Oxidative Stress in the Murine Model of Extraparenchymal Neurocysticercosis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design
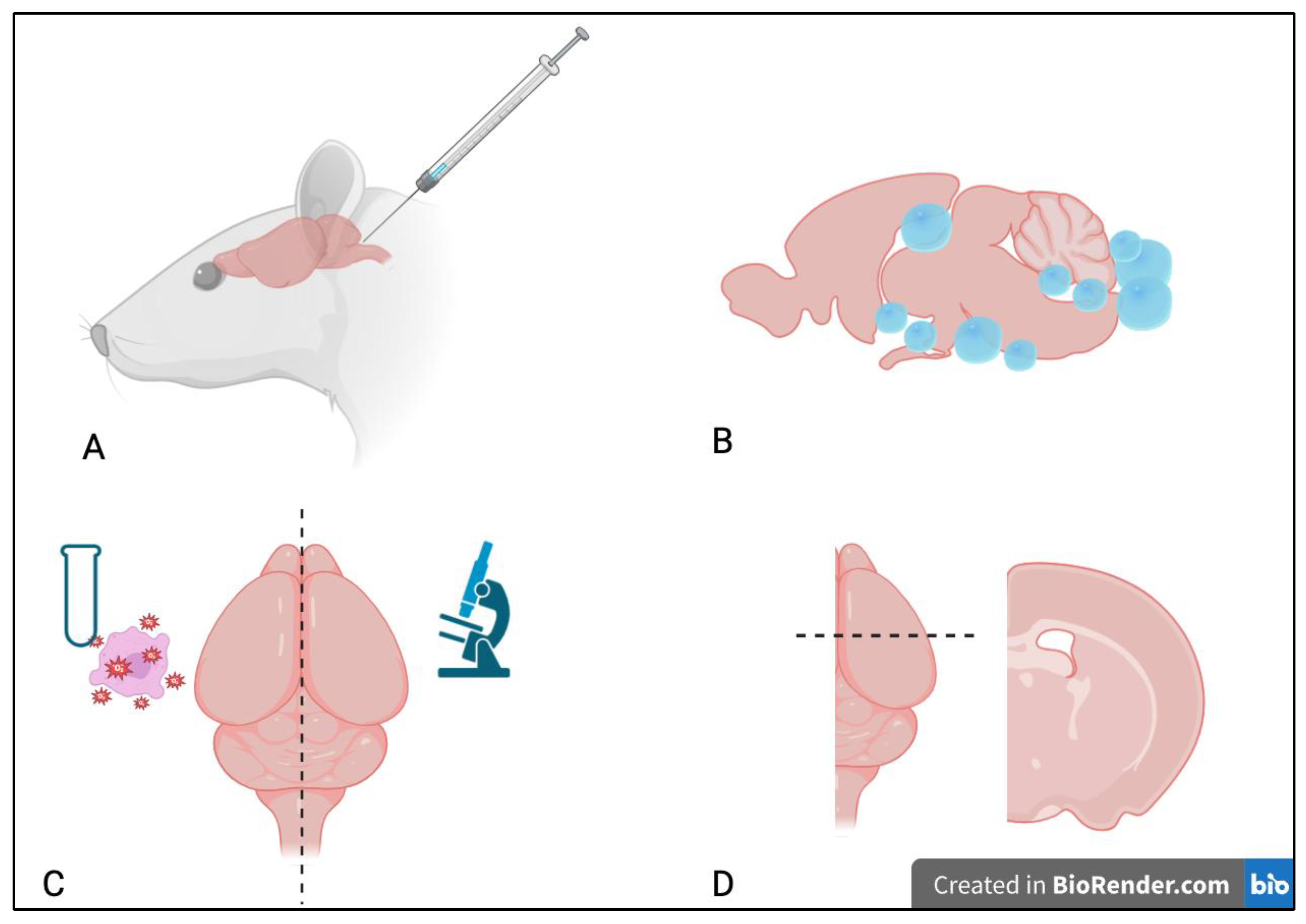
2.2. Parasites and Inoculations
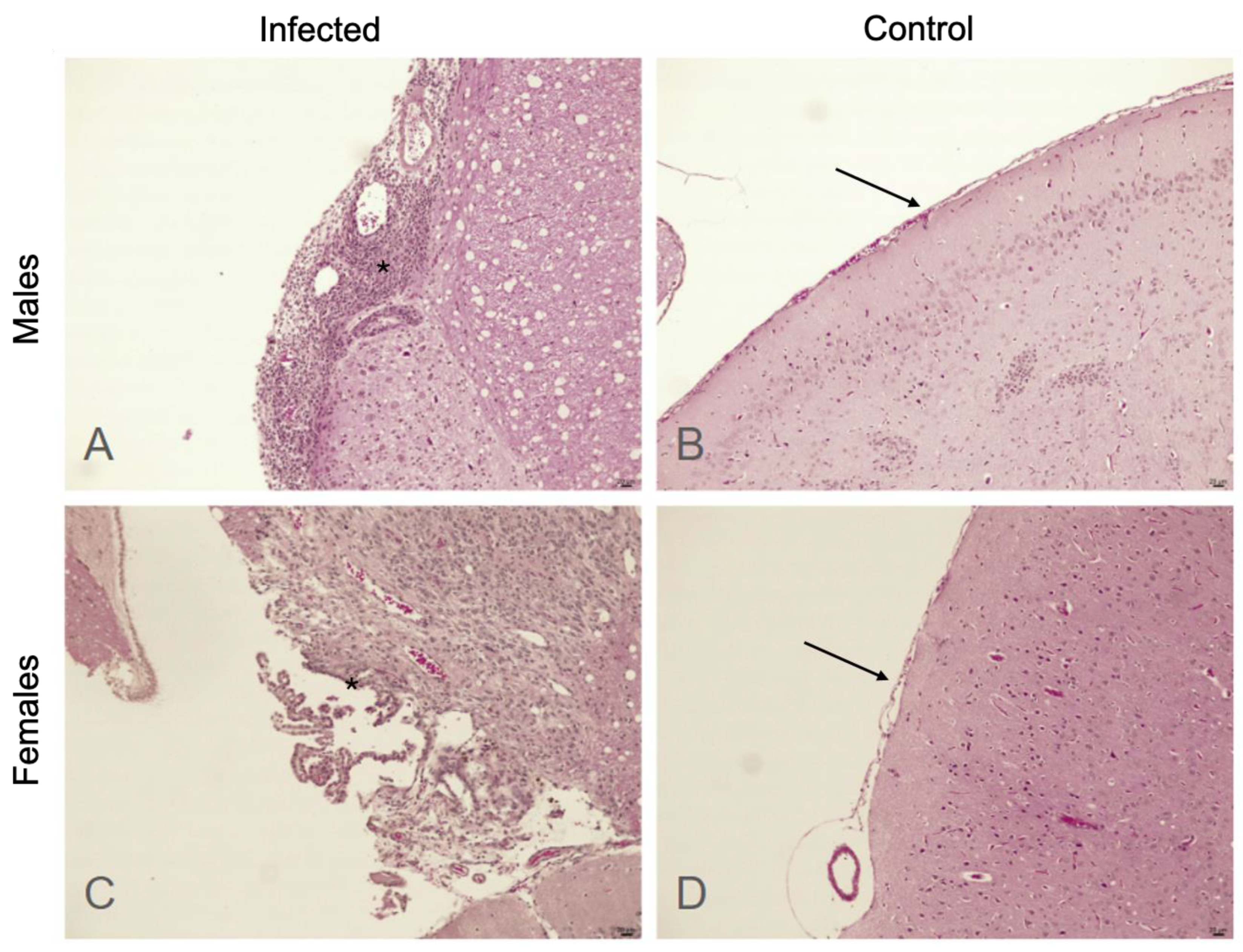
2.3. Histologic Assessment
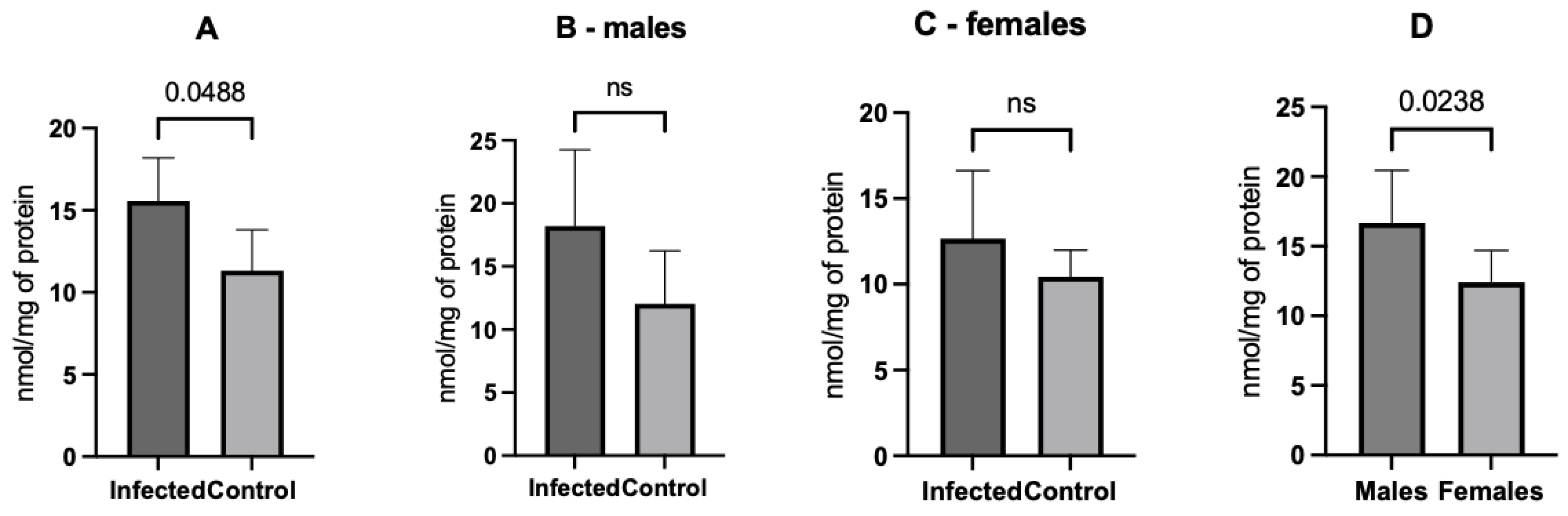
2.4. Oxidative Stress Assessment
2.5. Statistical Analysis
3. Results
4. Discussion
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Gripper, L.B.; Welburn, S.C. Neurocysticercosis infection and disease—A review. Acta Trop. 2017, 166, 218–224. [Google Scholar] [CrossRef]
- Rodríguez-Rivas, R.; Flisser, A.; Norcia, L.F.; Hamamoto Filho, P.T.; Bonilla-Aldana, D.K.; Rodriguez-Morales, A.J.; Carpio, A.; Romo, M.L.; Fleury, A. Neurocysticercosis in Latin America: Current epidemiological situation based on official statistics from four countries. PLOS Negl. Trop. Dis. 2022, 16, e0010652. [Google Scholar] [CrossRef]
- Singh, B.B.; Khatkar, M.S.; Gill, J.P.; Dhand, N.K. Estimation of the health and economic burden of neurocysticercosis in India. Acta Trop. 2017, 165, 161–169. [Google Scholar] [CrossRef] [PubMed]
- Serpa, J.A.; White, A.C., Jr. Neurocysticercosis in the United States. Pathog. Glob. Health 2012, 106, 256–260. [Google Scholar] [CrossRef] [PubMed]
- Abraham, A.; Schmidt, V.; Kaminski, M.; Stelzle, D.; De Meijere, R.; Bustos, J.; Sahu, P.S.; Garcia, H.H.; Bobić, B.; Cretu, C.; et al. Epidemiology and surveillance of human (neuro)cysticercosis in Europe: Is enhanced surveillance required? Trop. Med. Int. Health 2020, 25, 566–578. [Google Scholar] [CrossRef] [PubMed]
- Marcin Sierra, M.; Arroyo, M.; Cadena Torres, M.; Ramírez Cruz, N.; García Hernández, F.; Taboada, D.; Galicia Martínez, Á.; Govezensky, T.; Sciutto, E.; Toledo, A.; et al. Extraparenchymal neurocysticercosis: Demographic, clinicoradiological, and inflammatory features. PLoS Negl. Trop. Dis. 2017, 11, e0005646. [Google Scholar] [CrossRef]
