Identification of Virulence Genes Associated with Pathogenicity of Translocating Escherichia coli with Special Reference to the Type 6 Secretion System
Abstract
:1. Introduction
2. Materials and Methods
2.1. E. coli Strains
2.2. Testing for Adhesion and Invasion
2.3. Translocation Assay
2.4. DNA Extraction
2.5. PCR Detection of VGs
2.6. PCR Detection of Type 6 Secretion System
2.7. Gene Expression
2.8. RNA Extraction
2.9. Statistical Analysis
3. Results
3.1. Adhesion and Invasion Assays
3.2. Translocation Assays
3.3. Identification of VGs
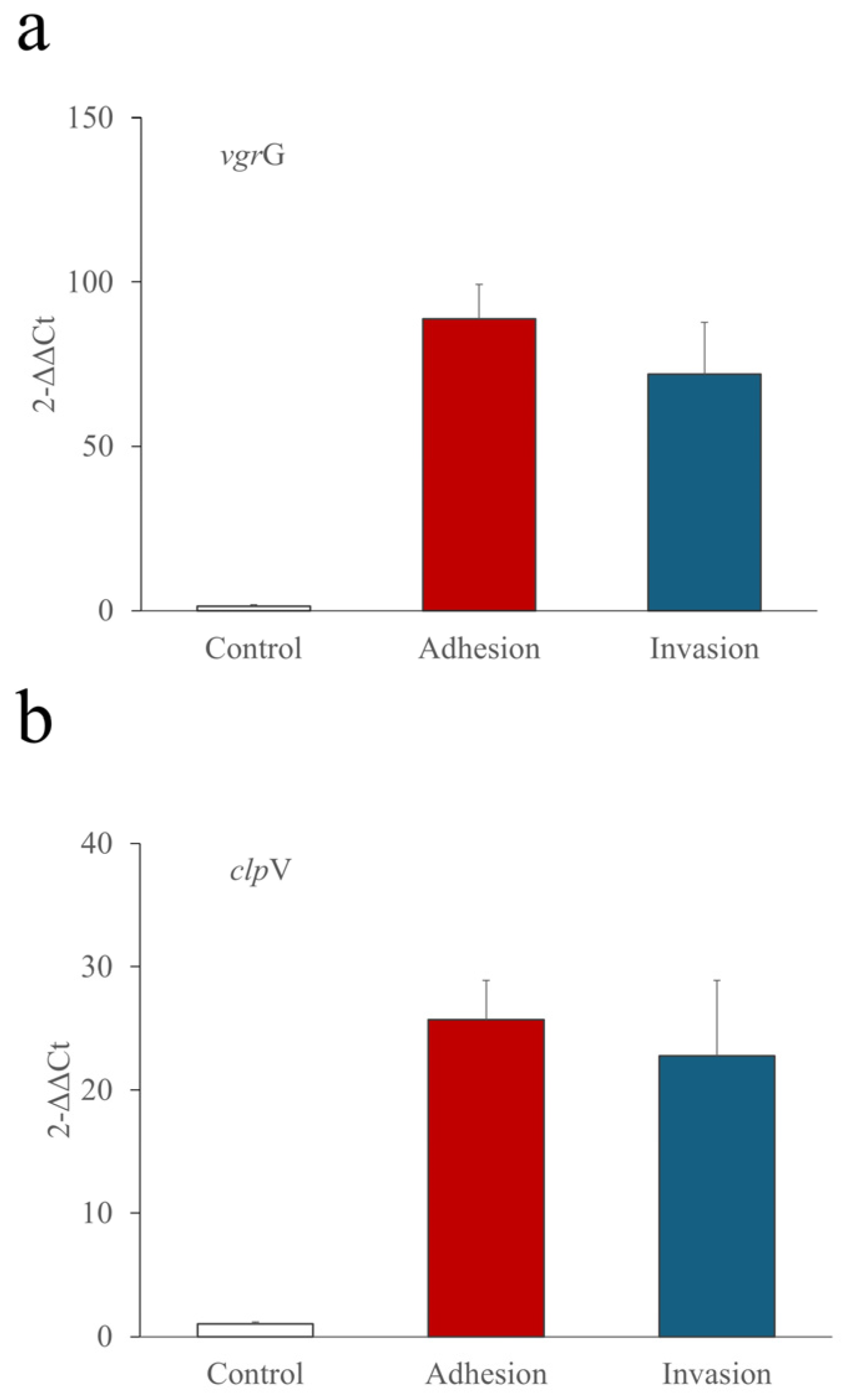
3.4. Prevalence of T6SS-1 among Clinical Strains of E. coli
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Poole, N.M.; Green, S.I.; Rajan, A.; Vela, L.E.; Zeng, X.L.; Estes, M.K.; Maresso, A.W. Role for FimH in extraintestinal pathogenic Escherichia coli invasion and translocation through the intestinal epithelium. Infect. Immun. 2017, 85. [Google Scholar] [CrossRef] [PubMed]
- MacFie, J. Current status of bacterial translocation as a cause of surgical sepsis. Br. Med. Bull. 2005, 71, 1–11. [Google Scholar] [CrossRef]
- Macutkiewicz, C.; Carlson, G.; Clark, E.; Dobrindt, U.; Roberts, I.; Warhurst, G. Characterisation of Escherichia coli strains involved in transcytosis across gut epithelial cells exposed to metabolic and inflammatory stress. Microbes Infect. 2008, 10, 424–431. [Google Scholar] [CrossRef]
- Cruz, N.; Lu, Q.; Alvarez, X.; Deitch, E.A. Bacterial translocation is bacterial species dependent: Results using the human CaCo-2 intestinal cell line. J. Trauma 1994, 36, 612–616. [Google Scholar] [CrossRef] [PubMed]
- Nagpal, R.; Yadav, H. Bacterial translocation from the gut to the distant organs: An overview. Ann. Nutr. Metab. 2017, 71 (Suppl. S1), 11–16. [Google Scholar] [CrossRef]
- Braithwaite, C.E.M.; Ross, S.E.; Nagele, R.; Mure, A.J.; O’Malley, K.F.; Garcia-Perez, F.A. Bacterial translocation occurs in humans after traumatic injury: Evidence using immunoflourescence. J. Trauma 1993, 34, 586–590. [Google Scholar] [CrossRef]
- Katouli, M.; Bark, T.; Ljungqvist, O.; Svenberg, T.; Mollby, R. Composition and Diversity of Intestinal Coliform Flora Influence Bacterial Translocation in Rats after Hemorrhagic Stress. Infect. Immun. 1994, 62, 4768–4774. [Google Scholar] [CrossRef]
- Katouli, M.; Nettebladt, C.G.; Muratov, V.; Ljungqvist, O.; Bark, T.; Svenberg, T.; Möllby, R. Selective Translocation of Coliform Bacteria Adhering to Caecal Epithelium of Rats during Catabolic Stress. J. Med. Microbiol. 1997, 46, 571–578. [Google Scholar] [CrossRef] [PubMed]
- Vollmerhausen, T.L.; Woods, J.L.; Faoagali, J.; Katouli, M. Interactions of uroseptic Escherichia coli with renal (A-498) and gastrointestinal (HT-29) cell lines. J. Med. Microbiol. 2014, 63, 1575–1583. [Google Scholar] [CrossRef]
- Kalita, A.; Hu, J.; Torres, A.G. Recent advances in adherence and invasion of pathogenic Escherichia coli. Curr. Opin. Infect. Dis. 2014, 27, 459–464. [Google Scholar] [CrossRef]
