Water Management-Mediated Changes in the Rhizosphere and Bulk Soil Microbial Communities Alter Their Utilization of Urea-Derived Carbon
Abstract
:1. Introduction
2. Materials and Methods
2.1. Soil Sampling
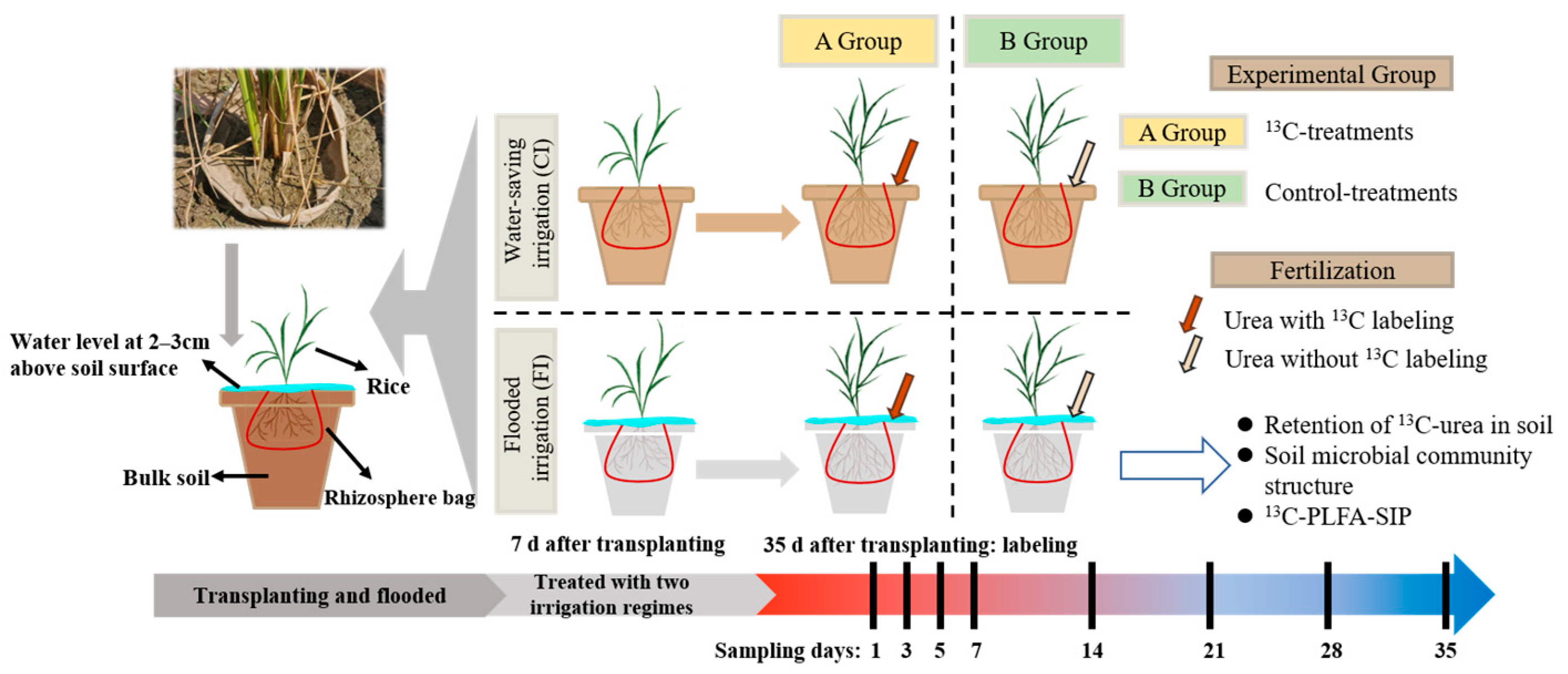
2.2. Experimental Design and Sampling
2.3. Soil PLFA Extraction and Analysis
2.4. 13C in Plant, Soil and PLFA
2.5. Statistical Analysis
3. Results
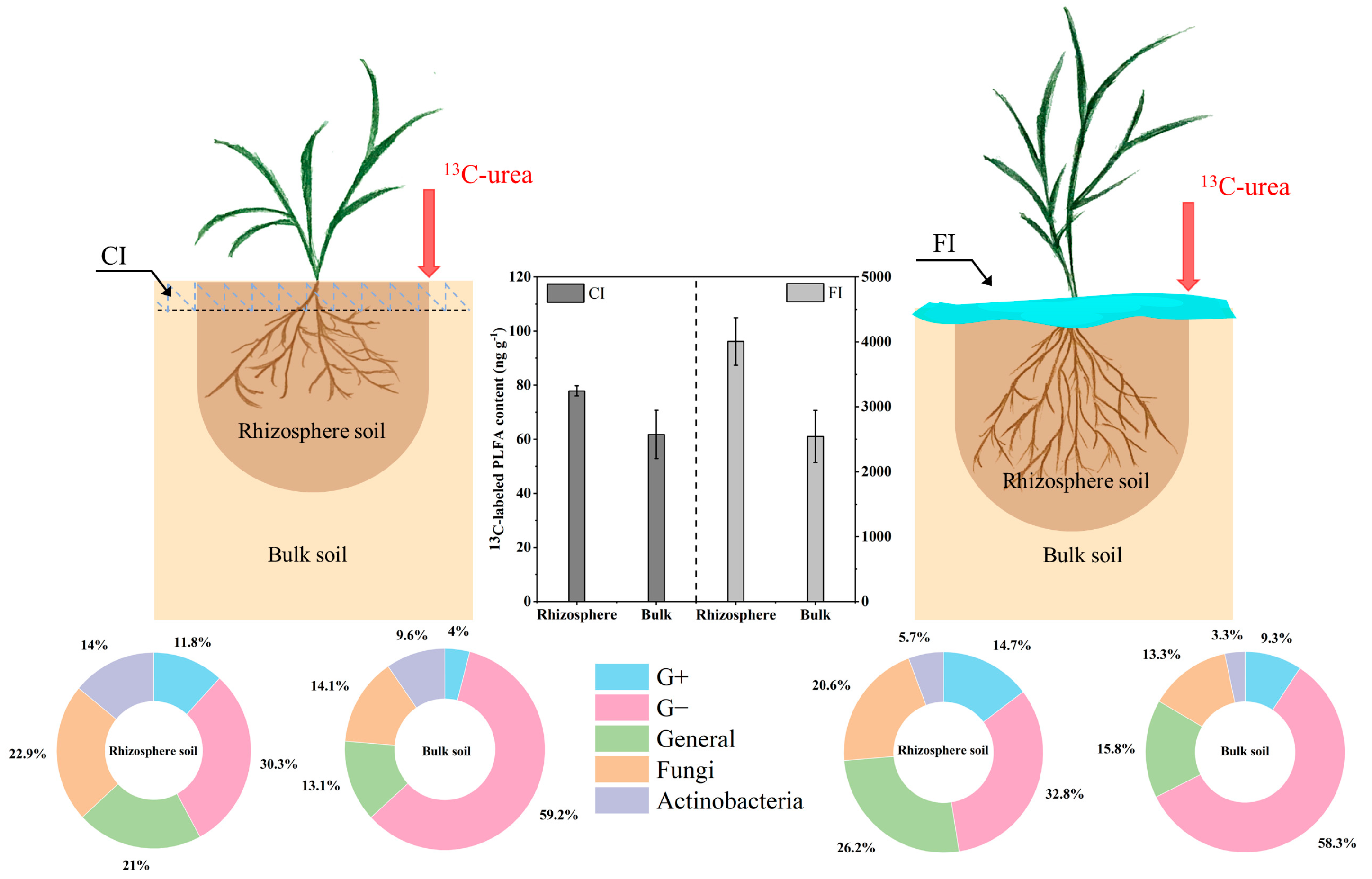
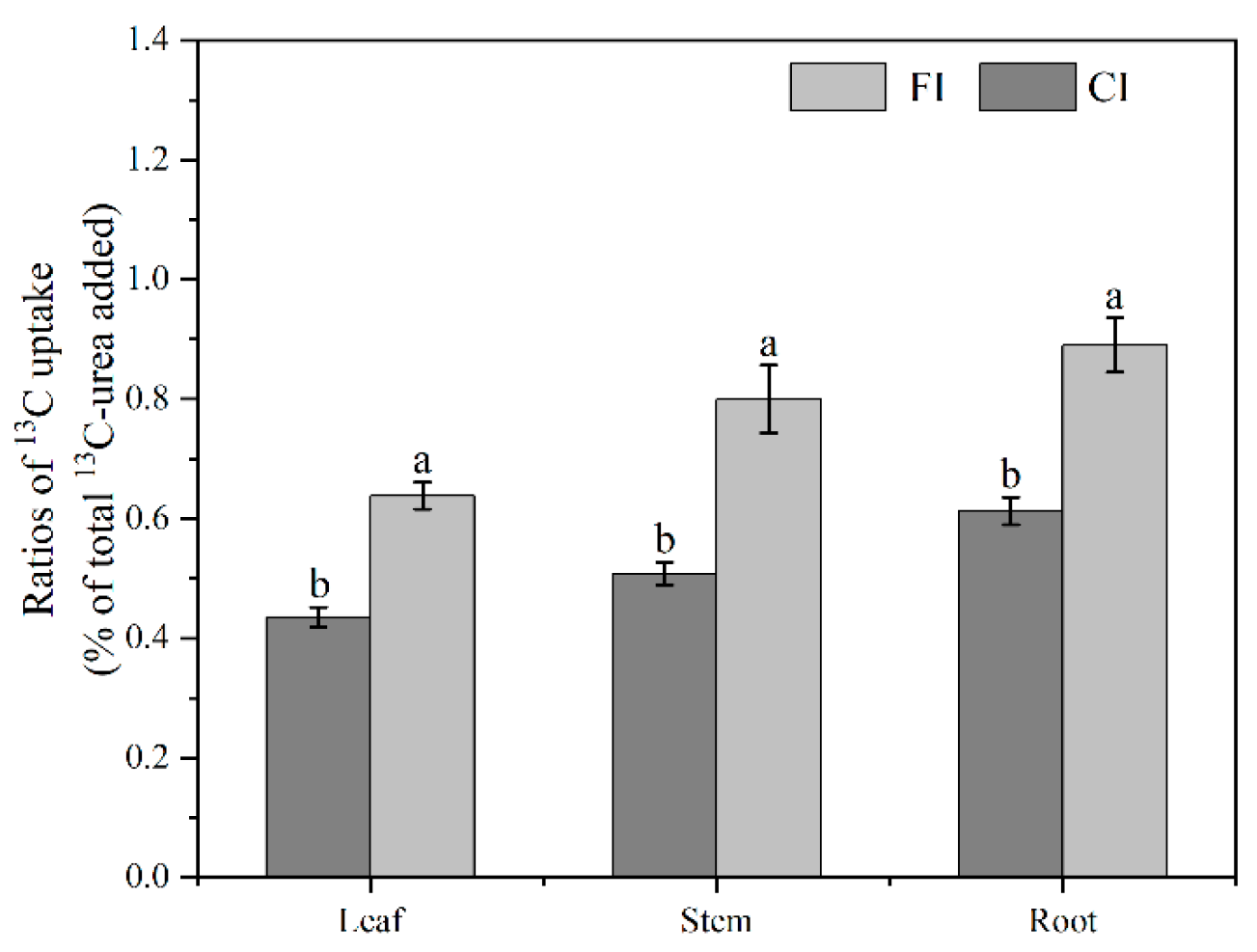
3.1. 13C-Urea Remained in Soil
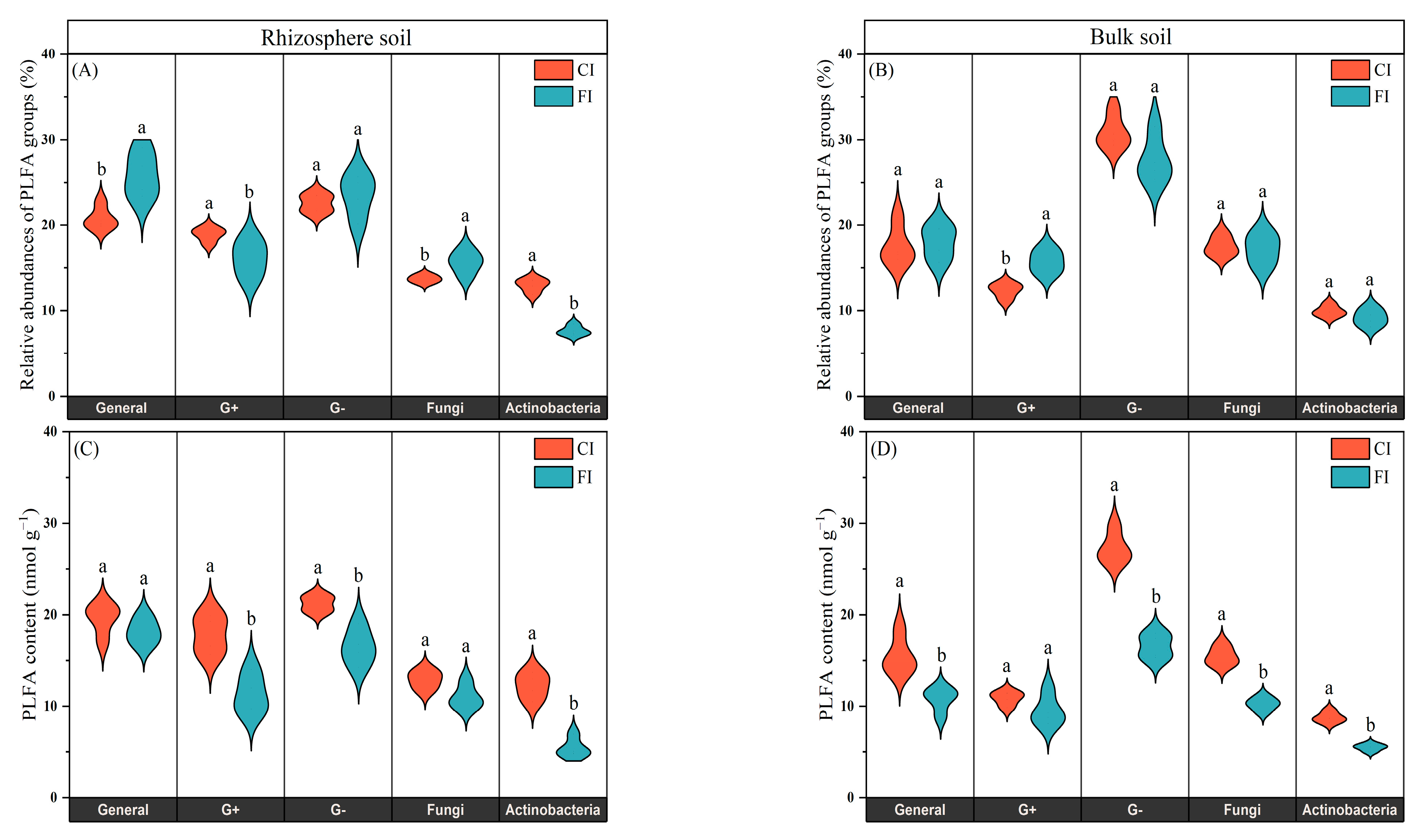
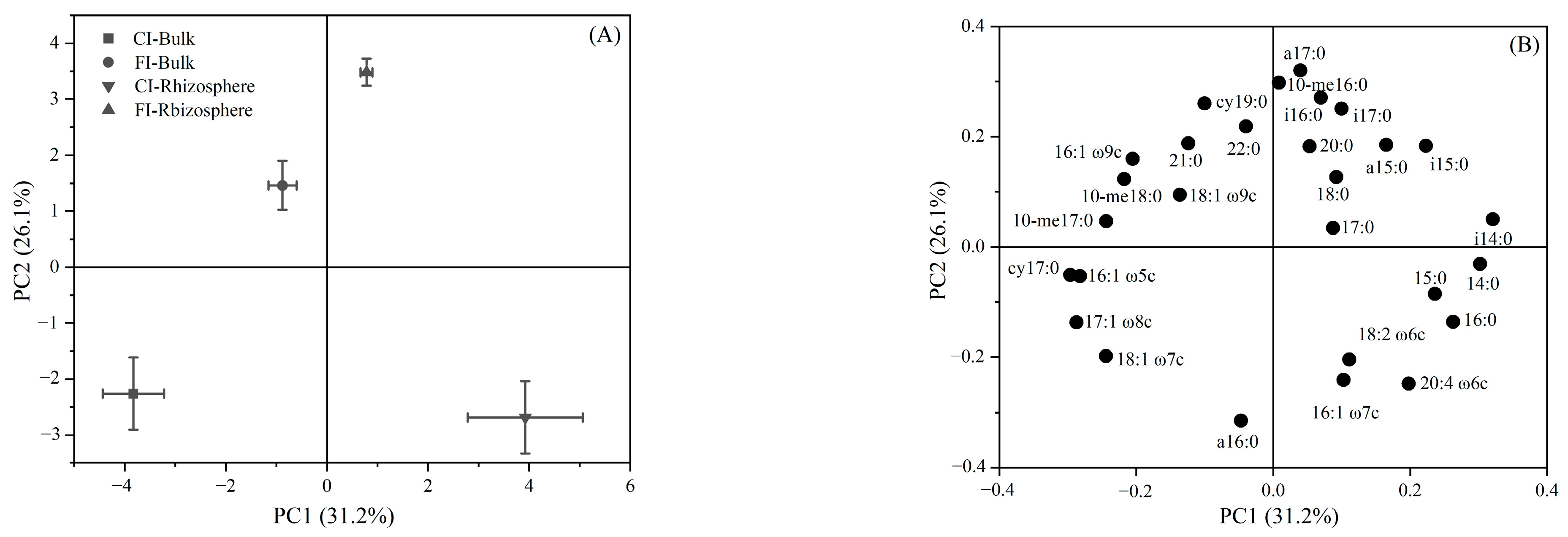
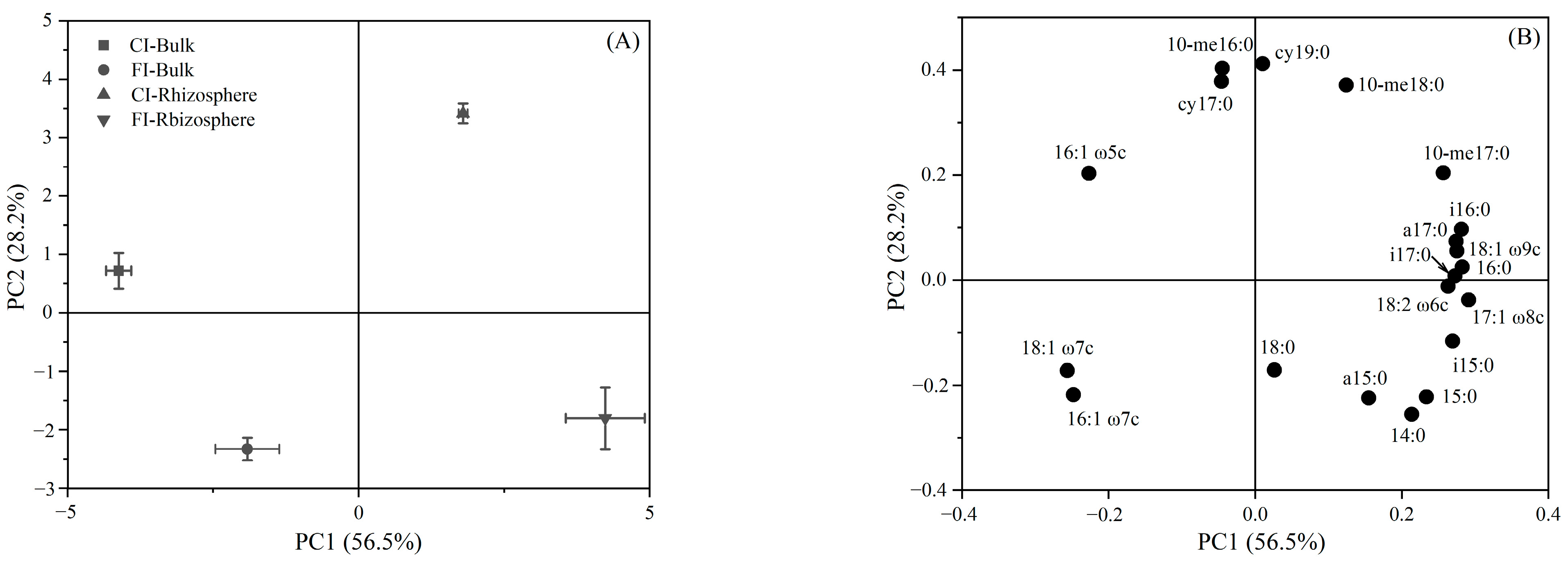
3.2. Soil Microbial Community Structure
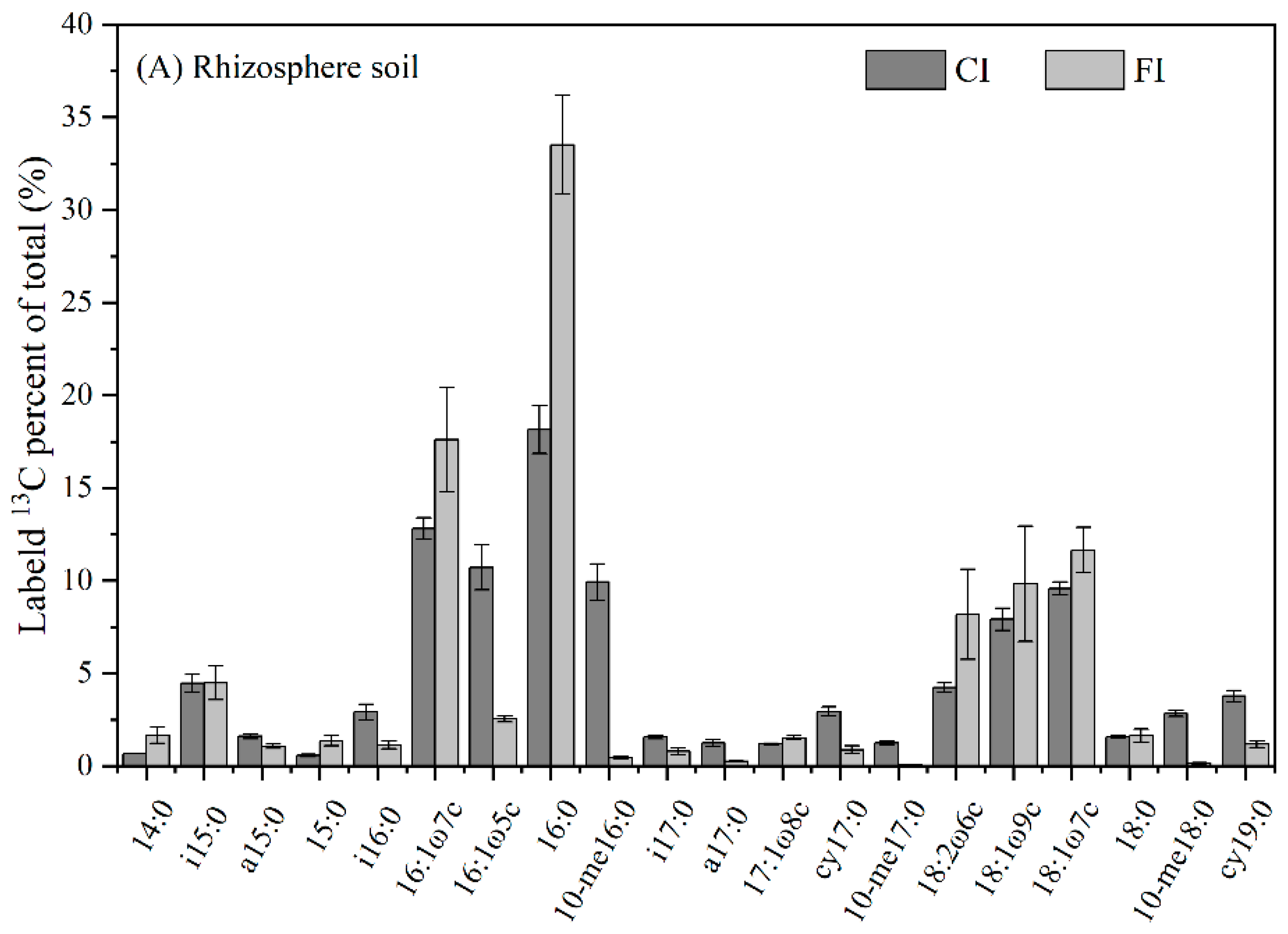
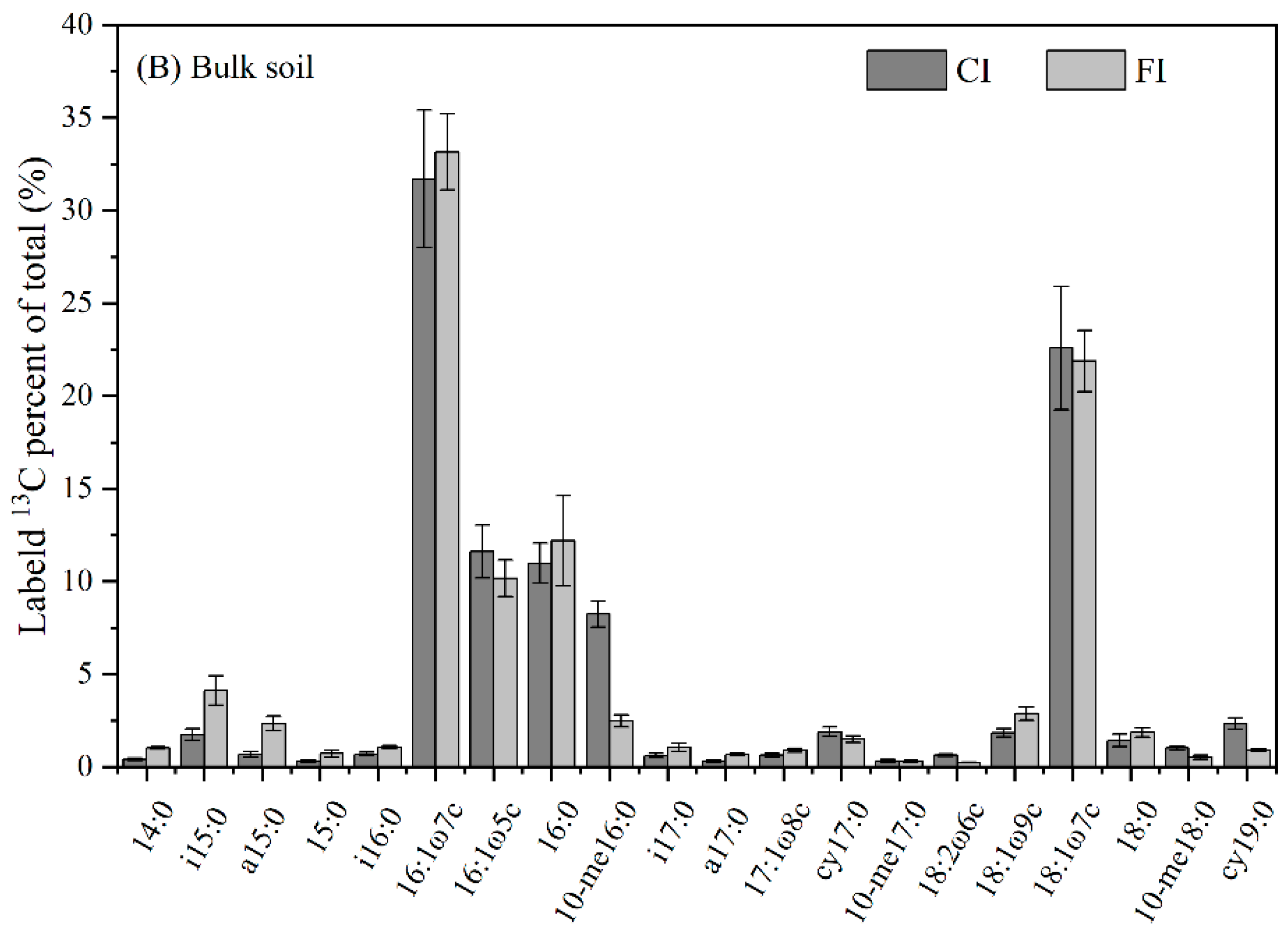
3.3. 13C-PLFA-SIP
4. Discussion
4.1. The Retention of Urea-C
4.2. Effect of Irrigation Regimes on Bulk and Rhizosphere Soil Microbial Community
4.3. Incorporation of Urea-C into Bulk and Rhizosphere Soil Microbial Groups under Different Irrigation Regimes
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Erisman, J.W.; Sutton, M.A.; Galloway, J.; Klimont, Z.; Winiwarter, W. How a century of ammonia synthesis changed the world. Nat. Geosci. 2008, 1, 636–639. [Google Scholar] [CrossRef]
- Heffer, P.; Prud’homme, M. Fertilizer Outlook 2014–2018; International Fertilizer Industry Association (IFA): Paris, France, 2014. [Google Scholar]
- Homme, P. Short-Term Fertilizer Outlook 2016–2017; International Fertilizer Industry Association (IFA): Paris, France, 2016. [Google Scholar]
- Chen, P.; Nie, T.; Chen, S.; Zhang, Z.; Qi, Z.; Liu, W. Recovery efficiency and loss of 15N-labelled urea in a rice-soil system under water saving irrigation in the Songnen Plain of Northeast China. Agric. Water Manag. 2019, 222, 139–153. [Google Scholar] [CrossRef]
- Dong, N.M.; Brandt, K.K.; Sørensen, J.; Hung, N.N.; Hach, C.V.; Tan, P.S.; Dalsgaard, T. Effects of alternating wetting and drying versus continuous flooding on fertilizer nitrogen fate in rice fields in the Mekong Delta, Vietnam. Soil Biol. Biochem. 2012, 47, 166–174. [Google Scholar] [CrossRef]
