Halotolerant Endophytic Bacteria Priestia flexa 7BS3110 with Hg2+ Tolerance Isolated from Avicennia germinans in a Caribbean Mangrove from Colombia
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Area and Sampling
2.2. Isolation of Endophytic Bacteria from Avicennia germinans
2.3. Tolerance to NaCl
2.4. Screening of Heavy Metal Tolerance in Strain 7BS3110
2.5. Genomic Analyses of Strain 7BS3110
2.6. Evaluation of the Effect of Mercury on the Cell Structure of P. flexa 7BS3110
2.7. Scanning Electron Microscope (SEM-EDX)
2.8. Transmission Electron Microscope (TEM)
2.9. Inductively Coupled Plasma Mass Spectrometry (ICP-MS)
2.10. Mercury Tolerance at Different Concentrations of NaCl
3. Results
3.1. Screening for NaCl Tolerance of Endophytic Bacterial Isolates
3.2. Screening of Heavy Metal Tolerance of Isolate 7BS3110
3.3. General Characteristics of the Genome and Taxonomic Identification of the Strain with Greater Tolerance to NaCl
3.4. Identification of Heavy Metal Tolerance Proteins Sequences in P. flexa 7BS3110
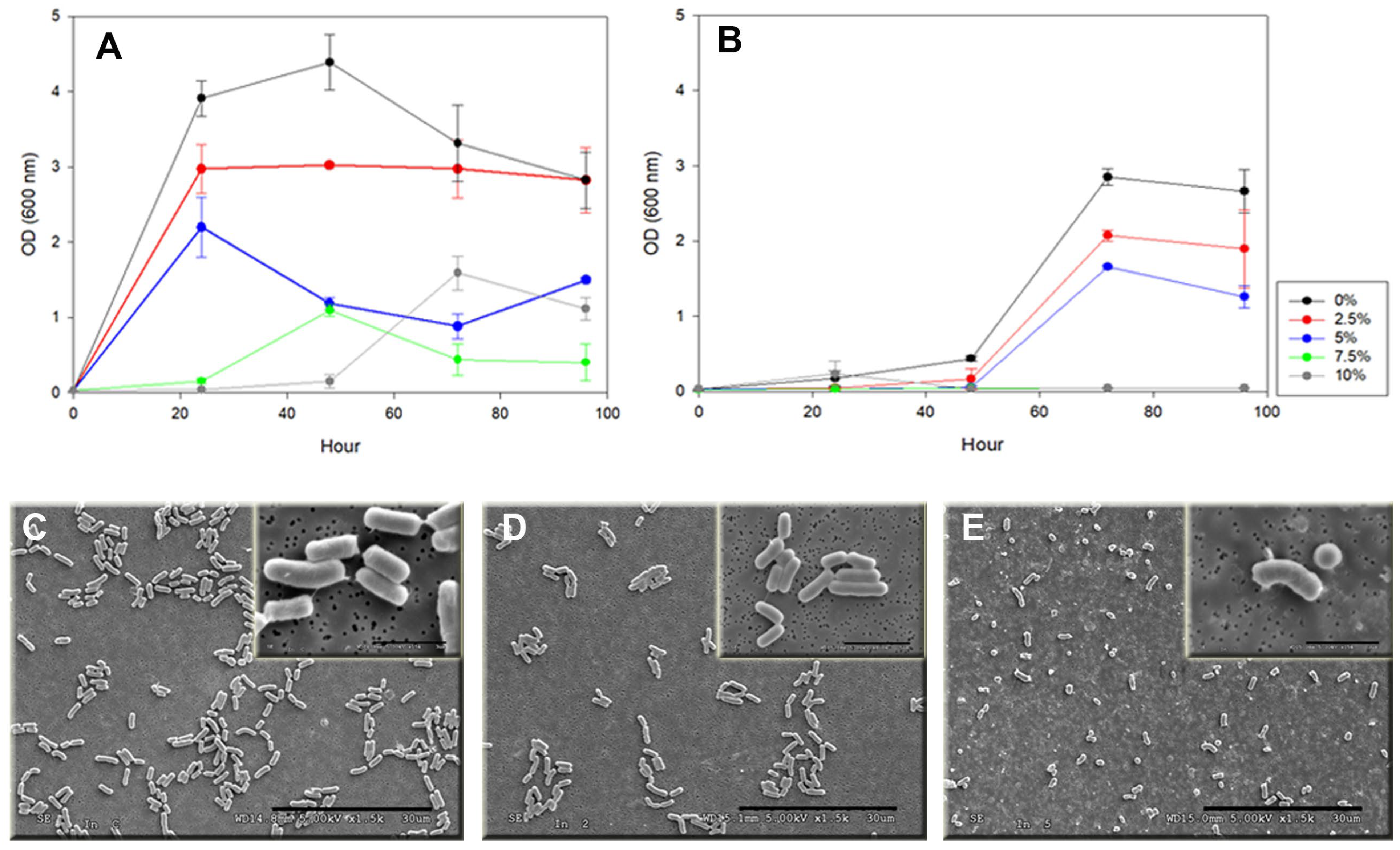
3.5. Effect of Hg2+ on Cell Morphology and Growth of P. flexa 7BS3110
3.6. Capacity to Reduce the Concentration of Hg2+ in Solution by P. flexa 7BS3110
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kochoni, B.I.; Avakoudjo, H.G.G.; Kamelan, T.M.; Sinsin, C.B.L.; Kouamelan, E.P. Contribution of Mangroves Ecosystems to Coastal Communities’ Resilience towards Climate Change: A Case Study in Southern Cote d’Ivoire. GeoJournal 2023, 88, 3935–3951. [Google Scholar] [CrossRef]
- Alongi, D.M. The Impact of Climate Change on Mangrove Forests. Curr. Clim. Chang. Rep. 2015, 1, 30–39. [Google Scholar] [CrossRef]
- Villate Daza, D.A.; Moreno, H.S.; Portz, L.; Manzolli, R.P.; Bolívar-Anillo, H.J.; Anfuso, G. Mangrove Forests Evolution and Threats in the Caribbean Sea of Colombia. Water 2020, 12, 1113. [Google Scholar] [CrossRef]
- Bolívar-Anillo, H.J.; Anfuso, G.; Chacón Abarca, S.; Badillo Romero, M.D.; Villate Daza, D.A.; Serrano, M.C.; Sánchez Moreno, H. Natural Processes and Human Actuations: Impacts on Mangrove Forests of South America. Rev. Costas 2020, 2, 211–232. [Google Scholar] [CrossRef]
- Agudelo, C.M.; Bolívar, J.; Polanía, J.; Urrego, L.E.; Yepes, A.; Sierra, A. Estructura y Composición Florística de Los Manglares de La Bahía de Cispatá, Caribe Colombiano. Rev. Biol. Trop. 2015, 63, 1137–1147. [Google Scholar] [CrossRef]
- Valero, N.O.; Barraza, B.; Medina, A.M. Un Escenario Para El Uso de Microorganismos Del Manglar Como Inoculantes Microbianos En Colombia. Biociencias 2011, 6, 97–103. [Google Scholar] [CrossRef]
- Sánchez-Moreno, H.; Bolívar-Anillo, H.J.; Villate-Daza, D.A.; Escobar-Olaya, G.; Anfuso, G. Influencia de Los Impactos Antrópicos Sobre La Evolución Del Bosque de Manglar En Puerto Colombia (Mar Caribe Colombiano). Rev. Latinoam. Recur. Nat. 2019, 15, 1–16. [Google Scholar]
- Kulkarni, R.; Deobagkar, D.; Zinjarde, S. Metals in Mangrove Ecosystems and Associated Biota: A Global Perspective. Ecotoxicol. Environ. Saf. 2018, 153, 215–228. [Google Scholar] [CrossRef] [PubMed]
- Milly, P.C.D.; Dunne, K.A.; Vecchia, A. V Global Pattern of Trends in Streamflow and Water Availability in a Changing Climate. Nature 2005, 438, 347–350. [Google Scholar] [CrossRef] [PubMed]
- Fuentes-Gandara, F.; Pinedo-Hernández, J.; Gutiérrez, E.; Marrugo-Negrete, J.; Díez, S. Heavy Metal Pollution and Toxicity Assessment in Mallorquin Swamp: A Natural Protected Heritage in the Caribbean Sea, Colombia. Mar. Pollut. Bull. 2021, 167, 112271. [Google Scholar] [CrossRef]
- Fuentes-Gandara, F.; Pinedo-Hernández, J.; Marrugo-Negrete, J.; Díez, S. Human Health Impacts of Exposure to Metals through Extreme Consumption of Fish from the Colombian Caribbean Sea. Environ. Geochem. Health 2018, 40, 229–242. [Google Scholar] [CrossRef] [PubMed]
- Alonso, D.; Pineda, P.; Olivero, J.; González, H.; Campos, N. Mercury Levels in Muscle of Two Fish Species and Sediments from the Cartagena Bay and the Cienaga Grande de Santa Marta, Colombia. Environ. Pollut. 2000, 109, 157–163. [Google Scholar] [CrossRef] [PubMed]
- Vareda, J.P.; Valente, A.J.M.; Durães, L. Assessment of Heavy Metal Pollution from Anthropogenic Activities and Remediation Strategies: A Review. J. Environ. Manag. 2019, 246, 101–118. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Zhou, K.; Qin, W.; Tian, C.; Qi, M.; Yan, X.; Han, W. A Review on Heavy Metals Contamination in Soil: Effects, Sources, and Remediation Techniques. Soil Sediment Contam. 2019, 28, 380–394. [Google Scholar] [CrossRef]
- Briffa, J.; Sinagra, E.; Blundell, R. Heavy Metal Pollution in the Environment and Their Toxicological Effects on Humans. Heliyon 2020, 6, e04691. [Google Scholar] [CrossRef] [PubMed]
- Akpor, O.B.; Muchie, M. Remediation of Heavy Metals in Drinking Water and Wastewater Treatment Systems: Processes and Applications. Int. J. Phys. Sci. 2010, 5, 1807–1817. [Google Scholar] [CrossRef]
- Tchounwou, P.B.; Yedjou, C.G.; Patlolla, A.K.; Sutton, D.J. Heavy Metal Toxicity and the Environment. In Molecular, Clinical and Environmental Toxicology, 1st ed.; Luch, A., Ed.; Springer: Basel, Switzerland, 2012; Volume 101, pp. 133–164. [Google Scholar] [CrossRef]