- Sedighi, I.; Nejad, A.S.M.; Amanati, A.; Nakhaei, S.; Alikhani, M.Y. Virulence factors and antibiotic resistance in uropathogenic and commensal Escherichia coli isolates. J. Krishna Inst. Med. Sci. Univ. 2016, 5, 50–57. [Google Scholar]
- Kim, B.Y.; Kang, J.; Kim, K.S. Invasion processes of pathogenic Escherichia coli. Int. J. Med. Microbiol. 2005, 295, 463–470. [Google Scholar] [CrossRef] [PubMed]
- Ramos, N.L.; Saayman, M.L.; Chapman, T.A.; Tucker, J.R.; Smith, H.V.; Faoagali, J.; Chin, J.C.; Brauner, A.; Katouli, M. Genetic relatedness and virulence gene profiles of Escherichia coli strains isolated from septicaemic and uroseptic patients. Eur. J. Clin. Microbiol. Infect. Dis. 2010, 29, 15–23. [Google Scholar] [CrossRef]
- Alexander, J.W.M.; Boyce, S.T.; Babcock, G.F.; Gianotti, L.; Peck, M.D.; Dunn, D.L.M.; Pyles, T.B.; Childress, C.P.M.; Ash, S.K.B. The process of microbial translocation. Ann. Surg. 1990, 212, 496–512. [Google Scholar] [CrossRef] [PubMed]
- Bachmann, N.L.; Katouli, M.; Polkinghorne, A. Genomic comparison of translocating and non-translocating Escherichia coli. PLoS ONE 2015, 10, e0137131. [Google Scholar] [CrossRef] [PubMed]
- Avalos Vizcarra, I.; Hosseini, V.; Kollmannsberger, P.; Meier, S.; Weber, S.S.; Arnoldini, M.; Ackermann, M.; Vogel, V. How type 1 fimbriae help Escherichia coli to evade extracellular antibiotics. Sci. Rep. 2016, 6, 18109. [Google Scholar] [CrossRef]
- Rouquet, G.; Porcheron, G.; Barra, C.; Répérant, M.; Chanteloup, N.K.; Schouler, C.; Gilot, P. A metabolic operon in extraintestinal pathogenic Escherichia coli promotes fitness under stressful conditions and invasion of eukaryotic cells. J. Bacteriol. 2009, 191, 4427–4440. [Google Scholar] [CrossRef]
- Garmendia, J.; Frankel, G.; Crepin, V.F. Enteropathogenic and enterohemorrhagic Escherichia coli infections: Translocation. Infect. Immun. 2005, 73, 2573–2585. [Google Scholar] [CrossRef]
- Nazli, A.; Wang, A.; Steen, O.; Prescott, D.; Lu, J.; Perdue, M.H.; Söderholm, J.D.; Sherman, P.M.; McKay, D.M. Enterocyte cytoskeleton changes are crucial for enhanced translocation of nonpathogenic Escherichia coli across metabolically stressed gut epithelia. Infect. Immun. 2006, 74, 192–201. [Google Scholar]
- Owrangi, B.; Masters, N.; Vollmerhausen, T.L.; O’Dea, C.; Kuballa, A.; Katouli, M. Comparison between virulence characteristics of dominant and non-dominant Escherichia coli strains of the gut and their interaction with Caco-2 cells. Microb. Pathog. 2017, 105, 171–176. [Google Scholar] [CrossRef]
- Katouli, M.; Ramos, N.L.; Nettelbladt, C.G.; Ljungdahl, M.; Robinson, W.; Ison, H.M.; Brauner, A.; Möllby, R. Host species-specific translocation of Escherichia coli. Eur. J. Clin. Microbiol. Infect. Dis. 2009, 28, 1095–1103. [Google Scholar] [CrossRef] [PubMed]
