Host Factor Rab4b Promotes Japanese Encephalitis Virus Replication
Abstract
:1. Introduction
2. Materials and Methods
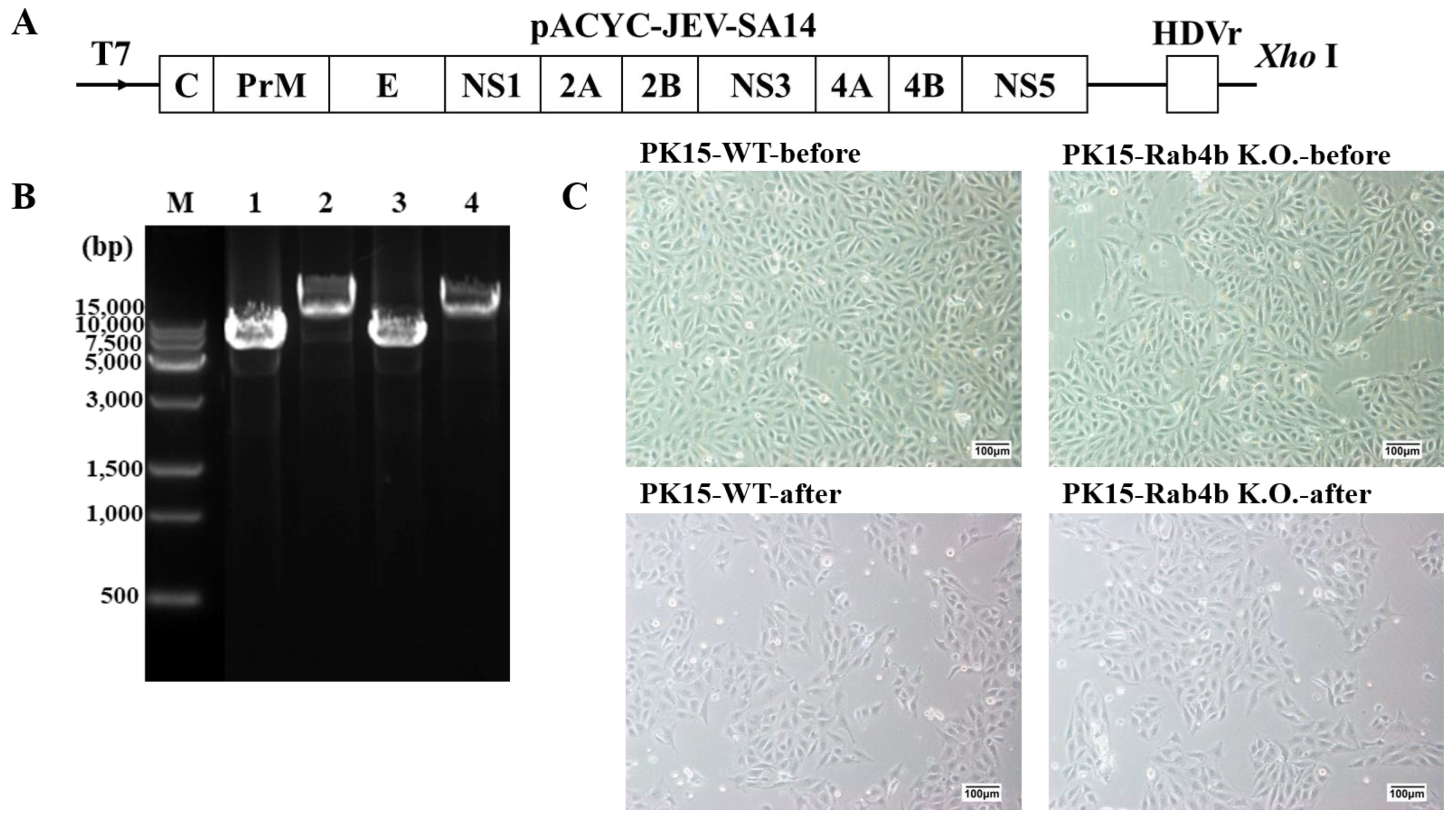
2.1. Cells, Virus, and Plasmid
2.2. An Assay for Determining Cell Viability Using the CCK-8 Kit
2.3. Real-Time–Quantitative PCR (RT‒qPCR) Assay for Virus Replication
2.4. Assay for Indirect Immunofluorescence (IFA)
2.5. Virus Adsorption Assay
2.6. Viral Internalization Assay
2.7. The Effect of Rab4b on JEV Assembly and Release
2.8. Western Blot
2.9. Statistical Analysis
3. Results
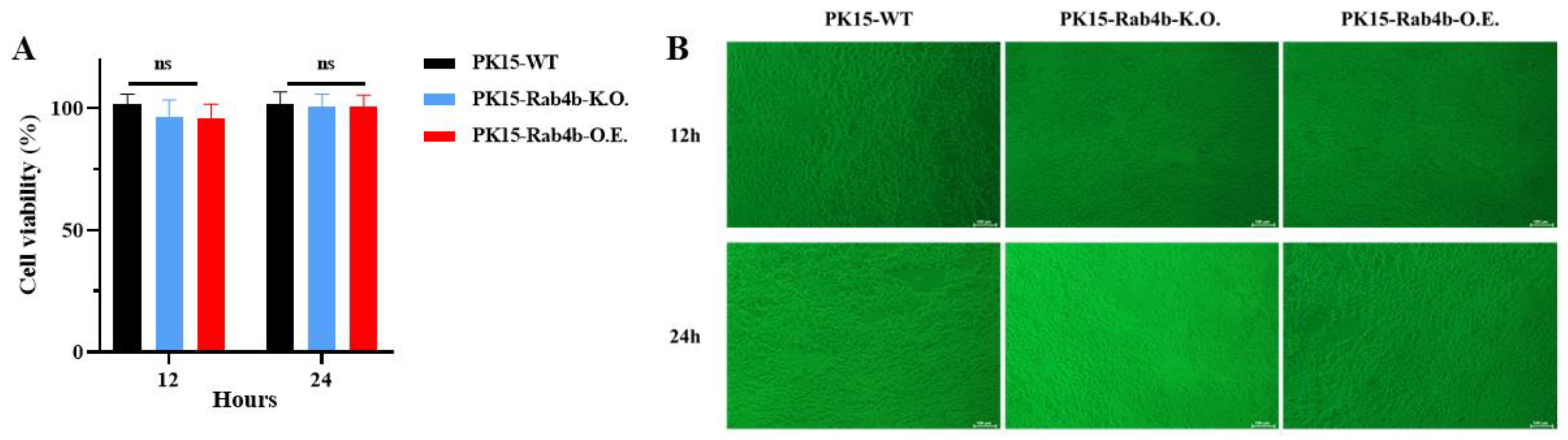
3.1. Effect of Rab4b Gene on Cell Viability
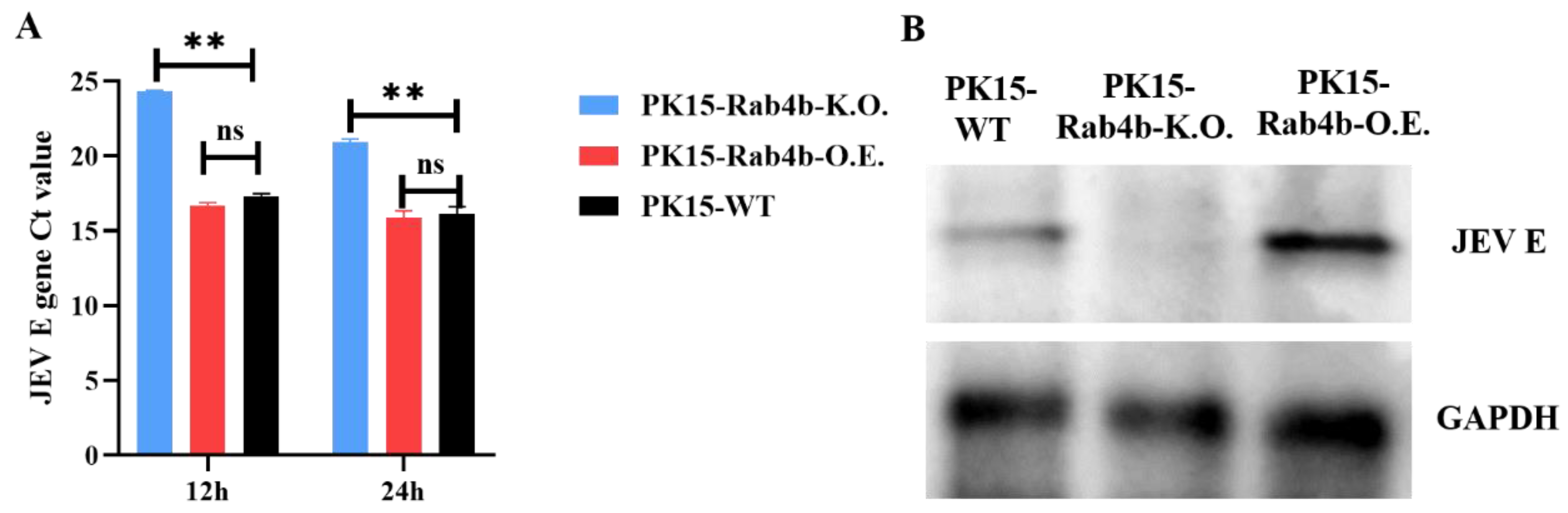
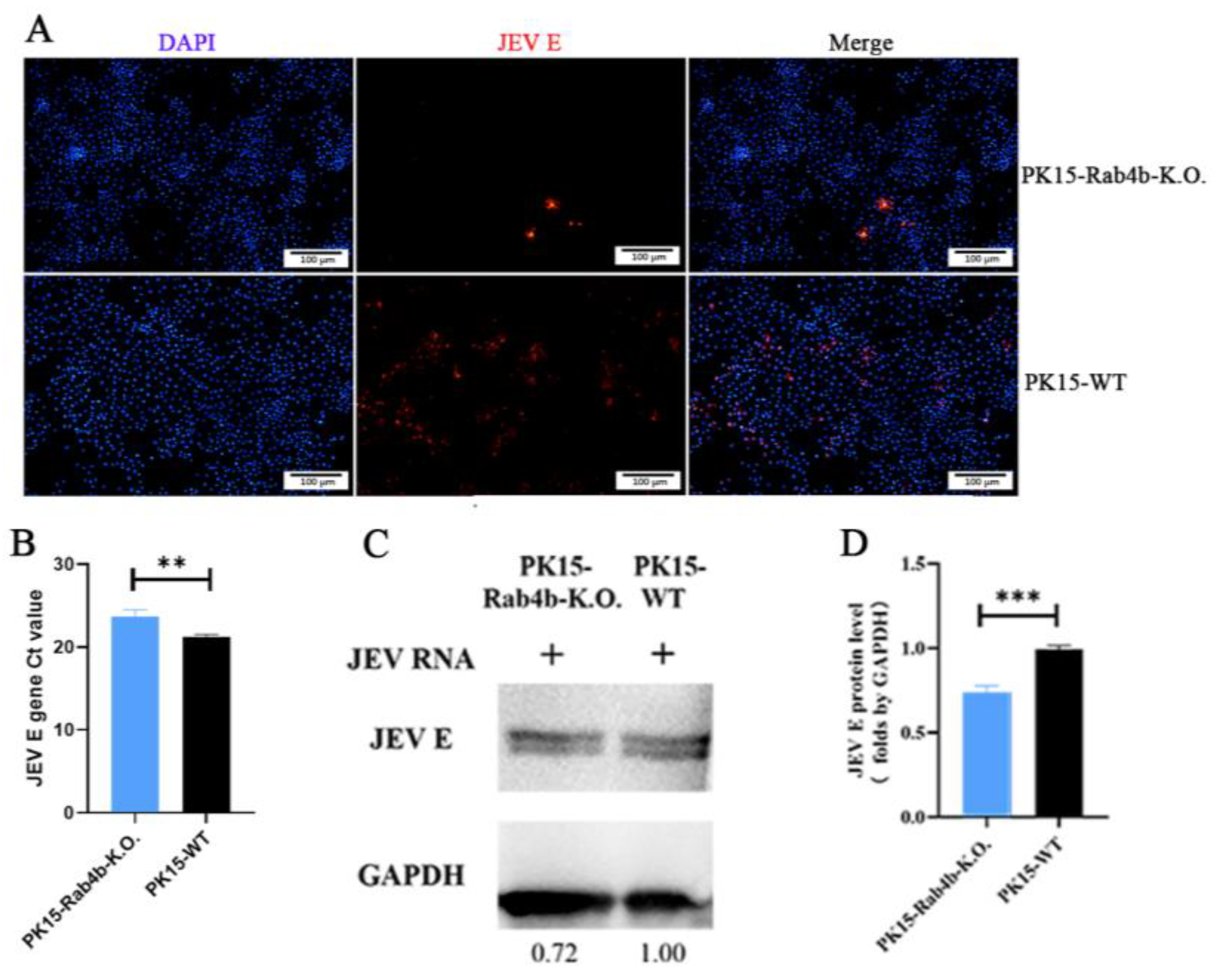
3.2. Effect of Rab4b on JEV Replication
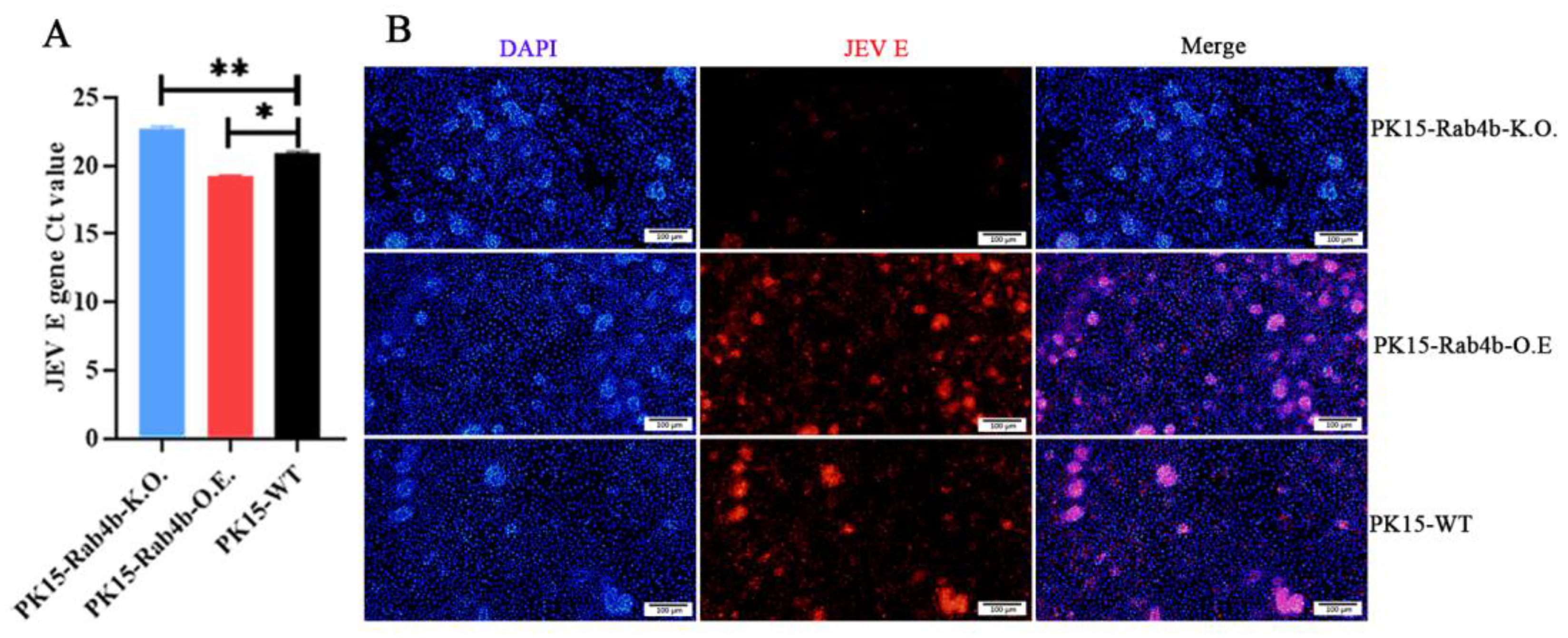
3.3. Effect of Rab4b on JEV Adsorption
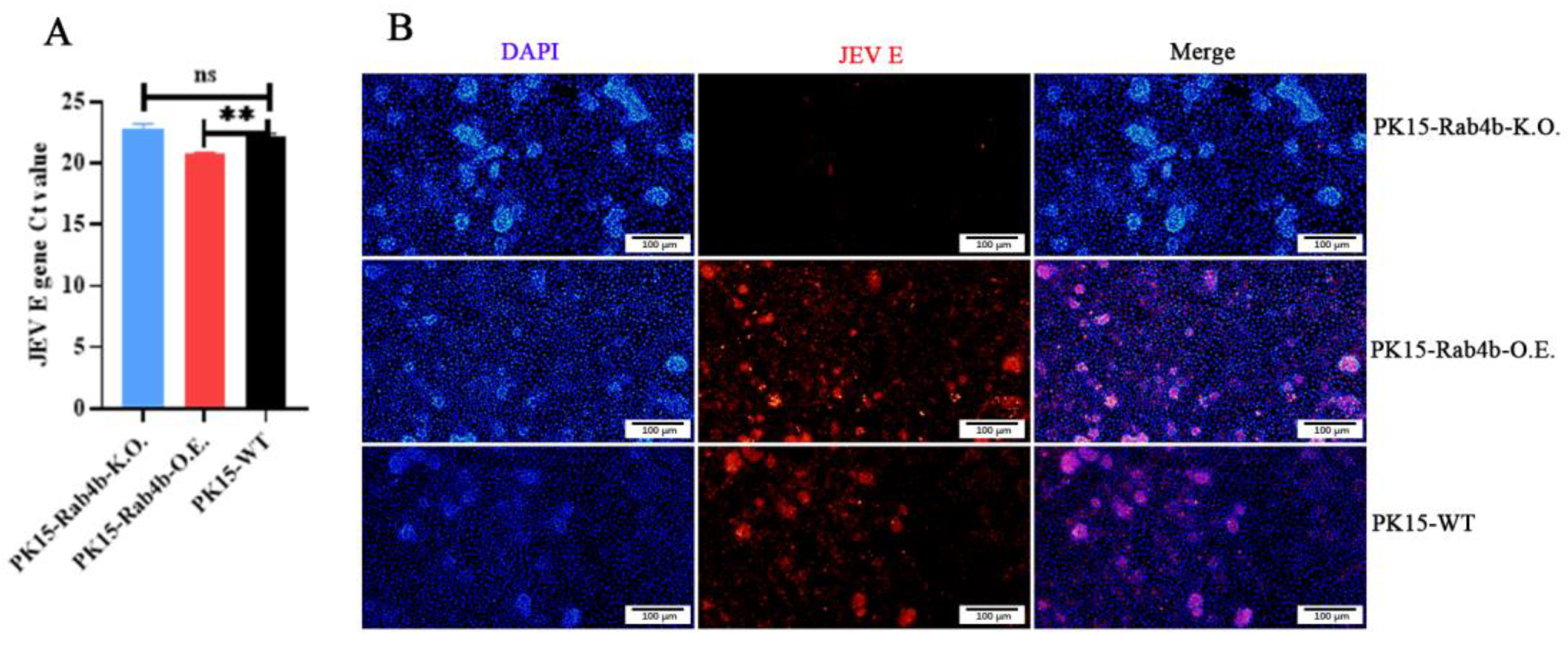
3.4. Effect of Rab4b on JEV Internalization
3.5. Effect of Rab4b on JEV Assembly and Release
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Redant, V.; Favoreel, H.W.; Dallmeier, K.; Van Campe, W.; De Regge, N. Efficient control of Japanese encephalitis virus in the central nervous system of infected pigs occurs in the absence of a pronounced inflammatory immune response. J. Neuroinflamm. 2020, 17, 315. [Google Scholar] [CrossRef]
- Kumar, K.; Ong, H.K.; Tan, W.S.; Arshad, S.S.; Ho, K.L. Immunological Analysis of Nodavirus Capsid Displaying the Domain III of Japanese Encephalitis Virus Envelope Protein. Pharmaceutics 2021, 13, 1826. [Google Scholar] [CrossRef]
- Itihas, A.; Jategaonkar, S.; Jain, M.; Narang, R.; Chauhan, V.; Tandale, B.V.; Tomar, S. Comparison of Clinical Profile and Outcomes of Japanese Encephalitis and Acute Encephalitis Syndrome among Rural Children. Indian J. Pediatr. 2023, 90, 1038–1040. [Google Scholar] [CrossRef] [PubMed]
- Sharma, K.B.; Vrati, S.; Kalia, M. Pathobiology of Japanese encephalitis virus infection. Mol. Asp. Med. 2021, 81, 100994. [Google Scholar] [CrossRef] [PubMed]
- Grewe, S.; Gliem, M.; Abrar, D.B.; Feldt, T.; Wojtecki, L.; Tan, V.; Lifea; Afzal, S.; Meuth, S.G.; Luedde, T.; et al. Myelitis with flaccid paralysis due to Japanese encephalitis: Case report and review of the literature. Infection 2022, 50, 1597–1630. [Google Scholar] [CrossRef]
- Shen, W.; Zhang, Y.; Zhou, C.; Shen, Y. Bilateral symmetrical deep gray matter involvement and leptomeningeal enhancement in a child with MOG-IgG-associated encephalomyelitis. BMC Neurol. 2021, 21, 10. [Google Scholar] [CrossRef]