- Hamamoto Filho, P.T.; Zanini, M.A.; Fleury, A. Hydrocephalus in neurocysticercosis: Challenges for Clinical Practice and Basic Research Perspectives. World Neurosurg. 2019, 126, 264–271. [Google Scholar] [CrossRef]
- Abanto, J.; Blanco, D.; Saavedra, H.; Gonzales, I.; Siu, D.; Pretell, E.J.; Bustos, J.A.; Garcia, H.H.; Cysticercosis Working Group in Peru. Mortality in parenchymal and subarachnoid neurocysticercosis. Am. J. Trop. Med. Hyg. 2021, 105, 176–180. [Google Scholar] [CrossRef]
- Hamamoto Filho, P.T.; Fragoso, G.; Sciutto, E.; Fleury, A. Inflammation in neurocysticercosis: Clinical relevance and impact on treatment decisions. Expert. Rev. Anti-Infect. Ther. 2021, 19, 1503–1518. [Google Scholar] [CrossRef]
- Bartsch, H.; Nair, J. Accumulation of lipid peroxidation-derived DNA lesions: Potential lead markers for chemoprevention of inflammation-driven malignancies. Mutat. Res. 2005, 591, 34–44. [Google Scholar] [CrossRef]
- Halliwell, B. Antioxidants in human health and disease. Annu. Rev. Nutr. 1996, 16, 33–50. [Google Scholar] [CrossRef]
- Rodríguez, U.; Ríos, C.; Corona, T.; Talayero, B.; Ostrosky-Wegman, P.; Herrera, L.A. Lipid peroxidation in the cerebrospinal fluid of patients with neurocysticercosis. Trans. R. Soc. Trop. Med. Hyg. 2008, 102, 1025–1031. [Google Scholar] [CrossRef]
- Alvarez, J.I.; Mishra, B.B.; Gundra, U.M.; Mishra, P.K.; Teale, J.M. Mesocestoides corti intracranial infection as a murine model for neurocysticercosis. Parasitology 2010, 137, 359–372. [Google Scholar] [CrossRef] [PubMed]
- Matos-Silva, H.; Reciputti, B.P.; Paula, E.C.; Oliveira, A.L.; Moura, V.B.L.; Vinaud, M.C.; Oliveira, M.A.P.; Lino-Júnior, L.J. Experimental encephalitis caused by Taenia crassiceps cysticerci in mice. Arq. Neuropsiquiatr. 2012, 70, 287–292. [Google Scholar] [CrossRef] [PubMed]