- Conte, M.P.; Longhi, C.; Marazzato, M.; Conte, A.L.; Aleandri, M.; Lepanto, M.S.; Zagaglia, C.; Nicoletti, M.; Aloi, M.; Totino, V.; et al. Adherent-invasive Escherichia coli (AIEC) in pediatric Crohn’s disease patients: Phenotypic and genetic pathogenic features. BMC Res. Notes 2014, 7, 748. [Google Scholar] [CrossRef] [PubMed]
- Bark, T.; Katouli, M.; Ljungqvist, O.; Möllby, R.; Svenberg, T. Bacterial translocation after non-lethal hemorrhage in the rat. Circ. Shock 1993, 41, 60–65. [Google Scholar] [PubMed]
- Angelis, I.D.; Turco, L. Caco-2 cells as a model for intestinal absorption. Curr. Protoc. Toxicol. 2011, 47, 20.6.1–20.6.15. [Google Scholar] [CrossRef]
- Hidalgo, I.J.; Raub, T.J.; Borchardt, R.T. Characterization of the human colon carcinoma cell line (Caco-2) as a model system for intestinal epithelial permeability. Gastroenterology 1989, 96, 36–49. [Google Scholar] [CrossRef]
- Gagnon, M.; Zihler Berner, A.; Chervet, N.; Chassard, C.; Lacroix, C. Comparison of the Caco-2, HT-29 and the mucus-secreting HT29-MTX intestinal cell models to investigate Salmonella adhesion and invasion. J. Microbiol. Methods 2013, 94, 274–279. [Google Scholar] [CrossRef]
- Lesuffleur, T.; Barbat, A.; Dussaulx, E.; Zweibaum, A. Growth adaptation to methotrexate of HT-29 human colon carcinoma cells is associated with their ability to differentiate into columnar absorptive and mucus-secreting cells. Cancer Res. 1990, 50, 6334–6343. [Google Scholar]
- Nettelbladt, C.-G.; Katouli, M.; Bark, T.; Svenberg, T.; Möllby, R.; Ljungqvist, O. Evidence of bacterial translocation in fatal hemorrhagic pancreatitis. Trauma Acute Care Surg. 2000, 48, 314. [Google Scholar] [CrossRef]
- Vollmerhausen, T.L.; Ramos, N.L.; Gündoğdu, A.; Robinson, W.; Brauner, A.; Katouli, M. Population structure and uropathogenic virulence-associated genes of faecal Escherichia coli from healthy young and elderly adults. J. Med. Microbiol. 2011, 60, 574–581. [Google Scholar] [CrossRef]
- Owrangi, B.; Masters, N.; Kuballa, A.; O’Dea, C.; Vollmerhausen, T.L.; Katouli, M. Invasion and translocation of uropathogenic Escherichia coli isolated from urosepsis and patients with community-acquired urinary tract infection. Eur. J. Clin. Microbiol. Infect. Dis. 2018, 37, 33–839. [Google Scholar] [CrossRef]
- Reddy, B.S.; MacFie, J.; Gatt, M.; Macfarlane-Smith, L.; Bitzopoulou, K.; Snelling, A.M. Commensal bacteria do translocate across the intestinal barrier in surgical patients. Clin. Nutr. 2007, 26, 208–215. [Google Scholar] [CrossRef]
- Astley, D.; Masters, N.; Kuballa, A.; Katouli, M. Commonality of adherent-invasive Escherichia coli isolated from patients with extraintestinal infections, healthy individuals, and the environment. Eur. J. Clin. Microbiol. Infect. Dis. 2021, 40, 181–192. [Google Scholar] [CrossRef]
- Gündoğdu, A.; Long, Y.B.; Katouli, M. Prevalence and pathogenesis of extended-spectrum beta-lactamase producing Escherichia coli causing urinary tract infection in hospitalized patients. Eur. J. Clin. Microbiol. Infect. Dis. 2012, 31, 3107–3116. [Google Scholar] [CrossRef] [PubMed]
