Treatment of Anaerobic Digester Liquids via Membrane Biofilm Reactors: Simultaneous Aerobic Methanotrophy and Nitrogen Removal
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reactor Setup
2.2. Reactor Startup and Operating Conditions
2.3. Analytical Methods
2.4. Batch NO2− Tests
2.5. Biomass and Biofilm Sampling
2.6. Nucleic Acid Extraction, Sequencing, Assembly, and Microbial Community Analyses
3. Results
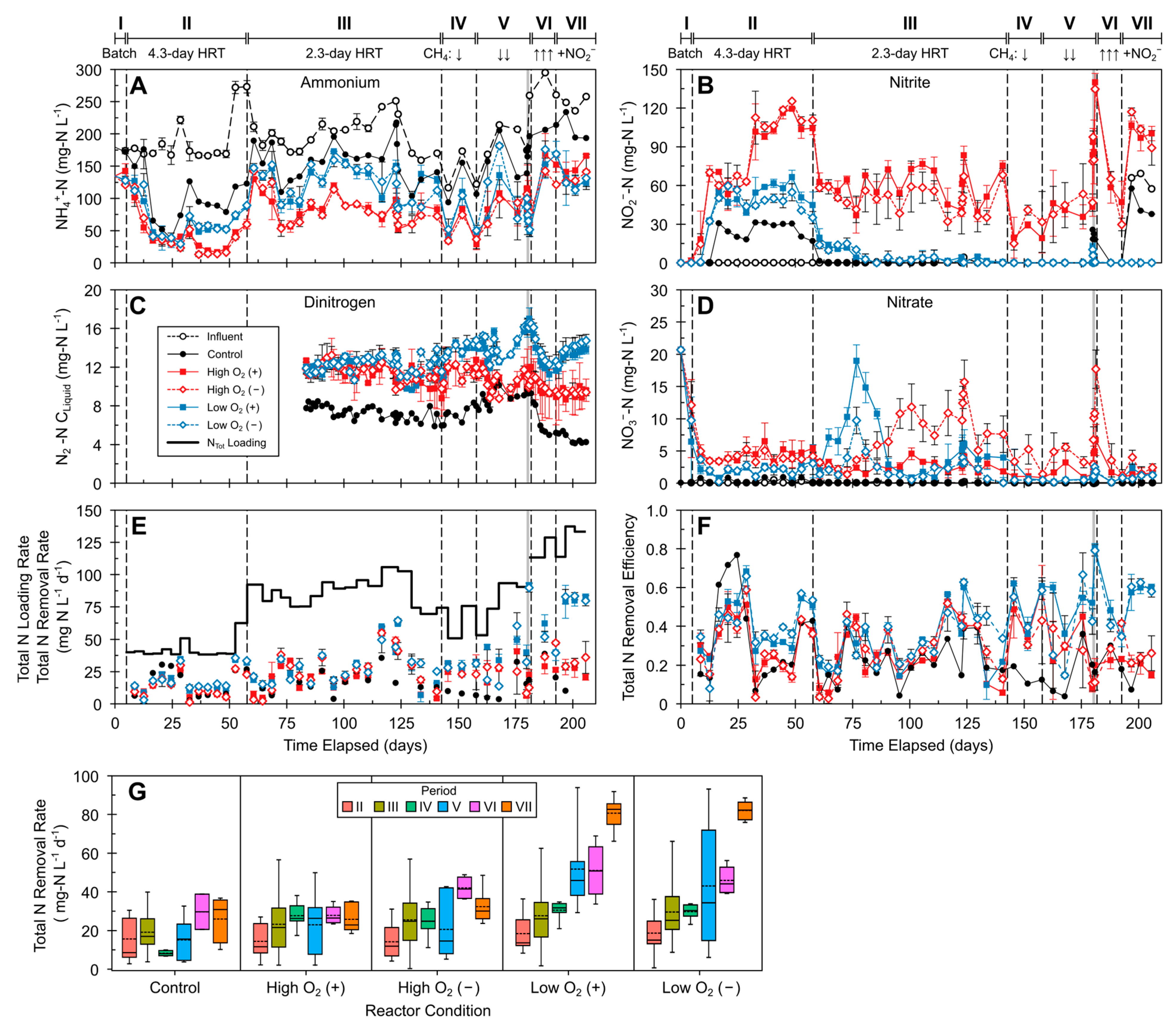
3.1. General Reactor Performance
3.2. Fixed Nitrogen Transformations and Removal
3.3. Total Inorganic Nitrogen (NTot) Removal
3.4. Short-Term NO2− Addition Tests
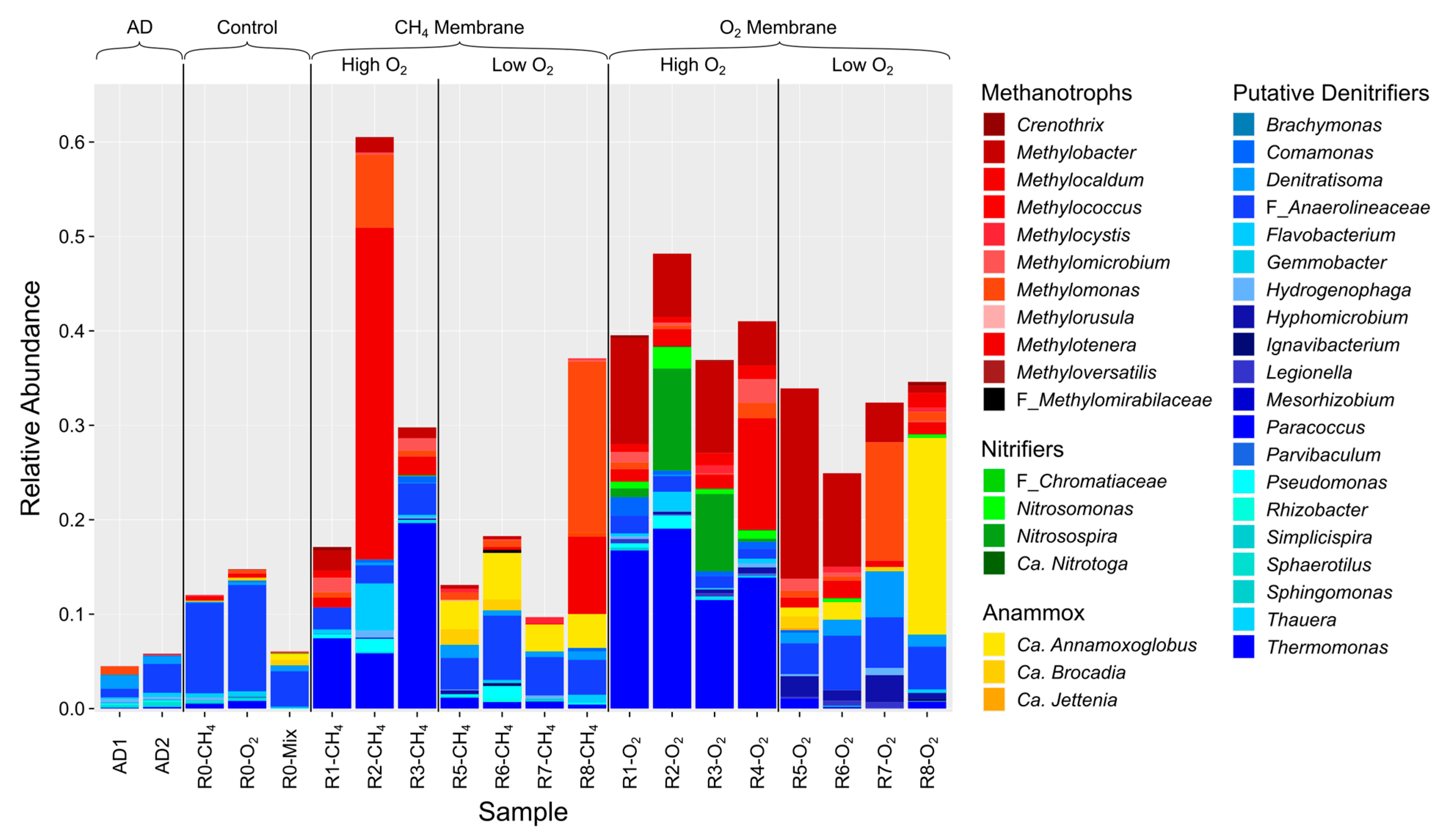
3.5. Membrane Biofilm Characteristics
3.6. Biofilm Microbial Community Structure and Diversity
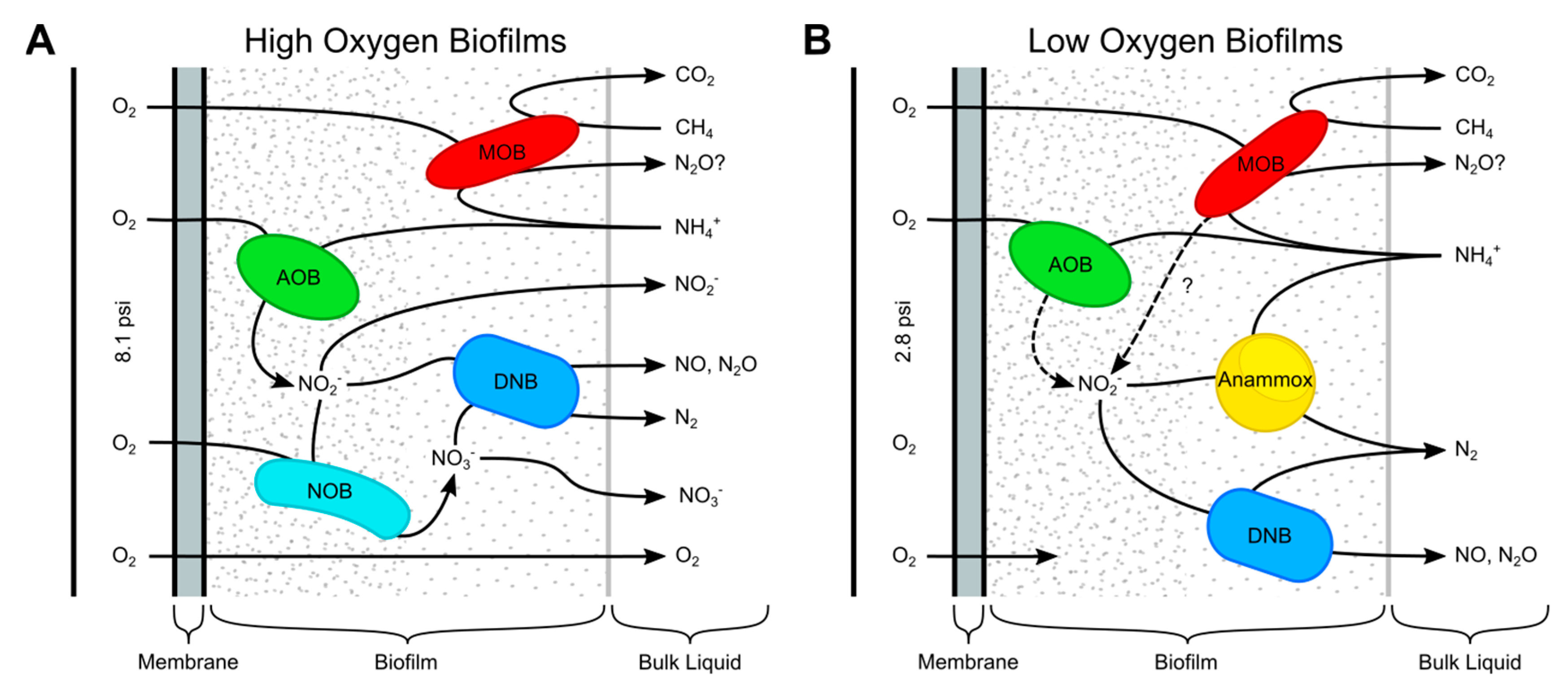
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Daelman, M.R.J.; van Voorthuizen, E.M.; van Dongen, U.G.J.M.; Volcke, E.I.P.; van Loosdrecht, M.C.M. Methane emission during municipal wastewater treatment. Water Res. 2012, 46, 3657–3670. [Google Scholar] [CrossRef] [PubMed]
- Delgado Vela, J.; Stadler, L.B.; Martin, K.J.; Raskin, L.; Bott, C.B.; Love, N.G. Prospects for biological nitrogen removal from anaerobic effluents during mainstream wastewater treatment. Environ. Sci. Technol. Lett. 2015, 2, 234–244. [Google Scholar] [CrossRef]
- Souza, C.L.; Chernicharo, C.A.L.; Aquino, S.F. Quantification of dissolved methane in UASB reactors treating domestic wastewater under different operating conditions. Water Sci. Technol. 2011, 64, 2259–2264. [Google Scholar] [CrossRef] [PubMed]
- IPCC. Climate Change 2021: The Physical Science Basis. Contribution of Working Group I to the Sixth Assessment Report (AR6) of the Intergovernmental Panel on Climate Change; Masson-Delmotte, V., Zhai, P., Pirani, A., Connors, S.L., Péan, C., Berger, S., Caud, N., Chen, Y., Goldfarb, L., Gomis, M.I., et al., Eds.; Cambridge University Press: Cambridge, UK; New York, NY, USA, 2021; p. 2391. [Google Scholar] [CrossRef]
- Li, J.; Feng, M.; Zheng, S.; Zhao, W.; Xu, X.; Yu, X. The membrane aerated biofilm reactor for nitrogen removal of wastewater treatment: Principles, performances, and nitrous oxide emissions. Chem. Eng. J. 2023, 460, 141693. [Google Scholar] [CrossRef]
- Song, C.; Zhu, J.; Willis, J.L.; Moore, D.P.; Zondlo, M.A.; Ren, Z.J. Methane Emissions from Municipal Wastewater Collection and Treatment Systems. Environ. Sci. Technol. 2023, 57, 2248–2261. [Google Scholar] [CrossRef]
- Masuda, S.; Suzuki, S.; Sano, I.; Li, Y.Y.; Nishimura, O. The seasonal variation of emission of greenhouse gases from a full-scale sewage treatment plant. Chemosphere 2015, 140, 167–173. [Google Scholar] [CrossRef]
- McCarty, P.L. What is the Best Biological Process for Nitrogen Removal: When and Why? Environ. Sci. Technol. 2018, 52, 3835–3841. [Google Scholar] [CrossRef]
- Kartal, B.; Kuenen, J.G.; Van Loosdrecht, M.C.M. Sewage treatment with anammox. Science 2010, 328, 702–703. [Google Scholar] [CrossRef]