- Fleury, A.; Trejo, A.; Cisneros, H.; García-Navarrete, R.; Villalobos, N.; Hernández, M.; Villeda Hernández, J.; Hernández, B.; Rosas, G.; Bobes, R.J.; et al. Taenia solium: Development of an Experimental Model of Porcine neurocysticercosis. PLoS Negl. Trop. Dis. 2015, 9, e0003980. [Google Scholar] [CrossRef] [PubMed]
- Verastegui, M.R.; Mejia, A.; Clark, T.; Gavidia, C.M.; Mamani, J.; Ccopa, F.; Angulo, N.; Chile, N.; Carmen, R.; Medina, R.; et al. Novel rat model for neurocysticercosis using Taenia solium. Am. J. Pathol. 2015, 185, 2259–2268. [Google Scholar] [CrossRef]
- de Lange, A.; Mahanty, S.; Raimondo, J.V. Model systems for investigating disease processes in neurocysticercosis. Parasitology 2019, 146, 553–562. [Google Scholar] [CrossRef]
- Hamamoto Filho, P.T.; Fogaroli, M.O.; Oliveira, M.A.C.; Oliveira, C.C.; Batah, S.S.; Fabro, A.T.; Vulcano, L.C.; Bazan, R.; Zanini, M.A. A rat model of neurocysticercosis-induced hydrocephalus: Chronic progressive hydrocephalus with mild clinical impairment. World Neurosurg. 2019, 132, e535–e544. [Google Scholar] [CrossRef]
- Espinosa-Cerón, A.; Méndez, A.; Hernández-Aceves, J.; Juárez-González, J.C.; Villalobos, N.; Hernández, M.; Díaz, G.; Soto, P.; Concha, L.; Pérez-Osorio, I.N.; et al. Standardizing an experimental murine model of extraparenchymal neurocysticercosis that immunologically resembles human infection. Brain Sci. 2023, 13, 1021. [Google Scholar] [CrossRef]
- Chernin, J. Maintenance of the metacestodes of Taenia crassiceps inn rats. J. Helminthol. 1975, 49, 91–92. [Google Scholar] [CrossRef]
- Willms, K.; Zurabian, R. Taenia crassiceps: In vivo and in vitro models. Parasitology 2010, 137, 335–346. [Google Scholar] [CrossRef] [PubMed]
