The Relative Importance of Cytotoxins Produced by Methicillin-Resistant Staphylococcus aureus Strain USA300 for Causing Human PMN Destruction
Abstract
:1. Introduction
2. Materials and Methods
2.1. Bacteria Strains and Culture Conditions
2.2. Human PMN Purification
2.3. Cytotoxicity Assays
2.4. Phagocytosis Assays
3. Results
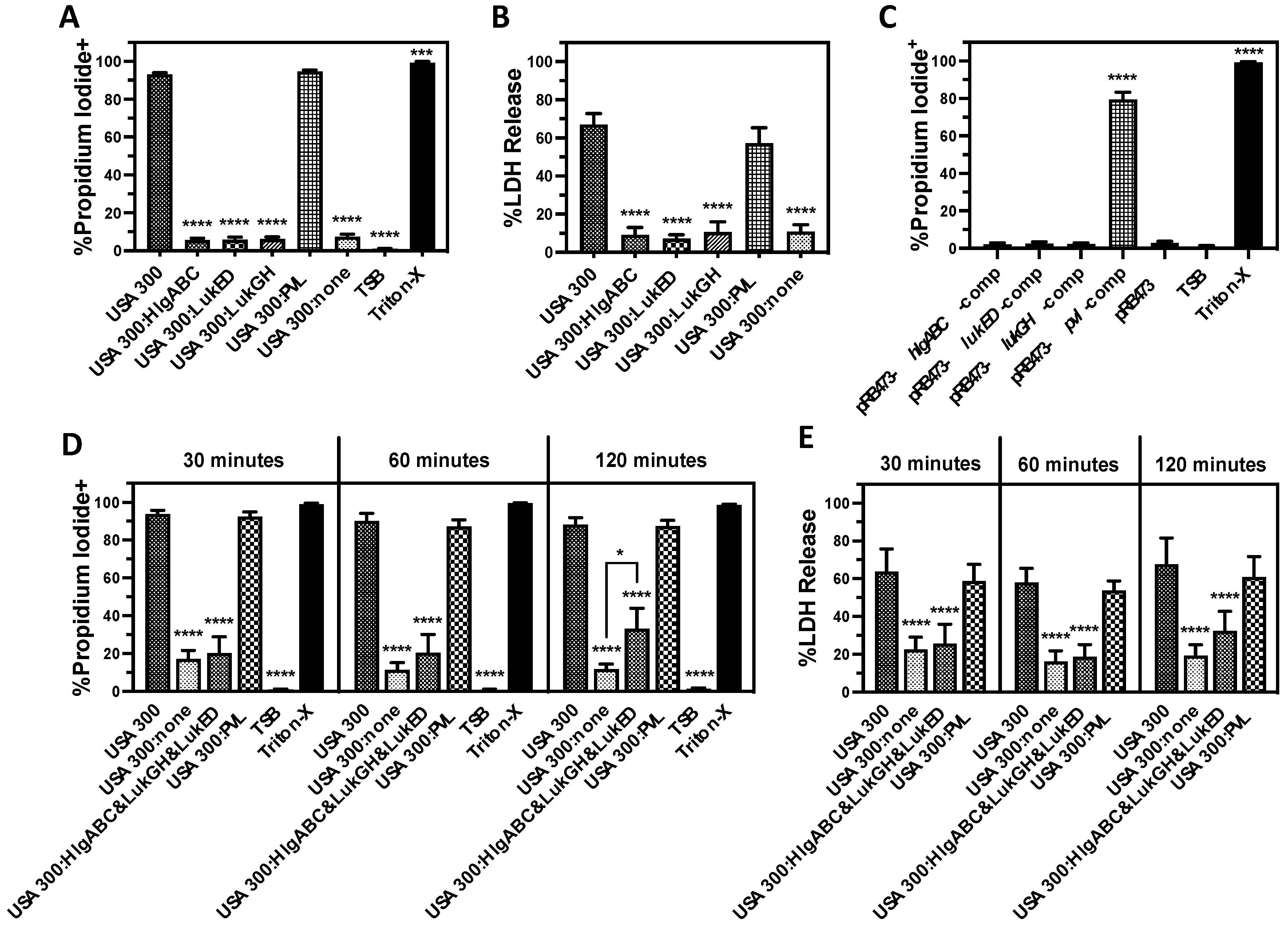
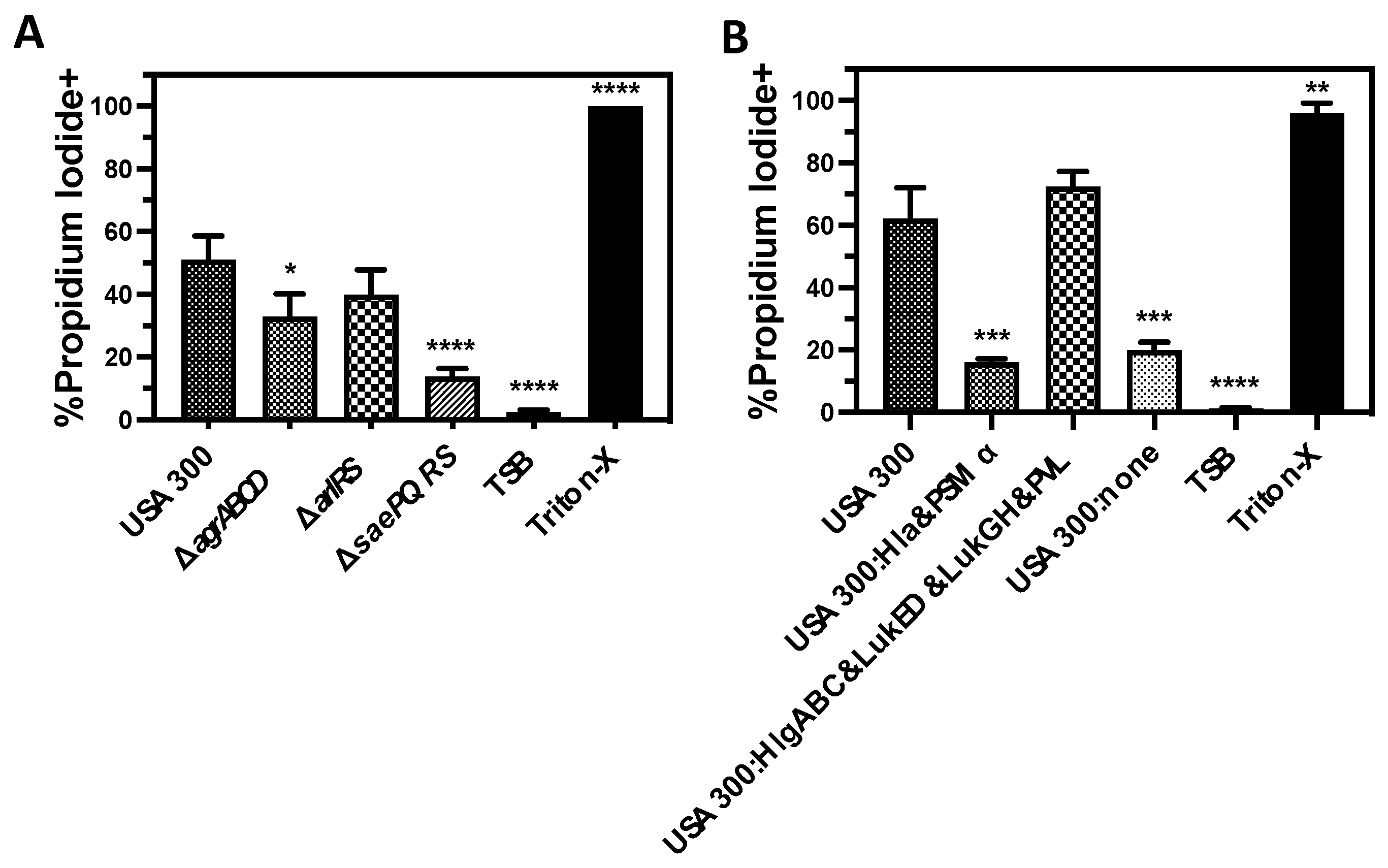
3.1. Bicomponent Leukocidins Are the Primary Extracellular Cytotoxic Component Produced by USA300 against Human PMNs
3.2. PVL Is the Prominent Cytotoxic Extracellular Factor Produced by USA300 That Causes Human PMN Destruction
3.3. Lysis of Human PMNs Following Phagocytosis of USA300 Is Primarily Mediated by Bicomponent Leukocidins
3.4. LukGH Is the Primary Initial Cause of Human PMN Destruction Following Phagocytosis of USA300
4. Discussion
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Ikuta, K.S.; Swetschinski, L.R.; Aguilar, G.R.; Sharara, F.; Mestrovic, T.; Gray, A.P.; Weaver, N.D.; Wool, E.E.; Han, C.; Hayoon, A.G.; et al. Global Mortality Associated with 33 Bacterial Pathogens in 2019: A Systematic Analysis for the Global Burden of Disease Study 2019. Lancet 2022, 400, 2221–2248. [Google Scholar] [CrossRef] [PubMed]
- CDC. CDC’s Antibiotic Resistance Threats Report; US Department of Health & Human Services: Washington, DC, USA, 2019.
- Nelson, R.E.; Hatfield, K.M.; Wolford, H.; Samore, M.H.; Scott, R.D.; Reddy, S.C.; Olubajo, B.; Paul, P.; Jernigan, J.A.; Baggs, J. National Estimates of Healthcare Costs Associated with Multidrug-Resistant Bacterial Infections among Hospitalized Patients in the United States. Clin. Infect. Dis. 2021, 72, S17–S26. [Google Scholar] [CrossRef]
- Moran, G.J.; Krishnadasan, A.; Gorwitz, R.J.; Fosheim, G.E.; McDougal, L.K.; Carey, R.B.; Talan, D.A.; EMERGEncy ID Net Study Group. Methicillin-Resistant S. aureus Infections among Patients in the Emergency Department. N. Engl. J. Med. 2006, 355, 666–674. [Google Scholar] [CrossRef] [PubMed]
- Seybold, U.; Kourbatova, E.V.; Johnson, J.G.; Halvosa, S.J.; Wang, Y.F.; King, M.D.; Ray, S.M.; Blumberg, H.M. Emergence of Community-Associated Methicillin-Resistant Staphylococcus aureus USA300 Genotype as a Major Cause of Health Care-Associated Blood Stream Infections. Clin. Infect. Dis. 2006, 42, 647–656. [Google Scholar] [CrossRef]
- Talan, D.A.; Krishnadasan, A.; Gorwitz, R.J.; Fosheim, G.E.; Limbago, B.; Albrecht, V.; Moran, G.J. EMERGEncy ID Net Study Group Comparison of Staphylococcus aureus From Skin and Soft-Tissue Infections in US Emergency Department Patients, 2004 and 2008. Clin. Infect. Dis. 2011, 53, 144–149. [Google Scholar] [CrossRef] [PubMed]
- Carrel, M.; Perencevich, E.N.; David, M.Z. USA300 Methicillin-Resistant Staphylococcus aureus, United States, 2000–2013. Emerg. Infect. Dis. 2015, 21, 1973–1980. [Google Scholar] [CrossRef]
- Diekema, D.J.; Richter, S.S.; Heilmann, K.P.; Dohrn, C.L.; Riahi, F.; Tendolkar, S.; McDanel, J.S.; Doern, G. V Continued Emergence of USA300 Methicillin-Resistant Staphylococcus aureus in the United States: Results from a Nationwide Surveillance Study. Infect. Control Hosp. Epidemiol. 2014, 35, 285–292. [Google Scholar] [CrossRef]
- Hofstetter, K.S.; Jacko, N.F.; Shumaker, M.J.; Talbot, B.M.; Petit, R.A.; Read, T.D.; David, M.Z. Strain Differences in Bloodstream and Skin Infection: Methicillin-Resistant Staphylococcus aureus Isolated in 2018-2021 in a Single Health System. Open Forum Infect. Dis. 2024, 11, ofae261. [Google Scholar] [CrossRef]
- Lowy, F.D. Staphylococcus aureus Infections. N. Engl. J. Med. 1998, 339, 520–532. [Google Scholar] [CrossRef]