- Lackner, S.; Gilbert, E.M.; Vlaeminck, S.E.; Joss, A.; Horn, H.; van Loosdrecht, M.C.M. Full-scale partial nitritation/anammox experiences—An application survey. Water Res. 2014, 55, 292–303. [Google Scholar] [CrossRef]
- Lee, H.J.; Bae, J.H.; Cho, K.M. Simultaneous nitrification and denitrification in a mixed methanotrophic culture. Biotechnol. Lett. 2001, 23, 935–941. [Google Scholar] [CrossRef]
- van Kessel, M.A.H.J.; Stultiens, K.; Pol, A.; Jetten, M.S.M.; Kartal, B.; den Camp, H.J.M.O. Simultaneous Anaerobic and Aerobic Ammonia and Methane Oxidation under Oxygen Limitation Conditions. Appl. Environ. Microbiol. 2021, 87, 1–11. [Google Scholar] [CrossRef]
- Cao, Q.; Li, X.; Jiang, H.; Wu, H.; Xie, Z.; Zhang, X.; Li, N.; Huang, X.; Li, Z.; Liu, X.; et al. Ammonia removal through combined methane oxidation and nitrification-denitrification and the interactions among functional microorganisms. Water Res. 2021, 188, 116555. [Google Scholar] [CrossRef]
- Vlaeminck, S.E.; Cloetens, L.F.F.; Carballa, M.; Boon, N.; Verstraete, W. Granular biomass capable of partial nitritation and anammox. Water Sci. Technol. 2008, 58, 1113–1120. [Google Scholar] [CrossRef] [PubMed]
- Martin, K.J.; Nerenberg, R. The membrane biofilm reactor (MBfR) for water and wastewater treatment: Principles, applications, and recent developments. Bioresour. Technol. 2012, 122, 83–94. [Google Scholar] [CrossRef]
- Lu, J.J.; Zhang, H.; Li, W.; Yi, J.B.; Sun, F.Y.; Zhao, Y.W.; Feng, L.; Li, Z.; Dong, W.Y. Biofilm stratification in counter-diffused membrane biofilm bioreactors (MBfRs) for aerobic methane oxidation coupled to aerobic/anoxic denitrification: Effect of oxygen pressure. Water Res. 2022, 226, 119243. [Google Scholar] [CrossRef] [PubMed]
- Silva-Teira, A.; Sánchez, A.; Buntner, D.; Rodríguez-Hernández, L.; Garrido, J.M. Removal of dissolved methane and nitrogen from anaerobically treated effluents at low temperature by MBR post-treatment. Chem. Eng. J. 2017, 326, 970–979. [Google Scholar] [CrossRef]
- Gilmore, K.R.; Terada, A.; Smets, B.F.; Love, N.G.; Garland, J.L. Autotrophic nitrogen removal in a membrane-aerated biofilm reactor under continuous aeration: A demonstration. Environ. Eng. Sci. 2013, 30, 38–45. [Google Scholar] [CrossRef]
- Pellicer-Nàcher, C.; Sun, S.; Lackner, S.; Terada, A.; Schreiber, F.; Zhou, Q.; Smets, B.F. Sequential aeration of membrane-aerated biofilm reactors for high-rate autotrophic nitrogen removal: Experimental demonstration. Environ. Sci. Technol. 2010, 44, 7628–7634. [Google Scholar] [CrossRef] [PubMed]
- Terada, A.; Hibiya, K.; Nagai, J.; Tsuneda, S.; Hirata, A. Nitrogen removal characteristics and biofilm analysis of a membrane-aerated biofilm reactor applicable to high-strength nitrogenous wastewater treatment. J. Biosci. Bioeng. 2003, 95, 170–178. [Google Scholar] [CrossRef]
- Helmer, C.; Kunst, S. Simultaneous nitrification/denitrification in an aerobic biofilm system. Water Sci. Technol. 1998, 37, 183–187. [Google Scholar] [CrossRef]