- Yin, R.; Yang, L.; Hao, Y.; Yang, Z.; Lu, T.; Jin, W.; Dan, M.; Peng, L.; Zhang, Y.; Wei, Y.; et al. Proteomic landscape subtype and clinical prognosis of patients with the cognitive impairment by Japanese encephalitis infection. J. Neuroinflamm. 2022, 19, 77. [Google Scholar] [CrossRef] [PubMed]
- Nie, M.; Zhou, Y.; Li, F.; Deng, H.; Zhao, M.; Huang, Y.; Jiang, C.; Sun, X.; Xu, Z.; Zhu, L. Epidemiological investigation of swine Japanese encephalitis virus based on RT-RAA detection method. Sci. Rep. 2022, 12, 9392. [Google Scholar] [CrossRef]
- Mansfield, K.L.; Hernández-Triana, L.M.; Banyard, A.C.; Fooks, A.R.; Johnson, N. Japanese encephalitis virus infection, diagnosis and control in domestic animals. Vet. Microbiol. 2017, 201, 85–92. [Google Scholar] [CrossRef]
- Ashraf, U.; Ding, Z.; Deng, S.; Ye, J.; Cao, S.; Chen, Z. Pathogenicity and virulence of Japanese encephalitis virus: Neuroinflammation and neuronal cell damage. Virulence 2021, 12, 968–980. [Google Scholar] [CrossRef]
- Hernández-Triana, L.M.; Folly, A.J.; Sewgobind, S.; Lean, F.Z.X.; Ackroyd, S.; Nuñez, A.; Delacour, S.; Drago, A.; Visentin, P.; Mansfield, K.L.; et al. Susceptibility of Aedes albopictus and Culex quinquefasciatus to Japanese encephalitis virus. Parasites Vectors 2022, 15, 210. [Google Scholar] [CrossRef] [PubMed]
- Li, C.-X.; Guo, X.-X.; Deng, Y.-Q.; Liu, Q.-M.; Xing, D.; Sun, A.-J.; Wu, Q.; Dong, Y.-D.; Zhang, Y.-M.; Zhang, H.-D.; et al. Susceptibility of Armigeres subalbatus Coquillett (Diptera: Culicidae) to Zika virus through oral and urine infection. PLoS Neglected Trop. Dis. 2020, 14, e0008450. [Google Scholar] [CrossRef] [PubMed]
- Bharucha, T.; Cleary, B.; Farmiloe, A.; Sutton, E.; Hayati, H.; Kirkwood, P.; Al Hamed, L.; van Ginneken, N.; Subramaniam, K.S.; Zitzmann, N.; et al. Mouse models of Japanese encephalitis virus infection: A systematic review and meta-analysis using a meta-regression approach. PLoS Neglected Trop. Dis. 2022, 16, e0010116. [Google Scholar] [CrossRef]
- Yang, H.; Wan, X.; Wang, Z.; Wang, G.; Fu, S.; Li, F.; Yang, L.; Yuan, Y.; Shen, K.; Wang, H.; et al. Peripheral Nerve Injury Induced by Japanese Encephalitis Virus in C57BL/6 Mouse. J. Virol. 2023, 97, e01658-22. [Google Scholar] [CrossRef] [PubMed]
- Boodhoo, N.; Kamble, N.; Sharif, S.; Behboudi, S.; Longnecker, R.M. Glutaminolysis and Glycolysis Are Essential for Optimal Replication of Marek’s Disease Virus. J. Virol. 2020, 94, 10–1128. [Google Scholar] [CrossRef]