- Nygaard, T.K.; DeLeo, F.R.; Voyich, J.M. Community-Associated Methicillin-Resistant Staphylococcus aureus Skin Infections: Advances toward Identifying the Key Virulence Factors. Curr. Opin. Infect. Dis. 2008, 21, 147–152. [Google Scholar] [CrossRef]
- Vandenesch, F.; Lina, G.; Henry, T. Staphylococcus aureus Hemolysins, Bi-Component Leukocidins, and Cytolytic Peptides: A Redundant Arsenal of Membrane-Damaging Virulence Factors? Front. Cell. Infect. Microbiol. 2012, 2, 12. [Google Scholar] [CrossRef]
- Spaan, A.N.; Van Strijp, J.A.G.; Torres, V.J. Leukocidins: Staphylococcal Bi-Component Pore-Forming Toxins Find Their Receptors. Nat. Rev. Microbiol. 2017, 15, 435–447. [Google Scholar] [CrossRef]
- Ahmad-Mansour, N.; Loubet, P.; Pouget, C.; Dunyach-Remy, C.; Sotto, A.; Lavigne, J.P.; Molle, V. Staphylococcus aureus Toxins: An Update on Their Pathogenic Properties and Potential Treatments. Toxins 2021, 13, 677. [Google Scholar] [CrossRef] [PubMed]
- Tam, K.; Torres, V.J. Staphylococcus aureus Secreted Toxins and Extracellular Enzymes. Microbiol. Spectr. 2019, 7, 10-1128. [Google Scholar] [CrossRef] [PubMed]
- Cheung, G.Y.C.; Bae, J.S.; Otto, M. Pathogenicity and Virulence of Staphylococcus aureus. Virulence 2021, 12, 547–569. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, D.; Borges, A.; Simões, M. Staphylococcus aureus Toxins and Their Molecular Activity in Infectious Diseases. Toxins 2018, 10, 252. [Google Scholar] [CrossRef]
- Alonzo, F.; Torres, V.J. The Bicomponent Pore-Forming Leucocidins of Staphylococcus aureus. Microbiol. Mol. Biol. Rev. 2014, 78, 199–230. [Google Scholar] [CrossRef]
- Nygaard, T.; Malachowa, N.; Kobayashi, S.D.; DeLeo, F.R. Phagocytes. In Management of Infections in the Immunocompromised Host; Segal, B.H., Ed.; Springer International Publishing: Cham, Switzerland, 2018; pp. 1–25. ISBN 978-3-319-77674-3. [Google Scholar]
- Lekstrom-Himes, J.A.; Gallin, J.I. Immunodeficiency Diseases Caused by Defects in Phagocytes. N. Engl. J. Med. 2000, 343, 1703–1714. [Google Scholar] [CrossRef]
- Guerra, F.E.; Borgogna, T.R.; Patel, D.M.; Sward, E.W.; Voyich, J.M. Epic Immune Battles of History: Neutrophils vs. Staphylococcus aureus. Front. Cell. Infect. Microbiol. 2017, 7, 286. [Google Scholar] [CrossRef]
- Zhang, Q.F.; Jiang, T.T.; Mao, X.; Kim, J.D.; Ahn, D.H.; Jung, Y.; Bae, T.; Lee, B.L. Development of Combination Vaccine Conferring Optimal Protection against Six Pore-Forming Toxins of Staphylococcus aureus. Infect. Immun. 2021, 89, IAI0034221. [Google Scholar] [CrossRef]
- Diep, B.A.; Le, V.T.M.; Visram, Z.C.; Rouha, H.; Stulik, L.; Dip, E.C.; Nagy, G.; Nagy, E. Improved Protection in a Rabbit Model of Community-Associated Methicillin-Resistant Staphylococcus aureus Necrotizing Pneumonia upon Neutralization of Leukocidins in Addition to Alpha-Hemolysin. Antimicrob. Agents Chemother. 2016, 60, 6333–6340. [Google Scholar] [CrossRef]
- Chan, R.; Buckley, P.T.; O’Malley, A.; Sause, W.E.; Alonzo, F.; Lubkin, A.; Boguslawski, K.M.; Payne, A.; Fernandez, J.; Strohl, W.R.; et al. Identification of Biologic Agents to Neutralize the Bicomponent Leukocidins of Staphylococcus aureus. Sci. Transl. Med. 2019, 11, eaat0882. [Google Scholar] [CrossRef] [PubMed]
- Staali, L.; Colin, D.A. Bi-Component HlgC/HlgB and HlgA/HlgB γ-Hemolysins from S. aureus: Modulation of Ca2+ Channels Activity through a Differential Mechanism. Toxicon 2021, 201, 74–85. [Google Scholar] [CrossRef] [PubMed]