- Snowden, L.; Wernbacher, L.; Stenzel, D.; Tucker, J.; McKay, D.; O’Brien, M.; Katouli, M. Prevalence of environmental Aeromonas in Southeast Queensland, Australia: A study of their interactions with human monolayer Caco-2 cells. J. Appl. Microbiol. 2006, 101, 964–975. [Google Scholar] [CrossRef]
- Chen, W.P.; Kuo, T.-T. A simple and rapid method for the preparation of gram-negative bacterial genomic DNA. Nucleic Acids Res. 1993, 21, 2260. [Google Scholar] [CrossRef] [PubMed]
- Prorok-Hamon, M.; Friswell, M.K.; Alswied, A.; Roberts, C.L.; Song, F.; Flanagan, P.K.; Knight, P.; Codling, C.; Marchesi, J.R.; Winstanley, C.; et al. Colonic mucosa-associated diffusely adherent afaC+ Escherichia coli expressing lpfA and pks are increased in inflammatory bowel disease and colon cancer. Gut 2014, 63, 761–770. [Google Scholar] [CrossRef]
- Chapman, T.A.; Wu, X.Y.; Barchia, I.; Bettelheim, K.A.; Driesen, S.; Trott, D.; Wilson, M.; Chin, J.J.C. Comparison of virulence gene profiles of Escherichia coli strains isolated from healthy and diarrheic swine. Appl. Environ. Microbiol. 2006, 72, 4782–4795. [Google Scholar] [CrossRef] [PubMed]
- Faïs, T.; Delmas, J.; Barnich, N.; Bonnet, R.; Dalmasso, G. Colibactin: More than a new bacterial toxin. Toxins 2018, 10, 151. [Google Scholar] [CrossRef]
- Johnson, J.R.; Stell, A.L. Extended virulence genotypes of Escherichia coli strains from patients with urosepsis in relation to phylogeny and host compromise. J. Infect. Dis. 2000, 181, 261–272. [Google Scholar] [CrossRef]
- Johnson, J.R.; Russo, T.A.; Tarr, P.I.; Carlino, U.; Bilge, S.S.; Vary, J.C., Jr.; Stell, A.L. Molecular epidemiological and phylogenetic associations of two novel putative virulence genes, iha and iroNE. coli, among Escherichia coli isolates from patients with urosepsis. Infect. Immun. 2000, 68, 3040–3047. [Google Scholar] [CrossRef]
- Li, Y.; Dai, J.; Zhuge, X.; Wang, H.; Hu, L.; Ren, J.; Chen, L.; Li, D.; Tang, F. Iron-regulated gene ireA in avian pathogenic Escherichia coli participates in adhesion and stress-resistance. BMC Vet. Res. 2016, 12, 167. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Sun, M.; Bao, Y.; Pan, Z.; Zhang, W.; Lu, C.; Yao, H. Genetic diversity and features analysis of type VI secretion systems loci in avian pathogenic Escherichia coli by wide genomic scanning. Infect. Genet. Evol. 2013, 20, 454–464. [Google Scholar] [CrossRef]
- De Pace, F.; Nakazato, G.; Pacheco, A.; Boldrin de Paiva, J.; Sperandio, V.; Dias da Silveira, W. The type VI secretion system plays a role in type 1 fimbria expression and pathogenesis of an avian pathogenic Escherichia coli strain. Infect. Immun. 2010, 78, 4990–4998. [Google Scholar] [CrossRef]
- Mirbagheri, V.S.; Alishahi, A.; Ahmadian, G.; Petroudi, S.H.H.; Ojagh, S.M.; Romanazzi, G. Recent findings in molecular reactions of Escherichia coli as exposed to alkylated, nano-and ordinary chitosans. Int. J. Biol. Macromol. 2023, 253, 127006. [Google Scholar] [CrossRef] [PubMed]