- Quan, Z.; Li, S.; Zhang, X.; Zhu, F.; Li, P.; Sheng, R.; Chen, X.; Zhang, L.-M.; He, J.-Z.; Wei, W.; et al. Fertilizer nitrogen use efficiency and fates in maize cropping systems across China: Field 15N tracer studies. Soil Tillage Res. 2020, 197, 104498. [Google Scholar] [CrossRef]
- Zhou, S.; Sun, H.; Bi, J.; Zhang, J.; Riya, S.; Hosomi, M. Effect of water-saving irrigation on the N2O dynamics and the contribution of exogenous and endogenous nitrogen to N2O production in paddy soil using 15N tracing. Soil Tillage Res. 2020, 200, 104610. [Google Scholar] [CrossRef]
- Wei, Q.; Xu, J.Z.; Liu, Y.Z.; Wang, D.; Chen, S.Y.; Qian, W.H.; He, M.; Chen, P.; Zhou, X.Y.; Qi, Z.M. Nitrogen losses from soil as affected by water and fertilizer management under drip irrigation: Development, hotspots and future per-spectives. Agric. Water Manag. 2024, 296, 108791. [Google Scholar] [CrossRef]
- Marsh, K.L.; Sims, G.K.; Mulvaney, R.L. Availability of urea to autotrophic ammonia-oxidizing bacteria as related to the fate of 14C- and 15N-labeled urea added to soil. Biol. Fertil. Soils 2005, 42, 137. [Google Scholar] [CrossRef]
- Liao, H.; Hao, X.; Qin, F.; Delgado-Baquerizo, M.; Liu, Y.; Zhou, J.; Cai, P.; Chen, W.; Huang, Q. Microbial autotrophy explains large-scale soil CO2 fixation. Glob. Chang. Biol. 2023, 29, 231–242. [Google Scholar] [CrossRef]
- Zheng, Z.; Liu, B.; Fang, X.; Fa, K.; Liu, Z. Dryland farm soil may fix atmospheric carbon through autotrophic microbial pathways. Catena 2022, 214, 106299. [Google Scholar] [CrossRef]
- Wei, L.; Ge, T.; Zhu, Z.; Ye, R.; Peñuelas, J.; Li, Y.; Lynn, T.M.; Jones, D.L.; Wu, J.; Kuzyakov, Y. Paddy soils have a much higher microbial biomass content than upland soils: A review of the origin, mechanisms, and drivers. Agric. Ecosyst. Environ. 2022, 326, 107798. [Google Scholar] [CrossRef]
- Chen, X.; Hu, Y.; Xia, Y.; Zheng, S.; Ma, C.; Rui, Y.; He, H.; Huang, D.; Zhang, Z.; Ge, T.; et al. Contrasting pathways of carbon sequestration in paddy and upland soils. Glob. Chang. Biol. 2021, 27, 2478–2490. [Google Scholar] [CrossRef]