- Calderón-Peláez, M.-A.; Velandia-Romero, M.L.; Bastidas-Legarda, L.Y.; Beltrán, E.O.; Camacho-Ortega, S.J.; Castellanos, J.E. Dengue Virus Infection of Blood-Brain Barrier Cells: Consequences of Severe Disease. Front. Microbiol. 2019, 10, 1435. [Google Scholar] [CrossRef]
- Chien, Y.-J.; Chen, W.-J.; Hsu, W.-L.; Chiou, S.-S. Bovine lactoferrin inhibits Japanese encephalitis virus by binding to heparan sulfate and receptor for low density lipoprotein. Virology 2008, 379, 143–151. [Google Scholar] [CrossRef]
- Nain, M.; Mukherjee, S.; Karmakar, S.P.; Paton, A.W.; Paton, J.C.; Abdin, M.Z.; Basu, A.; Kalia, M.; Vrati, S. GRP78 Is an Important Host Factor for Japanese Encephalitis Virus Entry and Replication in Mammalian Cells. J. Virol. 2017, 91, e02274-16. [Google Scholar] [CrossRef]
- Wang, Y.; Li, Y.; Ding, T. Heat shock protein 90β in the Vero cell membrane binds Japanese encephalitis virus. Int. J. Mol. Med. 2017, 40, 474–482. [Google Scholar] [CrossRef]
- Wang, P.; Liu, X.; Li, Q.; Wang, J.; Ruan, W. Proteomic analyses identify intracellular targets for Japanese encephalitis virus nonstructural protein 1 (NS1). Virus Res. 2021, 302, 198495. [Google Scholar] [CrossRef]
- Huang, L.; Li, H.; Ye, Z.; Xu, Q.; Fu, Q.; Sun, W.; Qi, W.; Yue, J. Berbamine inhibits Japanese encephalitis virus (JEV) infection by compromising TPRMLs-mediated endolysosomal trafficking of low-density lipoprotein receptor (LDLR). Emerg. Microbes Infect. 2021, 10, 1257–1271. [Google Scholar] [CrossRef]
- Lannes, N.; Neuhaus, V.; Scolari, B.; Kharoubi-Hess, S.; Walch, M.; Summerfield, A.; Filgueira, L. Interactions of human microglia cells with Japanese encephalitis virus. Virol. J. 2017, 14, 8. [Google Scholar] [CrossRef]
- Bian, P.; Ye, C.; Zheng, X.; Luo, C.; Yang, J.; Li, M.; Wang, Y.; Yang, J.; Zhou, Y.; Zhang, F.; et al. RIPK3 Promotes JEV Replication in Neurons via Downregulation of IFI44L. Front. Microbiol. 2020, 11, 368. [Google Scholar] [CrossRef]
- Anwar, M.N.; Akhtar, R.; Abid, M.; Khan, S.A.; Rehman, Z.U.; Tayyub, M.; Malik, M.I.; Shahzad, M.K.; Mubeen, H.; Qadir, M.S.; et al. The interactions of flaviviruses with cellular receptors: Implications for virus entry. Virology 2022, 568, 77–85. [Google Scholar] [CrossRef]