- Miajlovic, H.; Mac Aogain, M.; Collins, C.J.; Rogers, T.R.; Smith, S.G. Characterization of Escherichia coli bloodstream isolates associated with mortality. J. Med. Microbiol. 2016, 65, 71–79. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Ren, J.; Chen, G.; Wu, L.; Song, X.; Li, G.; Deng, Y.; Wang, G.; Gu, G.; Li, J. Systemic blockade of P2X7 receptor protects against sepsis-induced intestinal barrier disruption. Sci. Rep. 2017, 7, 4364. [Google Scholar] [CrossRef]
- Ramos, N.L.; Lamprokostopoulou, A.; Chapman, T.A.; Chin, J.C.; Römling, U.; Brauner, A.; Katouli, M. Virulence characteristics of translocating Escherichia coli and the interleukin-8 response to infection. Microb. Pathog. 2011, 50, 81–86. [Google Scholar] [CrossRef]
- Smith, S.N.; Hagan, E.C.; Lane, M.C.; Mobley, H.L. Dissemination and systemic colonization of uropathogenic Escherichia coli in a murine model of bacteremia. MBio 2010, 1. [Google Scholar] [CrossRef]
- Guarner, C.; Runyon, B.A.; Young, S.; Heck, M.; Sheikh, M.Y. Intestinal bacterial overgrowth and bacterial translocation in cirrhotic rats with ascites. J. Hepatol. 1997, 26, 372–1378. [Google Scholar] [CrossRef]
- Nettelbladt, C.-G.; Katouli, M.; Bark, T.; Svenberg, T.; Möllby, R.; Ljungqvist, O. Bulking fibre prevents translocation to mesenteric lymph nodes of an efficiently translocating Escherichia coli strain in rats. Clin. Nutr. 1998, 17, 185–190. [Google Scholar] [CrossRef]
- Dogan, B.; Fu, J.; Zhang, S.; Scherl, E.J.; Simpson, K.W. Rifaximin decreases virulence of Crohn’s disease-associated Escherichia coli and epithelial inflammatory responses. J. Antibiot. 2018, 71, 485–494. [Google Scholar] [CrossRef]
- Yang, Y.; Liao, Y.; Ma, Y.; Gong, W.; Zhu, G. The role of major virulence factors of AIEC involved in inflammatory bowl disease—A mini-review. Appl. Microbiol. Biotechnol. 2017, 101, 7781–7787. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.-M.; Elisia, I.; Kitts, D.D. Defining conditions for the co-culture of Caco-2 and HT29-MTX cells using Taguchi design. J. Pharmacol. Toxicol. Methods 2010, 61, 334–342. [Google Scholar] [CrossRef]
- Laparra, J.M.; Sanz, Y. Comparison of in vitro models to study bacterial adhesion to the intestinal epithelium. Lett. Appl. Microbiol. 2009, 49, 95–701. [Google Scholar] [CrossRef]
- Dostal, A.; Gagnon, M.; Chassard, C.; Zimmermann, M.B.; O’Mahony, L.; Lacroix, C. Salmonella adhesion, invasion and cellular immune responses are differentially affected by iron concentrations in a combined in vitro gut fermentation-cell model. PLoS ONE 2014, 9, e93549. [Google Scholar] [CrossRef]
- Arthur, J.C.; Perez-Chanona, E.; Mühlbauer, M.; Tomkovich, S.; Uronis, J.M.; Fan, T.J.; Campbell, B.J.; Abujamel, T.; Dogan, B.; Rogers, A.B.; et al. Intestinal inflammation targets cancer-inducing activity of the microbiota. Science 2012, 338, 120–123. [Google Scholar] [CrossRef] [PubMed]