- Ishfaq, M.; Farooq, M.; Zulfiqar, U.; Hussain, S.; Akbar, N.; Nawaz, A.; Anjum, S.A. Alternate wetting and drying: A water-saving and ecofriendly rice production system. Agric. Water Manag. 2020, 241, 106363. [Google Scholar] [CrossRef]
- Zhuang, Y.; Zhang, L.; Li, S.; Liu, H.; Zhai, L.; Zhou, F.; Ye, Y.; Ruan, S.; Wen, W. Effects and potential of water-saving irrigation for rice production in China. Agric. Water Manag. 2019, 217, 374–382. [Google Scholar] [CrossRef]
- Chen, M.M.; Zhu, Y.G.; Su, Y.H.; Chen, B.D.; Fu, B.J.; Marschner, P. Effects of soil moisture and plant interactions on the soil microbial community structure. Eur. J. Soil Biol. 2007, 43, 31–38. [Google Scholar] [CrossRef]
- Drenovsky, R.E.; Vo, D.; Graham, K.J.; Scow, K.M. Soil Water Content and Organic Carbon Availability Are Major Determinants of Soil Microbial Community Composition. Microb. Ecol. 2004, 48, 424–430. [Google Scholar] [CrossRef] [PubMed]
- Atere, C.T.; Ge, T.; Zhu, Z.; Tong, C.; Jones, D.L.; Shibistova, O.; Guggenberger, G.; Wu, J. Rice rhizodeposition and carbon stabilisation in paddy soil are regulated via drying-rewetting cycles and nitrogen fertilisation. Biol. Fertil. Soils 2017, 53, 407–417. [Google Scholar] [CrossRef]
- Tian, J.; Pausch, J.; Fan, M.; Li, X.; Tang, Q.; Kuzyakov, Y. Allocation and dynamics of assimilated carbon in rice-soil system depending on water management. Plant Soil 2013, 363, 273–285. [Google Scholar] [CrossRef]
- Long, X.-E.; Yao, H.; Wang, J.; Huang, Y.; Singh, B.K.; Zhu, Y.-G. Community Structure and Soil pH Determine Chemoautotrophic Carbon Dioxide Fixation in Drained Paddy Soils. Environ. Sci. Technol. 2015, 49, 7152–7160. [Google Scholar] [CrossRef] [PubMed]
- Tian, J.; Dippold, M.; Pausch, J.; Blagodatskaya, E.; Fan, M.; Li, X.; Kuzyakov, Y. Microbial response to rhizodeposition depending on water regimes in paddy soils. Soil Biol. Biochem. 2013, 65, 195–203. [Google Scholar] [CrossRef]
- Yao, H.; Thornton, B.; Paterson, E. Incorporation of 13C-labelled rice rhizodeposition carbon into soil microbial communities under different water status. Soil Biol. Biochem. 2012, 53, 72–77. [Google Scholar] [CrossRef]
