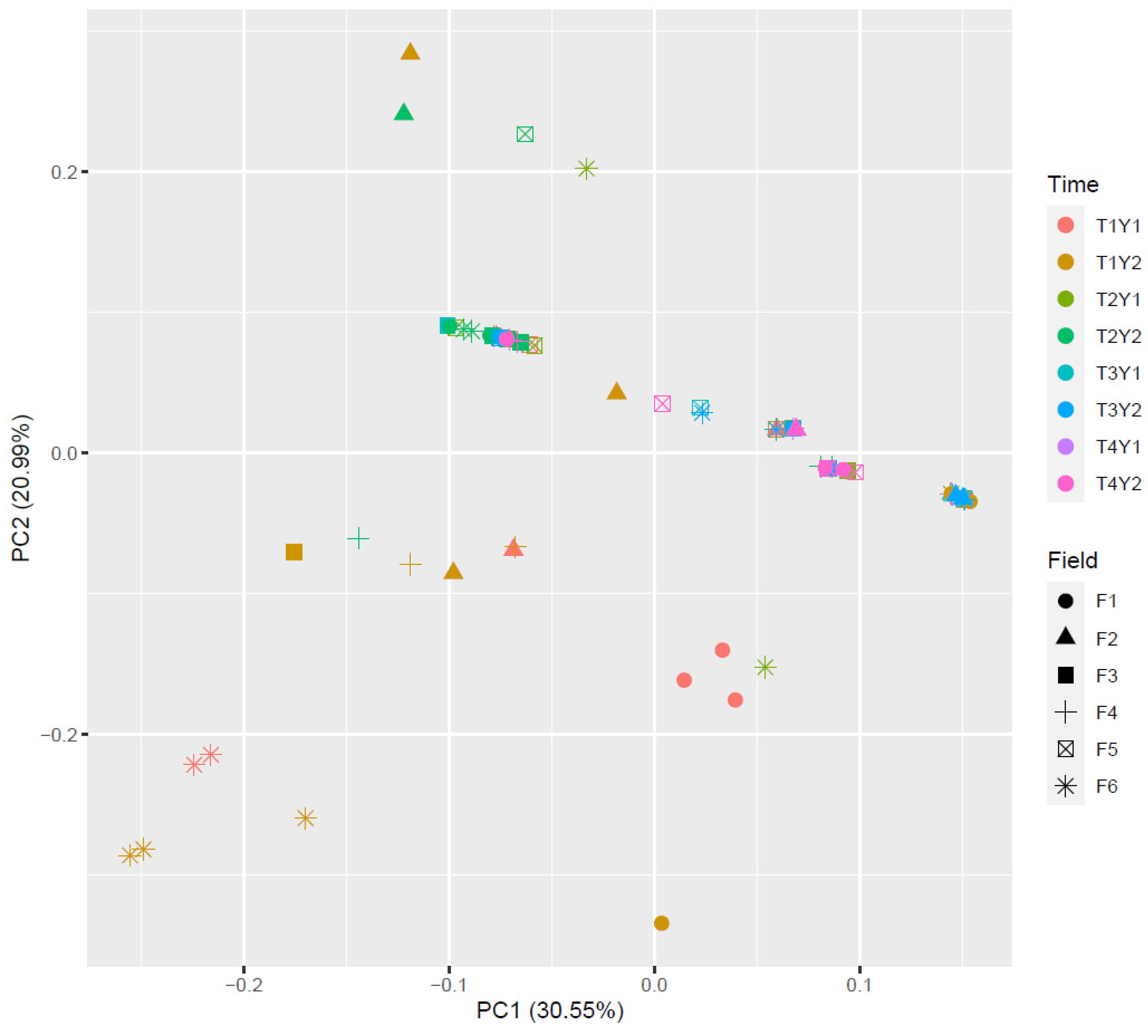
3.4. Fusarium Dispersal from Residues to Grains
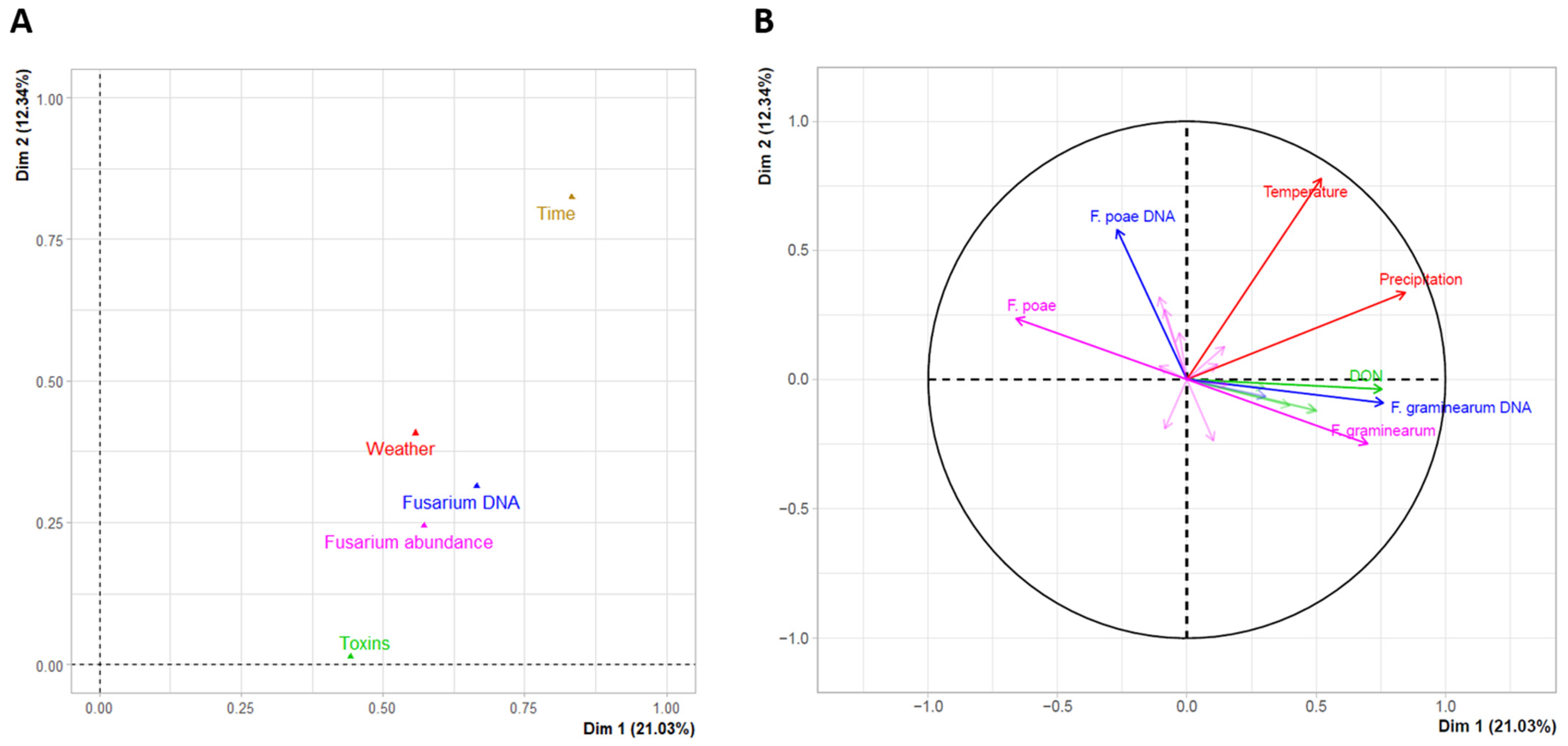
3.5. Climatic and Agronomic Factors Influencing Grain Contamination with Fusarium
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Summerell, B.A. Resolving Fusarium: Current Status of the Genus. Annu. Rev. Phytopathol. 2019, 57, 323–339. [Google Scholar] [CrossRef]
- Placinta, C.M.; D’Mello, J.P.F.; Macdonald, A.M.C. A Review of Worldwide Contamination of Cereal Grains and Animal Feed with Fusarium Mycotoxins. Anim. Feed. Sci. Technol. 1999, 78, 21–37. [Google Scholar] [CrossRef]
- Escrivá, L.; Font, G.; Manyes, L. In Vivo Toxicity Studies of Fusarium Mycotoxins in the Last Decade: A Review. Food Chem. Toxicol. 2015, 78, 185–206. [Google Scholar] [CrossRef] [PubMed]
- Ferrigo, D.; Raiola, A.; Causin, R. Fusarium Toxins in Cereals: Occurrence, Legislation, Factors Promoting the Appearance and Their Management. Molecules 2016, 21, 627. [Google Scholar] [CrossRef]
- Parry, D.W.; Jenkinson, P.; McLEOD, L. Fusarium Ear Blight (Scab) in Small Grain Cereals-a Review. Plant Pathol. 1995, 44, 207–238. [Google Scholar] [CrossRef]
- Aoki, T.; O’Donnell, K.; Geiser, D.M. Systematics of Key Phytopathogenic Fusarium Species: Current Status and Future Challenges. J. Gen. Plant Pathol. 2014, 80, 189–201. [Google Scholar] [CrossRef]
- Karlsson, I.; Persson, P.; Friberg, H. Fusarium Head Blight From a Microbiome Perspective. Front. Microbiol. 2021, 12, 628373. [Google Scholar] [CrossRef]
- Birr, T.; Hasler, M.; Verreet, J.-A.; Klink, H. Composition and Predominance of Fusarium Species Causing Fusarium Head Blight in Winter Wheat Grain Depending on Cultivar Susceptibility and Meteorological Factors. Microorganisms 2020, 8, 617. [Google Scholar] [CrossRef]
- Carbas, B.; Simões, D.; Soares, A.; Freitas, A.; Ferreira, B.; Carvalho, A.R.F.; Silva, A.S.; Pinto, T.; Diogo, E.; Andrade, E.; et al. Occurrence of Fusarium Spp. in Maize Grain Harvested in Portugal and Accumulation of Related Mycotoxins during Storage. Foods 2021, 10, 375. [Google Scholar] [CrossRef]
- Jestoi, M. Emerging Fusarium-Mycotoxins Fusaproliferin, Beauvericin, Enniatins, And Moniliformin—A Review. Crit. Rev. Food Sci. Nutr. 2008, 48, 21–49. [Google Scholar] [CrossRef] [PubMed]
- Battilani, P.; Palumbo, R.; Giorni, P.; Dall’Asta, C.; Dellafiora, L.; Gkrillas, A.; Toscano, P.; Crisci, A.; Brera, C.; De Santis, B.; et al. Mycotoxin Mixtures in Food and Feed: Holistic, Innovative, Flexible Risk Assessment Modelling Approach. EFSA Support. Publ. 2020, 17, 1757E. [Google Scholar] [CrossRef]
- Streit, E.; Schatzmayr, G.; Tassis, P.; Tzika, E.; Marin, D.; Taranu, I.; Tabuc, C.; Nicolau, A.; Aprodu, I.; Puel, O.; et al. Current Situation of Mycotoxin Contamination and Co-Occurrence in Animal Feed—Focus on Europe. Toxins 2012, 4, 788–809. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.J.; Ryu, D. Worldwide Occurrence of Mycotoxins in Cereals and Cereal-Derived Food Products: Public Health Perspectives of Their Co-Occurrence. J. Agric. Food Chem. 2017, 65, 7034–7051. [Google Scholar] [CrossRef] [PubMed]