- Zeng, R.J.; Lemaire, R.; Yuan, Z.; Keller, J. Simultaneous nitrification, denitrification, and phosphorus removal in a lab-scale sequencing batch reactor. Biotechnol. Bioeng. 2003, 84, 170–178. [Google Scholar] [CrossRef]
- Winkler, M.K.H.; Kleerebezem, R.; Van Loosdrecht, M.C.M. Integration of anammox into the aerobic granular sludge process for main stream wastewater treatment at ambient temperatures. Water Res. 2012, 46, 136–144. [Google Scholar] [CrossRef]
- Gilbert, E.M.; Agrawal, S.; Karst, S.M.; Horn, H.; Nielsen, P.H.; Lackner, S. Low temperature partial nitritation/anammox in a moving bed biofilm reactor treating low strength wastewater. Environ. Sci. Technol. 2014, 48, 8784–8792. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Liu, Y.; Cao, S.; Hao, Q.; Liu, C.; Li, Y. Anaerobic oxidation of methane driven by different electron acceptors: A review. Sci. Total Environ. 2024, 946, 174287. [Google Scholar] [CrossRef] [PubMed]
- Rajapakse, J.P.; Scutt, J.E. Denitrification with natural gas and various new growth media. Water Res. 1999, 33, 3723–3734. [Google Scholar] [CrossRef]
- Modin, O.; Fukushi, K.; Nakajima, F.; Yamamoto, K. A membrane biofilm reactor achieves aerobic methane oxidation coupled to denitrification (AME-D) with high efficiency. Water Sci. Technol. 2008, 58, 83–87. [Google Scholar] [CrossRef]
- Modin, O.; Fukushi, K.; Nakajima, F.; Yamamoto, K. Performance of a membrane biofilm reactor for denitrification with methane. Bioresour. Technol. 2008, 99, 8054–8060. [Google Scholar] [CrossRef]
- Kampman, C.; Hendrickx, T.L.G.; Luesken, F.A.; van Alen, T.A.; Op den Camp, H.J.M.; Jetten, M.S.M.; Zeeman, G.; Buisman, C.J.N.; Temmink, H. Enrichment of denitrifying methanotrophic bacteria for application after direct low-temperature anaerobic sewage treatment. J. Hazard. Mater. 2012, 227–228, 164–171. [Google Scholar] [CrossRef]
- Castro-Barros, C.M.; Ho, L.T.; Winkler, M.K.H.; Volcke, E.I.P. Integration of methane removal in aerobic anammox-based granular sludge reactors. Environ. Technol. 2017, 39, 1615–1625. [Google Scholar] [CrossRef]
- Cai, C.; Hu, S.; Guo, J.; Shi, Y.; Xie, G.J.; Yuan, Z. Nitrate reduction by denitrifying anaerobic methane oxidizing microorganisms can reach a practically useful rate. Water Res. 2015, 87, 211–217. [Google Scholar] [CrossRef]
- Shi, Y.; Hu, S.; Lou, J.; Lu, P.; Keller, J.; Yuan, Z. Nitrogen Removal from Wastewater by Coupling Anammox and Methane-Dependent Denitrification in a Membrane Biofilm Reactor. Environ. Sci. Technol. 2013, 47, 11577–11583. [Google Scholar] [CrossRef]
- Fan, S.Q.; Xie, G.-J.; Lu, Y.; Liu, B.-F.; Xing, D.F.; Han, H.J.; Yuan, Z.; Ren, N.Q. Granular Sludge Coupling Nitrate/Nitrite Dependent Anaerobic Methane Oxidation with Anammox: From Proof-of-Concept to High Rate Nitrogen Removal. Environ. Sci. Technol. 2020, 54, 297–305. [Google Scholar] [CrossRef]
- Zhu, J.; Wang, Q.; Yuan, M.; Tan, G.Y.A.; Sun, F.; Wang, C.; Wu, W.; Lee, P.H. Microbiology and potential applications of aerobic methane oxidation coupled to denitrification (AME-D) process: A review. Water Res. 2016, 90, 203–215. [Google Scholar] [CrossRef] [PubMed]