- Thakur, A.K.; Rath, S.; Patil, D.U.; Kumar, A. Effects on rice plant morphology and physiology of water and associated management practices of the system of rice intensification and their implications for crop performance. Paddy Water Environ. 2011, 9, 13–24. [Google Scholar] [CrossRef]
- Finzi, A.C.; Abramoff, R.Z.; Spiller, K.S.; Brzostek, E.R.; Darby, B.A.; Kramer, M.A.; Phillips, R.P. Rhizosphere processes are quantitatively important components of terrestrial carbon and nutrient cycles. Glob. Chang. Biol. 2015, 21. [Google Scholar] [CrossRef]
- Singh, B.K.; Millard, P.; Whiteley, A.S.; Murrell, J.C. Unravelling rhizosphere-microbial interactions: Opportunities and limitations. Trends Microbiol. 2004, 12, 386–393. [Google Scholar] [CrossRef]
- Wei, X.; Zhu, Z.; Wei, L.; Wu, J.; Ge, T. Biogeochemical cycles of key elements in the paddy-rice rhizosphere: Microbial mechanisms and coupling processes. Rhizosphere 2019, 10, 100145. [Google Scholar] [CrossRef]
- Xu, J.Z.; Peng, S.Z.; Yang, S.H.; Wang, W.G. Ammonia volatilization losses from a rice paddy with different irrigation and nitrogen managements. Agric. Water Manag. 2012, 104, 184–192. [Google Scholar] [CrossRef]
- Nie, T.; Chen, P.; Zhang, Z.; Qi, Z.; Lin, Y.; Xu, D. Effects of Different Types of Water and Nitrogen Fertilizer Management on Greenhouse Gas Emissions, Yield, and Water Consumption of Paddy Fields in Cold Region of China. Int. J. Environ. Res. Public Health 2019, 16, 1639. [Google Scholar] [CrossRef]
- Butler, J.L.; Williams, M.A.; Bottomley, P.J.; Myrold, D.D. Microbial Community Dynamics Associated with Rhizosphere Carbon Flow. Appl. Environ. Microbiol. 2003, 69, 6793–6800. [Google Scholar] [CrossRef]
- Liua, Y.; Geb, T.; Yec, J.; Liub, S.; Shibistovad, O. Initial utilization of rhizodeposits with rice growth in paddy soils: Rhizosphere and N fertilization effects. Geoderma 2019, 338, 30–39. [Google Scholar] [CrossRef]
- Frostegård, Å.; Tunlid, A.; Bååth, E. Microbial biomass measured as total lipid phosphate in soils of different organic content. J. Microbiol. Methods 1991, 14, 151–163. [Google Scholar] [CrossRef]