- Ellermann, M.; Arthur, J.C. Siderophore-mediated iron acquisition and modulation of host-bacterial interactions. Free Radic. Biol. Med. 2017, 105, 68–78. [Google Scholar] [CrossRef] [PubMed]
- Johnsen, N.; Hamilton, A.D.; Greve, A.S.; Christensen, M.G.; Therkildsen, J.R.; Wehmöller, J.; Skals, M.; Praetorius, H.A. α-Haemolysin production, as a single factor, causes fulminant sepsis in a model of Escherichia coli-induced bacteraemia. Cell. Microbiol. 2019, 21, e13017. [Google Scholar] [CrossRef] [PubMed]
- Bingen-Bidois, M.; Clermont, O.; Bonacorsi, S.; Terki, M.; Brahimi, N.; Loukil, C.; Barraud, D.; Bingen, E. Phylogenetic analysis and prevalence of urosepsis strains of Escherichia coli bearing pathogenicity island-like domains. Infect. Immun. 2002, 70, 3216–3226. [Google Scholar] [CrossRef]
- Zhong, H.; Wang, P.; Chen, Y.; Wang, H.; Li, J.; Li, J.; Zhu, G.; Cui, L.; Meng, X. ClpV1 in avian pathogenic Escherichia coli is a crucial virulence factor contributing to meningitis in a mouse model in vivo. Vet. Microbiol. 2021, 263, 109273. [Google Scholar] [CrossRef]
- Pukatzki, S.; Ma, A.T.; Sturtevant, D.; Krastins, B.; Sarracino, D.; Nelson, W.C.; Heidelberg, J.F.; Mekalanos, J.J. Identification of a conserved bacterial protein secretion system in Vibrio cholerae using the Dictyostelium host model system. Proc. Natl. Acad. Sci. USA 2006, 103, 1528–1533. [Google Scholar] [CrossRef] [PubMed]
- Zoued, A.; Brunet, Y.R.; Durand, E.; Aschtgen, M.-S.; Logger, L.; Douzi, B.; Journet, L.; Cambillau, C.; Cascales, E. Architecture and assembly of the Type VI secretion system. Biochim. Biophys. Acta 2014, 1843, 1664–1673. [Google Scholar] [CrossRef]
- Russell, A.B.; Wexler, A.G.; Harding, B.N.; Whitney, J.C.; Bohn, A.J.; Goo, Y.A.; Tran, B.Q.; Barry, N.A.; Zheng, H.; Peterson, S.B.; et al. A type VI secretion-related pathway in Bacteroidetes mediates interbacterial antagonism. Cell Host Microbe 2014, 16, 227–236. [Google Scholar] [CrossRef] [PubMed]
- Czaja, C.A.; Stamm, W.E.; Stapleton, A.E.; Roberts, P.L.; Hawn, T.R.; Scholes, D.; Samadpour, M.; Hultgren, S.J.; Hooton, T.M. Prospective cohort study of microbial and inflammatory events immediately preceding Escherichia coli recurrent urinary tract infection in women. J. Infect. Dis. 2009, 200, 528–536. [Google Scholar] [CrossRef] [PubMed]
- Moreno, E.; Andreu, A.; Perez, T.; Sabaté, M.; Johnson, J.; Prats, G. Relationship between Escherichia coli strains causing urinary tract infection in women and the dominant faecal flora of the same hosts. Epidemiol. Infect. 2006, 134, 1015–1023. [Google Scholar] [CrossRef]
 , JM109
, JM109  , 46-4
, 46-4  , 73–89
, 73–89  and KIC-1
and KIC-1  . Only significant values are shown. * At two hours, E. coli HMLN-1 vs. KIC-1, JM109, 46-4 and 73–89 (p = 0.0243). All tests were conducted in triplicate, and the results were expresses as mean ± SEM.
