A Novel Bi-Directional Channel for Nutrient Uptake across Mycobacterial Outer Envelope
Abstract
:1. Introduction
2. Materials and Methods
2.1. Mycolicibacterium Smegmatis Strains and Clone Construction
2.2. Bacteria Culture Conditions
2.3. Transcriptome Sequencing
2.4. Protein Extraction from Outer Envelope of M. smegmatis
2.5. Saccharide Uptake Assay
2.6. Anti-TB Drug Sensitivity Experiments
3. Results
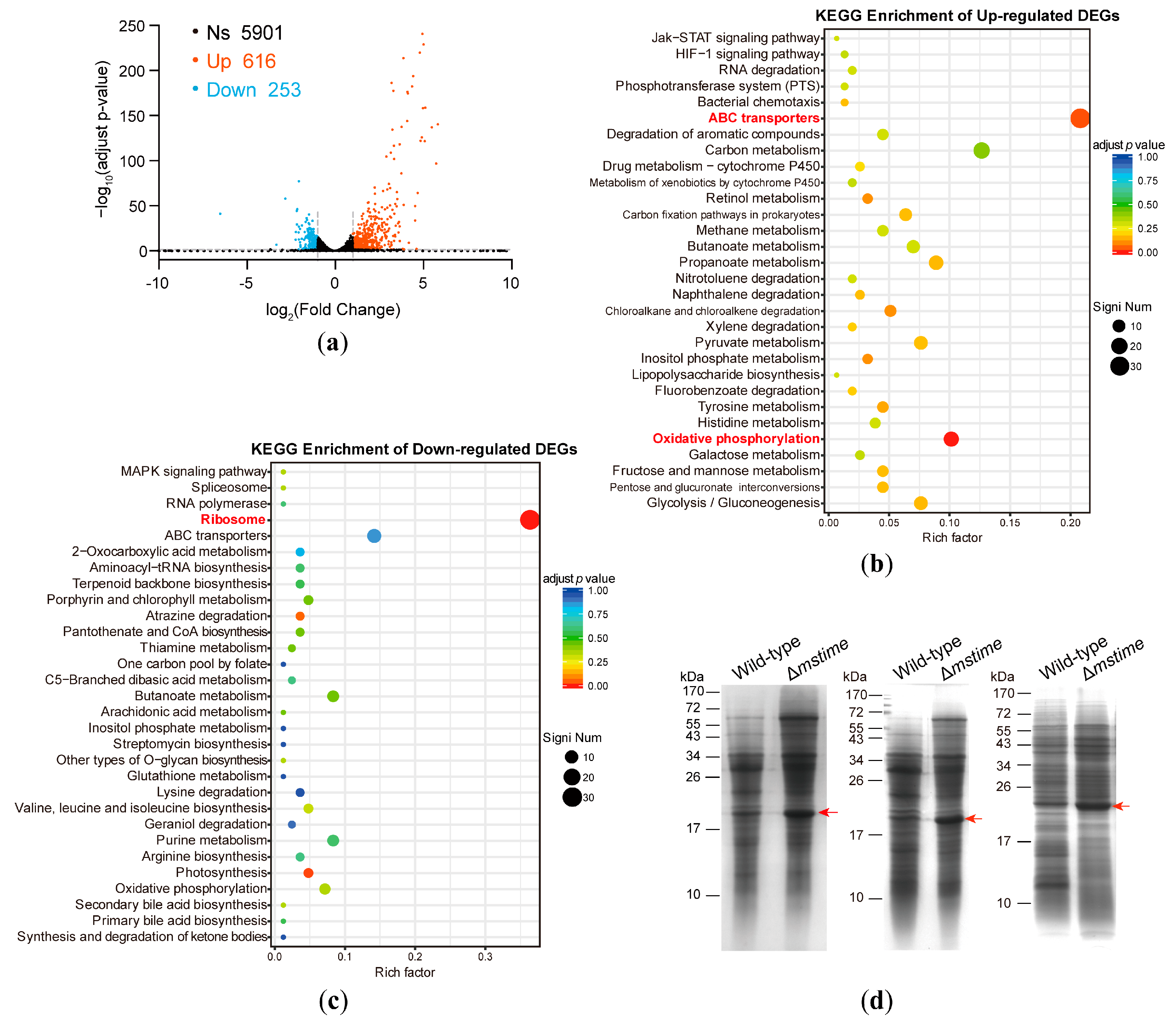
3.1. Transcriptome Analysis of Wild-Type and Δmstime M. smegmatis Strains
3.2. TiME Deletion Triggers Elevated Expression of MspB or Its Paralogues
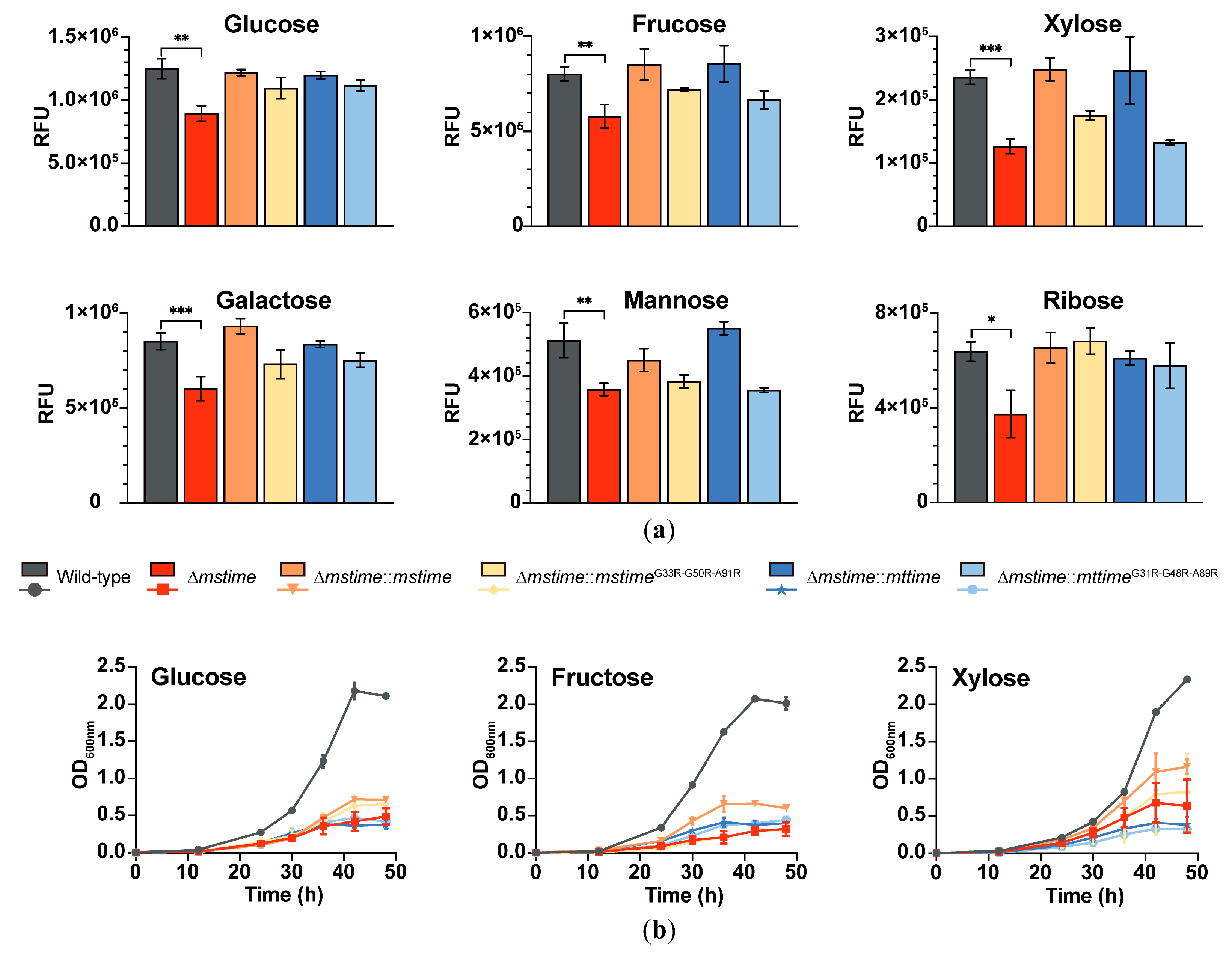
3.3. TiME Benefits Monosaccharide Uptake in M. smegmatis
3.4. TiME Facilitates Growth of M. smegmatis on Different Monosaccharides
3.5. TiME Is Required for Transportation of Amino Acids by M. smegmatis
3.6. TiME Aids the Resistance of M. smegmatis against Anti-TB Drugs
3.7. TiME Is Essential for Efficient Growth by M. smegmatis at Acidic pH
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Daniel, T.M. The history of tuberculosis. Respir. Med. 2006, 100, 1862–1870. [Google Scholar] [CrossRef]
- Pai, M.; Behr, M.A.; Dowdy, D.; Dheda, K.; Divangahi, M.; Boehme, C.C.; Ginsberg, A.; Swaminathan, S.; Spigelman, M.; Getahun, H.; et al. Tuberculosis. Nat. Rev. Dis. Primers 2016, 2, 16076. [Google Scholar] [CrossRef] [PubMed]
- Wulandari, D.A.; Hartati, Y.W.; Ibrahim, A.U.; Pitaloka, D.A.E.; Irkham. Multidrug-resistant tuberculosis. Clin. Chim. Acta 2024, 559, 119701. [Google Scholar] [CrossRef]
- Farhat, M.; Cox, H.; Ghanem, M.; Denkinger, C.M.; Rodrigues, C.; Abd El Aziz, M.S.; Enkh-Amgalan, H.; Vambe, D.; Ugarte-Gil, C.; Furin, J.; et al. Drug-resistant tuberculosis: A persistent global health concern. Nat. Rev. Microbiol. 2024. [Google Scholar] [CrossRef]
- Daffé, M. The cell envelope of tubercle bacilli. Tuberculosis 2015, 95 (Suppl. S1), S155–S158. [Google Scholar] [CrossRef]
- Mittal, E.; Roth, A.T.; Seth, A.; Singamaneni, S.; Beatty, W.; Philips, J.A. Single cell preparations of Mycobacterium tuberculosis damage the mycobacterial envelope and disrupt macrophage interactions. eLife 2023, 12, e85416. [Google Scholar] [CrossRef]