- Sharma, P.; Kumar, S. Bioremediation of Heavy Metals from Industrial Effluents by Endophytes and Their Metabolic Activity: Recent Advances. Bioresour. Technol. 2021, 339, 125589. [Google Scholar] [CrossRef] [PubMed]
- Lata, R.; Chowdhury, S.; Gond, S.K.; White, J.F. Induction of Abiotic Stress Tolerance in Plants by Endophytic Microbes. Lett. Appl. Microbiol. 2018, 66, 268–276. [Google Scholar] [CrossRef]
- Khare, E.; Mishra, J.; Arora, N.K. Multifaceted Interactions between Endophytes and Plant: Developments and Prospects. Front. Microbiol. 2018, 9, 2732. [Google Scholar] [CrossRef]
- Tiwari, S.; Lata, C. Heavy Metal Stress, Signaling, and Tolerance Due to Plant-Associated Microbes: An Overview. Front. Plant Sci. 2018, 9, 452. [Google Scholar] [CrossRef]
- Srivastava, S.; Verma, P.C.; Chaudhry, V.; Singh, N.; Abhilash, P.C.; Kumar, K.V.; Sharma, N.; Singh, N. Influence of Inoculation of Arsenic-Resistant Staphylococcus arlettae on Growth and Arsenic Uptake in Brassica juncea (L.) Czern. Var. R-46. J. Hazard. Mater. 2013, 262, 1039–1047. [Google Scholar] [CrossRef] [PubMed]
- Narayanan, M.; Ma, Y. Mitigation of Heavy Metal Stress in the Soil through Optimized Interaction between Plants and Microbes. J. Environ. Manag. 2023, 345, 118732. [Google Scholar] [CrossRef] [PubMed]
- Tiwari, P.; Bae, H. Trends in Harnessing Plant Endophytic Microbiome for Heavy Metal Mitigation in Plants: A Perspective. Plants 2023, 12, 1515. [Google Scholar] [CrossRef] [PubMed]
- Ghuge, S.A.; Nikalje, G.C.; Kadam, U.S.; Suprasanna, P.; Hong, J.C. Comprehensive Mechanisms of Heavy Metal Toxicity in Plants, Detoxification, and Remediation. J. Hazard. Mater. 2023, 450, 131039. [Google Scholar] [CrossRef] [PubMed]
- Priya, E.M.; Tamilselvi, K.S. Biotransformation of Heavy Metals by Plant Growth Promoting Endophytic Bacteria: An Assessment. Indones. J. Soc. Environ. Issues 2023, 4, 36–44. [Google Scholar] [CrossRef]
- Roy, R.; Samanta, S.; Pandit, S.; Naaz, T.; Banerjee, S.; Rawat, J.M.; Chaubey, K.K.; Saha, R.P. An Overview of Bacteria-Mediated Heavy Metal Bioremediation Strategies. Appl. Biochem. Biotechnol. 2023, 196, 1712–1751. [Google Scholar] [CrossRef] [PubMed]
- Nanda, M.; Kumar, V.; Sharma, D.K. Multimetal Tolerance Mechanisms in Bacteria: The Resistance Strategies Acquired by Bacteria That Can Be Exploited to ‘Clean-up’ Heavy Metal Contaminants from Water. Aquat. Toxicol. 2019, 212, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Dave, D.; Sarma, S.; Parmar, P.; Shukla, A.; Goswami, D.; Shukla, A.; Saraf, M. Microbes as a Boon for the Bane of Heavy Metals. Environ. Sustain. 2020, 3, 233–255. [Google Scholar] [CrossRef]
- Liaqat, I.; Muhammad, N.; Ara, C.; Hanif, U.; Andleeb, S.; Arshad, M.; Aftab, M.N.; Raza, C.; Mubin, M. Bioremediation of Heavy Metals Polluted Environment and Decolourization of Black Liquor Using Microbial Biofilms. Mol. Biol. Rep. 2023, 50, 3985–3997. [Google Scholar] [CrossRef] [PubMed]
- Andy, A.K.; Rajput, V.D.; Burachevskaya, M.; Gour, V.S. Exploring the Identity and Properties of Two Bacilli Strains and Their Potential to Alleviate Drought and Heavy Metal Stress. Horticulturae 2023, 9, 46. [Google Scholar] [CrossRef]
- Lu, H.; Xia, C.; Chinnathambi, A.; Nasif, O.; Narayanan, M.; Shanmugam, S.; Lan Chi, N.T.; Pugazhendhi, A.; On-uma, R.; Jutamas, K.; et al. Optimistic Influence of Multi-Metal Tolerant Bacillus Species on Phytoremediation Potential of Chrysopogon zizanioides on Metal Contaminated Soil. Chemosphere 2023, 311, 136889. [Google Scholar] [CrossRef]
- Gupta, R.S.; Patel, S.; Saini, N.; Chen, S. Robust Demarcation of 17 Distinct Bacillus Species Clades, Proposed as Novel Bacillaceae Genera, by Phylogenomics and Comparative Genomic Analyses: Description of Robertmurraya kyonggiensis sp. Nov. and Proposal for an Emended Genus Bacillus Limiting It Only to the Members of the Subtilis and Cereus Clades of Species. Int. J. Syst. Evol. Microbiol. 2020, 70, 5753–5798. [Google Scholar] [CrossRef]
- Chathalingath, N.; Kingsly, J.S.; Gunasekar, A. Biosynthesis and biodegradation of poly(3-hydroxybutyrate) from Priestia flexa; A promising mangrove halophyte towards the development of sustainable eco-friendly bioplastics. Microbiol. Res. 2023, 267, 127270. [Google Scholar] [CrossRef]
- Shukla, A.; Gupta, A.; Srivastava, S. Bacterial Consortium (Priestia endophytica NDAS01F, Bacillus licheniformis NDSA24R, and Priestia flexa NDAS28R) and Thiourea Mediated Amelioration of Arsenic Stress and Growth Improvement of Oryza sativa L. Plant Physiol. Biochem. 2023, 195, 14–24. [Google Scholar] [CrossRef]
- Abou-Shanab, R.A.I.; van Berkum, P.; Angle, J.S. Heavy Metal Resistance and Genotypic Analysis of Metal Resistance Genes in Gram-Positive and Gram-Negative Bacteria Present in Ni-Rich Serpentine Soil and in the Rhizosphere of Alyssum murale. Chemosphere 2007, 68, 360–367. [Google Scholar] [CrossRef]
- Ugboma, C.J.; Sampson, T.; Mbonu, N.E. Bioremediation of Heavy Metals from Artisanal Crude Oil Refinery (Kpo-Fire) Impacted Soil Using Bacillus flexus and Pseudomonas aeruginosa in Ngie Community, Degema Local Government Area, Rivers State, Nigeria. J. Appl. Sci. Environ. Manag. 2021, 24, 2049–2054. [Google Scholar] [CrossRef]