- White, D.C.; Davis, W.M.; Nickels, J.S.; King, J.D.; Bobbie, R.J. Determination of the sedimentary microbial biomass by extractible lipid phosphate. Oecologia 1979, 40, 51–62. [Google Scholar] [CrossRef]
- Jin, V.L.; Evans, R.D. Microbial 13C utilization patterns via stable isotope probing of phospholipid biomarkers in Mojave Desert soils exposed to ambient and elevated atmospheric CO2. Glob. Chang. Biol. 2010, 16, 2334–2344. [Google Scholar] [CrossRef]
- Zheng, T.; Miltner, A.; Liang, C.; Nowak, K.M.; Kästner, M. Turnover of gram-negative bacterial biomass-derived carbon through the microbial food web of an agricultural soil. Soil Biol. Biochem. 2021, 152, 108070. [Google Scholar] [CrossRef]
- Dong, W.Y.; Zhang, X.Y.; Dai, X.Q.; Fu, X.L.; Yang, F.T.; Liu, X.Y.; Sun, X.M.; Wen, X.F.; Schaeffer, S. Changes in soil microbial community composition in response to fertilization of paddy soils in subtropical China. Appl. Soil Ecol. 2014, 84, 140–147. [Google Scholar] [CrossRef]
- Pan, F.; Li, Y.; Chapman, S.J.; Khan, S.; Yao, H. Microbial utilization of rice straw and its derived biochar in a paddy soil. Sci. Total Environ. 2016, 559, 15–23. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.-J.; Chen, X.; Shamsi, I.H.; Fang, P.; Lin, X.-Y. Effects of Irrigation Patterns and Nitrogen Fertilization on Rice Yield and Microbial Community Structure in Paddy Soil. Pedosphere 2012, 22, 661–672. [Google Scholar] [CrossRef]
- Robie, J.V.; White, D.C. Lipid analysis in microbial ecology-quantitative approaches to the study of microbial communities. Bioscience 1989, 39, 535–541. [Google Scholar] [CrossRef]
- Frostegård, Å.; Bååth, E.; Tunlio, A. Shifts in the structure of soil microbial communities in limed forests as revealed by phospholipid fatty acid analysis. Soil Biol. Biochem. 1993, 25, 723–730. [Google Scholar] [CrossRef]
- Olsson, P.A. Signature fatty acids provide tools for determination of the distribution and interactions of mycorrhizal fungi in soil. FEMS Microbiol. Ecol. 1999, 29, 303–310. [Google Scholar] [CrossRef]