- Zhai, W.; Wu, F.; Zhang, Y.; Fu, Y.; Liu, Z. The Immune Escape Mechanisms of Mycobacterium Tuberculosis. Int. J. Mol. Sci. 2019, 20, 340. [Google Scholar] [CrossRef] [PubMed]
- Holzheimer, M.; Buter, J.; Minnaard, A.J. Chemical Synthesis of Cell Wall Constituents of Mycobacterium tuberculosis. Chem. Rev. 2021, 121, 9554–9643. [Google Scholar] [CrossRef]
- David, E.M.; Oona, Y.C.L.; Houdini, H.T.W.; Vijayashankar, N.; Helen, D.D.; Malin, R.; Motoko, W.; Luke, A.; Apoorva, B.; Gurdyal, S.B. Pathophysiological Implications of Cell Envelope Structure in Mycobacterium tuberculosis and Related Taxa. In Tuberculosis; Wellman, R., Ed.; In Tech Open Access Publisher: London, UK, 2015; pp. 145–175. [Google Scholar]
- Kalscheuer, R.; Palacios, A.; Anso, I.; Cifuente, J.; Anguita, J.; Jacobs, W.R., Jr.; Guerin, M.E.; Prados-Rosales, R. The Mycobacterium tuberculosis capsule: A cell structure with key implications in pathogenesis. Biochem. J. 2019, 476, 1995–2016. [Google Scholar] [CrossRef]
- Sani, M.; Houben, E.N.; Geurtsen, J.; Pierson, J.; de Punder, K.; van Zon, M.; Wever, B.; Piersma, S.R.; Jiménez, C.R.; Daffé, M.; et al. Direct visualization by cryo-EM of the mycobacterial capsular layer: A labile structure containing ESX-1-secreted proteins. PLoS Pathog. 2010, 6, e1000794. [Google Scholar] [CrossRef]
- Jackson, M.; Stevens, C.M.; Zhang, L.; Zgurskaya, H.I.; Niederweis, M. Transporters Involved in the Biogenesis and Functionalization of the Mycobacterial Cell Envelope. Chem. Rev. 2021, 121, 5124–5157. [Google Scholar] [CrossRef]
- Faller, M.; Niederweis, M.; Schulz, G.E. The structure of a mycobacterial outer-membrane channel. Science 2004, 303, 1189–1192. [Google Scholar] [CrossRef] [PubMed]
- Cai, X.; Liu, L.; Qiu, C.; Wen, C.; He, Y.; Cui, Y.; Li, S.; Zhang, X.; Zhang, L.; Tian, C.; et al. Identification and architecture of a putative secretion tube across mycobacterial outer envelope. Sci. Adv. 2021, 7, eabg5656. [Google Scholar] [CrossRef] [PubMed]