- Jebeli, M.A.; Maleki, A.; Amoozegar, M.A.; Kalantar, E.; Izanloo, H.; Gharibi, F. Bacillus flexus Strain As-12, a New Arsenic Transformer Bacterium Isolated from Contaminated Water Resources. Chemosphere 2017, 169, 636–641. [Google Scholar] [CrossRef]
- Fundación Guardaguas de Ecosistemas Marinos y Costeros “Bocas de Ceniza Waterkeeper”. Programa de Conservación y Restauración de los Ecosistemas Costeros del Departamento del Atlántico: Medidas de Prevención, Control y Reducción de Fuentes Terrestres de Contaminación y Procesos de Erosión Costera en una Primera Fase. Available online: https://www.bocasdecenizawaterkeeper.org/sites/default/files/EBOOK%20FUNDACION%20GUARDAGUAS.pdf (accessed on 22 July 2023).
- Instituto de Investigaciones Marinas y Costeras (INVEMAR). Ordenamiento Ambiental de La Zona Costera Del Departamento Del Atlántico 2007. Available online: http://www.invemar.org.co/redcostera1/invemar/docs/zcatlantico.pdf (accessed on 8 November 2023).
- Instituto de Investigaciones Marinas y Costeras (INVEMAR). Informe del Estado de los Ambientes Marinos y Costeros en Colombia: Año 2000. Available online: https://www.invemar.org.co/redcostera1/invemar/docs/EAMC_2000/INVEMAR_INF_EAMC_2000_02.pdf (accessed on 4 December 2023).
- Potshangbam, M.; Devi, I.; Sahoo, D.; Strobel, G. Functional Characterization of Endophytic Fungal Community Associated with Oryza sativa L. and Zea mays L. Front. Microbiol. 2017, 8, 325. [Google Scholar] [CrossRef]
- Shahid, M.; Hameed, S.; Tariq, M.; Zafar, M.; Ali, A.; Ahmad, N. Characterization of Mineral Phosphate-Solubilizing Bacteria for Enhanced Sunflower Growth and Yield-Attributing Traits. Ann. Microbiol. 2015, 65, 1525–1536. [Google Scholar] [CrossRef]
- Wilson, K. Preparation of Genomic DNA from Bacteria. Curr. Protoc. Mol. Biol. 2001, 56, 2–4. [Google Scholar] [CrossRef]
- Bolger, A.M.; Lohse, M.; Usadel, B. Trimmomatic: A Flexible Trimmer for Illumina Sequence Data. Bioinformatics 2014, 30, 2114–2120. [Google Scholar] [CrossRef]
- Rozov, R.; Kav, A.B.; Bogumil, D.; Shterzer, N.; Halperin, E.; Mizrahi, I.; Shamir, R. Recycler: An Algorithm for Detecting Plasmids from de Novo Assembly Graphs. Bioinformatics 2017, 33, 475–482. [Google Scholar] [CrossRef]
- Boetzer, M.; Henkel, C.V.; Jansen, H.J.; Butler, D.; Pirovano, W. Scaffolding Pre-Assembled Contigs Using SSPACE. Bioinformatics 2011, 27, 578–579. [Google Scholar] [CrossRef]
- Nadalin, F.; Vezzi, F.; Policriti, A. GapFiller: A de Novo Assembly Approach to Fill the Gap within Paired Reads. BMC Bioinform. 2012, 13, S8. [Google Scholar] [CrossRef]
- Mikheenko, A.; Prjibelski, A.; Saveliev, V.; Antipov, D.; Gurevich, A. Versatile Genome Assembly Evaluation with QUAST-LG. Bioinformatics 2018, 34, i142–i150. [Google Scholar] [CrossRef]
- Rodriguez-R, L.M.; Gunturu, S.; Harvey, W.T.; Rosselló-Mora, R.; Tiedje, J.M.; Cole, J.R.; Konstantinidis, K.T. The Microbial Genomes Atlas (MiGA) Webserver: Taxonomic and Gene Diversity Analysis of Archaea and Bacteria at the Whole Genome Level. Nucleic Acids Res. 2018, 46, W282–W288. [Google Scholar] [CrossRef]
- Meier-Kolthoff, J.P.; Auch, A.F.; Klenk, H.-P.; Göker, M. Genome Sequence-Based Species Delimitation with Confidence Intervals and Improved Distance Functions. BMC Bioinform. 2013, 14, 60. [Google Scholar] [CrossRef]
- Chun, J.; Oren, A.; Ventosa, A.; Christensen, H.; Arahal, D.R.; da Costa, M.S.; Rooney, A.P.; Yi, H.; Xu, X.W.; De Meyer, S.; et al. Proposed Minimal Standards for the Use of Genome Data for the Taxonomy of Prokaryotes. Int. J. Syst. Evol. Microbiol. 2018, 68, 461–466. [Google Scholar] [CrossRef]
- Seemann, T. Prokka: Rapid Prokaryotic Genome Annotation. Bioinformatics 2014, 30, 2068–2069. [Google Scholar] [CrossRef]
- Aziz, R.K.; Bartels, D.; Best, A.; DeJongh, M.; Disz, T.; Edwards, R.A.; Formsma, K.; Gerdes, S.; Glass, E.M.; Kubal, M.; et al. The RAST Server: Rapid Annotations Using Subsystems Technology. BMC Genom. 2008, 9, 75. [Google Scholar] [CrossRef]
- Paysan-Lafosse, T.; Blum, M.; Chuguransky, S.; Grego, T.; Pinto, B.L.; Salazar, G.A.; Bileschi, M.L.; Bork, P.; Bridge, A.; Colwell, L.; et al. InterPro in 2022. Nucleic Acids Res. 2023, 51, D418–D427. [Google Scholar] [CrossRef]
- Reynolds, E.S. The Use of Lead Citrate at High pH as an Electron-Opaque Stain in Electron Microscopy. J. Cell. Biol. 1963, 17, 208–212. [Google Scholar] [CrossRef]
- Bagchi, D.; Stohs, S.J.; Downs, B.W.; Bagchi, M.; Preuss, H.G. Cytotoxicity and Oxidative Mechanisms of Different Forms of Chromium. Toxicology 2002, 180, 5–22. [Google Scholar] [CrossRef]
- Mehdi, Z.; Jamshid, G.G.; Yahya, E.; Fayyaz, E. Effects of Different Levels of Copper Sulfate on Performance in Japanese Quail (Coturnix coturnix japonica). Int. J. Biosci. 2014, 4, 387–392. [Google Scholar] [CrossRef]
- Jakubowski, M. Zinc and Cadmium Compounds. In Patty’s Toxicology; Bingham, E., Cohrssen, B., Eds.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2012; ISBN 978-0-470-41081-3. [Google Scholar]
- U.S. Environmental Protection Agency (EPA). Reregistration Eligibility Decision (RED) for Coppers; Office of Pesticide Programs, Ed.; U.S. Government Printing Office: Washington, DC, USA, 2009.