- Suga, H.; Karugia, G.W.; Ward, T.; Gale, L.R.; Tomimura, K.; Nakajima, T.; Miyasaka, A.; Koizumi, S.; Kageyama, K.; Hyakumachi, M. Molecular Characterization of the Fusarium Graminearum Species Complex in Japan. Phytopathology 2008, 98, 159–166. [Google Scholar] [CrossRef]
- Lindblad, M.; Gidlund, A.; Sulyok, M.; Börjesson, T.; Krska, R.; Olsen, M.; Fredlund, E. Deoxynivalenol and Other Selected Fusarium Toxins in Swedish Wheat—Occurrence and Correlation to Specific Fusarium Species. Int. J. Food Microbiol. 2013, 167, 284–291. [Google Scholar] [CrossRef]
- Karlsson, I.; Edel-Hermann, V.; Gautheron, N.; Durling, M.B.; Kolseth, A.-K.; Steinberg, C.; Persson, P.; Friberg, H. Genus-Specific Primers for Study of Fusarium Communities in Field Samples. Appl. Environ. Microbiol. 2016, 82, 491–501. [Google Scholar] [CrossRef] [PubMed]
- Bass, D.; Stentiford, G.D.; Wang, H.-C.; Koskella, B.; Tyler, C.R. The Pathobiome in Animal and Plant Diseases. Trends Ecol. Evol. 2019, 34, 996–1008. [Google Scholar] [CrossRef] [PubMed]
- Cobo-Díaz, J.F.; Baroncelli, R.; Le Floch, G.; Picot, A. A Novel Metabarcoding Approach to Investigate Fusarium Species Composition in Soil and Plant Samples. FEMS Microbiol. Ecol. 2019, 95, fiz084. [Google Scholar] [CrossRef]
- Gilbert, J.; Haber, S. Overview of Some Recent Research Developments in Fusarium Head Blight of Wheat. Can. J. Plant Pathol. 2013, 35, 149–174. [Google Scholar] [CrossRef]
- Keller, M.D.; Bergstrom, G.C.; Shields, E.J. The Aerobiology of Fusarium Graminearum. Aerobiologia 2014, 30, 123–136. [Google Scholar] [CrossRef]
- Sutton, J.C. Epidemiology of Wheat Head Blight and Maize Ear Rot Caused by Fusarium Graminearum. Can. J. Plant Pathol. 1982, 4, 195–209. [Google Scholar] [CrossRef]
- Dill-Macky, R.; Jones, R.K. The Effect of Previous Crop Residues and Tillage on Fusarium Head Blight of Wheat. Plant Dis. 2000, 84, 71–76. [Google Scholar] [CrossRef]
- Fernando, W.G.D.; Miller, J.D.; Seaman, W.L.; Seifert, K.; Paulitz, T.C. Daily and Seasonal Dynamics of Airborne Spores of Fusarium Graminearum and Other Fusarium Species Sampled over Wheat Plots. Can. J. Bot. 2000, 78, 497–505. [Google Scholar] [CrossRef]
- Fernando, W.G.D.; Paulitz, T.C.; Seaman, W.L.; Dutilleul, P.; Miller, J.D. Head Blight Gradients Caused by Gibberella Zeae from Area Sources of Inoculum in Wheat Field Plots. Phytopathology 1997, 87, 414–421. [Google Scholar] [CrossRef]
- Trail, F. For Blighted Waves of Grain: Fusarium Graminearum in the Postgenomics Era. Plant Physiol. 2009, 149, 103–110. [Google Scholar] [CrossRef]
- Blandino, M.; Pilati, A.; Reyneri, A.; Scudellari, D. Effect of Maize Crop Residue Density on Fusarium Head Blight and on Deoxynivalenol Contamination of Common Wheat Grains. Cereal Res. Commun. 2010, 38, 550–559. [Google Scholar] [CrossRef]
- Leplat, J.; Friberg, H.; Abid, M.; Steinberg, C. Survival of Fusarium Graminearum, the Causal Agent of Fusarium Head Blight. A Review. Agron. Sustain. Dev. 2013, 33, 97–111. [Google Scholar] [CrossRef]
- Edwards, S.G.; Jennings, P. Impact of Agronomic Factors on Fusarium Mycotoxins in Harvested Wheat. Food Addit. Contam. Part A 2018, 35, 2443–2454. [Google Scholar] [CrossRef]