- Chiou, S.S.; Liu, H.; Chuang, C.K.; Lin, C.C.; Chen, W.J. Fitness of Japanese encephalitis virus to Neuro-2a cells is determined by interactions of the viral envelope protein with highly sulfated glycosaminoglycans on the cell surface. J. Med. Virol. 2005, 76, 583–592. [Google Scholar] [CrossRef] [PubMed]
- Yiu, C.C.; Sasano, H.; Ono, K.; Chow, L.W. Changes in protein expression after neoadjuvant use of aromatase inhibitors in primary breast cancer: A proteomic approach to search for potential biomarkers to predict response or resistance. Expert Opin. Investig. Drugs 2010, 19 (Suppl. 1), S79–S89. [Google Scholar] [CrossRef]
- Schweitzer, J.K.; Sedgwick, A.E.; D’Souza-Schorey, C. ARF6-mediated endocytic recycling impacts cell movement, cell division and lipid homeostasis. Semin. Cell Dev. Biol. 2011, 22, 39–47. [Google Scholar] [CrossRef]
- McCormick, D.; Lin, Y.-T.; Greya, F. Identification of Host Factors Involved in Human Cytomegalovirus Replication, Assembly, and Egress Using a Two-Step Small Interfering RNA Screen. mBio 2018, 9, e00716-18. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Zhang, Y.; Liu, S.; Li, C.; Sun, L.; Bao, L.; Feng, J.; Liu, Z. Analysis of 52 Rab GTPases from channel catfish and their involvement in immune responses after bacterial infections. Dev. Comp. Immunol. 2014, 45, 21–34. [Google Scholar] [CrossRef] [PubMed]
- Coombes, B.K.; Mölleken, K.; Hegemann, J.H. Acquisition of Rab11 and Rab11-Fip2—A novel strategy for Chlamydia pneumoniae early survival. PLoS Pathog. 2017, 13, e1006556. [Google Scholar] [CrossRef]
- Liu, Y.-G.; Chen, Y.; Wang, X.; Zhao, P.; Zhu, Y.; Qi, Z. Ezrin is essential for the entry of Japanese encephalitis virus into the human brain microvascular endothelial cells. Emerg. Microbes Infect. 2020, 9, 1330–1341. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; He, M.; Liu, X.; Li, X.; Fan, B.; Zhao, S. Japanese encephalitis virus infects porcine kidney epithelial PK15 cells via clathrin- and cholesterol-dependent endocytosis. Virol. J. 2013, 10, 258. [Google Scholar] [CrossRef] [PubMed]
- Niu, J.; Jiang, Y.; Xu, H.; Zhao, C.; Zhou, G.; Chen, P.; Cao, R. TIM-1 Promotes Japanese Encephalitis Virus Entry and Infection. Viruses 2018, 10, 630. [Google Scholar] [CrossRef] [PubMed]
- Chandan, K.; Gupta, M.; Sarwat, M. Role of Host and Pathogen-Derived MicroRNAs in Immune Regulation During Infectious and Inflammatory Diseases. Front. Immunol. 2020, 10, 3081. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Yang, K.; Zheng, Y.; Cao, S.; Yan, Q.; Huang, X.; Wen, Y.; Zhao, Q.; Du, S.; Lang, Y.; et al. BAK-Mediated Pyroptosis Promotes Japanese Encephalitis Virus Proliferation in Porcine Kidney 15 Cells. Viruses 2023, 15, 974. [Google Scholar] [CrossRef]