- Al-Zubaidi, E.S.; Rabee, A.M. Effect of Mercuric Chloride on Biochemical and Hematological Parameters in Male Albino Mice. Int. J. Sci. Res. 2015, 6, 2319–7064. [Google Scholar] [CrossRef]
- Apaydin, F.G.; Kalender, S.; Bas, H.; Demir, F.; Kalender, Y. Lead Nitrate Induced Testicular Toxicity in Diabetic and Non-Diabetic Rats: Protective Role of Sodium Selenite. Braz. Arch. Biol. Technol. 2015, 58, 68–74. [Google Scholar] [CrossRef]
- Henderson, R.G.; Durando, J.; Oller, A.R.; Merkel, D.J.; Marone, P.A.; Bates, H.K. Acute Oral Toxicity of Nickel Compounds. Regul. Toxicol. Pharmacol. 2012, 62, 425–432. [Google Scholar] [CrossRef]
- Diaby, V.; Adou Yapo, F.; Arsène Mousan, A.; Dosso, M.; Allico Djama, J.; Vandjiguiba, D.; Adou Francis, Y.; Mireille, D.; Allico Joseph, D. Renal, Hepatic and Splenic Biotoxicity of Cadmium Sulphate in the Wistar Rats. Int. J. Environ. Sci. Technol. Res. 2016, 4, 103–110. [Google Scholar]
- Toropov, V.; Demyanova, E.; Shalaeva, O.; Sitkin, S.; Vakhitov, T. Whole-Genome Sequencing of Lactobacillus Helveticus D75 and D76 Confirms Safety and Probiotic Potential. Microorganisms 2020, 8, 329. [Google Scholar] [CrossRef]
- Wei, X.; Chen, Z.; Liu, A.; Yang, L.; Xu, Y.; Cao, M.; He, N. Advanced Strategies for Metabolic Engineering of Bacillus to Produce Extracellular Polymeric Substances. Biotechnol. Adv. 2023, 67, 108199. [Google Scholar] [CrossRef]
- Priyadarshanee, M.; Chatterjee, S.; Rath, S.; Dash, H.R.; Das, S. Cellular and Genetic Mechanism of Bacterial Mercury Resistance and Their Role in Biogeochemistry and Bioremediation. J. Hazard. Mater. 2022, 423, 126985. [Google Scholar] [CrossRef]
- Van Kranenburg, R.; Marugg, J.D.; Van Swam, I.I.; Willem, N.J.; De Vos, W.M. Molecular Characterization of the Plasmid-encoded Eps Gene Cluster Essential for Exopolysaccharide Biosynthesis in Lactococcus lactis. Mol. Microbiol. 1997, 24, 387–397. [Google Scholar] [CrossRef]
- Wu, R.; Qin, Y.; Shen, Q.; Li, P. The Complete Genome Sequence of Bacillus Velezensis LPL061, an Exopolysaccharide-Producing Bacterium. 3 Biotech 2020, 10, 243. [Google Scholar] [CrossRef]
- Newman, J.A.; Rodrigues, C.; Lewis, R.J. Molecular Basis of the Activity of SinR Protein, the Master Regulator of Biofilm Formation in Bacillus subtilis. J. Biol. Chem. 2013, 288, 10766–10778. [Google Scholar] [CrossRef]
- Palmiter, R.D. Molecular Biology of Metallothionein Gene Expression. In Metallothionein II; Kägi, J.H.R., Kojima, Y., Eds.; Birkhäuser: Basel, Switzerland, 1987; Volume 52, pp. 63–80. [Google Scholar] [CrossRef]
- Domı́nguez-Solı́s, J.R.; Gutiérrez-Alcalá, G.; Romero, L.C.; Gotor, C. The Cytosolic O-Acetylserine (Thiol)Lyase Gene Is Regulated by Heavy Metals and Can Function in Cadmium Tolerance. J. Biol. Chem. 2001, 276, 9297–9302. [Google Scholar] [CrossRef]
- Ndu, U.; Barkay, T.; Mason, R.P.; Schartup, A.T.; Al-Farawati, R.; Liu, J.; Reinfelder, J.R. The Use of a Mercury Biosensor to Evaluate the Bioavailability of Mercury-Thiol Complexes and Mechanisms of Mercury Uptake in Bacteria. PLoS ONE 2015, 10, e0138333. [Google Scholar] [CrossRef]
- Chandrangsu, P.; Van Loi, V.; Antelmann, H.; Helmann, J.D. The Role of Bacillithiol in Gram-Positive Firmicutes. Antioxid. Redox Signal. 2018, 28, 445–462. [Google Scholar] [CrossRef]
- Hobman, J.L.; Julian, D.J.; Brown, N.L. Cysteine Coordination of Pb(II) Is Involved in the PbrR-Dependent Activation of the Lead-Resistance Promoter, PpbrA, from Cupriavidus metallidurans CH34. BMC Microbiol. 2012, 12, 109. [Google Scholar] [CrossRef]
- Nong, Q.; Yuan, K.; Li, Z.; Chen, P.; Huang, Y.; Hu, L.; Jiang, J.; Luan, T.; Chen, B. Bacterial Resistance to Lead: Chemical Basis and Environmental Relevance. J. Environ. Sci. 2019, 85, 46–55. [Google Scholar] [CrossRef]
- Jarosławiecka, A.; Piotrowska-Seget, Z. Lead Resistance in Micro-Organisms. Microbiology 2014, 160, 12–25. [Google Scholar] [CrossRef]
- Maglangit, F.; Alrashdi, S.; Renault, J.; Trembleau, L.; Victoria, C.; Tong, M.H.; Wang, S.; Kyeremeh, K.; Deng, H. Characterization of the Promiscuous N -Acyl CoA Transferase, LgoC, in Legonoxamine Biosynthesis. Org. Biomol. Chem. 2020, 18, 2219–2222. [Google Scholar] [CrossRef]
- Rajkumar, M.; Ae, N.; Prasad, M.N.V.; Freitas, H. Potential of Siderophore-Producing Bacteria for Improving Heavy Metal Phytoextraction. Trends Biotechnol. 2010, 28, 142–149. [Google Scholar] [CrossRef]
- Carroll, C.S.; Moore, M.M. Ironing out Siderophore Biosynthesis: A Review of Non-Ribosomal Peptide Synthetase (NRPS)-Independent Siderophore Synthetases. Crit. Rev. Biochem. Mol. Biol. 2018, 53, 356–381. [Google Scholar] [CrossRef]
- He, Y.; Dong, L.; Zhou, S.; Jia, Y.; Gu, R.; Bai, Q.; Gao, J.; Li, Y.; Xiao, H. Chromium Resistance Characteristics of Cr(VI) Resistance Genes ChrA and ChrB in Serratia sp. S2. Ecotoxicol. Environ. Saf. 2018, 157, 417–423. [Google Scholar] [CrossRef]
- Ramírez-Díaz, M.I.; Díaz-Pérez, C.; Vargas, E.; Riveros-Rosas, H.; Campos-García, J.; Cervantes, C. Mechanisms of Bacterial Resistance to Chromium Compounds. BioMetals 2008, 21, 321–332. [Google Scholar] [CrossRef]
- Viti, C.; Marchi, E.; Decorosi, F.; Giovannetti, L. Molecular Mechanisms of Cr(VI) Resistance in Bacteria and Fungi. FEMS Microbiol. Rev. 2014, 38, 633–659. [Google Scholar] [CrossRef]
- Fathima, A.; Rao, J.R. Is Cr(III) Toxic to Bacteria: Toxicity Studies Using Bacillus subtilis and Escherichia coli as Model Organism. Arch. Microbiol. 2018, 200, 453–462. [Google Scholar] [CrossRef]
- Reclus, É. Viaje a La Sierra Nevada de Santa Marta, 1st ed.; Editorial Unimagdalena: Santa Marta, Colombia, 2020. [Google Scholar] [CrossRef]
- Restrepo, J.D.; López, S.A. Morphodynamics of the Pacific and Caribbean Deltas of Colombia, South America. J. S. Am. Earth Sci. 2008, 25, 1–21. [Google Scholar] [CrossRef]
- Anfuso, G.; Rangel-Buitrago, N.; Correa Arango, I.D. Evolution of Sandspits Along the Caribbean Coast of Colombia: Natural and Human Influences. In Sand and Gravel Spits, 1st ed.; Randazzo, G., Jackson, D.W.T., Cooper, J.A.G., Eds.; Springer International Publishing: Cham, Switzerland, 2015; pp. 1–19. [Google Scholar] [CrossRef]
- Ortiz-Romero, L.T.; Delgado-Tascón, J.G.; Pardo-Rodríguez, D.A.; Murillo-Perea, E.; Guio Duque, A.J. Heavy Metals Determination and Quality Indexes in Water and Sediments from Magdalena River–Tolima Tract, Colombia. Rev. Tumbaga 2015, 2, 43–60. [Google Scholar]
- Instituto de Investigaciones Marinas y Costeras (INVEMAR). Diagnóstico y Evaluación de La Calidad de Las Aguas Marinas y Costeras En El Caribe y Pacífico Colombianos 2007. Available online: https://alfresco.invemar.org.co/share/s/q1nxpommtz-f0khzp_kh6a (accessed on 5 June 2023).