- Heděnec, P.; Nilsson, L.O.; Zheng, H.; Gundersen, P.; Vesterdal, L. Mycorrhizal association of common European tree species shapes biomass and growth of bacterial and fungal communities in soil. Soil Biol. Biochem. 2020, 149, 107933. [Google Scholar] [CrossRef]
- Wang, J.; Thornton, B.; Yao, H. Incorporation of urea-derived 13C into microbial communities in four different agriculture soils. Biol. Fertil. Soils 2014, 50, 603–612. [Google Scholar] [CrossRef]
- Dungait, J.A.J.; Kemmitt, S.J.; Michallon, L.; Guo, S.; Wen, Q.; Brookes, P.C.; Evershed, R.P. Variable responses of the soil microbial biomass to trace concentrations of 13C-labelled glucose, using 13C-PLFA analysis. Eur. J. Soil Sci. 2011, 62, 117–126. [Google Scholar] [CrossRef]
- Tavi, N.M.; Martikainen, P.J.; Lokko, K.; Kontro, M.; Wild, B.; Richter, A.; Biasi, C. Linking microbial community structure and allocation of plant-derived carbon in an organic agricultural soil using 13CO2 pulse-chase labelling combined with 13C-PLFA profiling. Soil Biol. Biochem. 2013, 58, 207–215. [Google Scholar] [CrossRef]
- Bagheri Novair, S.; Mirseyed Hosseini, H.; Etesami, H.; Razavipour, T. Rice straw and composted azolla alter carbon and nitrogen mineralization and microbial activity of a paddy soil under drying–rewetting cycles. Appl. Soil Ecol. 2020, 154, 103638. [Google Scholar] [CrossRef]
- Yang, S.; Sun, X.; Ding, J.; Jiang, Z.; Liu, X.; Xu, J. Effect of biochar addition on CO2 exchange in paddy fields under water-saving irrigation in Southeast China. J. Environ. Manag. 2020, 271, 111029. [Google Scholar] [CrossRef] [PubMed]
- Witte, C.-P. Urea metabolism in plants. Plant Sci. 2011, 180, 431–438. [Google Scholar] [CrossRef]
- Rao, S.; Wu, Y.; Wang, R. Bicarbonate stimulates non-structural carbohydrate pools of Camptotheca acuminata. Physiol. Plant. 2019, 165, 780–789. [Google Scholar] [CrossRef]
- Chen, P.; Xu, J.; Zhang, Z.; Wang, K.; Li, T.; Wei, Q.; Li, Y. Carbon pathways in aggregates and density fractions in Mollisols under water and straw management: Evidence from 13C natural abundance. Soil Biol. Biochem. 2022, 169, 108684. [Google Scholar] [CrossRef]