- Sparks, I.L.; Derbyshire, K.M.; Jacobs, W.R., Jr.; Morita, Y.S. Mycobacterium smegmatis: The Vanguard of Mycobacterial Research. J. Bacteriol. 2023, 205, e0033722. [Google Scholar] [CrossRef] [PubMed]
- Bendre, A.D.; Peters, P.J.; Kumar, J. Recent Insights into the Structure and Function of Mycobacterial Membrane Proteins Facilitated by Cryo-EM. J. Membr. Biol. 2021, 254, 321–341. [Google Scholar] [CrossRef] [PubMed]
- Mailaender, C.; Reiling, N.; Engelhardt, H.; Bossmann, S.; Ehlers, S.; Niederweis, M. The MspA porin promotes growth and increases antibiotic susceptibility of both Mycobacterium bovis BCG and Mycobacterium tuberculosis. Microbiology 2004, 150, 853–864. [Google Scholar] [CrossRef]
- Stahl, C.; Kubetzko, S.; Kaps, I.; Seeber, S.; Engelhardt, H.; Niederweis, M. MspA provides the main hydrophilic pathway through the cell wall of Mycobacterium smegmatis. Mol. Microbiol. 2001, 40, 451–464. [Google Scholar] [CrossRef]
- Dechow Shelby, J.; Baker Jacob, J.; Murto, M.; Abramovitch Robert, B. ppe51 Variants Enable Growth of Mycobacterium tuberculosis at Acidic pH by Selectively Promoting Glycerol Uptake. J. Bacteriol. 2022, 204, e00212–e00222. [Google Scholar] [CrossRef]
- Wang, Q.; Boshoff, H.I.M.; Harrison, J.R.; Ray, P.C.; Green, S.R.; Wyatt, P.G.; Barry, C.E., 3rd. PE/PPE proteins mediate nutrient transport across the outer membrane of Mycobacterium tuberculosis. Science 2020, 367, 1147–1151. [Google Scholar] [CrossRef]
- Mitra, A.; Speer, A.; Lin, K.; Ehrt, S.; Niederweis, M. PPE Surface Proteins Are Required for Heme Utilization by Mycobacterium tuberculosis. mBio 2017, 8, e01720-16. [Google Scholar] [CrossRef]
- Korycka-Machała, M.; Pawełczyk, J.; Borówka, P.; Dziadek, B.; Brzostek, A.; Kawka, M.; Bekier, A.; Rykowski, S.; Olejniczak, A.B.; Strapagiel, D.; et al. PPE51 Is Involved in the Uptake of Disaccharides by Mycobacterium tuberculosis. Cells 2020, 9, 603. [Google Scholar] [CrossRef] [PubMed]
- Tak, U.; Dokland, T.; Niederweis, M. Pore-forming Esx proteins mediate toxin secretion by Mycobacterium tuberculosis. Nat. Commun. 2021, 12, 394. [Google Scholar] [CrossRef] [PubMed]