- Alrashed, W.; Chandra, R.; Abbott, T.; Lee, H.S. Nitrite reduction using a membrane biofilm reactor (MBfR) in a hypoxic environment with dilute methane under low pressures. Sci. Total Environ. 2022, 841, 156757. [Google Scholar] [CrossRef]
- Xu, X.; Qin, Y.; Li, X.; Ma, Z.; Wu, W. Heterogeneity of CH4-derived carbon induced by O2:CH4 mediates the bacterial community assembly processes. Sci. Total Environ. 2022, 829, 154442. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Liu, Y.; Peng, L.; Yuan, Z.; Ni, B.J. Model-Based Feasibility Assessment of Membrane Biofilm Reactor to Achieve Simultaneous Ammonium, Dissolved Methane, and Sulfide Removal from Anaerobic Digestion Liquor. Sci. Rep. 2016, 6, 25114. [Google Scholar] [CrossRef] [PubMed]
- Tentori, E.F.; Richardson, R.E. Methane Monooxygenase Gene Transcripts as Quantitative Biomarkers of Methanotrophic Activity in Methylosinus trichosporium OB3b. Appl. Environ. Microbiol. 2020, 86, e01048-20. [Google Scholar] [CrossRef] [PubMed]
- Whittenbury, R.; Phillips, K.; Wilkinson, J. Enrichment, Isolation and Some Properties of Methane-utilizing Bacteria. J. Gen. Microbiol. 1970, 61, 205–218. [Google Scholar] [CrossRef]
- McGuire, P.M.; Reid, M.C. Nitrous Oxide and Methane Dynamics in Woochip Bioreactors: Effects of Water Level Fluctuations on Partitioning into Trapped Gas Phases. Environ. Sci. Technol. 2019, 53, 14348–14356. [Google Scholar] [CrossRef]
- Bower, C.E.; Holm-Hansen, T. A Salicylate–Hypochlorite Method for Determining Ammonia in Seawater. Can. J. Fish. Aquat. Sci. 1980, 37, 794–798. [Google Scholar] [CrossRef]
- Fernández-Baca, C.P.; Omar, A.E.H.; Pollard, J.T.; Richardson, R.E. Microbial communities controlling methane and nutrient cycling in leach field soils. Water Res. 2019, 151, 456–467. [Google Scholar] [CrossRef]
- APHA; AWWA; WEF. Standard Methods for the Examination of Water and Wastewater, 21st ed.; American Public Health Association: Washington, DC, USA, 2005. [Google Scholar]
- HACH. Phenolphthalein and Total Alkalinity. Method 8221. Buret Titration. DOC316.53.01151, 9th ed.; HACH Company: Loveland, CO, USA, 2017. [Google Scholar]
- Wasimuddin; Schlaeppi, K.; Ronchi, F.; Leib, S.L.; Erb, M.; Ramette, A. Evaluation of primer pairs for microbiome profiling from soils to humans within the One Health framework. Mol. Ecol. Resour. 2020, 20, 1558–1571. [Google Scholar] [CrossRef]
- Bolyen, E.; Rideout, J.R.; Dillon, M.R.; Bokulich, N.A.; Abnet, C.C.; Al-Ghalith, G.A.; Alexander, H.; Alm, E.J.; Arumugam, M.; Asnicar, F.; et al. Reproducible, interactive, scalable and extensible microbiome data science using QIIME 2. Nat. Biotechnol. 2019, 37, 852–857. [Google Scholar] [CrossRef]
- Callahan, B.J.; McMurdie, P.J.; Rosen, M.J.; Han, A.W.; Johnson, A.J.A.; Holmes, S.P. DADA2: High-resolution sample inference from Illumina amplicon data. Nat. Methods 2016, 13, 581–583. [Google Scholar] [CrossRef] [PubMed]