- Zhou, X.; Yuan, Q.; Zhang, C.; Dai, Z.; Du, C.; Wang, H.; Li, X.; Yang, S.; Zhao, A. Inhibition of Japanese encephalitis virus proliferation by long non-coding RNA SUSAJ1 in PK-15 cells. Virol. J. 2021, 18, 29. [Google Scholar] [CrossRef]
- Zhao, C.; Liu, H.; Xiao, T.; Wang, Z.; Nie, X.; Li, X.; Qian, P.; Qin, L.; Han, X.; Zhang, J.; et al. CRISPR screening of porcine sgRNA library identifies host factors associated with Japanese encephalitis virus replication. Nat. Commun. 2020, 11, 5178. [Google Scholar] [CrossRef]
- Zhang, Y.-T. Effect of Host Factor Rab4b on Japanese Encephalitis Virus Infection. Master’s Thesis, Sichuan Agricultural University, Chengdu, China, 2022. [Google Scholar]
- Li, X.-F.; Ye, H.-Q.; Deng, C.-L.; Ye, Q.; Shan, C.; Shang, B.-D.; Xu, L.-L.; Li, S.-H.; Cao, S.-B.; Yuan, Z.-M.; et al. Recovery of a chemically synthesized Japanese encephalitis virus reveals two critical adaptive mutations in NS2B and NS4A. J. Gen. Virol. 2014, 94, 806–815. [Google Scholar] [CrossRef]
- Taniguchi, M.; Tasaki, T.; Ninomiya, H.; Ueda, Y.; Kuremoto, K.-I.; Mitsutake, S.; Igarashi, Y.; Okazaki, T.; Takegami, T. Sphingomyelin generated by sphingomyelin synthase 1 is involved in attachment and infection with Japanese encephalitis virus. Sci. Rep. 2016, 6, 37829. [Google Scholar] [CrossRef]
- Sun, Y.; Ding, H.; Zhao, F.; Yan, Q.; Li, Y.; Niu, X.; Zeng, W.; Wu, K.; Ling, B.; Fan, S.; et al. Genomic Characteristics and E Protein Bioinformatics Analysis of JEV Isolates from South China from 2011 to 2018. Vaccines 2022, 10, 1303. [Google Scholar] [CrossRef]
- Mukhopadhyay, S.; Kuhn, R.J.; Rossmann, M.G. A structural perspective of the flavivirus life cycle. Nat. Rev. Microbiol. 2005, 3, 13–22. [Google Scholar] [CrossRef] [PubMed]
- Nain, M.; Abdin, M.Z.; Kalia, M.; Vrati, S. Japanese encephalitis virus invasion of cell: Allies and alleys. Rev. Med. Viology 2016, 26, 129–141. [Google Scholar] [CrossRef]
- Luo, S.Q.; Cao, S.J.; Zhao, Q. CRISPR/Cas9-Mediated Knockout of the HuR Gene in U251 Cell Inhibits Japanese Encephalitis Virus Replication. Microorganisms 2024, 12, 314. [Google Scholar] [CrossRef]
- Verawan Boonsanay, D.R.S. Entry into and production of the Japanese encephalitis virus from C6/36 cells. Intervirology 2007, 50, 85–92. [Google Scholar] [CrossRef]