- Spaan, A.N.; Vrieling, M.; Wallet, P.; Badiou, C.; Reyes-Robles, T.; Ohneck, E.A.; Benito, Y.; De Haas, C.J.C.; Day, C.J.; Jennings, M.P.; et al. The Staphylococcal Toxins γ-Haemolysin AB and CB Differentially Target Phagocytes by Employing Specific Chemokine Receptors. Nat. Commun. 2014, 5, 5438. [Google Scholar] [CrossRef]
- Malachowa, N.; Whitney, A.R.; Kobayashi, S.D.; Sturdevant, D.E.; Kennedy, A.D.; Braughton, K.R.; Shabb, D.W.; Diep, B.A.; Chambers, H.F.; Otto, M.; et al. Global Changes in Staphylococcus aureus Gene Expression in Human Blood. PLoS ONE 2011, 6, e18617. [Google Scholar] [CrossRef] [PubMed]
- Yang, D.; Ho, Y.X.; Cowell, L.M.; Jilani, I.; Foster, S.J.; Prince, L.R. A Genome-Wide Screen Identifies Factors Involved in S. aureus—Induced Human Neutrophil Cell Death and Pathogenesis. Front. Immunol. 2019, 10, 45. [Google Scholar] [CrossRef] [PubMed]
- Yanai, M.; Rocha, M.A.; Matolek, A.Z.; Chintalacharuvu, A.; Taira, Y.; Chintalacharuvu, K.; Beenhouwer, D.O. Separately or Combined, LukG/LukH Is Functionally Unique Compared to Other Staphylococcal Bicomponent Leukotoxins. PLoS ONE 2014, 9, e89308. [Google Scholar] [CrossRef]
- Janesch, P.; Rouha, H.; Weber, S.; Malafa, S.; Gross, K.; Maierhofer, B.; Badarau, A.; Visram, Z.C.; Stulik, L.; Nagy, E. Selective Sensitization of Human Neutrophils to LukGH Mediated Cytotoxicity by Staphylococcus aureus and IL-8. J. Infect. 2017, 74, 473–483. [Google Scholar] [CrossRef]
- Meyer, F.; Girardot, R.; Piémont, Y.; Prévost, G.; Colin, D.A. Analysis of the Specificity of Panton-Valentine Leucocidin and Gamma-Hemolysin F Component Binding. Infect. Immun. 2009, 77, 266–273. [Google Scholar] [CrossRef]
- Tromp, A.T.; Van Gent, M.; Abrial, P.; Martin, A.; Jansen, J.P.; De Haas, C.J.C.; Van Kessel, K.P.M.; Bardoel, B.W.; Kruse, E.; Bourdonnay, E.; et al. Human CD45 Is an F-Component-Specific Receptor for the Staphylococcal Toxin Panton-Valentine Leukocidin. Nat. Microbiol. 2018, 3, 708–717. [Google Scholar] [CrossRef]
- Spaan, A.N.; Schiepers, A.; de Haas, C.J.C.; van Hooijdonk, D.D.J.J.; Badiou, C.; Contamin, H.; Vandenesch, F.; Lina, G.; Gerard, N.P.; Gerard, C.; et al. Differential Interaction of the Staphylococcal Toxins Panton–Valentine Leukocidin and γ-Hemolysin CB with Human C5a Receptors. J. Immunol. 2015, 195, 1034–1043. [Google Scholar] [CrossRef]
- Hongo, I.; Baba, T.; Oishi, K.; Morimoto, Y.; Ito, T.; Hiramatsu, K. Phenol-Soluble Modulin A3 Enhances the Human Neutrophil Lysis Mediated by Panton-Valentine Leukocidin. J. Infect. Dis. 2009, 200, 715–723. [Google Scholar] [CrossRef] [PubMed]
- Malachowa, N.; Kobayashi, S.D.; Braughton, K.R.; Whitney, A.R.; Parnell, M.J.; Gardner, D.J.; Deleo, F.R. Staphylococcus aureus Leukotoxin GH Promotes Inflammation. J. Infect. Dis. 2012, 206, 1185–1193. [Google Scholar] [CrossRef] [PubMed]
- Graves, S.F.; Kobayashi, S.D.; Braughton, K.R.; Diep, B.A.; Chambers, H.F.; Otto, M.; DeLeo, F.R. Relative Contribution of Panton-Valentine Leukocidin to PMN Plasma Membrane Permeability and Lysis Caused by USA300 and USA400 Culture Supernatants. Microbes Infect. 2010, 12, 446–456. [Google Scholar] [CrossRef] [PubMed]
- Löffler, B.; Hussain, M.; Grundmeier, M.; Brück, M.; Holzinger, D.; Varga, G.; Roth, J.; Kahl, B.C.; Proctor, R.A.; Peters, G. Staphylococcus aureus Panton-Valentine Leukocidin Is a Very Potent Cytotoxic Factor for Human Neutrophils. PLoS Pathog. 2010, 6, e1000715. [Google Scholar] [CrossRef]
- Genestier, A.L.; Michallet, M.C.; Prévost, G.; Bellot, G.; Chalabreysse, L.; Peyrol, S.; Thivolet, F.; Etienne, J.; Lina, G.; Vallette, F.M.; et al. Staphylococcus aureus Panton-Valentine Leukocidin Directly Targets Mitochondria and Induces Bax-Independent Apoptosis of Human Neutrophils. J. Clin. Investig. 2005, 115, 3117–3127. [Google Scholar] [CrossRef]