- Quast, C.; Pruesse, E.; Yilmaz, P.; Gerken, J.; Schweer, T.; Yarza, P.; Peplies, J.; Glöckner, F.O. The SILVA ribosomal RNA gene database project: Improved data processing and web-based tools. Nucleic Acids Res. 2013, 41, D590–D596. [Google Scholar] [CrossRef] [PubMed]
- McMurdie, P.J.; Holmes, S. Phyloseq: An R Package for Reproducible Interactive Analysis and Graphics of Microbiome Census Data. PLoS ONE 2013, 8, e61217. [Google Scholar] [CrossRef]
- Law, Y.; Ye, L.; Pan, Y.; Yuan, Z. Nitrous oxide emissions from wastewater treatment processes. Philos. Trans. R. Soc. B Biol. Sci. 2012, 367, 1265–1277. [Google Scholar] [CrossRef] [PubMed]
- Kosonen, H.; Heinonen, M.; Mikola, A.; Haimi, H.; Mulas, M.; Corona, F.; Vahala, R. Nitrous Oxide Production at a Fully Covered Wastewater Treatment Plant: Results of a Long-Term Online Monitoring Campaign. Environ. Sci. Technol. 2016, 50, 5547–5554. [Google Scholar] [CrossRef]
- Yamada, T.; Sekiguchi, Y.; Imachi, H.; Kamagata, Y.; Ohashi, A.; Harada, H. Diversity, localization, and physiological properties of filamentous microbes belonging to Chloroflexi subphylum I in mesophilic and thermophilic methanogenic sludge granules. Appl. Environ. Microbiol. 2005, 71, 7493–7503. [Google Scholar] [CrossRef]
- Yamada, T.; Sekiguchi, Y.; Hanada, S.; Imachi, H.; Ohashi, A.; Harada, H.; Kamagata, Y. Anaerolinea thermolimosa sp. nov., Levilinea saccharolytica gen. nov., sp. nov. and Leptolinea tardivitalis gen. nov., sp. nov., novel filamentous anaerobes, and description of the new classes Anaerolineae classis nov. and Caldilineae classis nov. in the bacterial phylum Chloroflexi. Int. J. Syst. Evol. Microbiol. 2006, 56, 1331–1340. [Google Scholar] [CrossRef]
- McIlroy, S.J.; Karst, S.M.; Nierychlo, M.; Dueholm, M.S.; Albertsen, M.; Kirkegaard, R.H.; Seviour, R.J.; Nielsen, P.H. Genomic and in situ investigations of the novel uncultured Chloroflexi associated with 0092 morphotype filamentous bulking in activated sludge. ISME J. 2016, 10, 2223–2234. [Google Scholar] [CrossRef]
- Shu, D.; He, Y.; Yue, H.; Yang, S. Effects of Fe(II) on microbial communities, nitrogen transformation pathways and iron cycling in the anammox process: Kinetics, quantitative molecular mechanism and metagenomic analysis. RSC Adv. 2016, 6, 68005–68016. [Google Scholar] [CrossRef]
- Xie, G.J.; Liu, T.; Cai, C.; Hu, S.; Yuan, Z. Achieving high-level nitrogen removal in mainstream by coupling anammox with denitrifying anaerobic methane oxidation in a membrane biofilm reactor. Water Res. 2018, 131, 196–204. [Google Scholar] [CrossRef]
- Sánchez, A.; Rodríguez-Hernández, L.; Buntner, D.; Esteban-García, A.L.; Tejero, I.; Garrido, J.M. Denitrification coupled with methane oxidation in a membrane bioreactor after methanogenic pre-treatment of wastewater. J. Chem. Technol. Biotechnol. 2016, 91, 2950–2958. [Google Scholar] [CrossRef]
- Martínez-Quintela, M.; Arias, A.; Alvarino, T.; Suarez, S.; Garrido, J.M.; Omil, F. Cometabolic removal of organic micropollutants by enriched nitrite-dependent anaerobic methane oxidizing cultures. J. Hazard. Mater. 2021, 402, 123450. [Google Scholar] [CrossRef] [PubMed]