- Chacón Serrano, S.; Serrano, M.C.; Bolívar-Anillo, H.J.; Villate Daza, D.A.; Sánchez Moreno, H.; Anfuso, G. Bosques de Manglar Del Caribe Norte Colombiano: Análisis, Evolución y Herramientas de Gestión. Rev. Latinoam. Recur. Nat. 2020, 16, 31–54. [Google Scholar]
- Arumugam, G.; Rajendran, R.; Ganesan, A.; Sethu, R. Bioaccumulation and Translocation of Heavy Metals in Mangrove Rhizosphere Sediments to Tissues of Avicennia Marina–A Field Study from Tropical Mangrove Forest. Environ. Nanotechnol. Monit. Manag. 2018, 10, 272–279. [Google Scholar] [CrossRef]
- Abeywardhana, D.C.; Adikaram, N.M.; Kularatne, R.K.A. Are Mangrove Forests Reliable Sinks of Heavy Metals Due to Phytoremediation and Other Mechanisms? A Sri Lankan Perspective. Mar. Pollut. Bull. 2022, 177, 113453. [Google Scholar] [CrossRef]
- Analuddin, K.; Sharma, S.; Jamili; Septiana, A.; Sahidin, I.; Rianse, U.; Nadaoka, K. Heavy Metal Bioaccumulation in Mangrove Ecosystem at the Coral Triangle Ecoregion, Southeast Sulawesi, Indonesia. Mar. Pollut. Bull. 2017, 125, 472–480. [Google Scholar] [CrossRef]
- Yan, Z.; Sun, X.; Xu, Y.; Zhang, Q.; Li, X. Accumulation and Tolerance of Mangroves to Heavy Metals: A Review. Curr. Pollut. Rep. 2017, 3, 302–317. [Google Scholar] [CrossRef]
- Almahasheer, H.; Serrano, O.; Duarte, C.M.; Irigoien, X. Remobilization of Heavy Metals by Mangrove Leaves. Front. Mar. Sci. 2018, 5, 484. [Google Scholar] [CrossRef]
- Margesin, R.; Schinner, F. Potential of Halotolerant and Halophilic Microorganisms for Biotechnology. Extremophiles 2001, 5, 73–83. [Google Scholar] [CrossRef]
- Sebastianes, F.L.S.; de Azevedo, J.L.; Lacava, P.T. Diversity and Biotechnological Potential of Endophytic Microorganisms Associated with Tropical Mangrove Forests. In Diversity and Benefits of Microorganisms from the Tropics, 1st ed.; De Azevedo, J.L., Quecine, M.C., Eds.; Springer: Cham, Switzerland, 2017; pp. 37–56. [Google Scholar] [CrossRef]
- Munawar, M.; Hakeem, K.R.; Alharby, H.F.; Anwar, Y.; Bamagoos, A.; Shah, M.; Abdul Sattar, F.; Sayeed Akhtar, M. Isolation and Identification of Salt-Tolerant Mangrove Endophyte from Red Sea Coast Jeddah, Saudi Arabia. Energy Environ. Focus 2023, 7, 48–53. [Google Scholar] [CrossRef]
- Li, F.N.; Zheng, Z.Q.; Chen, M.S.; Chen, X.H.; Tuo, L. Ancylobacter mangrovi sp. Nov., a Novel Endophytic Bacterium Isolated Form Mangrove Plant. Syst. Appl. Microbiol. 2023, 46, 126419. [Google Scholar] [CrossRef]
- Deepika, D.S.; Lavanya, J.; Sridevi, M. Screening of Salt Tolerant Endophytic Bacteria with Plant Growth Promoting Characters Isolated from Acanthus ilicifolius L., a Species of Mangrove Ecosystem Located at Corangi Wildlife Sanctuary, Andhra Pradesh. J. Appl. Nat. Sci. 2023, 15, 518–525. [Google Scholar] [CrossRef]
- Soldan, R.; Mapelli, F.; Crotti, E.; Schnell, S.; Daffonchio, D.; Marasco, R.; Fusi, M.; Borin, S.; Cardinale, M. Bacterial Endophytes of Mangrove Propagules Elicit Early Establishment of the Natural Host and Promote Growth of Cereal Crops under Salt Stress. Microbiol. Res. 2019, 223–225, 33–43. [Google Scholar] [CrossRef]
- Lavanya, J.; Sandhya Deepika, D.; Sridevi, M. Screening and Isolation of Plant Growth Promoting, Halotolerant Endophytic Bacteria from Mangrove Plant Avicennia officinalis L. at Coastal Region of Corangi Andhra Pradesh. Agric. Sci. Dig. 2023, 43, 51–56. [Google Scholar] [CrossRef]
- Chen, M.S.; Li, F.N.; Chen, X.H.; Yan, X.R.; Tuo, L. Brachybacterium halotolerans sp. Nov., a Halotolerant, Endophytic Actinomycete Isolated from Branch of Bruguiera gymnoirhiza. Antonie van Leeuwenhoek 2021, 114, 875–884. [Google Scholar] [CrossRef]
- Shaw, D.R.; Dussan, J. Mathematical Modelling of Toxic Metal Uptake and Efflux Pump in Metal-Resistant Bacterium Bacillus cereus Isolated from Heavy Crude Oil. Water Air Soil Pollut. 2015, 226, 112. [Google Scholar] [CrossRef]
- Guo, H.; Luo, S.; Chen, L.; Xiao, X.; Xi, Q.; Wei, W.; Zeng, G.; Liu, C.; Wan, Y.; Chen, J.; et al. Bioremediation of Heavy Metals by Growing Hyperaccumulaor Endophytic Bacterium bacillus sp. L14. Bioresour. Technol. 2010, 101, 8599–8605. [Google Scholar] [CrossRef]
- Luo, S.; Wan, Y.; Xiao, X.; Guo, H.; Chen, L.; Xi, Q.; Zeng, G.; Liu, C.; Chen, J. Isolation and Characterization of Endophytic Bacterium LRE07 from Cadmium Hyperaccumulator Solanum nigrum L. and Its Potential for Remediation. Appl. Microbiol. Biotechnol. 2011, 89, 1637–1644. [Google Scholar] [CrossRef]
- Zhang, Y.F.; He, L.Y.; Chen, Z.J.; Zhang, W.H.; Wang, Q.Y.; Qian, M.; Sheng, X.F. Characterization of Lead-Resistant and ACC Deaminase-Producing Endophytic Bacteria and Their Potential in Promoting Lead Accumulation of Rape. J. Hazard. Mater. 2011, 186, 1720–1725. [Google Scholar] [CrossRef]
- Mello, I.S.; Pietro-Souza, W.; Barros, B.M.; da Silva, G.F.; Campos, M.L.; Soares, M.A. Endophytic Bacteria Mitigate Mercury Toxicity to Host Plants. Symbiosis 2019, 79, 251–262. [Google Scholar] [CrossRef]
- Salmi, A.; Boulila, F. Heavy Metals Multi-Tolerant Bradyrhizobium Isolated from Mercury Mining Region in Algeria. J. Environ. Manag. 2021, 289, 112547. [Google Scholar] [CrossRef]
- Nithya, C.; Gnanalakshmi, B.; Pandian, S.K. Assessment and Characterization of Heavy Metal Resistance in Palk Bay Sediment Bacteria. Mar. Environ. Res. 2011, 71, 283–294. [Google Scholar] [CrossRef]