- Del Rio, D.; Stewart, A.J.; Pellegrini, N. A review of recent studies on malondialdehyde as toxic molecule and biological marker of oxidative stress. Nutr. Metab. Cardiovasc. Dis. 2005, 15, 316–328. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, Y.J.; Carini, M.; Butterfield, D.A. Protein carbonylation. Antioxid. Redox Signal 2010, 12, 323–325. [Google Scholar] [CrossRef]
- Toledo, A.; Osorio, R.; Matus, C.; Martinez Lopez, Y.; Ramirez Cruz, N.; Sciutto, E.; Fragoso, G.; Arauz, A.; Carrillo-Mezo, R.; Fleury, A. Human extraparenchymal neurocysticercosis: The control of inflammation favors the Host…but Also the Parasite. Front. Immunol. 2018, 9, 2652. [Google Scholar] [CrossRef]
- Munk, M.; Poulsen, F.R.; Larsen, L.; Nordström, C.H.; Nielsen, T.H. Cerebral metabolic changes related to oxidative metabolism in a model of bacterial meningitis induced by lipopolysaccharide. Neurocrit Care 2018, 29, 496–503. [Google Scholar] [CrossRef]
- Trist, B.G.; Hare, D.J.; Double, K.L. Oxidative stress in the aging substantia nigra and the etiology of Parkinson’s disease. Aging Cell 2019, 18, e13031. [Google Scholar] [CrossRef]
- Principi, N.; Esposito, S. Bacterial meningitis: New treatment options to reduce the risk of brain damage. Expert. Opin. Pharmacother. 2020, 21, 97–105. [Google Scholar] [CrossRef]
- Bai, R.; Guo, J.; Ye, X.Y.; Xie, Y.; Xie, T. Oxidative stress: The core pathogenesis and mechanism of Alzheimer’s disease. Ageing Res. Rev. 2022, 77, 101619. [Google Scholar] [CrossRef] [PubMed]
- Prasad, R.; Anil, M.O.P.; Mishra, O.P.; Mishra, S.P.; Upadhyay, R.S.; Singh, T.B. Oxidative stress in children with neurocysticercosis. Pediatr. Infect. Dis. J. 2012, 31, 1012–1015. [Google Scholar] [CrossRef]
- Ivanov, A.V.; Bartosch, B.; Isaguliants, M.G. Oxidative stress in infection and consequent disease. Oxid. Med. Cell Longev. 2017, 2017, 3496043. [Google Scholar] [CrossRef]