- Zhu, Y.-Z.; Xu, Q.-Q.; Wu, D.-G.; Ren, H.; Zhao, P.; Lao, W.-G.; Wang, Y.; Tao, Q.-Y.; Qian, X.-J.; Wei, Y.-H.; et al. Japanese encephalitis virus enters rat neuroblastoma cells via a pH-dependent, dynamin and caveola-mediated endocytosis pathway. J. Virol. 2012, 86, 13407–13422. [Google Scholar] [CrossRef]
- Xu, Q.; Zhu, N.; Chen, S.; Zhao, P.; Ren, H.; Zhu, S.; Tang, H.; Zhu, Y.; Qi, Z. E3 Ubiquitin Ligase Nedd4 Promotes Japanese Encephalitis Virus Replication by Suppressing Autophagy in Human Neuroblastoma Cells. Sci. Rep. 2017, 7, 45375. [Google Scholar] [CrossRef]
- Wang, Q.; Yang, S.; Yang, K.; Li, X.; Dai, Y.; Zheng, Y.; Cao, S.; Yan, Q.; Huang, X.; Wen, Y.; et al. CD4 is an important host factor for Japanese encephalitis virus entry and replication in PK-15 cells. Vet. Microbiol. 2023, 287, 109913. [Google Scholar] [CrossRef] [PubMed]
- Tan, T.; Xia, L. TRIM21 Aggravates Herpes Simplex Virus Epithelial Keratitis by Attenuating STING-IRF3-Mediated Type I Interferon Signaling. Front. Microbiol. 2020, 11, 703. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.; Li, F.; Zhang, J.-W.; Li, W.; Zhao, D.-M.; Wang, H.; Hua, R.-H.; Bu, Z.-G. Host Factor SPCS1 Regulates the Replication of Japanese Encephalitis Virus through Interactions with Transmembrane Domains of NS2B. J. Virol. 2018, 92, e00197-18. [Google Scholar] [CrossRef]
- Li, Q.; Zhou, D.; Jia, F.; Zhang, L.; Ashraf, U.; Li, Y.; Duan, H.; Song, Y.; Chen, H.; Cao, S.; et al. Japanese Encephalitis Virus NS1’ Protein Interacts with Host CDK1 Protein to Regulate Antiviral Response. Microbiol. Spectr. 2021, 9, e01661-21. [Google Scholar] [CrossRef]
- Sergeeva, O.; Abakumova, T.; Kurochkin, I.; Ialchina, R.; Kosyreva, A.; Prikazchikova, T.; Varlamova, V.; Shcherbinina, E.; Zatsepin, T. Level of Murine DDX3 RNA Helicase Determines Phenotype Changes of Hepatocytes In Vitro and In Vivo. Int. J. Mol. Sci. 2021, 22, 6958. [Google Scholar] [CrossRef] [PubMed]
- Kung, Y.-A.; Chiang, H.-J.; Li, M.-L.; Gong, Y.-N.; Chiu, H.-P.; Hung, C.-T.; Huang, P.-N.; Huang, S.-Y.; Wang, P.-Y.; Hsu, T.-A.; et al. Acyl-Coenzyme A Synthetase Long-Chain Family Member 4 Is Involved in Viral Replication Organelle Formation and Facilitates Virus Replication via Ferroptosis. mBio 2022, 13, e02717-21. [Google Scholar] [CrossRef]
- Elgner, F.; Hildt, E.; Bender, D. Relevance of Rab Proteins for the Life Cycle of Hepatitis C Virus. Front. Cell Dev. Biol. 2018, 6, 166. [Google Scholar] [CrossRef] [PubMed]