- Zulaika, E.; Sembiring, L.; Soegianto, A. Characterization and Identification of Mercury-Resistant Bacteria from Kalimas River Surabaya-Indonesia By Numerical Phenetic Taxonomy. J. Basic Appl. Sci. Res. 2012, 2, 7263–7269. [Google Scholar]
- Bisane, K.D. Population diversity and cyclicity of fruit fly (Bactrocera spp.) in sapota orchard under South Gujarat condition. Indian J. Ecol. 2017, 44, 369–374. [Google Scholar]
- Orji, O.U.; Awoke, J.N.; Aja, P.M.; Aloke, C.; Obasi, O.D.; Alum, E.U.; Udu-Ibiam, O.E.; Oka, G.O. Halotolerant and Metalotolerant Bacteria Strains with Heavy Metals Biorestoration Possibilities Isolated from Uburu Salt Lake, Southeastern, Nigeria. Heliyon 2021, 7, e07512. [Google Scholar] [CrossRef] [PubMed]
- Boyd, E.S.; Barkay, T. The Mercury Resistance Operon: From an Origin in a Geothermal Environment to an Efficient Detoxification Machine. Front. Microbiol. 2012, 3, 349. [Google Scholar] [CrossRef] [PubMed]
- Oyetibo, G.O.; Miyauchi, K.; Suzuki, H.; Ishikawa, S.; Endo, G. Extracellular Mercury Sequestration by Exopolymeric Substances Produced by Yarrowia spp.: Thermodynamics, Equilibria, and Kinetics Studies. J. Biosci. Bioeng. 2016, 122, 701–707. [Google Scholar] [CrossRef] [PubMed]
- Kailasam, S.; Arumugam, S.; Balaji, K.; Vinodh Kanth, S. Adsorption of Chromium by Exopolysaccharides Extracted from Lignolytic Phosphate Solubilizing Bacteria. Int. J. Biol. Macromol. 2022, 206, 788–798. [Google Scholar] [CrossRef] [PubMed]
- Abdelnasser, S.M.; Yahya, S.M.M.; Mohamed, W.F.; Asker, M.M.S.; Abu Shady, H.M.; Mahmoud, M.G.; Gadallah, M.A. Antitumor Exopolysaccharides Derived from Novel Marine Bacillus: Isolation, Characterization Aspect and Biological Activity. Asian Pac. J. Cancer Prev. 2017, 18, 1847–1854. [Google Scholar] [CrossRef] [PubMed]
- Dash, H.R.; Das, S. Interaction between Mercuric Chloride and Extracellular Polymers of Biofilm-Forming Mercury Resistant Marine Bacterium: Bacillus thuringiensis PW-05. RSC Adv. 2016, 6, 109793–109802. [Google Scholar] [CrossRef]
- Dash, H.R.; Basu, S.; Das, S. Evidence of Mercury Trapping in Biofilm-EPS and mer Operon-Based Volatilization of Inorganic Mercury in a Marine Bacterium Bacillus cereus BW-201B. Arch. Microbiol. 2017, 199, 445–455. [Google Scholar] [CrossRef] [PubMed]
- Dash, H.R.; Das, S. Diversity, Community Structure, and Bioremediation Potential of Mercury-Resistant Marine Bacteria of Estuarine and Coastal Environments of Odisha, India. Environ. Sci. Pollut. Res. 2016, 23, 6960–6971. [Google Scholar] [CrossRef]
- Mathivanan, K.; Uthaya Chandirika, J.; Srinivasan, R.; Emmanuel Charles, P.; Rajaram, R.; Zhang, R. Exopolymeric Substances Production by Bacillus cereus KMS3-1 Enhanced Its Biosorption Efficiency in Removing Cd2+ and Pb2+ in Single and Binary Metal Mixtures. Environ. Res. 2023, 228, 115917. [Google Scholar] [CrossRef]
- Martis, B.S.; Mohan, A.K.; Chiplunkar, S.; Kamath, S.; Goveas, L.C.; Rao, C.V. Bacterium Isolated from Coffee Waste Pulp Biosorps Lead: Investigation of EPS Mediated Mechanism. Curr. Res. Microb. Sci. 2021, 2, 100029. [Google Scholar] [CrossRef] [PubMed]
- Guibaud, G.; Bordas, F.; Saaid, A.; D’abzac, P.; Van Hullebusch, E. Effect of pH on Cadmium and Lead Binding by Extracellular Polymeric Substances (EPS) Extracted from Environmental Bacterial Strains. Colloids Surf. B 2008, 63, 48–54. [Google Scholar] [CrossRef] [PubMed]
- Roskova, Z.; Skarohlid, R.; McGachy, L. Siderophores: An Alternative Bioremediation Strategy? Sci. Total Environ. 2022, 819, 153144. [Google Scholar] [CrossRef] [PubMed]
- He, Z.; Shen, J.; Li, Q.; Yang, Y.; Zhang, D.; Pan, X. Bacterial Metal(Loid) Resistance Genes (MRGs) and Their Variation and Application in Environment: A Review. Sci. Total Environ. 2023, 871, 162148. [Google Scholar] [CrossRef] [PubMed]
- Kandegedara, A.; Thiyagarajan, S.; Kondapalli, K.C.; Stemmler, T.L.; Rosen, B.P. Role of Bound Zn(II) in the CadC Cd(II)/Pb(II)/Zn(II)-Responsive Repressor. J. Biol. Chem. 2009, 284, 14958–14965. [Google Scholar] [CrossRef] [PubMed]
- Busenlehner, L.S.; Giedroc, D.P. Kinetics of Metal Binding by the Toxic Metal-Sensing Transcriptional Repressor Staphylococcus aureus PI258 CadC. J. Inorg. Biochem. 2006, 100, 1024–1034. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Wong, M.D.; Rosen, B.P. Role of Cysteinyl Residues in Sensing Pb(II), Cd(II), and Zn(II) by the Plasmid PI258 CadC Repressor. J. Biol. Chem. 2001, 276, 14955–14960. [Google Scholar] [CrossRef] [PubMed]
- Sundar, K.; Mukherjee, A.; Sadiq, M.; Chandrasekaran, N. Cr (III) Bioremoval Capacities of Indigenous and Adapted Bacterial Strains from Palar River Basin. J. Hazard. Mater. 2011, 187, 553–561. [Google Scholar] [CrossRef] [PubMed]
- Plaper, A.; Jenko-Brinovec, Š.; Premzl, A.; Kos, J.; Raspor, P. Genotoxicity of Trivalent Chromium in Bacterial Cells. Possible Effects on DNA Topology. Chem. Res. Toxicol. 2002, 15, 943–949. [Google Scholar] [CrossRef]
- Mushtaq, Z.; Liaquat, M.; Nazir, A.; Liaquat, R.; Iftikhar, H.; Anwar, W.; Itrat, N. Potential of Plant Growth Promoting Rhizobacteria to Mitigate Chromium Contamination. Environ. Technol. Innov. 2022, 28, 102826. [Google Scholar] [CrossRef]