- Kampman, C.; Temmink, H.; Hendrickx, T.L.G.; Zeeman, G.; Buisman, C.J.N. Enrichment of denitrifying methanotrophic bacteria from municipal wastewater sludge in a membrane bioreactor at 20 °C. J. Hazard Mater. 2014, 274, 428–435. [Google Scholar] [CrossRef]
- Ding, Z.W.; Ding, J.; Fu, L.; Zhang, F.; Zeng, R.J. Simultaneous enrichment of denitrifying methanotrophs and anammox bacteria. Appl. Microbiol. Biotechnol. 2014, 98, 10211–10221. [Google Scholar] [CrossRef]
- Ding, Z.W.; Lu, Y.Z.; Fu, L.; Ding, J.; Zeng, R.J. Simultaneous enrichment of denitrifying anaerobic methane-oxidizing microorganisms and anammox bacteria in a hollow-fiber membrane biofilm reactor. Appl. Microbiol. Biotechnol. 2017, 101, 437–446. [Google Scholar] [CrossRef]
- Hu, S.; Zeng, R.J.; Burow, L.C.; Lant, P.; Keller, J.; Yuan, Z. Enrichment of denitrifying anaerobic methane oxidizing microorganisms. Environ. Microbiol. Rep. 2009, 1, 377–384. [Google Scholar] [CrossRef]
- Cao, Q.; Li, X.; Xie, Z.; Li, C.; Huang, S.; Zhu, B.; Li, D.; Liu, X. Compartmentation of microbial communities in structure and function for methane oxidation coupled to nitrification–denitrification. Bioresour. Technol. 2021, 341, 125761. [Google Scholar] [CrossRef]
- Liu, T.; Li, J.; Khai Lim, Z.; Chen, H.; Hu, S.; Yuan, Z.; Guo, J. Simultaneous Removal of Dissolved Methane and Nitrogen from Synthetic Mainstream Anaerobic Effluent. Environ. Sci. Technol. 2020, 54, 7629–7638. [Google Scholar] [CrossRef]
- Stein, L.Y.; Arp, D.J. Loss of ammonia monooxygenase activity in Nitrosomonas europaea upon exposure to nitrite. Appl. Environ. Microbiol. 1998, 64, 4098–4102. [Google Scholar] [CrossRef]
- Cua, L.S.; Stein, L.Y. Effects of nitrite on ammonia-oxidizing activity and gene regulation in three ammonia-oxidizing bacteria. FEMS Microbiol. Lett. 2011, 319, 169–175. [Google Scholar] [CrossRef] [PubMed]
- Jin, R.C.; Yang, G.F.; Yu, J.J.; Zheng, P. The inhibition of the Anammox process: A review. Chem. Eng. J. 2012, 197, 67–79. [Google Scholar] [CrossRef]
- Strous, M.; Kuenen, J.G.; Jetten, M.S.M. Key physiology of anaerobic ammonium oxidation. Appl. Environ. Microbiol. 1999, 65, 3248–3250. [Google Scholar] [CrossRef] [PubMed]
- Lotti, T.; van der Star, W.R.L.; Kleerebezem, R.; Lubello, C.; van Loosdrecht, M.C.M. The effect of nitrite inhibition on the anammox process. Water Res. 2012, 46, 2559–2569. [Google Scholar] [CrossRef]
- Strous, M.; Van Gerven, E.; Kuenen, J.G.; Jetten, M. Effects of aerobic and microaerobic conditions on anaerobic ammonium-oxidizing (anammox) sludge. Appl. Environ. Microbiol. 1997, 63, 2446–2448. [Google Scholar] [CrossRef]
- Oshiki, M.; Satoh, H.; Okabe, S. Ecology and physiology of anaerobic ammonium oxidizing bacteria. Environ. Microbiol. 2016, 18, 2784–2796. [Google Scholar] [CrossRef] [PubMed]