- Rungelrath, V.; Porter, A.R.; Malachowa, N.; Freedman, B.A.; Leung, J.M.; Voyich, J.M.; Otto, M.; Kobayashi, S.D.; DeLeo, F.R. Further Insight into the Mechanism of Human PMN Lysis Following Phagocytosis of Staphylococcus aureus. Microbiol. Spectr. 2021, 9, e0088821. [Google Scholar] [CrossRef]
- DuMont, A.L.; Yoong, P.; Day, C.J.; Alonzo, F.; McDonald, W.H.; Jennings, M.P.; Torres, V.J. Staphylococcus aureus LukAB Cytotoxin Kills Human Neutrophils by Targeting the CD11b Subunit of the Integrin Mac-1. Proc. Natl. Acad. Sci. USA 2013, 110, 10794–10799. [Google Scholar] [CrossRef]
- Dumont, A.L.; Nygaard, T.K.; Watkins, R.L.; Smith, A.; Kozhaya, L.; Kreiswirth, B.N.; Shopsin, B.; Unutmaz, D.; Voyich, J.M.; Torres, V.J. Characterization of a New Cytotoxin That Contributes to Staphylococcus aureus Pathogenesis. Mol. Microbiol. 2011, 79, 814–825. [Google Scholar] [CrossRef]
- DuMont, A.L.; Yoong, P.; Surewaard, B.G.J.; Benson, M.A.; Nijland, R.; van Strijp, J.A.G.; Torres, V.J. Staphylococcus aureus Elaborates Leukocidin AB to Mediate Escape from within Human Neutrophils. Infect. Immun. 2013, 81, 1830–1841. [Google Scholar] [CrossRef]
- Perelman, S.S.; James, D.B.A.; Boguslawski, K.M.; Nelson, C.W.; Ilmain, J.K.; Zwack, E.E.; Prescott, R.A.; Mohamed, A.; Tam, K.; Chan, R.; et al. Genetic Variation of Staphylococcal LukAB Toxin Determines Receptor Tropism. Nat. Microbiol. 2021, 6, 731–745. [Google Scholar] [CrossRef]
- Trstenjak, N.; Milić, D.; Graewert, M.A.; Rouha, H.; Svergun, D.; Djinović-Carugo, K.; Nagy, E.; Badarau, A. Molecular Mechanism of Leukocidin GH–Integrin CD11b/CD18 Recognition and Species Specificity. Proc. Natl. Acad. Sci. USA 2020, 117, 317–327. [Google Scholar] [CrossRef] [PubMed]
- Ventura, C.L.; Malachowa, N.; Hammer, C.H.; Nardone, G.A.; Robinson, M.A.; Kobayashi, S.D.; DeLeo, F.R. Identification of a Novel Staphylococcus aureus Two-Component Leukotoxin Using Cell Surface Proteomics. PLoS ONE 2010, 5, e11634. [Google Scholar] [CrossRef]
- Reyes-Robles, T.; Alonzo, F.; Kozhaya, L.; Lacy, D.B.; Unutmaz, D.; Torres, V.J. Staphylococcus aureus Leukotoxin ED Targets the Chemokine Receptors CXCR1 and CXCR2 to Kill Leukocytes and Promote Infection. Cell Host Microbe 2013, 14, 453–459. [Google Scholar] [CrossRef]
- Pang, Y.Y.; Schwartz, J.; Thoendel, M.; Ackermann, L.W.; Horswill, A.R.; Nauseef, W.M. Agr-Dependent Interactions of Staphylococcus aureus USA300 with Human Polymorphonuclear Neutrophils. J. Innate Immun. 2010, 2, 546–559. [Google Scholar] [CrossRef]
- Surewaard, B.G.J.; Nijland, R.; Spaan, A.N.; Kruijtzer, J.A.W.; de Haas, C.J.C.; van Strijp, J.A.G. Inactivation of Staphylococcal Phenol Soluble Modulins by Serum Lipoprotein Particles. PLoS Pathog. 2012, 8, e1002606. [Google Scholar] [CrossRef]
- Hommes, J.W.; Kratofil, R.M.; Wahlen, S.; de Haas, C.J.C.; Hildebrand, R.B.; Hovingh, G.K.; Otto, M.; van Eck, M.; Hoekstra, M.; Korporaal, S.J.A.; et al. High Density Lipoproteins Mediate in Vivo Protection against Staphylococcal Phenol-Soluble Modulins. Sci. Rep. 2021, 11, 15357. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Niu, C.; Ma, B.; Xue, X.; Li, Z.; Chen, Z.; Li, F.; Zhou, S.; Luo, X.; Hou, Z. Inhibiting PSMα-Induced Neutrophil Necroptosis Protects Mice with MRSA Pneumonia by Blocking the Agr System. Cell Death Dis. 2018, 9, 362. [Google Scholar] [CrossRef] [PubMed]
- Surewaard, B.G.J.; De Haas, C.J.C.; Vervoort, F.; Rigby, K.M.; Deleo, F.R.; Otto, M.; Van Strijp, J.A.G.; Nijland, R. Staphylococcal Alpha-Phenol Soluble Modulins Contribute to Neutrophil Lysis after Phagocytosis. Cell. Microbiol. 2013, 15, 1427–1437. [Google Scholar] [CrossRef]