- Sankey, N.; Merrick, H.; Singh, P.; Rogers, J.; Reddi, A.; Hartson, S.D.; Mitra, A. Role of the Mycobacterium tuberculosis ESX-4 Secretion System in Heme Iron Utilization and Pore Formation by PPE Proteins. mSphere 2023, 8, e0057322. [Google Scholar] [CrossRef]
- Ehtram, A.; Shariq, M.; Ali, S.; Quadir, N.; Sheikh, J.A.; Ahmad, F.; Sharma, T.; Ehtesham, N.Z.; Hasnain, S.E. Teleological cooption of Mycobacterium tuberculosis PE/PPE proteins as porins: Role in molecular immigration and emigration. Int. J. Med. Microbiol. 2021, 311, 151495. [Google Scholar] [CrossRef]
- Rodríguez, D.C.; Ocampo, M.; Reyes, C.; Arévalo-Pinzón, G.; Munoz, M.; Patarroyo, M.A.; Patarroyo, M.E. Cell-Peptide Specific Interaction Can Inhibit Mycobacterium tuberculosis H37Rv Infection. J. Cell Biochem. 2016, 117, 946–958. [Google Scholar] [CrossRef]
- Jaisinghani, N.; Previti, M.L.; Andrade, J.; Askenazi, M.; Ueberheide, B.; Seeliger, J.C. Proteomics from compartment-specific APEX2 labeling in Mycobacterium tuberculosis reveals Type VII secretion substrates in the cell wall. Cell Chem. Biol. 2023, 31, 523–533.e4. [Google Scholar] [CrossRef] [PubMed]
- Mao, X.J.; Yan, M.Y.; Zhu, H.; Guo, X.P.; Sun, Y.C. Efficient and simple generation of multiple unmarked gene deletions in Mycobacterium smegmatis. Sci. Rep. 2016, 6, 22922. [Google Scholar] [CrossRef]
- Cascioferro, A.; Boldrin, F.; Serafini, A.; Provvedi, R.; Palù, G.; Manganelli, R. Xer site-specific recombination, an efficient tool to introduce unmarked deletions into mycobacteria. Appl. Environ. Microbiol. 2010, 76, 5312–5316. [Google Scholar] [CrossRef]
- Gouzy, A.; Healy, C.; Black, K.A.; Rhee, K.Y.; Ehrt, S. Growth of Mycobacterium tuberculosis at acidic pH depends on lipid assimilation and is accompanied by reduced GAPDH activity. Proc. Natl. Acad. Sci. USA 2021, 118, e2024571118. [Google Scholar] [CrossRef]
- Lee, W.; VanderVen, B.C.; Fahey, R.J.; Russell, D.G. Intracellular Mycobacterium tuberculosis exploits host-derived fatty acids to limit metabolic stress. J. Biol. Chem. 2013, 288, 6788–6800. [Google Scholar] [CrossRef]
- Bachmann, N.L.; Salamzade, R.; Manson, A.L.; Whittington, R.; Sintchenko, V.; Earl, A.M.; Marais, B.J. Key Transitions in the Evolution of Rapid and Slow Growing Mycobacteria Identified by Comparative Genomics. Front. Microbiol. 2019, 10, 3019. [Google Scholar] [CrossRef] [PubMed]