- Carvajal-Arroyo, J.M.; Sun, W.; Sierra-Alvarez, R.; Field, J.A. Inhibition of anaerobic ammonium oxidizing (anammox) enrichment cultures by substrates, metabolites and common wastewater constituents. Chemosphere 2013, 91, 22–27. [Google Scholar] [CrossRef]
- Egli, K.; Fanger, U.; Alvarez, P.J.J.; Siegrist, H.; Van der Meer, J.R.; Zehnder, A.J.B. Enrichment and characterization of an anammox bacterium from a rotating biological contactor treating ammonium-rich leachate. Arch. Microbiol. 2001, 175, 198–207. [Google Scholar] [CrossRef]
- Li, Y.; Wang, J.; Hua, M.; Yao, X.; Zhao, Y.; Hu, J.; Xi, C.; Hu, B. Strategy for denitrifying anaerobic methane-oxidizing bacteria growing under the oxygen-present condition. Sci. Total Environ. 2020, 742, 140476. [Google Scholar] [CrossRef] [PubMed]
- Cai, C.; Hu, S.; Chen, X.; Ni, B.J.; Pu, J.; Yuan, Z. Effect of methane partial pressure on the performance of a membrane biofilm reactor coupling methane-dependent denitrification and anammox. Sci. Total Environ. 2018, 639, 278–285. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Guo, J.; Xie, G.J.; Liu, Y.; Yuan, Z.; Ni, B.J. A new approach to simultaneous ammonium and dissolved methane removal from anaerobic digestion liquor: A model-based investigation of feasibility. Water Res. 2015, 85, 295–303. [Google Scholar] [CrossRef] [PubMed]
- Hanson, R.; Hanson, T. Methanotrophic bacteria. Microbiol. Rev. 1996, 60, 439–471. [Google Scholar] [CrossRef] [PubMed]
- King, G.M.; Schnell, S. Ammonium and nitrite inhibition of methane oxidation by Methylobacter albus BG8 and Methylosinus trichosporium OB3b at low methane concentrations. Appl. Environ. Microbiol. 1994, 60, 3508–3513. [Google Scholar] [CrossRef]
- Bédard, C.; Knowles, R. Physiology, biochemistry, and specific inhibitors of CH4, NH4+, and CO oxidation by methanotrophs and nitrifiers. Microbiol. Rev. 1989, 53, 68–84. [Google Scholar] [CrossRef]
- Kits, K.D.; Klotz, M.G.; Stein, L.Y. Methane oxidation coupled to nitrate reduction under hypoxia by the Gammaproteobacterium Methylomonas denitrificans, sp. nov. type strain FJG1. Environ. Microbiol. 2015, 17, 3219–3232. [Google Scholar] [CrossRef]
- Kits, K.D.; Campbell, D.J.; Rosana, A.R.; Stein, L.Y. Diverse electron sources support denitrification under hypoxia in the obligate methanotroph Methylomicrobium album strain BG8. Front. Microbiol. 2015, 6, 1072. [Google Scholar] [CrossRef]
- Nyerges, G.; Han, S.K.; Stein, L.Y. Effects of ammonium and nitrite on growth and competitive fitness of cultivated methanotrophic bacteria. Appl. Environ. Microbiol. 2010, 76, 5648–5651. [Google Scholar] [CrossRef]
- Modin, O.; Fukushi, K.; Nakajima, F.; Yamamoto., K. Aerobic methane oxidation coupled to denitrification: Kinetics and effect of oxygen supply. J. Environ. Eng. 2010, 136, 211–219. [Google Scholar] [CrossRef]