- Grosz, M.; Kolter, J.; Paprotka, K.; Winkler, A.C.; Schäfer, D.; Chatterjee, S.S.; Geiger, T.; Wolz, C.; Ohlsen, K.; Otto, M.; et al. Cytoplasmic Replication of Staphylococcus aureus upon Phagosomal Escape Triggered by Phenol-Soluble Modulin α. Cell. Microbiol. 2014, 16, 451–465. [Google Scholar] [CrossRef]
- Wang, R.; Braughton, K.R.; Kretschmer, D.; Bach, T.-H.L.; Queck, S.Y.; Li, M.; Kennedy, A.D.; Dorward, D.W.; Klebanoff, S.J.; Peschel, A.; et al. Identification of Novel Cytolytic Peptides as Key Virulence Determinants for Community-Associated MRSA. Nat. Med. 2007, 13, 1510–1514. [Google Scholar] [CrossRef] [PubMed]
- Diep, B.A.; Gill, S.R.; Chang, R.F.; Van Phan, T.H.; Chen, J.H.; Davidson, M.G.; Lin, F.; Lin, J.; Carleton, H.A.; Mongodin, E.F.; et al. Complete Genome Sequence of USA300, an Epidemic Clone of Community-Acquired Meticillin-Resistant Staphylococcus aureus. Lancet 2006, 367, 731–739. [Google Scholar] [CrossRef]
- Bae, T.; Schneewind, O. Allelic Replacement in Staphylococcus aureus with Inducible Counter-Selection. Plasmid 2006, 55, 58–63. [Google Scholar] [CrossRef] [PubMed]
- Nygaard, T.K.; Borgogna, T.R.; Sward, E.W.; Guerra, F.E.; Dankoff, J.G.; Collins, M.M.; Pallister, K.B.; Chen, L.; Kreiswirth, B.N.; Voyich, J.M. Aspartic Acid Residue 51 of SaeR Is Essential for Staphylococcus aureus Virulence. Front. Microbiol. 2018, 9, 3085. [Google Scholar] [CrossRef] [PubMed]
- Collins, M.M.; Behera, R.K.; Pallister, K.B.; Evans, T.J.; Burroughs, O.; Flack, C.; Guerra, F.E.; Pullman, W.; Cone, B.; Dankoff, J.G.; et al. The Accessory Gene SaeP of the SaeR/S Two-Component Gene Regulatory System Impacts Staphylococcus aureus Virulence During Neutrophil Interaction. Front. Microbiol. 2020, 11, 561. [Google Scholar] [CrossRef]
- Nygaard, T.K.; Pallister, K.B.; DuMont, A.L.; DeWald, M.; Watkins, R.L.; Pallister, E.Q.; Malone, C.; Griffith, S.; Horswill, A.R.; Torres, V.J.; et al. Alpha-Toxin Induces Programmed Cell Death of Human T Cells, B Cells, and Monocytes during USA300 Infection. PLoS ONE 2012, 7, e36532. [Google Scholar] [CrossRef]
- Nygaard, T.K.K.; Pallister, K.B.B.; Ruzevich, P.; Griffith, S.; Vuong, C.; Voyich, J.M.M. SaeR Binds a Consensus Sequence within Virulence Gene Promoters to Advance USA300 Pathogenesis. J. Infect. Dis. 2010, 201, 241–254. [Google Scholar] [CrossRef]
- Deatherage, D.E.; Barrick, J.E. Identification of Mutations in Laboratory-Evolved Microbes from next-Generation Sequencing Data Using Breseq. Methods Mol. Biol. 2014, 1151, 165–188. [Google Scholar] [CrossRef]
- Voyich, J.M.; Vuong, C.; DeWald, M.; Nygaard, T.K.; Kocianova, S.; Griffith, S.; Jones, J.; Iverson, C.; Sturdevant, D.E.; Braughton, K.R.; et al. The SaeR/S Gene Regulatory System Is Essential for Innate Immune Evasion by Staphylococcus aureus. J. Infect. Dis. 2009, 199, 1698–1706. [Google Scholar] [CrossRef]
- Crosby, H.A.; Tiwari, N.; Kwiecinski, J.M.; Xu, Z.; Dykstra, A.; Jenul, C.; Fuentes, E.J.; Horswill, A.R. The Staphylococcus aureus ArlRS Two-Component System Regulates Virulence Factor Expression through MgrA. Mol. Microbiol. 2020, 113, 103–122. [Google Scholar] [CrossRef]
- Dankoff, J.G.; Pallister, K.B.; Guerra, F.E.; Parks, A.J.; Gorham, K.; Mastandrea, S.; Voyich, J.M.; Nygaard, T.K. Quantifying the Cytotoxicity of Staphylococcus aureus against Human Polymorphonuclear Leukocytes. J. Vis. Exp. 2019, e60681. [Google Scholar] [CrossRef]