. Only significant values are shown. * At two hours, E. coli HMLN-1 vs. KIC-1, JM109, 46-4 and 73–89 (p = 0.0243). All tests were conducted in triplicate, and the results were expresses as mean ± SEM.
 , JM109
, JM109  , 46-4
, 46-4  , 73–89
, 73–89  and KIC-1
and KIC-1  . Only significant values are shown. * At two hours, E. coli HMLN-1 vs. KIC-1, JM109, 46-4 and 73–89 (p = 0.0243). All tests were conducted in triplicate, and the results were expresses as mean ± SEM.
. Only significant values are shown. * At two hours, E. coli HMLN-1 vs. KIC-1, JM109, 46-4 and 73–89 (p = 0.0243). All tests were conducted in triplicate, and the results were expresses as mean ± SEM.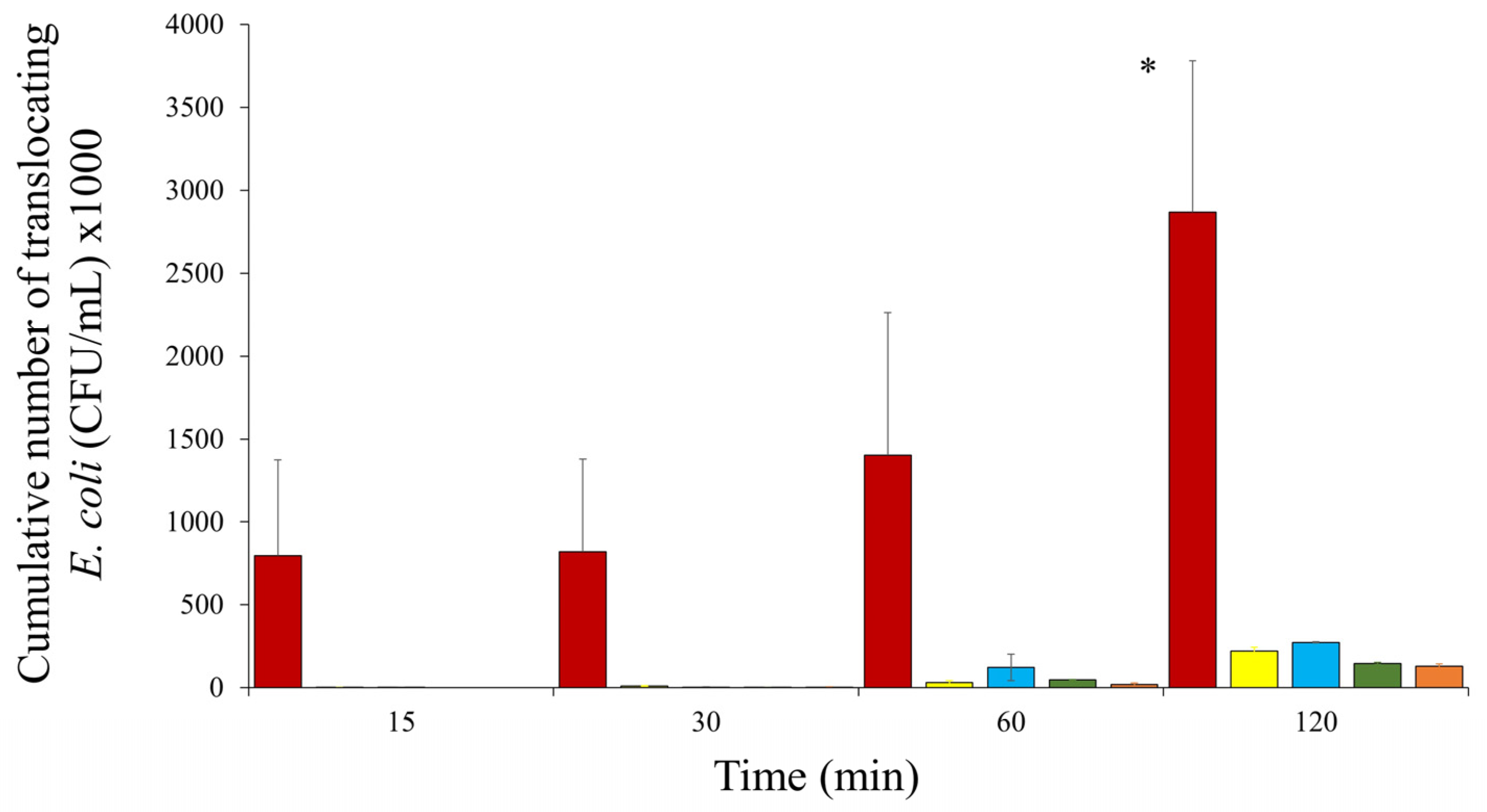
| Strains | Percentage Colonisation of Co-Culture Cells | No. of Diffusely Adhering Bacteria (CFU) per Cell | No. of Invading Bacteria (CFU) per Well | No. of Intra-Macrophagic Surviving Bacteria (CFU) per Well |
|---|---|---|---|---|
| HMLN-1 | 79% ± 1.2% | 5.7 ± 0.4 | 1.1 × 104 ± 1.1 × 103 | 475 ± 60 |
| E. coli JM109 | 23.3% ± 2.1% | 2.0 ± 0.2 | 1700 ± 245 | 90 ± 7 |
| p value | p < 0.0001 | p < 0.0001 | p = 0.0012 | p = 0.0238 |
| AIEC-Associated VGs | Iron Acquisition VGs | T6SS VGs | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| E. coli | htrA | ompC | lpfA | dsbA | clbA | afaC | hlyA | fyuA | iutA | ireA | iroN | clpV | vgrG |
| HMLN-1 | + | + | − | + | − | + | + | + | − | + | + | + | + |
| KIC-1 | + | + | − | + | − | + | − | + | + | − | − | − | − |
| 73–89 | − | + | − | − | − | − | − | + | + | − | − | − | − |
| 46-4 | − | − | − | − | − | − | − | − | + | − | − | − | − |