- Vogelgsang, S.; Beyer, M.; Pasquali, M.; Jenny, E.; Musa, T.; Bucheli, T.D.; Wettstein, F.E.; Forrer, H.-R. An Eight-Year Survey of Wheat Shows Distinctive Effects of Cropping Factors on Different Fusarium Species and Associated Mycotoxins. Eur. J. Agron. 2019, 105, 62–77. [Google Scholar] [CrossRef]
- Wegulo, S.N.; Baenziger, P.S.; Hernandez Nopsa, J.; Bockus, W.W.; Hallen-Adams, H. Management of Fusarium Head Blight of Wheat and Barley. Crop Prot. 2015, 73, 100–107. [Google Scholar] [CrossRef]
- Holland, J.M. The Environmental Consequences of Adopting Conservation Tillage in Europe: Reviewing the Evidence. Agric. Ecosyst. Environ. 2004, 103, 1–25. [Google Scholar] [CrossRef]
- Kassam, A.; Friedrich, T.; Derpsch, R. Global Spread of Conservation Agriculture. Int. J. Environ. Stud. 2019, 76, 29–51. [Google Scholar] [CrossRef]
- Kenngott, K.G.J.; Albert, J.; Meyer-Wolfarth, F.; Schaumann, G.E.; Muñoz, K. Fusarium Mycotoxins in Maize Field Soils: Method Validation and Implications for Sampling Strategy. Toxins 2022, 14, 130. [Google Scholar] [CrossRef] [PubMed]
- ARVALIS Choisir & Décider—Synthèse Nationale 2021. Available online: https://www.google.com/url?sa=t&source=web&rct=j&opi=89978449&url=https://www.arvalis.fr/sites/default/files/imported_files/choisir1_cap_2021_national1799701216705264424.pdf&ved=2ahUKEwj38YLei5GIAxV9TaQEHcq5EFkQFnoECBUQAQ&usg=AOvVaw1_eB_8C1ZQKbp7gwCVe1f3 (accessed on 26 May 2023).
- Stack, R.; McMullen, M. A Visual Scale to Estimate Severity of Fusarium Head Blight in Wheat; North Dakota State University: Fargo, ND, USA, 1994; p. 1905. [Google Scholar]
- Callahan, B.J.; McMurdie, P.J.; Rosen, M.J.; Han, A.W.; Johnson, A.J.A.; Holmes, S.P. DADA2: High-Resolution Sample Inference from Illumina Amplicon Data. Nat. Methods 2016, 13, 581–583. [Google Scholar] [CrossRef] [PubMed]
- Weinstein, M.M.; Prem, A.; Jin, M.; Tang, S.; Bhasin, J.M. FIGARO: An Efficient and Objective Tool for Optimizing Microbiome rRNA Gene Trimming Parameters. bioRxiv 2019, bioRxiv:610394. [Google Scholar] [CrossRef]
- Caporaso, J.G.; Kuczynski, J.; Stombaugh, J.; Bittinger, K.; Bushman, F.D.; Costello, E.K.; Fierer, N.; Peña, A.G.; Goodrich, J.K.; Gordon, J.I.; et al. QIIME Allows Analysis of High-Throughput Community Sequencing Data. Nat. Methods 2010, 7, 335–336. [Google Scholar] [CrossRef]
- Elbelt, S.; Siou, D.; Gelisse, S.; Cruaud, C.; Lannou, C.; Lebrun, M.H.; Laval, V. Optimized Real Time QPCR Assays for Detection and Quantification of Fusarium and Microdochium Species Involved in Wheat Head Blight as Defined by MIQE Guidelines. bioRxiv 2018, bioRxiv:272534. [Google Scholar] [CrossRef]
- Sulyok, M.; Berthiller, F.; Krska, R.; Schuhmacher, R. Development and Validation of a Liquid Chromatography/Tandem Mass Spectrometric Method for the Determination of 39 Mycotoxins in Wheat and Maize. Rapid Commun. Mass. Spectrom. 2006, 20, 2649–2659. [Google Scholar] [CrossRef] [PubMed]
- Scarpino, V.; Reyneri, A.; Blandino, M. Development and Comparison of Two Multiresidue Methods for the Determination of 17 Aspergillus and Fusarium Mycotoxins in Cereals Using HPLC-ESI-TQ-MS/MS. Front. Microbiol. 2019, 10, 1–12. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2022; Available online: https://www.R-project.org/ (accessed on 13 April 2022).