- Robins, K.J.; Hooks, D.O.; Rehm, B.H.A.; Ackerley, D.F. Escherichia coli Nema Is an Efficient Chromate Reductase That Can Be Biologically Immobilized to Provide a Cell Free System for Remediation of Hexavalent Chromium. PLoS ONE 2013, 8, e59200. [Google Scholar] [CrossRef]
- Kwak, Y.H.; Lee, D.S.; Kim, H.B. Vibrio harveyi Nitroreductase Is Also a Chromate Reductase. Appl. Environ. Microbiol. 2003, 69, 4390–4395. [Google Scholar] [CrossRef] [PubMed]
- Aguilar-Barajas, E.; Díaz-Pérez, C.; Ramírez-Díaz, M.I.; Riveros-Rosas, H.; Cervantes, C. Bacterial Transport of Sulfate, Molybdate, and Related Oxyanions. BioMetals 2011, 24, 687–707. [Google Scholar] [CrossRef] [PubMed]
- Hu, P.; Brodie, E.L.; Suzuki, Y.; McAdams, H.H.; Andersen, G.L. Whole-Genome Transcriptional Analysis of Heavy Metal Stresses in Caulobacter crescentus. J. Bacteriol. 2005, 187, 8437–8449. [Google Scholar] [CrossRef] [PubMed]
- Rosario-Cruz, Z.; Boyd, J.M. Physiological Roles of Bacillithiol in Intracellular Metal Processing. Curr. Genet. 2016, 62, 59–65. [Google Scholar] [CrossRef] [PubMed]
- Al-Ansari, M.M. Biodetoxification Mercury by Using a Marine Bacterium Marinomonas sp. RS3 and Its MerA Gene Expression under Mercury Stress. Environ. Res. 2022, 205, 112452. [Google Scholar] [CrossRef] [PubMed]
- Yao, H.; Wang, H.; Ji, J.; Tan, A.; Song, Y.; Chen, Z. Isolation and Identification of Mercury-Tolerant Bacteria LBA119 from Molybdenum-Lead Mining Soils and Their Removal of Hg2+. Toxics 2023, 11, 261. [Google Scholar] [CrossRef] [PubMed]
- Naguib, M.M.; Khairalla, A.S.; El-Gendy, A.O.; Elkhatib, W.F. Isolation and Characterization of Mercury-Resistant Bacteria from Wastewater Sources in Egypt. Can. J. Microbiol. 2019, 65, 308–321. [Google Scholar] [CrossRef] [PubMed]
- Abbas, S.Z.; Yee, C.J.; Hossain, K.; Ahmad, A.; Rafatullah, M. Isolation and Characterization of Mercury-Resistant Bacteria from Industrial Wastewater. Desalin. Water Treat. 2019, 138, 128–133. [Google Scholar] [CrossRef]
- Imron, M.F.; Kurniawan, S.B.; Soegianto, A. Characterization of Mercury-Reducing Potential Bacteria Isolated from Keputih Non-Active Sanitary Landfill Leachate, Surabaya, Indonesia under Different Saline Conditions. J. Environ. Manag. 2019, 241, 113–122. [Google Scholar] [CrossRef]


| Isolates | Gram Stain Result and Morphology | Maximum Concentration NaCl Tolerance (%) |
|---|---|---|
| 1BS3110 | Gram-stain-positive bacilli | 7.5 |
| 2BS3110 | Gram-stain-positive bacilli | 10 |
| 3BS3110 | Gram-stain-positive cocci | 7.5 |
| 4BS3110 | Gram-stain-positive cocci | 10 |
| 5BS3110 | Gram-stain-negative bacilli | 10 |
| 6BS3110 | Gram-stain-positive cocci | 10 |
| 7BS3110 | Gram-stain-positive bacilli | 12.5 |
| Heavy Metals | ||||||
|---|---|---|---|---|---|---|
| Name | Compound | LD50 (mg/Kg) | Ref. | P. flexa | Ref. | |
| MIC (mM) | BMC (mM) | |||||
| Chrome | CrCl3 · 6H20 | 1790 | [57] | 15 | 50 | This study |
| Copper | CuSO4 · 5H20 | 384 | [58] | 5 | 30 | |
| Zinc | ZnCl2 | 528 | [59] | 1 | 30 | |
| Cobalt | CoSO4 · 7H2O | 450 | [60] | 5 | 1000 | |
| Mercury | HgCl2 | 0.25–2.25 kg | [61] | 0.01 | 0.75 | |
| Lead | Pb(NO3)2 | 2250 | [62] | 10 | 1000 | |
| Nickel | NiCl2 · 7H2O | 500 | [63] | 5 | 5 | |
| Cadmium | CdSO4 | 200 | [64] | 1 | 1 | |
| Mercury | |||||
|---|---|---|---|---|---|
| Activity | Protein Product | Gene | Protein Code | Similarity Protein Results | Ref. |
| Extracellular sequestration | Exopolysaccharide biosynthesis EpsA | epsA | AEP92472.1 | SFu, SDo, SCu | [65,66,67] |
| Tyrosine kinase | epsB | WFA11311.1 | Presence | [66,67,68] | |
| Glycosyltransferase EpsD | epsD | SIQ94561.1 | Presence | [66,67,68] | |
| Glycosytransferase EpsF | epsF | CAH0138532.1 | Presence | [65,66,67] | |
| Exopolysaccharide biosynthesis EpsG | epsG | WFA11306.1 | Presence | [65,66,67] | |
| Putative sugar transferase EpsL | epsL | CAH0206043.1 | SFu, SDo, SCu | [65,66,67] | |
| Acetyltransferase EpsM | epsM | SIQ94782.1 | Presence | [66,67,69] | |
| Aminotransferase EpsN | epsN | SIQ94819.1 | Presence | [66,67,69] | |
| Transcriptional regulator SinR | SinR | KFM97474.1 | Presence | [66,67,70] | |
| Wzz N-terminal domain | Wzz | WP_049163267.1 | SFu, SDo, SCu | [65,67] | |
| mer Operon | Mercuric reductase | merA | WP_138116160.1 | Presence | [67] |
| Broad mercury transporter merE | merE | BAS29549.1 | SCu | [67] | |
| Mercuric transport | merP | QCS51148.1 | SFa, SDo, SFu | [67] | |
| Mercuric resistance operon | merR | WP_289521299.1 | Presence | [67] | |
| Intra cellular accumulation | Metalloregulator smtB | smtB | WP_195783159.1 | SFa, SDo, SFu | [71] |
| Thiol | L-malate glycosyltransferase BshA | bshA | WP_210608563.1 | Presence | [28,72,73,74] |
| Glucosaminide deacetylase BshB1 | bshB1 | AIC44925.1 | Presence | [28,72,73] | |
| Glucosaminide deacetylase BshB2 | bshB2 | WEZ07453.1 | Presence | [28,72,73,74] | |
| Bacillithiol synthase | bshC | WP_034649136.1 | Presence | [28,72,73,74] | |
| Lead | |||||
| Activity | Protein Product | Gene | Protein code | Similarity protein results | Ref. |
| Specific Efflux | Pb-efflux protein PbrA | pbrA | CAI11271.1 | SFa, SDo, SFu, SCu | [75,76] |
| Non-specific efflux | Transcriptional regulator CadC | cadC | CAH0174756.1 | Presence | [29] |
| Transcriptional regulator CmtR | cmtR | WP_308474560.1 | SFa, SDo, SFu | [77] | |