- Krishnan, M.N.; Sukumaran, B.; Pal, U.; Agaisse, H.; Murray, J.L.; Hodge, T.W.; Fikrig, E. Rab 5 Is Required for the Cellular Entry of Dengue and West Nile Viruses. J. Virol. 2007, 81, 4881–4885. [Google Scholar] [CrossRef] [PubMed]
- Barbosa, M.D.; Johnson, S.A.; Achey, K.; Gutierrez, M.J.; Wakeland, E.K.; Zerial, M.; Kingsmore, S.F. The Rab protein family: Genetic mapping of six Rab genes in the mouse. Genomics 1995, 30, 439–444. [Google Scholar] [CrossRef]
- Krawczyk, M.; Leimgruber, E.; Seguín-Estévez, Q.; Dunand-Sauthier, I.; Barras, E.; Reith, W. Expression of RAB4B, a protein governing endocytic recycling, is co-regulated with MHC class II genes. Nucleic Acid Res. 2007, 35, 595–605. [Google Scholar] [CrossRef]
- Mohrmann, K.; Gerez, L.; Oorschot, V.; Klumperman, J.; van der Sluijs, P. Rab4 Function in Membrane Recycling from Early Endosomes Depends on a Membrane to Cytoplasm Cycle. J. Biol. Chem. 2002, 277, 32029–32035. [Google Scholar] [CrossRef] [PubMed]
- Gilleron, J.; Bouget, G.; Ivanov, S.; Meziat, C.; Ceppo, F.; Vergoni, B.; Djedaini, M.; Soprani, A.; Dumas, K.; Jacquel, A.; et al. Rab4b Deficiency in T Cells Promotes Adipose Treg/Th17 Imbalance, Adipose Tissue Dysfunction, and Insulin Resistance. Cell Rep. 2018, 25, 3329–3341.e3325. [Google Scholar] [CrossRef] [PubMed]
- Turner, D.L.; Korneev, D.V.; Purdy, J.G.; de Marco, A.; A Mathias, R. The host exosome pathway underpins biogenesis of the human cytomegalovirus virion. eLife 2020, 9, e58288. [Google Scholar] [CrossRef]
- William, J.; Cisneros, D.C.; Judd, F. Hultquist. Application of CRISPR-Cas9 Gene Editing for HIV Host Factor Discovery and Validation. Pathogens 2022, 11, 891. [Google Scholar]
- Liu, H.; Liu, Y.; Wang, S.; Zhang, Y.; Zu, X.; Zhou, Z.; Zhang, B.; Xiao, G. Structure-Based Mutational Analysis of Several Sites in the E Protein: Implications for Understanding the Entry Mechanism of Japanese Encephalitis Virus. J. Virol. 2015, 89, 5668–5686. [Google Scholar] [CrossRef]
- Han, X.; Cai, Z.; Dai, Y.; Huang, H.; Cao, X.; Wang, Y.; Fang, Y.; Liu, G.; Zhang, M.; Zhang, Y.; et al. Re-burying Artificially Exposed Surface of Viral Subunit Vaccines Through Oligomerization Enhances Vaccine Efficacy. Front. Cell. Infect. Microbiol. 2022, 12, 927674. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, T.; Yi, Y.; Song, M.; Jin, M.; Guo, K.; Zhang, Y. ARF1 with Sec7 Domain-Dependent GBF1 Activates Coatomer Protein I To Support Classical Swine Fever Virus Entry. J. Virol. 2022, 96, e02193-21. [Google Scholar] [CrossRef] [PubMed]