- Xu, X.; Zhu, J.; Thies, J.E.; Wu, W. Methanol-linked synergy between aerobic methanotrophs and denitrifiers enhanced nitrate removal efficiency in a membrane biofilm reactor under a low O2:CH4 ratio. Water Res. 2020, 174, 115595. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Wu, W.; Li, X.; Zhao, C.; Qin, Y. Metagenomics coupled with thermodynamic analysis revealed a potential way to improve the nitrogen removal efficiency of the aerobic methane oxidation coupled to denitrification process under the hypoxic condition. Sci. Total Environ. 2024, 912, 168953. [Google Scholar] [CrossRef] [PubMed]
- Rueden, C.T.; Schindelin, J.; Hiner, M.C.; DeZonia, B.E.; Walter, A.E.; Arena, E.T.; Eliceiri, K.W. ImageJ2: ImageJ for the next generation of scientific image data. BMC Bioinform. 2017, 18, 529. [Google Scholar] [CrossRef] [PubMed]
- Wunderlin, P.; Mohn, J.; Joss, A.; Emmenegger, L.; Siegrist, H. Mechanisms of N2O production in biological wastewater treatment under nitrifying and denitrifying conditions. Water Res. 2012, 46, 1027–1037. [Google Scholar] [CrossRef] [PubMed]
- Zumft, W.G. Cell biology and molecular basis of denitrification. Microbiol. Mol. Biol. Rev. MMBR 1997, 61, 533–616. [Google Scholar] [CrossRef]
- Lourenço, K.S.; Cassman, N.A.; Pijl, A.S.; van Veen, J.A.; Cantarella, H.; Kuramae, E.E. Nitrosospira sp. govern nitrous oxide emissions in a tropical soil amended with residues of bioenergy crop. Front. Microbiol. 2018, 9, 674. [Google Scholar] [CrossRef]
- Pereira, M.O.; Kuehn, M.; Wuertz, S.; Neu, T.; Melo, L.F. Effect of flow regime on the architecture of a Pseudomonas fluorescens biofilm. Biotechnol. Bioeng. 2002, 78, 164–171. [Google Scholar] [CrossRef]
- dos Santos, L.M.F.; Livingston, A.G. Membrane-attached biofilms for VOC wastewater treatment I: Novel in situ biofilm thickness measurement technique. Biotechnol. Bioeng. 1995, 47, 82–89. [Google Scholar] [CrossRef]
- Rishell, S.; Casey, E.; Glennon, B.; Hamer, G. Characteristics of a methanotrophic culture in a membrane-aerated biofilm reactor. Biotechnol. Prog. 2004, 20, 1082–1090. [Google Scholar] [CrossRef]
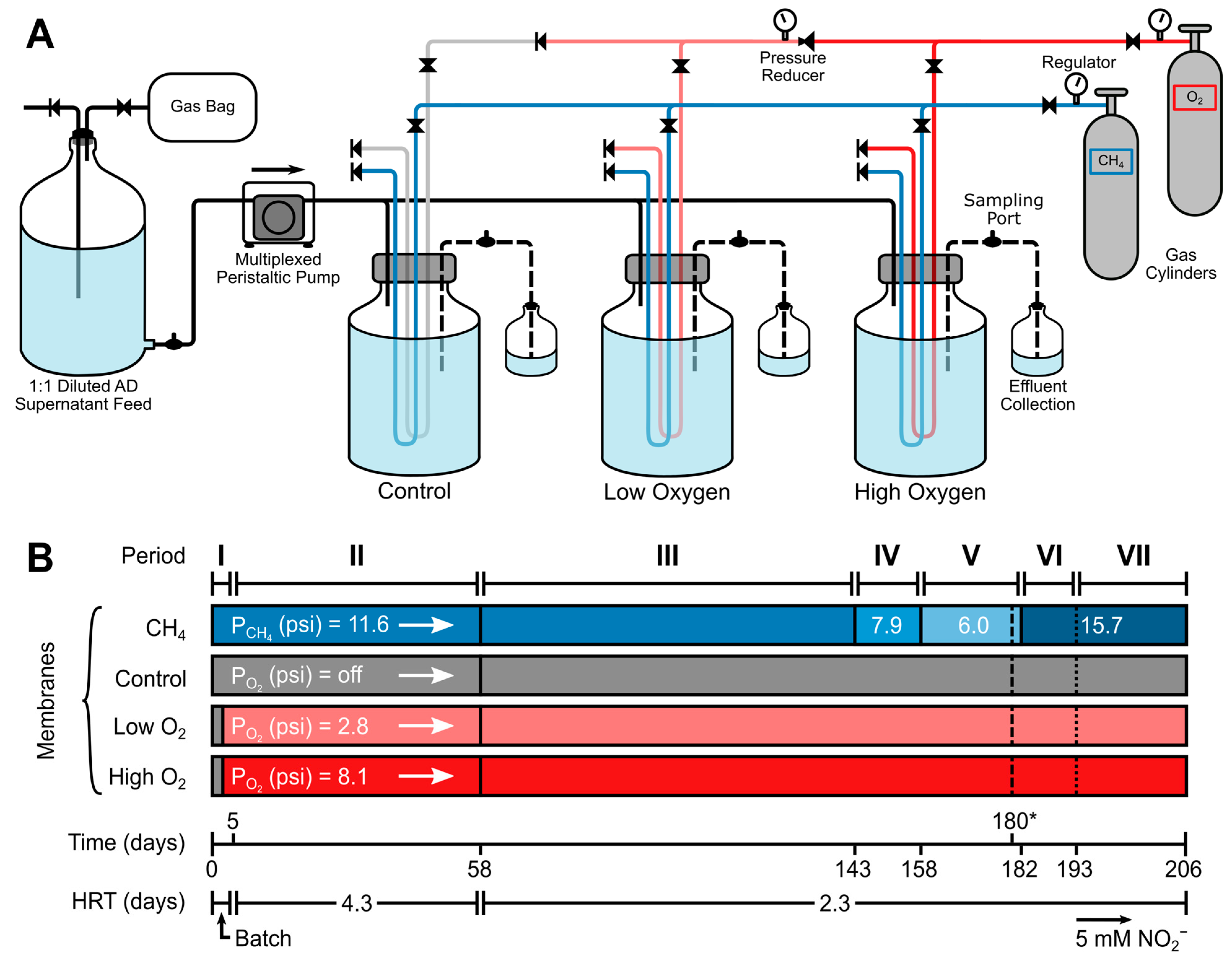


| Reactor Number | Reactor Condition | Methanotroph-Inoculated 1 | O2 Membrane Pressure (psig) 2 |
|---|---|---|---|
| R0 | Control | − | N/A |
| R1, R2 | High O2 (+) | + | 8.1 |
| R3, R4 | High O2 (−) | − | |
| R5, R6 | Low O2 (+) | + | 2.8 |
| R7, R8 | Low O2 (−) | − |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tentori, E.F.; Wang, N.; Devin, C.J.; Richardson, R.E. Treatment of Anaerobic Digester Liquids via Membrane Biofilm Reactors: Simultaneous Aerobic Methanotrophy and Nitrogen Removal. Microorganisms 2024, 12, 1841. https://doi.org/10.3390/microorganisms12091841
Tentori EF, Wang N, Devin CJ, Richardson RE. Treatment of Anaerobic Digester Liquids via Membrane Biofilm Reactors: Simultaneous Aerobic Methanotrophy and Nitrogen Removal. Microorganisms. 2024; 12(9):1841. https://doi.org/10.3390/microorganisms12091841
Chicago/Turabian StyleTentori, Egidio F., Nan Wang, Caroline J. Devin, and Ruth E. Richardson. 2024. "Treatment of Anaerobic Digester Liquids via Membrane Biofilm Reactors: Simultaneous Aerobic Methanotrophy and Nitrogen Removal" Microorganisms 12, no. 9: 1841. https://doi.org/10.3390/microorganisms12091841