| ATP-binding protein AztA | aztA | WP_078614428.1 | SFa, SDo, SFu | [77] | |
| Metalloregulator AztR | aztR | WP_042466054.1 | SFa, SDo, SFu | [77] | |
| Cation efflux P1-ATPase | czcP | ABF12829.1 | SFa, SDo, SFu, SCu | [77] | |
| Transporting Cd2+, Zn2+, Co ATPase | cadA | OFC98076.1 | Presence | [29] | |
| Intra cellular accumulation | Metalloregulator SmtB | smtB | WP_195783159.1 | SFa, SDo, SFu | [71,76] |
| Siderophores | Lysine decarboxylase DesA | desA | WP_302000531.1 | SFa | [76,78,79] |
| Cadaverine hydroxylase | desB | AOC37727.1 | SFa | [76,78,79] | |
| Alcaligin biosynthesis enzyme | alcA | AAA97596.1 | SFa | [76,79,80] | |
| Acetyltransferase | alcB | WP_013084704.1 | SFa, SDo, SFu | [76,79,80] | |
| Siderophore transport | STra | QLK07663.1 | SFa, SDo, SFu, SCu | [76] | |
| Siderophore related permease | Sperm | QLK04956.1 | SFa | [76] | |
| Siderophore transport system | FeSB | WP_055992581.1 | SDo, SUc | [76] | |
| Siderophore transport system | FeP | CBS89604.1 | SFa, SFu, SCu | [76] | |
| Siderophore transport system | FeATP | SPR94826.1 | SFa, SDo, SFu | [76] | |
| Siderophore transport system | ABCp | QLK08254.1 | SFa, SFu, SCu | [76] | |
| Siderophore transport system | ABCatp | QLK04041.1 | SFa, SDo, DFu | [76] | |
| Siderophore transport system | ABCsb | QLK04038.1 | SDo, SUc | [76] | |
| Siderophore transport system | ABCp2 | QLK04040.1 | SFa, SFu, SCu | [76] | |
| Chrome | |||||
| Activity | Protein Product | Gene | Protein code | Similarity protein results | Ref. |
| Efflux | Chromate resistance efflux | chrA | GMG74543.1 | SFa, SFu, SCu | [81,82] |
| Chromate efflux transporter | chrB | GMG74544.1 | SFa, SFu, SCu | [81,82] | |
| Sulfur or iron homeostasis | Adenylyl sulfate kinase | cysC | WKU24108.1 | Presence | [82,83] |
| Sulfite reductase | cysL | KNH16506.1 | Presence | [82,83] | |
| Ferritin | fntA | WP_283880548.1 | SDo | [82,83] | |
| Sulfate/thiosulfate import | CysA | CAH0220131.1 | SFa, SDo | [82,83] | |
| Sulfate transporter permease | CysT | WP_013082952.1 | Presence | [82,83] | |
| Reduction of chromium VI to chromium III | NADPH nitroreductase | NfsA | WP_028412760.1 | SFa, SDo, SFu | [83] |
| NAD(P)H oxidoreductase | NfsB | WP_013083136.1 | SFa, SDo, SFu, SCu | [83] | |
| N-ethylmaleimide reductase | NemA | CAH0175675.1 | Presence | [83] | |
| NADH-azoreductase | AzoR | ADF40836.1 | Presence | [83] | |
| NADPH nitroreductase | Frp | WP_098524731.1 | SFa, SDo, SFu | [83] | |
| Oxidoreductase | YcnD | AEN89027.1 | SFa, SDo, SFu | [83] | |
| AD(P)H oxidoreductase | NfoR | WP_000069098.1 | SFa, SDo, SFu, SCu | [83] | |
| Oxidative stress reduction | Thioredoxin | trxA | WP_277717273.1 | Presence | [83] |
| Glutaredoxin | grxA | PVE91831.1 | SFa | [83] | |
| Superoxide dismutase SodA | sodA | WP_195674987.1 | Presence | [84] | |
| Bacteria | Compound/ Metal | Tolerance | Source | Ref. |
|---|---|---|---|---|
| Bacillus halotolerans | NaCl | 3.2% (w/v) | Avicennia germinans | [98] |
| Ancylobacter mangrovi sp. | NaCl | 7% (w/v) | Bruguiera gymnorrhiza and Sonneratia apetala | [99] |
| Bacillus altitudinis | NaCl | 8% (w/v) | Acanthus ilicifolius L. | [100] |
| Acinetobacter (Pseudomonadota) | NaCl | 5% (w/v) | Avicennia marina propagules | [101] |
| Staphylococcus (Bacillota) | NaCl | 10% (w/v) | Avicennia marina propagules | [101] |
| Staphylococcus (Bacillota) | NaCl | 10% (w/v) | Avicennia marina propagules | [101] |
| Salinicola salaries | NaCl | 10% (w/v) | Avicennia officinalis L. | [102] |
| Brachybacterium halotolerans sp. | NaCl | 20% (w/v) | Bruguiera gymnoirhiza | [103] |
| Bacillus sp. | Pb2+ | MIC of 4 mM | Solanum nigrum L. | [105] |
| Cr6+ | MIC of 12 mM | |||
| Serratia sp. | Pb2+ | MIC of 4 mM | Solanum nigrum | [106] |
| Cr6+ | MIC of 12 mM | |||
| Bacillus sp. | Pb2+ | MIC of 3.6 mM | Commelina communis | [107] |
| Cu2+ | MIC of 1.6 mM | |||
| Cd2+ | MIC of 0.9 mM | |||
| Not specified | Hg2+ | MIC of up to 5 mM | Aeschynomene fluminensis and Polygonum acuminatum | [108] |
| Bradyrhizobium spp. | Hg2+ | MIC > 150 μM | Calicotome spinosa roots | [109] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Soto-Varela, Z.E.; Orozco-Sánchez, C.J.; Bolívar-Anillo, H.J.; Martínez, J.M.; Rodríguez, N.; Consuegra-Padilla, N.; Robledo-Meza, A.; Amils, R. Halotolerant Endophytic Bacteria Priestia flexa 7BS3110 with Hg2+ Tolerance Isolated from Avicennia germinans in a Caribbean Mangrove from Colombia. Microorganisms 2024, 12, 1857. https://doi.org/10.3390/microorganisms12091857
Soto-Varela ZE, Orozco-Sánchez CJ, Bolívar-Anillo HJ, Martínez JM, Rodríguez N, Consuegra-Padilla N, Robledo-Meza A, Amils R. Halotolerant Endophytic Bacteria Priestia flexa 7BS3110 with Hg2+ Tolerance Isolated from Avicennia germinans in a Caribbean Mangrove from Colombia. Microorganisms. 2024; 12(9):1857. https://doi.org/10.3390/microorganisms12091857
Chicago/Turabian StyleSoto-Varela, Zamira E., Christian J. Orozco-Sánchez, Hernando José Bolívar-Anillo, José M. Martínez, Nuria Rodríguez, Natalia Consuegra-Padilla, Alfredo Robledo-Meza, and Ricardo Amils. 2024. "Halotolerant Endophytic Bacteria Priestia flexa 7BS3110 with Hg2+ Tolerance Isolated from Avicennia germinans in a Caribbean Mangrove from Colombia" Microorganisms 12, no. 9: 1857. https://doi.org/10.3390/microorganisms12091857