- Chappell, K.J.; Stoermer, M.J.; Fairlie, D.P.; Young, P.R. West Nile Virus NS2B/NS3 Protease As An Antiviral Target. Curr. Med. Chem. 2008, 15, 2771–2784. [Google Scholar] [CrossRef] [PubMed]
- Qin, F.; Xu, C.; Hu, J.; Lei, C.; Zheng, Z.; Peng, K.; Wang, H.; Sun, X. Dissecting the Cell Entry Pathway of Baculovirus by Single-Particle Tracking and Quantitative Electron Microscopic Analysis. J. Virol. 2019, 93, e00033-19. [Google Scholar] [CrossRef] [PubMed]
- Spearman, P. Viral interactions with host cell Rab GTPases. Small GTPases 2018, 9, 192–201. [Google Scholar] [CrossRef]
- Uddin, K.; Watanabe, T.; Arata, M.; Sato, Y.; Kimura, H.; Murata, T. Epstein-Barr Virus BBLF1 Mediates Secretory Vesicle Transport to Facilitate Mature Virion Release. J. Virol. 2023, 97, e00437-23. [Google Scholar] [CrossRef]
- Saha, S.; Chatterjee, P.; Halder, A.K.; Nasipuri, M.; Basu, S.; Plewczynski, D. ML-DTD: Machine Learning-Based Drug Target Discovery for the Potential Treatment of COVID-19. Vaccines 2022, 10, 1643. [Google Scholar] [CrossRef]
- Freije, C.A.; Sabeti, P.C. Detect and destroy: CRISPR-based technologies for the response against viruses. Cell Host Microbe 2021, 29, 689–703. [Google Scholar] [CrossRef]
| Target Names | (5′→3′) Primer Sequences |
|---|---|
| JEV-E1223-F | CAGTGGAGCCACTTGGGTG |
| JEV-E1223-R | TTGTGAGCTTCTCCTGTCG |
| GAPDH-PF | TATGATTCCACCCACGGCAAG |
| GAPDH-PR | ATACGTAGCACCAGCATCACC |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, Q.; Miao, C.; Chen, Y.-T.; Zhu, L.-Y.; Zhang, Y.-T.; Luo, S.-Q.; Wang, Y.-L.; Zhu, Z.-M.; Han, X.; Wen, Y.; et al. Host Factor Rab4b Promotes Japanese Encephalitis Virus Replication. Microorganisms 2024, 12, 1804. https://doi.org/10.3390/microorganisms12091804
Zhao Q, Miao C, Chen Y-T, Zhu L-Y, Zhang Y-T, Luo S-Q, Wang Y-L, Zhu Z-M, Han X, Wen Y, et al. Host Factor Rab4b Promotes Japanese Encephalitis Virus Replication. Microorganisms. 2024; 12(9):1804. https://doi.org/10.3390/microorganisms12091804
Chicago/Turabian StyleZhao, Qin, Chang Miao, Yi-Ting Chen, Long-Yue Zhu, Ya-Ting Zhang, Sai-Qi Luo, Yu-Luo Wang, Zhu-Ming Zhu, Xinfeng Han, Yiping Wen, and et al. 2024. "Host Factor Rab4b Promotes Japanese Encephalitis Virus Replication" Microorganisms 12, no. 9: 1804. https://doi.org/10.3390/microorganisms12091804