- Friedman, J.; Alm, E.J. Inferring Correlation Networks from Genomic Survey Data. PLoS Comput. Biol. 2012, 8, e1002687. [Google Scholar] [CrossRef] [PubMed]
- Crous, P.W.; Cowan, D.A.; Maggs-Kölling, G.; Yilmaz, N.; Thangavel, R.; Wingfield, M.J.; Noordeloos, M.E.; Dima, B.; Brandrud, T.E.; Jansen, G.M.; et al. Fungal Planet Description Sheets: 1182–1283. Persoonia-Mol. Phylogeny Evol. Fungi 2021, 46, 313–528. [Google Scholar] [CrossRef] [PubMed]
- Dong, F.; Qiu, J.; Xu, J.; Yu, M.; Wang, S.; Sun, Y.; Zhang, G.; Shi, J. Effect of Environmental Factors on Fusarium Population and Associated Trichothecenes in Wheat Grain Grown in Jiangsu Province, China. Int. J. Food Microbiol. 2016, 230, 58–63. [Google Scholar] [CrossRef] [PubMed]
- Scala, V.; Aureli, G.; Cesarano, G.; Incerti, G.; Fanelli, C.; Scala, F.; Reverberi, M.; Bonanomi, G. Climate, Soil Management, and Cultivar Affect Fusarium Head Blight Incidence and Deoxynivalenol Accumulation in Durum Wheat of Southern Italy. Front. Microbiol. 2016, 7, 1014. [Google Scholar] [CrossRef]
- Schöneberg, T.; Musa, T.; Forrer, H.-R.; Mascher, F.; Bucheli, T.D.; Bertossa, M.; Keller, B.; Vogelgsang, S. Infection Conditions of Fusarium Graminearum in Barley Are Variety Specific and Different from Those in Wheat. Eur. J. Plant Pathol. 2018, 151, 975–989. [Google Scholar] [CrossRef]
- Subedi, K.D.; Ma, B.L.; Xue, A.G. Planting Date and Nitrogen Effects on Fusarium Head Blight and Leaf Spotting Diseases in Spring Wheat. Agron. J. 2007, 99, 113–121. [Google Scholar] [CrossRef]
- Gorczyca, A.; Oleksy, A.; Gala-Czekaj, D.; Urbaniak, M.; Laskowska, M.; Waśkiewicz, A.; Stępień, Ł. Fusarium Head Blight Incidence and Mycotoxin Accumulation in Three Durum Wheat Cultivars in Relation to Sowing Date and Density. Sci. Nat. 2017, 105, 2. [Google Scholar] [CrossRef]
- Xu, X.-M.; Nicholson, P.; Thomsett, M.A.; Simpson, D.; Cooke, B.M.; Doohan, F.M.; Brennan, J.; Monaghan, S.; Moretti, A.; Mule, G.; et al. Relationship Between the Fungal Complex Causing Fusarium Head Blight of Wheat and Environmental Conditions. Phytopathology 2008, 98, 69–78. [Google Scholar] [CrossRef] [PubMed]
- Nazari, L.; Pattori, E.; Manstretta, V.; Terzi, V.; Morcia, C.; Somma, S.; Moretti, A.; Ritieni, A.; Rossi, V. Effect of Temperature on Growth, Wheat Head Infection, and Nivalenol Production by Fusarium Poae. Food Microbiol. 2018, 76, 83–90. [Google Scholar] [CrossRef]
- Martínez, M.; Ramirez Albuquerque, L.D.; Dinolfo, M.I.; Biganzoli, F.; F Pinto, V.; Stenglein, S.A. Effects of Fusarium Graminearum and Fusarium Poae on Disease Parameters, Grain Quality and Mycotoxin Contamination in Barley (Part II). J. Sci. Food Agric. 2020, 100, 3182–3191. [Google Scholar] [CrossRef]
- Xu, X.; Nicholson, P. Community Ecology of Fungal Pathogens Causing Wheat Head Blight. Annu. Rev. Phytopathol. 2009, 47, 83–103. [Google Scholar] [CrossRef]
- Covarelli, L.; Beccari, G.; Prodi, A.; Generotti, S.; Etruschi, F.; Juan, C.; Ferrer, E.; Mañes, J. Fusarium Species, Chemotype Characterisation and Trichothecene Contamination of Durum and Soft Wheat in an Area of Central Italy. J. Sci. Food Agric. 2015, 95, 540–551. [Google Scholar] [CrossRef] [PubMed]
- Martínez, M.; Ramírez Albuquerque, L.; Arata, A.F.; Biganzoli, F.; Fernández Pinto, V.; Stenglein, S.A. Effects of Fusarium Graminearum and Fusarium Poae on Disease Parameters, Grain Quality and Mycotoxins Contamination in Bread Wheat (Part I). J. Sci. Food Agric. 2020, 100, 863–873. [Google Scholar] [CrossRef]
- Audenaert, K.; Van Broeck, R.; Bekaert, B.; De Witte, F.; Heremans, B.; Messens, K.; Höfte, M.; Haesaert, G. Fusarium Head Blight (FHB) in Flanders: Population Diversity, Inter-Species Associations and DON Contamination in Commercial Winter Wheat Varieties. Eur. J. Plant Pathol. 2009, 125, 445–458. [Google Scholar] [CrossRef]
- Munkvold, G.P. Fusarium Species and Their Associated Mycotoxins. Methods Mol. Biol. 2017, 1542, 51–106. [Google Scholar] [CrossRef]
- Boutigny, A.-L.; Ward, T.J.; Ballois, N.; Iancu, G.; Ioos, R. Diversity of the Fusarium Graminearum Species Complex on French Cereals. Eur. J. Plant Pathol. 2014, 138, 133–148. [Google Scholar] [CrossRef]
- Yli-Mattila, T. Ecology and Evolution of Toxigenic Fusarium Species in Cereals in Northern Europe and Asia. J. Plant Pathol. 2010, 92, 7–18. [Google Scholar]
- Imathiu, S.M.; Edwards, S.G.; Ray, R.V.; Back, M.A. Fusarium Langsethiae—A HT-2 and T-2 Toxins Producer That Needs More Attention. J. Phytopathol. 2013, 161, 1–10. [Google Scholar] [CrossRef]
- Infantino, A.; Santori, A.; Aureli, G.; Belocchi, A.; De Felice, S.; Tizzani, L.; Lattanzio, V.M.T.; Haidukowski, M.; Pascale, M. Occurrence of Fusarium Langsethiae Strains Isolated from Durum Wheat in Italy. J. Phytopathol. 2015, 163, 612–619. [Google Scholar] [CrossRef]