- Voyich, J.M.; Braughton, K.R.; Sturdevant, D.E.; Whitney, A.R.; Saïd-Salim, B.; Porcella, S.F.; Long, R.D.; Dorward, D.W.; Gardner, D.J.; Kreiswirth, B.N.; et al. Insights into Mechanisms Used by Staphylococcus aureus to Avoid Destruction by Human Neutrophils. J. Immunol. 2005, 175, 3907–3919. [Google Scholar] [CrossRef]
- Zurek, O.W.; Nygaard, T.K.; Watkins, R.L.; Pallister, K.B.; Torres, V.J.; Horswill, A.R.; Voyich, J.M. The Role of Innate Immunity in Promoting SaeR/S-Mediated Virulence in Staphylococcus aureus. J. Innate Immun. 2014, 6, 21–30. [Google Scholar] [CrossRef] [PubMed]
- Sward, E.W.; Fones, E.M.; Spaan, R.R.; Pallister, K.B.; Haller, B.L.; Guerra, F.E.; Zurek, O.W.; Nygaard, T.K.; Voyich, J.M. Staphylococcus aureus SaeR/S-Regulated Factors Decrease Monocyte-Derived Tumor Necrosis Factor-α to Reduce Neutrophil Bactericidal Activity. J. Infect. Dis. 2018, 217, 943–952. [Google Scholar] [CrossRef] [PubMed]
- Queck, S.Y.; Jameson-Lee, M.; Villaruz, A.E.; Bach, T.H.L.; Khan, B.A.; Sturdevant, D.E.; Ricklefs, S.M.; Li, M.; Otto, M. RNAIII-Independent Target Gene Control by the Agr Quorum-Sensing System: Insight into the Evolution of Virulence Regulation in Staphylococcus aureus. Mol. Cell 2008, 32, 150–158. [Google Scholar] [CrossRef]
- Kwiecinski, J.M.; Kratofil, R.M.; Parlet, C.P.; Surewaard, B.G.J.; Kubes, P.; Horswill, A.R. Staphylococcus aureus Uses the ArlRS and MgrA Cascade to Regulate Immune Evasion during Skin Infection. Cell Rep. 2021, 36, 109462. [Google Scholar] [CrossRef]
- Woodin, A.M. Purification of the Two Components of Leucocidin from Staphylococcus aureus. Biochem. J. 1960, 75, 158–165. [Google Scholar] [CrossRef]
- Woodin, A.M. Fractionation of a Leucocidin from Staphylococcus aureus. Biochem. J. 1959, 73, 225–237. [Google Scholar] [CrossRef]
- Brown, M.L.; O’Hara, F.P.; Close, N.M.; Mera, R.M.; Miller, L.A.; Suaya, J.A.; Amrine-Madsen, H. Prevalence and Sequence Variation of Panton-Valentine Leukocidin in Methicillin-Resistant and Methicillin-Susceptible Staphylococcus aureus Strains in the United States. J. Clin. Microbiol. 2012, 50, 86–90. [Google Scholar] [CrossRef]
- Tong, S.Y.C.; Davis, J.S.; Eichenberger, E.; Holland, T.L.; Fowler, V.G. Staphylococcus aureus Infections: Epidemiology, Pathophysiology, Clinical Manifestations, and Management. Clin. Microbiol. Rev. 2015, 28, 603–661. [Google Scholar] [CrossRef]
- Voyich, J.M.; Otto, M.; Mathema, B.; Braughton, K.R.; Whitney, A.R.; Welty, D.; Long, R.D.; Dorward, D.W.; Gardner, D.J.; Lina, G.; et al. Is Panton-Valentine Leukocidin the Major Virulence Determinant in Community-Associated Methicillin-Resistant Staphylococcus aureus Disease? J. Infect. Dis. 2006, 194, 1761–1770. [Google Scholar] [CrossRef]
- Pivard, M.; Caldelari, I.; Brun, V.; Croisier, D.; Jaquinod, M.; Anzala, N.; Gilquin, B.; Teixeira, C.; Benito, Y.; Couzon, F.; et al. Complex Regulation of Gamma-Hemolysin Expression Impacts Staphylococcus aureus Virulence. Microbiol. Spectr. 2023, 11, 1–15. [Google Scholar] [CrossRef]
- DuMont, A.L.; Yoong, P.; Liu, X.; Day, C.J.; Chumbler, N.M.; James, D.B.A.; Alonzo, F.; Bode, N.J.; Borden Lacy, D.; Jennings, M.P.; et al. Identification of a Crucial Residue Required for Staphylococcus aureus LukAB Cytotoxicity and Receptor Recognition. Infect. Immun. 2014, 82, 1268–1276. [Google Scholar] [CrossRef] [PubMed]
- Badarau, A.; Rouha, H.; Malafa, S.; Logan, D.T.; Håkansson, M.; Stulik, L.; Dolezilkova, I.; Teubenbacher, A.; Gross, K.; Maierhofer, B.; et al. Structure-Function Analysis of Heterodimer Formation, Oligomerization, and Receptor Binding of the Staphylococcus aureus Bi-Component Toxin LukGH. J. Biol. Chem. 2015, 290, 142–156. [Google Scholar] [CrossRef] [PubMed]