- Mohamed, I.; Bassouny, M.A.; Abbas, M.H.H.; Ming, Z.; Cougui, C.; Fahad, S.; Saud, S.; Khan Khattak, J.Z.; Ali, S.; Salem, H.M.S.; et al. Rice straw application with different water regimes stimulate enzymes activity and improve aggregates and their organic carbon contents in a paddy soil. Chemosphere 2021, 274, 129971. [Google Scholar] [CrossRef]
- Zhao, Z.; Xia, l.; Qin, Z.; Cao, J.; Omer Mohammed, A.A.; Toland, H. The environmental fate of phenanthrene in paddy field system and microbial responses in rhizosphere interface: Effect of water-saving patterns. Chemosphere 2021, 269, 128774. [Google Scholar] [CrossRef] [PubMed]
- Paterson, E.; Gebbing, T.; Abel, C.; Sim, A.; Telfer, G. Rhizodeposition shapes rhizosphere microbial community structure in organic soil. New Phytol. 2007, 173, 600–610. [Google Scholar] [CrossRef]
- Liu, Y.; Ge, T.; Zhu, Z.; Liu, S.; Luo, Y.; Li, Y.; Wang, P.; Gavrichkova, O.; Xu, X.; Wang, J. Carbon input and allocation by rice into paddy soils: A review. Soil Biol. Biochem. 2019, 133, 97–107. [Google Scholar] [CrossRef]
- Wen, T.; Yu, G.-H.; Hong, W.-D.; Yuan, J.; Niu, G.-Q.; Xie, P.-H.; Sun, F.-S.; Guo, L.-D.; Kuzyakov, Y.; Shen, Q.-R. Root exudate chemistry affects soil carbon mobilization via microbial community reassembly. Fundam. Res. 2022, 2, 697–707. [Google Scholar] [CrossRef]
- Liang, C.; Schimel, J.P.; Jastrow, J.D. The importance of anabolism in microbial control over soil carbon storage. Nat. Microbiol. 2017, 2, 17105. [Google Scholar] [CrossRef] [PubMed]
- Denef, K.; Bubenheim, H.; Lenhart, K.; Vermeulen, J.; Van Cleemput, O.; Boeckx, P.; Mueller, C. Community shifts and carbon translocation within metabolically-active rhizosphere microorganisms in grasslands under elevated CO2. Biogeosciences 2007, 4, 769–779. [Google Scholar] [CrossRef]
- Jiao, P.; Li, Z.; Yang, L.; He, J.; Chang, X.; Xiao, H.; Nie, X.; Tong, D. Bacteria are more sensitive than fungi to moisture in eroded soil by natural grass vegetation restoration on the Loess Plateau. Sci. Total Environ. 2021, 756, 143899. [Google Scholar] [CrossRef]
- Manzoni, S.; Schimel, J.P.; Porporato, A. Responses of soil microbial communities to water stress: Results from a meta-analysis. Ecology 2012, 93, 930–938. [Google Scholar] [CrossRef]