- Morcia, C.; Tumino, G.; Ghizzoni, R.; Badeck, F.W.; Lattanzio, V.M.T.; Pascale, M.; Terzi, V. Occurrence of Fusarium Langsethiae and T-2 and HT-2 Toxins in Italian Malting Barley. Toxins 2016, 8, 247. [Google Scholar] [CrossRef]
- Orlando, B.; Grignon, G.; Vitry, C.; Kashefifard, K.; Valade, R. Fusarium Species and Enniatin Mycotoxins in Wheat, Durum Wheat, Triticale and Barley Harvested in France. Mycotoxin Res. 2019, 35, 369–380. [Google Scholar] [CrossRef]
- Witte, T.E.; Hicks, C.; Hermans, A.; Shields, S.; Overy, D.P. Debunking the Myth of Fusarium Poae T-2/HT-2 Toxin Production. J. Agric. Food Chem. 2024, 72, 3949–3957. [Google Scholar] [CrossRef]
- Ioos, R.; Belhadj, A.; Menez, M. Occurrence and Distribution of Microdochium Nivale and Fusarium Species Isolated from Barley, Durum and Soft Wheat Grains in France from 2000 to 2002. Mycopathologia 2004, 158, 351–362. [Google Scholar] [CrossRef] [PubMed]
- Jenkinson, P.; Parry, D.W. Splash Dispersal of Conidia of Fusarium Culmorum and Fusarium Avenaceum. Mycol. Res. 1994, 98, 506–510. [Google Scholar] [CrossRef]
- Hörberg, H.M. Patterns of Splash Dispersed Conidia of Fusarium Poae and Fusarium Culmorum. Eur. J. Plant Pathol. 2002, 108, 73–80. [Google Scholar] [CrossRef]
- Fernandez, M.R.; Huber, D.; Basnyat, P.; Zentner, R.P. Impact of Agronomic Practices on Populations of Fusarium and Other Fungi in Cereal and Noncereal Crop Residues on the Canadian Prairies. Soil. Tillage Res. 2008, 100, 60–71. [Google Scholar] [CrossRef]
- Landschoot, S.; Audenaert, K.; Waegeman, W.; Pycke, B.; Bekaert, B.; De Baets, B.; Haesaert, G. Connection between Primary Fusarium Inoculum on Gramineous Weeds, Crop Residues and Soil Samples and the Final Population on Wheat Ears in Flanders, Belgium. Crop Prot. 2011, 30, 1297–1305. [Google Scholar] [CrossRef]
- Wagacha, J.M.; Muthomi, J.W. Fusarium Culmorum: Infection Process, Mechanisms of Mycotoxin Production and Their Role in Pathogenesis in Wheat. Crop Prot. 2007, 26, 877–885. [Google Scholar] [CrossRef]
- Alabouvette, C.; Olivain, C.; Migheli, Q.; Steinberg, C. Microbiological Control of Soil-Borne Phytopathogenic Fungi with Special Emphasis on Wilt-Inducing Fusarium Oxysporum. New Phytol. 2009, 184, 529–544. [Google Scholar] [CrossRef] [PubMed]
- Jones, R.K. Assessments of Fusarium Head Blight of Wheat and Barley in Response to Fungicide Treatment. Plant Dis. 2000, 84, 1021–1030. [Google Scholar] [CrossRef]
- Venkatesh, N.; Keller, N.P. Mycotoxins in Conversation With Bacteria and Fungi. Front. Microbiol. 2019, 10, 403. [Google Scholar] [CrossRef]
- Abid, M.; Fayolle, L.; Edel-Hermann, V.; Gautheron, N.; Héraud, C.; Leplat, J.; Steinberg, C. Fate of Deoxynivalenol (DON) and Impact on the Soil Microflora and Soil Fauna. Appl. Soil. Ecol. 2021, 162, 103898. [Google Scholar] [CrossRef]
- Wambacq, E.; Vanhoutte, I.; Audenaert, K.; De Gelder, L.; Haesaert, G. Occurrence, Prevention and Remediation of Toxigenic Fungi and Mycotoxins in Silage: A Review. J. Sci. Food Agric. 2016, 96, 2284–2302. [Google Scholar] [CrossRef] [PubMed]
- Ogunade, I.M.; Martinez-Tuppia, C.; Queiroz, O.C.M.; Jiang, Y.; Drouin, P.; Wu, F.; Vyas, D.; Adesogan, A.T. Silage Review: Mycotoxins in Silage: Occurrence, Effects, Prevention, and Mitigation. J. Dairy. Sci. 2018, 101, 4034–4059. [Google Scholar] [CrossRef] [PubMed]
- Köhl, J.; de Haas, B.H.; Kastelein, P.; Burgers, S.L.G.E.; Waalwijk, C. Population Dynamics of Fusarium Spp. and Microdochium Nivale in Crops and Crop Residues of Winter Wheat. Phytopathology 2007, 97, 971–978. [Google Scholar] [CrossRef]
- Mourelos, C.A.; Malbrán, I.; Mengual Gómez, D.; Ghiringhelli, P.D.; Lori, G.A. Dynamics of Fusarium Graminearum Inoculum on Residues of Naturally Infected Winter and Summer Crops. Eur. J. Plant Pathol. 2024, 169, 543–553. [Google Scholar] [CrossRef]
- Scauflaire, J.; Mahieu, O.; Louvieaux, J.; Foucart, G.; Renard, F.; Munaut, F. Biodiversity of Fusarium Species in Ears and Stalks of Maize Plants in Belgium. Eur. J. Plant Pathol. 2011, 131, 59–66. [Google Scholar] [CrossRef]
- Oldenburg, E.; Höppner, F.; Ellner, F.; Weinert, J. Fusarium Diseases of Maize Associated with Mycotoxin Contamination of Agricultural Products Intended to Be Used for Food and Feed. Mycotoxin Res. 2017, 33, 167–182. [Google Scholar] [CrossRef]
- Audenaert, K.; Vanheule, A.; Höfte, M.; Haesaert, G. Deoxynivalenol: A Major Player in the Multifaceted Response of Fusarium to Its Environment. Toxins 2014, 6, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Sherif, M.; Kirsch, N.; Splivallo, R.; Pfohl, K.; Karlovsky, P. The Role of Mycotoxins in Interactions between Fusarium Graminearum and F. Verticillioides Growing in Saprophytic Cultures and Co-Infecting Maize Plants. Toxins 2023, 15, 575. [Google Scholar] [CrossRef] [PubMed]