- Stephan, J.; Bender, J.; Wolschendorf, F.; Hoffmann, C.; Roth, E.; Mailänder, C.; Engelhardt, H.; Niederweis, M. The growth rate of Mycobacterium smegmatis depends on sufficient porin-mediated influx of nutrients. Mol. Microbiol. 2005, 58, 714–730. [Google Scholar] [CrossRef]
- Danilchanka, O.; Sun, J.; Pavlenok, M.; Maueröder, C.; Speer, A.; Siroy, A.; Marrero, J.; Trujillo, C.; Mayhew, D.L.; Doornbos, K.S.; et al. An outer membrane channel protein of Mycobacterium tuberculosis with exotoxin activity. Proc. Natl. Acad. Sci. USA 2014, 111, 6750–6755. [Google Scholar] [CrossRef]
- Speer, A.; Sun, J.; Danilchanka, O.; Meikle, V.; Rowland, J.L.; Walter, K.; Buck, B.R.; Pavlenok, M.; Hölscher, C.; Ehrt, S.; et al. Surface hydrolysis of sphingomyelin by the outer membrane protein Rv0888 supports replication of Mycobacterium tuberculosis in macrophages. Mol. Microbiol. 2015, 97, 881–897. [Google Scholar] [CrossRef] [PubMed]
- Lamrabet, O.; Ghigo, E.; Mège, J.L.; Lepidi, H.; Nappez, C.; Raoult, D.; Drancourt, M. MspA-Mycobacterium tuberculosis-transformant with reduced virulence: The “unbirthday paradigm”. Microb. Pathog. 2014, 76, 10–18. [Google Scholar] [CrossRef]
- Chinison, J.J.; Danelishvili, L.; Gupta, R.; Rose, S.J.; Babrak, L.M.; Bermudez, L.E. Identification of Mycobacterium avium subsp. hominissuis secreted proteins using an in vitro system mimicking the phagosomal environment. BMC Microbiol. 2016, 16, 270. [Google Scholar] [CrossRef] [PubMed]
- Kruse, E.; Uehlein, N.; Kaldenhoff, R. The aquaporins. Genome Biol. 2006, 7, 206. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Pan, T.; Wang, K.; Wang, Y.; Yan, S.; Wang, L.; Zhang, S.; Du, X.; Jia, W.; Zhang, P.; et al. Allosteric Switching of Calmodulin in a Mycobacterium smegmatis porin A (MspA) Nanopore-Trap. Angew. Chem. Int. Edit. 2021, 60, 23863–23870. [Google Scholar] [CrossRef]