- Wang, K.; Xu, J.; Guo, H.; Min, Z.; Wei, Q.; Chen, P.; Sleutel, S. Reuse of straw in the form of hydrochar: Balancing the carbon budget and rice production under different irrigation management. Waste Manag. 2024, 189, 77–87. [Google Scholar] [CrossRef]


| Functional Group and Fatty Acid Markers | ||
|---|---|---|
| General | 14:0, 15:0, 16:0, 17:0, 18:0, 20:0, 21:0, 22:0 | [33] |
| Gram-positive bacteria (G+) | i14:0, i15:0, a15:0, i16:0, a16:0, i17:0, a17:0 | [34,35] |
| Gram-negative bacteria (G−) | 16:1ω7c, 16:1ω9c, cy17:0, 17:1ω8c, 18:1ω7c, cy19:0 | [34,36,37] |
| Actinobacteria | 10-me16:0, 10-me17:0, 10-me18:0 | [36,38] |
| Fungi | 16:1ω5c, 18:1ω9c, 18:2ω6,9c | [39,40,41] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, P.; Li, Y.; Lv, Y.; Xu, J.; Zhang, Z.; Liu, X.; Luan, Y.; Wei, Q.; Zheng, E.; Wang, K. Water Management-Mediated Changes in the Rhizosphere and Bulk Soil Microbial Communities Alter Their Utilization of Urea-Derived Carbon. Microorganisms 2024, 12, 1829. https://doi.org/10.3390/microorganisms12091829
Chen P, Li Y, Lv Y, Xu J, Zhang Z, Liu X, Luan Y, Wei Q, Zheng E, Wang K. Water Management-Mediated Changes in the Rhizosphere and Bulk Soil Microbial Communities Alter Their Utilization of Urea-Derived Carbon. Microorganisms. 2024; 12(9):1829. https://doi.org/10.3390/microorganisms12091829
Chicago/Turabian StyleChen, Peng, Yawei Li, Yuping Lv, Junzeng Xu, Zhongxue Zhang, Xiaoyin Liu, Yajun Luan, Qi Wei, Ennan Zheng, and Kechun Wang. 2024. "Water Management-Mediated Changes in the Rhizosphere and Bulk Soil Microbial Communities Alter Their Utilization of Urea-Derived Carbon" Microorganisms 12, no. 9: 1829. https://doi.org/10.3390/microorganisms12091829
APA StyleChen, P., Li, Y., Lv, Y., Xu, J., Zhang, Z., Liu, X., Luan, Y., Wei, Q., Zheng, E., & Wang, K. (2024). Water Management-Mediated Changes in the Rhizosphere and Bulk Soil Microbial Communities Alter Their Utilization of Urea-Derived Carbon. Microorganisms, 12(9), 1829. https://doi.org/10.3390/microorganisms12091829