- Tunali, B.; Obanor, F.; Erginbaş, G.; Westecott, R.A.; Nicol, J.; Chakraborty, S. Fitness of Three Fusarium Pathogens of Wheat. FEMS Microbiol. Ecol. 2012, 81, 596–609. [Google Scholar] [CrossRef]
- Weaver, A.C.; Weaver, D.M.; Adams, N.; Yiannikouris, A. Co-Occurrence of 35 Mycotoxins: A Seven-Year Survey of Corn Grain and Corn Silage in the United States. Toxins 2021, 13, 516. [Google Scholar] [CrossRef]
- Vandicke, J.; De Visschere, K.; Croubels, S.; De Saeger, S.; Audenaert, K.; Haesaert, G. Mycotoxins in Flanders’ Fields: Occurrence and Correlations with Fusarium Species in Whole-Plant Harvested Maize. Microorganisms 2019, 7, 571. [Google Scholar] [CrossRef] [PubMed]
- Schiro, G.; Müller, T.; Verch, G.; Sommerfeld, T.; Mauch, T.; Koch, M.; Grimm, V.; Müller, M.E.H. The Distribution of Mycotoxins in a Heterogeneous Wheat Field in Relation to Microclimate, Fungal and Bacterial Abundance. J. Appl. Microbiol. 2019, 126, 177–190. [Google Scholar] [CrossRef] [PubMed]
- Cooney, J.M.; Lauren, D.R.; Di Menna, M.E. Impact of Competitive Fungi on Trichothecene Production by Fusarium Graminearum. J. Agric. Food Chem. 2001, 49, 522–526. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Nicholson, P.; Ritieni, A. Effects of Fungal Interactions among Fusarium Head Blight Pathogens on Disease Development and Mycotoxin Accumulation. Int. J. Food Microbiol. 2007, 119, 67–71. [Google Scholar] [CrossRef]
- Dawidziuk, A.; Koczyk, G.; Popiel, D. Adaptation and Response to Mycotoxin Presence in Pathogen-Pathogen Interactions within the Fusarium Genus. World Mycotoxin J. 2016, 9, 565–575. [Google Scholar] [CrossRef]
- Perincherry, L.; Lalak-Kańczugowska, J.; Stępień, Ł. Fusarium-Produced Mycotoxins in Plant-Pathogen Interactions. Toxins 2019, 11, 664. [Google Scholar] [CrossRef]
- Schrader, S.; Meyer-Wolfarth, F.; Oldenburg, E. Biological Control of Soil-Borne Phytopathogenic Fungi and Their Mycotoxins by Soil Fauna-a Review. Bull. Univ. Agric. Sci. Vet. Med. Cluj-Napoca. Agric. 2013, 70, 291–298. [Google Scholar] [CrossRef]
- Goncharov, A.A.; Glebova, A.A.; Tiunov, A.V. Trophic Interactions between Fusarium Species and Soil Fauna: A Meta-Analysis of Experimental Studies. Appl. Soil. Ecol. 2020, 145, 103302. [Google Scholar] [CrossRef]
- Wolfarth, F.; Schrader, S.; Oldenburg, E.; Brunotte, J. Mycotoxin Contamination and Its Regulation by the Earthworm Species Lumbricus Terrestris in Presence of Other Soil Fauna in an Agroecosystem. Plant Soil. 2016, 402, 331–342. [Google Scholar] [CrossRef]
- Meyer-Wolfarth, F.; Oldenburg, E.; Meiners, T.; Muñoz, K.; Schrader, S. Effects of Temperature and Soil Fauna on the Reduction and Leaching of Deoxynivalenol and Zearalenone from Fusarium Graminearum-Infected Maize Stubbles. Mycotoxin Res. 2021, 37, 249–263. [Google Scholar] [CrossRef] [PubMed]
- Völkl, A.; Vogler, B.; Schollenberger, M.; Karlovsky, P. Microbial Detoxification of Mycotoxin Deoxynivalenol. J. Basic Microbiol. 2004, 44, 147–156. [Google Scholar] [CrossRef] [PubMed]
- Karlovsky, P. Biological Detoxification of the Mycotoxin Deoxynivalenol and Its Use in Genetically Engineered Crops and Feed Additives. Appl. Microbiol. Biotechnol. 2011, 91, 491–504. [Google Scholar] [CrossRef] [PubMed]
- Sato, I.; Ito, M.; Ishizaka, M.; Ikunaga, Y.; Sato, Y.; Yoshida, S.; Koitabashi, M.; Tsushima, S. Thirteen Novel Deoxynivalenol-Degrading Bacteria Are Classified within Two Genera with Distinct Degradation Mechanisms. FEMS Microbiol. Lett. 2012, 327, 110–117. [Google Scholar] [CrossRef] [PubMed]
- Tan, H.; Hu, Y.; He, J.; Wu, L.; Liao, F.; Luo, B.; He, Y.; Zuo, Z.; Ren, Z.; Zhong, Z.; et al. Zearalenone Degradation by Two Pseudomonas Strains from Soil. Mycotoxin Res. 2014, 30, 191–196. [Google Scholar] [CrossRef]
- Calvo, A.M.; Wilson, R.A.; Bok, J.W.; Keller, N.P. Relationship between Secondary Metabolism and Fungal Development. Microbiol. Mol. Biol. Rev. 2002, 66, 447–459. [Google Scholar] [CrossRef] [PubMed]
- Brodhagen, M.; Keller, N.P. Signalling Pathways Connecting Mycotoxin Production and Sporulation. Mol. Plant Pathol. 2006, 7, 285–301. [Google Scholar] [CrossRef] [PubMed]





| Field | Location | Wheat Cycle | Previous Crop: Maize Silage or Grain | Maize Cultivar | Maize Residue Management | Wheat Cultivar | Wheat Sowing Date | Resistance Level to DON * | Wheat Flowering Date | Number of Fungicide Applications before Complete Flowering |
|---|---|---|---|---|---|---|---|---|---|---|