- Cheng, A.G.; DeDent, A.C.; Schneewind, O.; Missiakas, D. A Play in Four Acts: Staphylococcus aureus Abscess Formation. Trends Microbiol. 2011, 19, 225–232. [Google Scholar] [CrossRef]
- Shallcross, L.J.; Fragaszy, E.; Johnson, A.M.; Hayward, A.C. The Role of the Panton-Valentine Leucocidin Toxin in Staphylococcal Disease: A Systematic Review and Meta-Analysis. Lancet Infect. Dis. 2013, 13, 43–54. [Google Scholar] [CrossRef] [PubMed]



| Strain | Genes Deleted; Complemented | Toxin Genes Present (↓ = Downregulated) |
|---|---|---|
| USA300 strain LAC | none | hlgABC, lukED, lukGH, pvl, hla, and psmα [54] |
| USA300ΔagrABCD | agrABCD | ↓hlgABC, ↓lukED, ↓lukGH, ↓pvl, ↓hla, and ↓psm [11,13,16] |
| USA300ΔsaePQRS | saePQRS | ↓hlgABC, ↓lukED, ↓lukGH, ↓pvl, ↓hla, and psmα [13,59] |
| USA300ΔarlRS | arlRS | ↓hlgABC, lukED, ↓lukGH, ↓pvl, hla, and psmα [62] |
| USA300ΔhlgABC | hlgABC | lukED, lukGH, pvl, hla, and psmα |
| USA300ΔlukGH | lukGH | hlgABC, lukED, pvl, hla, and psmα |
| USA300ΔlukED | lukED | hlgABC, lukGH, pvl, hla, and psmα |
| USA300Δpvl | pvl | hlgABC, lukED, lukGH, hla, and psmα |
| USA300Δpsm-aΔhla | psm-a and hla | hlgABC, lukED, lukGH, and pvl |
| USA300ΔhlgABCΔlukGHΔpvl | hlgABC, lukGH, and PVL | lukED, hla, and psmα |
| USA300ΔhlgABCΔlukGHΔlukED | hlgABC, lukGH, and lukED | pvl, hla, and psmα |
| USA300ΔpvlΔlukGHΔlukED | pvl, lukGH, and lukED | hlgABC, hla, and psmα |
| USA300ΔpvlΔhlgABCΔlukED | pvl, hlgABC, and lukED | lukGH, hla, and psmα |
| USA300ΔhlgABCΔlukGHΔpvlΔlukED | hlgABC, lukGH, PVL, and lukED | hla and psmα |
| USA300ΔhlgABCΔlukGHΔpvlΔlukEDΔhlaΔpsm | hlgABC, lukAB, pvl, lukED, hla and psm-a | none |
| USA300ΔhlgABCΔlukGHΔpvlΔlukED pRB473-pvl-comp | hlgABC, lukGH, pvl, lukED; pvl complemented | pvl, hla, and psmα |
| USA300ΔhlgABCΔlukGHΔpvlΔlukED pRB473-hlgABC-comp | hlgABC, lukGH, pvl, lukED; hlgABC complemented | hlgABC, hla, and psmα |
| USA300ΔhlgABCΔlukGHΔpvlΔlukED pRB473-lukED-comp | hlgABC, lukGH, pvl, lukED; lukED complemented | lukED, hla, and psmα |
| USA300ΔhlgABCΔlukABΔpvlΔlukED pRB473-lukGH-comp | hlgABC, lukGH, pvl, lukED; lukGH complemented | lukGH, hla, and psmα |
| Primer | Sequence |
|---|---|
| agrABCD-Top_fwd | 5′ - GGG GAC AAG TTT GTA CAA AAA AGC AGG CGA AGC GCC CGA AAT AAT ATT TAA CAC - 3′ |
| agrABCD-SphI-Top_rvs | 5′ - GGT GGT GCA TGC CTC CTC ACT GTC ATT ATA CGA TTT AG - 3′ |
| agrABCD-SphI-Bot_fwd | 5′ - GGT GGT GCA TGC GTC AGT TAA CGG CGT ATT CAA TTG - 3′ |
| agrABCD-Bot_rvs | 5′ - GGG GAC CAC TTT GTA CAA GAA AGC TGG GTG TAA GCC CTC TGC TGA TAT G - 3′ |
| SaePQRS-Top_Fwd | 5′ - GGG GAC AAG TTT GTA CAA AAA AGC AGG CGA AGG GGA AGT CAT TAC ACA AAC - 3′ |
| SaePQRS-SphI-Top_Rvs | 5′ - GGT GGT GCA TGC CTC CCA TTA ATG AGG GCT TC - 3′ |
| saePQRS-SphI-Bot_fwd | 5′ - GGT GGT GCA TGC CTC GGA GAG ATT GCA ATT GG - 3′ |
| saePQRS-Bot_Rvs | 5′ - GGG GAC AAG TTT GTA CAA AAA AGC AGG CGT CAT ATG GCC GTT AAA CCA CA - 3′ |
| arlRS-SalI-Top_fwd | 5′ - TGT CGA CCT CAT ATT ACG ACT TTT TC - 3′ |
| arlRS-PstI-Top_rvs | 5′ - CTG CAG TAA ACC TAA AGT GTC GTA AG - 3′ |
| arlRS-SacI-Bot_fwd | 5′ - TCA CTA TTG AGC TCT TTG TTA AAG TAG - 3′ |
| arlRS-BamHI-Bot_rvs | 5′ - AAA TGG ATC CTA TCA TAA AAT TAG TCG AAG - 3′ |
| hlgABC-SphI-Top_Fwd | 5′ - GGG GAC AAG TTT GTA CAA AAA AGC AGG CGT TCG TCA TGA TGA GCG TG - 3′ |
| hlgABC-SphI-Top_rvs | 5′ - GGT GGT GCA TGC GGT CGC AGG CGT TTA TAT AG - 3′ |
| hlgABC-SphI-Bot_Fwd | 5′ - GGT GGT GCA TGC GTG ACG ACC GTG - 3′ |
| hlgABC-SphI-Bot_rvs | 5′ - GGG GAC CAC TTT GTA CAA GAA AGC TGG GTG CGC TAA ATC AAG GGA TG - 3′ |