- Barichello, T.; Lemos, J.L.; Generoso, J.S.; Cipriano, A.L.; Milioli, G.L.; Marcelino, D.M.; Vuolo, F.; Petronilho, F.; Dal-Pizzol, F.; Vilela, M.C.; et al. Oxidative Stress, Cytokine/Chemokine and Disruption of Blood–Brain Barrier in Neonate Rats After Meningitis by Streptococcus agalactiae. Neurochem. Res. 2011, 36, 1922–1930. [Google Scholar] [CrossRef]
- Giridharan, V.V.; Simões, L.R.; Dagostin, V.S.; Generoso, J.S.; Rezin, G.T.; Florentino, D.; Muniz, J.P.; Collodel, A.; Petronilho, F.; Quevedo, J.; et al. Temporal changes of oxidative stress markers in Escherichia coli K1-induced experimental meningitis in a neonatal rat model. Neurosci. Lett. 2017, 653, 288–295. [Google Scholar] [CrossRef] [PubMed]
- Geronzi, U.; Lotti, F.; Grosso, S. Oxidative stress in epilepsy. Expert. Rev. Neurother. 2018, 18, 427–434. [Google Scholar] [CrossRef] [PubMed]
- Eastman, C.L.; D’Ambrosio, R.; Ganesh, T. Modulating neuroinflammation and oxidative stress to prevent epilepsy and improve outcomes after traumatic brain injury. Neuropharmacology 2020, 172, 107907. [Google Scholar] [CrossRef] [PubMed]
- Szewczyk-Golec, K.; Pawłowska, M.; Wesołowski, R.; Wróblewski, M.; Mila-Kierzenkowska, C. Oxidative Stress as a Possible Target in the Treatment of Toxoplasmosis: Perspectives and Ambiguities. Int. J. Mol. Sci. 2021, 22, 5705. [Google Scholar] [CrossRef]
- Rekate, H. The definition and classification of hydrocephalus: A personal recommendation to stimulate debate. Cerebrospinal Fluid. Res. 2008, 5, 2. [Google Scholar] [CrossRef]
- Di Curzio, D.L.; Turner-Brannen, E.; Del Bigio, M.R. Oral antioxidant therapy for juvenile rats with kaolin-induced hydrocephalus. Fluid. Barriers CNS 2014, 11, 23. [Google Scholar] [CrossRef]