| F1Y1 | Gennes sur Seiche, Ille-Et-Vilaine | 2020–2021 | Maize silage | LBS 2964 et DKC 3450 | Tillage | Complice | 5 November 2020 | 3.5 | 20 May 2021 | 3 |
| F2Y1 | Villamée, Ille-Et-Vilaine | 2020–2021 | Maize grain | Belami | Min-till | Vyckor, LG Absalon | 17 October 2020 | Vyckor 5.5 LG Absalon 5 | 3 June 2021 | 3 |
| F3Y1 | Pleslin-Trigavou, Côtes d’Amor | 2020–2021 | Maize grain | Kolossalis | Min-till | KWS Extase | 3 November 2020 | 4 | 31 May 2021 | 1 |
| F4Y1 | Loudéac, Côtes d’Amor | 2020–2021 | Maize silage | Pontivy | Min-till | Vyckor | 29 October 2020 | 5.5 | 1 June 2021 | 3 |
| F5Y1 | Guilers, Finistère | 2020–2021 | Maize silage | Franceen, Kilomeris, LG 31.295 | Min-till | Chevignon, KWS Extasse, | 7 November 2020 | Chevignon 5 KWS Extasse 4 RGT Perkussio 3.5 | 21 May 2021 | 3 |
| RGT Perkussio | ||||||||||
| F6Y1 | Loudéac, Côtes d’Amor | 2020–2021 | Maize grain | RGT Synfonixx | Min-till | Fructidor, Vyckor | 20 November 2020 | Fructidor 5 Vyckor 5.5 | 4 June 2021 | 3 |
| F1Y2 | Gennes sur Seiche, Ille-Et-Vilaine | 2021–2022 | Maize silage | Franceen | Min-till | Complice | 29 October 2021 | 3.5 | 15 May 2022 | 2 |
| F2Y2 | Villamée, Ille-Et-Vilaine | 2021–2022 | Maize grain | Kolossalis kws | Tillage | LG Absalon | 16 November 2021 | 5 | 21 May 2022 | 1 |
| F3Y2 | Plelin-Trigavou, Côtes d’Amor | 2021–2022 | Maize grain | DKC3888 | Min-till | Hyking | 28 October 2021 | 4 | 18 May 2022 | 1 |
| F4Y2 | Loudéac, Côtes d’Amor | 2021–2022 | Maize silage | LG 31.272 | Min-till | Fructidor | 27 November 2021 | 5 | 25 May 2022 | 0 |
| F5Y2 | Milizac, Finistère | 2021–2022 | Maize silage | Figaro, Milkmax, LG 31.259 | Min-till | Chevignon, Winner, Vyckor | 10 November 2021 | Chevignon 5 Winner 4.5 Vyckor 5.5 | 20 May 2022 | 2 |
| F6Y2 | Loudéac, Côtes d’Amor | 2021–2022 | Maize grain | RGT Synfonixx | Min-till | Vyckor, Chevignon, Fructidor | 18 November 2021 | Vyckor 5.5 Chevignon 5 Fructidor 5 | 25 May 2022 | 2 |
| Nom | Sequence | Reporter/ Quencher (5′/3′) | Final Concentration (nM) | Annealing Temperature (°C) | |
|---|---|---|---|---|---|
| F. graminearum | EF1-FCFG_F | TCGATACGCGCCTGTTACC | FAM/TAMRA | 300 | 62 |
| EF1-FG_R | ATGAGCGCCCAGGGAATG | 300 | |||
| grami2-EF1_rev | AGCCCCACCGGGAAAAAAATTACGACA | 100 | |||
| F. poae | EF1-FP2_F | CTCGAGCGATTGCATTTCTTT | FAM/TAMRA | 300 | 60 |
| EF1_FP2_R | GGCTTCCTATTGACAGGTGGTT | 300 | |||
| EF1-FP | CGCGAATCGTCACGTGTCAATCAGTT | 100 | |||
| F. avenaceum | EF1-FA_F2 | CATCTTGCTAACTCTTGACAGACCG | FAM/TAMRA | 300 | 64 |
| EF1-FA_R3 | GGGTAATGAATGCGTTTCGAA | 300 | |||
| EF1-FA | AGCGAGTCGTGGGAATCGATGGG | 150 |
| Mycotoxin | Retention Time (Min) | Quantifier | Qualifier | Extraction Recovery (%) | Range (ng/g) | R2 | LOD (ng/g) | LOQ (ng/g) | ||
|---|---|---|---|---|---|---|---|---|---|---|
| Ion | m/z | Ion | m/z | |||||||
| ZEA | 14.06 | [M-H]− | 317.1394 | / | / | 91.18 ± 6.10 | 1–250 | 0.978 | 11.92 | 36.13 |
| DON | 4.65 | [M+CH3COO]− | 355.1398 | [M-H]− | 295.1187 | 126.01 ± 20.57 | 100–2000 | 0.989 | 96.21 | 291.53 |
| 15ADON/3ADON | 12.07 | [M-H]− | 337.1293 | [M+CH3COO]− | 397.1504 | 91.26 ± 10.57 | 100–2000 | 0.998 | 41.69 | 126.33 |
| NIV | 2.31 | [M+CH3COO]− | 371.1348 | [M-H]− | 311.1136 | 131.77 ± 2.84 | 50–1000 | 0.985 | 58.02 | 175.81 |
| ENN B | 14.70 | [M+NH4]+ | 657.4433 | [M+Na]+ | 662.3987 | 111.96 ± 10.48 | 1–500 | 0.998 | 7.10 | 21.51 |
| ENN B1 | 14.82 | [M+NH4]+ | 671.4590 | [M+Na]+ | 676.4144 | 118.16 ± 13.47 | 1–500 | 0.997 | 8.16 | 24.74 |
| ENN A | 15.05 | [M+NH4]+ | 699.4903 | [M+Na]+ | 704.4457 | 105.03 ± 10.02 | 1–500 | 0.998 | 5.85 | 17.73 |
| ENN A1 | 14.94 | [M+NH4]+ | 685.4746 | [M+Na]+ | 690.4300 | 108.85 ± 14.29 | 1–500 | 0.997 | 7.94 | 24.07 |
| FB1 | 13.33 | [M+H]+ | 722.3957 | [M+Na]+ | 744.3777 | 80.73 ± 1.26 | 1–2500 | 0.993 | 61.46 | 186.25 |
| FB2 | 13.74 | [M+H]+ | 706.4008 | [M+Na]+ | 728.3828 | 95.89 ± 4.89 | 1–2500 | 0.995 | 53.36 | 161.70 |
| MON | 2.63 | [M-H]− | 96.9931 | / | / | 119.87 ± 2.68 | 100–2000 | 0.980 | 135.47 | 410.50 |
| BEA | 14.79 | [M+NH4]+ | 801.4483 | [M+Na]+ | 806.3987 | 154.07 ± 15.92 | 1–250 | 0.987 | 9.06 | 27.46 |
| HT-2 | 13.47 | [M+Na]+ | 447.1989 | / | / | 96.63 ± 3.50 | 1–2000 | 0.992 | 40.28 | 122.06 |
| T-2 | 13.71 | [M+Na]+ | 489.2095 | [M+NH4]+ | 484.2541 | 69.45 ± 18.83 | 5–500 | 0.965 | 32.52 | 98.55 |
| Year | Field | Severity Index (%) | Levels of Fusarium DNA(Log10 (1 + DNA) pg/20 ng DNA) | ||
|---|---|---|---|---|---|
| Fg | Fp | Fa | |||
| Y1 | F1Y1 | 0.00 ± 0.00 a | 0.000 ± 0.000 a | 0.000 ± 0.000 a | 0.000 ± 0.000 a |
| F2Y1 | 2.05 ± 1.23 bd | 0.000 ± 0.000 a | 0.000 ± 0.000 a | 0.000 ± 0.000 a | |
| F3Y1 | 10.90 ± 3.96 d | 0.000 ± 0.000 a | 1.102 ± 0.277 bc | 0.000 ± 0.000 a | |