| JM109 | − | − | − | − | − | − | − | − | − | − | − | − | - |
| Source of Infection | E. coli Type | No. of C-Types Or s-Types | No. Isolates Represented | clpV | vgrG | Total (%) Positive |
|---|---|---|---|---|---|---|
| Adult CA-UTI | ExPEC | C1-C11 | 75 | 67 | 67 | 89% |
| Child HA-UTI | ExPEC | C12-C25 | 75 | 60 ** | 59 ** | 83% |
| Adult septicaemia | ExPEC | C26-C37 | 74 | 47 | 49 | 66% |
| Adult HA-UTI | ExPEC | S1-S39 | 40 | 17 | 17 | 43% |
| Child diarrhoea | IPEC | S1-S30 | 30 | 3 * | 3 * | 10% |
| Adult IBD | IPEC | S1-S24 | 25 | 8 | 8 | 32% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Asgari, B.; Burke, J.R.; Quigley, B.L.; Bradford, G.; Hatje, E.; Kuballa, A.; Katouli, M. Identification of Virulence Genes Associated with Pathogenicity of Translocating Escherichia coli with Special Reference to the Type 6 Secretion System. Microorganisms 2024, 12, 1851. https://doi.org/10.3390/microorganisms12091851
Asgari B, Burke JR, Quigley BL, Bradford G, Hatje E, Kuballa A, Katouli M. Identification of Virulence Genes Associated with Pathogenicity of Translocating Escherichia coli with Special Reference to the Type 6 Secretion System. Microorganisms. 2024; 12(9):1851. https://doi.org/10.3390/microorganisms12091851
Chicago/Turabian StyleAsgari, Behnoush, Jarred R. Burke, Bonnie L. Quigley, Georgia Bradford, Eva Hatje, Anna Kuballa, and Mohammad Katouli. 2024. "Identification of Virulence Genes Associated with Pathogenicity of Translocating Escherichia coli with Special Reference to the Type 6 Secretion System" Microorganisms 12, no. 9: 1851. https://doi.org/10.3390/microorganisms12091851