- Shorkey, S.A.; Du, J.; Pham, R.; Strieter, E.R.; Chen, M. Real-Time and Label-Free Measurement of Deubiquitinase Activity with a MspA Nanopore. Chembiochem 2021, 22, 2688–2692. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, K.; Wang, Y.; Wang, L.; Yan, S.; Du, X.; Zhang, P.; Chen, H.Y.; Huang, S. Machine Learning Assisted Simultaneous Structural Profiling of Differently Charged Proteins in a Mycobacterium smegmatis Porin A (MspA) Electroosmotic Trap. J. Am. Chem. Soc. 2022, 144, 757–768. [Google Scholar] [CrossRef]
- Mohan, A.; Padiadpu, J.; Baloni, P.; Chandra, N. Complete Genome Sequences of a Mycobacterium smegmatis Laboratory Strain (MC2 155) and Isoniazid-Resistant (4XR1/R2) Mutant Strains. Genome Announc. 2015, 3, e01520-14. [Google Scholar] [CrossRef] [PubMed]
- Rieck, B.; Degiacomi, G.; Zimmermann, M.; Cascioferro, A.; Boldrin, F.; Lazar-Adler, N.R.; Bottrill, A.R.; le Chevalier, F.; Frigui, W.; Bellinzoni, M.; et al. PknG senses amino acid availability to control metabolism and virulence of Mycobacterium tuberculosis. PLOS Pathog. 2017, 13, e1006399. [Google Scholar] [CrossRef] [PubMed]
- Baker, J.J.; Johnson, B.K.; Abramovitch, R.B. Slow growth of Mycobacterium tuberculosis at acidic pH is regulated by phoPR and host-associated carbon sources. Mol. Microbiol. 2014, 94, 56–69. [Google Scholar] [CrossRef] [PubMed]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, L.; Wen, C.; Cai, X.; Gong, W. A Novel Bi-Directional Channel for Nutrient Uptake across Mycobacterial Outer Envelope. Microorganisms 2024, 12, 1827. https://doi.org/10.3390/microorganisms12091827
Liu L, Wen C, Cai X, Gong W. A Novel Bi-Directional Channel for Nutrient Uptake across Mycobacterial Outer Envelope. Microorganisms. 2024; 12(9):1827. https://doi.org/10.3390/microorganisms12091827
Chicago/Turabian StyleLiu, Lei, Chongzheng Wen, Xiaoying Cai, and Weimin Gong. 2024. "A Novel Bi-Directional Channel for Nutrient Uptake across Mycobacterial Outer Envelope" Microorganisms 12, no. 9: 1827. https://doi.org/10.3390/microorganisms12091827
APA StyleLiu, L., Wen, C., Cai, X., & Gong, W. (2024). A Novel Bi-Directional Channel for Nutrient Uptake across Mycobacterial Outer Envelope. Microorganisms, 12(9), 1827. https://doi.org/10.3390/microorganisms12091827