| F4Y1 | 1.35 ± 0.61 bcd | 0.973 ± 0.785 b | 0.000 ± 0.000 a | 0.000 ± 0.000 a | |
| F5Y1 | 0.44 ± 0.43 ac | 0.000 ± 0.000 a | 0.000 ± 0.000 a | 0.000 ± 0.000 a | |
| F6Y1 | 2.30 ± 1.25 bd | 0.082 ± 0.164 ab | 2.192 ± 0.444 c | 0.000 ± 0.000 a | |
| Y2 | F1Y2 | 0.82 ± 0.91 abc | 0.000 ± 0.000 a | 0.000 ± 0.000 a | 0.000 ± 0.000 a |
| F2Y2 | 0.75 ± 0.48 abc | 0.000 ± 0.000 a | 0.000 ± 0.000 a | 0.000 ± 0.000 a | |
| F3Y2 | 0.45 ± 0.15 ac | 0.000 ± 0.000 a | 0.387 ± 0.585 ab | 0.000 ± 0.000 a | |
| F4Y2 | 1.03 ± 0.59 abc | 0.000 ± 0.000 a | 0.158 ± 0.256 ab | 0.000 ± 0.000 a | |
| F5Y2 | 1.37 ± 0.58 bcd | 0.000 ± 0.000 a | 0.000 ± 0.000 a | 0.378 ± 0.587 a | |
| F6Y2 | 1.33 ± 0.51 bcd | 0.000 ± 0.000 a | 0.096 ± 0.194 ab | 0.000 ± 0.000 a | |
| Year | Field | Percentage of Grains Contaminated by | Levels of Fusarium DNA (Log10 (1 + DNA) pg/20 ng DNA) | Toxin Accumulation (ng g−1 Dry Weight) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Fg | Fp | Fa | Micro | Fg | Fp | Fa | DON | NIV | ENNB | ENNB1 | ENNA1 | ENNA | ZEA | ||
| Y1 | F1Y1 | 18.67 ± 4.16 ab | 0.00 ± 0.00 a | 4.00 ± 4.00 a | 7.33 ± 2.31 a | 0.724 ± 1.092 ab | 0.000 ± 0.000 a | 0.145 ± 0.291 a | 425.35 ± 638.03 ab | <LOD | <LOD | <LOD | <LOD | <LOD | <LOQ |
| F2Y1 | 4.00 ± 3.46 ac | 0.00 ± 0.00 a | 0.67 ± 1.15 a | 0.00 ± 0.00 b | 0.696 ± 0.956 abc | 0.000 ± 0.000 a | 0.000 ± 0.000 a | 847.81 ± 1271.71 ab | <LOD | <LOD | <LOD | <LOD | 80.84 ± 7.68 | <LOQ | |
| F3Y1 | 14.67 ± 8.33 ab | 4.67 ± 1.15 b | 1.33 ± 2.31 a | 0.00 ± 0.00 b | 2.080 ± 1.361 bc | 1.250 ± 1.061 abc | 0.318 ± 0.480 a | 4493.61 ± 4672.66 bc | <LOD | <LOQ | <LOD | <LOD | <LOD | <LOQ | |
| F4Y1 | 19.33 ± 1.15 ab | 1.33 ± 1.15 ab | 8.67 ± 9.02 a | 10.00 ± 4.00 a | 0.742 ± 1.128 ab | 0.000 ± 0.000 a | 0.559 ± 0.513 a | 1570.11 ± 2355.17 ab | <LOD | <LOQ | <LOQ | <LOQ | <LOD | <LOQ | |
| F5Y1 | 2.67 ± 2.31 ac | 0.00 ± 0.00 a | 0.67 ± 1.15 a | 2.00 ± 2.00 ab | 0.062 ± 0.186 a | 0.000 ± 0.000 a | 0.000 ± 0.000 a | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD | |
| F6Y1 | 39.33 ± 11.02 b | 1.33 ± 1.15 ab | 2.00 ± 3.46 a | 2.00 ± 0.00 a | 3.117 ± 0.392 c | 0.546 ± 0.819 ac | 0.172 ± 0.267 a | 9247.49 ± 1857.51 c | 183.05 ± 274.58 | 101.37 ± 116.48 | 20.22 ± 15.47 | <LOQ | <LOD | <LOQ | |
| Y2 | F1Y2 | 0.00 ± 0.00 c | 0.00 ± 0.00 a | 0.00 ± 0.00 a | 0.00 ± 0.00 b | 0.000 ± 0.000 a | 0.000 ± 0.000 a | 0.000 ± 0.000 a | <LOD | <LOD | <LOD | <LOQ | <LOQ | <LOD | <LOQ |
| F2Y2 | 0.00 ± 0.00 c | 0.51 ± 0.89 a | 0.00 ± 0.00 a | 0.00 ± 0.00 b | 0.000 ± 0.000 a | 0.555 ± 0.558 abc | 0.000 ± 0.000 a | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD | |
| F3Y2 | 0.00 ± 0.00 c | 2.56 ± 2.35 ab | 0.51 ± 0.89 a | 0.00 ± 0.00 b | 0.105 ± 0.210 a | 2.066 ± 0.810 b | 0.000 ± 0.000 a | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD | |
| F4Y2 | 0.00 ± 0.00 c | 0.00 ± 0.00 a | 0.00 ± 0.00 a | 0.00 ± 0.00 b | 0.000 ± 0.000 a | 0.115 ± 0.235 a | 0.289 ± 0.440 a | <LOD | <LOD | <LOD | <LOD | <LOQ | <LOD | <LOQ | |
| F5Y2 | 0.00 ± 0.00 c | 1.54 ± 2.67 ab | 0.00 ± 0.00 a | 0.00 ± 0.00 b | 0.000 ± 0.000 a | 0.490 ± 0.504 abc | 0.052 ± 0.156 a | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD | |
| F6Y2 | 0.00 ± 0.00 c | 4.62 ± 2.67 b | 0.00 ± 0.00 a | 0.00 ± 0.00 b | 0.037 ± 0.112 a | 1.945 ± 0.577 bc | 0.031 ± 0.094 a | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nguyen, T.B.H.; Henri-Sanvoisin, A.; Coton, M.; Le Floch, G.; Picot, A. Shifts in Fusarium Communities and Mycotoxins in Maize Residues, Soils, and Wheat Grains throughout the Wheat Cycle: Implications for Fusarium Head Blight Epidemiology. Microorganisms 2024, 12, 1783. https://doi.org/10.3390/microorganisms12091783
Nguyen TBH, Henri-Sanvoisin A, Coton M, Le Floch G, Picot A. Shifts in Fusarium Communities and Mycotoxins in Maize Residues, Soils, and Wheat Grains throughout the Wheat Cycle: Implications for Fusarium Head Blight Epidemiology. Microorganisms. 2024; 12(9):1783. https://doi.org/10.3390/microorganisms12091783
Chicago/Turabian StyleNguyen, Toan Bao Hung, Amandine Henri-Sanvoisin, Monika Coton, Gaétan Le Floch, and Adeline Picot. 2024. "Shifts in Fusarium Communities and Mycotoxins in Maize Residues, Soils, and Wheat Grains throughout the Wheat Cycle: Implications for Fusarium Head Blight Epidemiology" Microorganisms 12, no. 9: 1783. https://doi.org/10.3390/microorganisms12091783
APA StyleNguyen, T. B. H., Henri-Sanvoisin, A., Coton, M., Le Floch, G., & Picot, A. (2024). Shifts in Fusarium Communities and Mycotoxins in Maize Residues, Soils, and Wheat Grains throughout the Wheat Cycle: Implications for Fusarium Head Blight Epidemiology. Microorganisms, 12(9), 1783. https://doi.org/10.3390/microorganisms12091783