| lukGH-SphI-Top_fwd | 5′ - GGG GAC AAG TTT GTA CAA AAA AGC AGG CCA ATC AGG GTG GGA CAA AAC - 3′ |
| lukGH-SphI-Top_rvs | 5′ - GGG GGT GGT GCA TGC GAC GTG CAG TGT ATG AAT CTT G - 3′ |
| lukGH-SphI-Bot_fwd | 5′ - GGT GGT GCA TGC GAT TGA TAT TTG TTG ATA TGT ATC GAC ATG TG - 3′ |
| lukGH-SphI-Bot_rvs | 5′ - GGG GAC CAC TTT GTA CAA GAA AGC TGG GTC AAT GAT TTG AAC ATA GGC GCA AC - 3′ |
| lukED-SphI-Top_fwd | 5′ - GGG GAC AAG TTT GTA CAA AAA AGC AGG CGA AGT TAA GGC CTA CTT CAA TTG TC - 3′ |
| lukED-SphI-Top_rvs | 5′ - GGT GGT GCA TGC GAA ACT AAT CCT GGA GTA TAA CTG TTA G - 3′ |
| lukED-SphI-Bot_fwd | 5′ - GGT GGT GCA TGC CTA CTG ACA AAG TTG CAG CTA AC - 3′ |
| lukED-SphI-Bot_rvs | 5′ - GGG GAC CAC TTT GTA CAA GAA AGC TGG GTG TGC TCG TCG TCA AGA C - 3′ |
| PVL-SphI-Top_fwd | 5′ - GGG GAC AAG TTT GTA CAA AAA AGC AGG CCT CAT ATC ATC GCC TTT GTC C - 3′ |
| PVL-SphI-Top_rvs | 5′ - GGT GGT GCA TGC GGA ATC AAC TTC ACT GGA TAG G - 3′ |
| PVL-SphI-Bot_fwd | 5′ - GGT GGT GCA TGC CTA ACG ACA ATG TTG CAG CTA ATA G - 3′ |
| PVL-SphI-Bot_rvs | 5′ - GGG GAC CAC TTT GTA CAA GAA AGC TGG GTG AGA AAG CGC AAG TGG TG - 3′ |
| PSMa-Top_fwd | 5’′ - GGG GAC AAG TTT GTA CAA AAA AGC AGG CGT CGT CTA CCT TTC CAT GC - 3′ |
| PSMa-SphI-Top_rvs | 5′ - GGT GGT GCA TGC CTC AGG CCA CTA TAC CAA TAG - 3′ |
| PSMa-SphI-Bot_fwd | 5′ - GGT GGT GCA TGC CAG CGA TGA TAC CCA TTA AGA TTA CC - 3′ |
| PSMa-Bot_rvs | 5′ - GGG GAC CAC TTT GTA CAA GAA AGC TGG GTC GAA TGC AAG CCA ACC AC - 3′ |
| hla-Top_fwd | 5′ - GGG GAC AAG TTT GTA CAA AAA AGC AGG CGA AGT CCA TAC AAA ATC CGC ATC - 3′ |
| hla-BamHI-Top_rvs | 5′ - GGT GGT GGA TCC CTA TCT ACT TGA TTT GCT TTC CTG AC - 3′ |
| hla-BamHI-Bot_fwd | 5′ - GGT GGT GGA TCC CAA TTT CGA GGG TTA GTC AAA GTT G - 3′ |
| hla-Bot_rvs | 5′ - GGG GAC CAC TTT GTA CAA GAA AGC TGG GTG CAA TAC TTT ATT GTC CCA TGA TTA GTG - 3′ |
| pvl-EcoRI-comp_fwd | 5′ - AGG AGG GAA TTC GTT TGG TAA TGA ACG GGT TTT TTT CG - 3′ |
| pvl-BamHI-comp_rvs | 5′ - GGT GGT GGA TCC CAA TTA AGA CGT GGT TAC CCT AAT ATA G - 3′ |
| hlgABC-SacI-comp_fwd | 5′ - GGT GGT GAG CTC CAG TTA ATT CGA AAA CGC TTA CAA ATG G - 3′ |
| hlgABC-BamHI-comp_rvs | 5′ - GGT GGT GGA TCC CTG TTG GCG ACC GTG - 3′ |
| lukED-SacI-Comp_fwd | 5′ - GGT GGT GAG CTC CCA TGA GAG TAG AAG CTT CAG - 3′ |
| lukED-BamHI-Comp_rvs | 5′ - GGT GGT GGA TCC GAA GTT AAG ACC CAC TTC AAT TGT C - 3′ |
| lukGH-EcoRI-comp_fwd | 5′ - GGT GGT GAA TTC GTA TCA ACG ATC TTA TTA ACG CTG - 3′ |
| lukGH-BamHI-comp_rvs | 5′ - GGT GGT GGA TCC CTA CAT TCT ATG TAG CAG GCA AC - 3′ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nygaard, T.K.; Borgogna, T.R.; Pallister, K.B.; Predtechenskaya, M.; Burroughs, O.S.; Gao, A.; Lubick, E.G.; Voyich, J.M. The Relative Importance of Cytotoxins Produced by Methicillin-Resistant Staphylococcus aureus Strain USA300 for Causing Human PMN Destruction. Microorganisms 2024, 12, 1782. https://doi.org/10.3390/microorganisms12091782
Nygaard TK, Borgogna TR, Pallister KB, Predtechenskaya M, Burroughs OS, Gao A, Lubick EG, Voyich JM. The Relative Importance of Cytotoxins Produced by Methicillin-Resistant Staphylococcus aureus Strain USA300 for Causing Human PMN Destruction. Microorganisms. 2024; 12(9):1782. https://doi.org/10.3390/microorganisms12091782
Chicago/Turabian StyleNygaard, Tyler K., Timothy R. Borgogna, Kyler B. Pallister, Maria Predtechenskaya, Owen S. Burroughs, Annika Gao, Evan G. Lubick, and Jovanka M. Voyich. 2024. "The Relative Importance of Cytotoxins Produced by Methicillin-Resistant Staphylococcus aureus Strain USA300 for Causing Human PMN Destruction" Microorganisms 12, no. 9: 1782. https://doi.org/10.3390/microorganisms12091782