- Wang, C.; Wang, X.; Tan, C.; Wang, Y.; Tang, Z.; Zhang, Z.; Liu, J.; Xiao, G. Novel therapeutics for hydrocephalus: Insights from animal models. CNS Neurosci. Ther. 2021, 27, 1012–1022. [Google Scholar] [CrossRef]
- Silva, S.C.; Beggiora, P.S.; Catalão, C.H.R.; Dutra, M.; Matias Júnior, I.; Santos, M.V.; Machado, H.R.; Lopes, L.S. Hyperbaric Oxygen Therapy Associated with Ventricular-Subcutaneous Shunt Promotes Neuroprotection in Young Hydrocephalic Rats. Neuroscience 2022, 488, 77–95. [Google Scholar] [CrossRef]
- Moreira, C.A.A.; Murayama, L.H.V.M.; Martins, T.C.; Oliveira, V.T.; Generoso, D.; Machado, V.M.V.; Batah, S.S.; Fabro, A.T.; Bazan, R.; Zanini, M.A.; et al. Sexual dimorphism in the murine model of extraparenchymal neurocysticercosis. Parasitol. Res. 2023, 122, 2147–2154. [Google Scholar] [CrossRef]
- Ambrosio, J.R.; Valverde-Islas, L.; Nava-Castro, K.E.; Palacios-Arreola, M.I.; Ostoa-Saloma, P.; Reynoso-Ducoing, O.; Escobedo, G.; Ruíz-Rosado, A.; Dominguez-Ramírez, L.; Morales-Montor, J. Androgens exert a cysticidal effect upon Taenia crassiceps by disrupting flame cell morphology and function. PLoS ONE 2015, 10, e0127928. [Google Scholar] [CrossRef]
- Chainy, G.B.N.; Sahoo, D.K. Hormones and oxidative stress: An overview. Free Radic. Res. 2020, 54, 1–26. [Google Scholar] [CrossRef] [PubMed]
- Del Brutto, O.H.; García, E.; Talámas, O.; Sotelo, J. Sex-related severity of inflammation in parenchymal brain cysticercosis. Arch. Intern. Med. 1988, 148, 544–546. [Google Scholar] [CrossRef] [PubMed]
- Fleury, A.; Dessein, A.; Preux, P.M.; Dumas, M.; Tapia, G.; Larralde, C.; Sciutto, E. Symptomatic human neurocysticercosis—Age, sex and exposure factors relating with disease heterogeneity. J. Neurol. 2004, 251, 830–837. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Generoso, D.; Martins, T.d.C.; Camacho, C.R.C.; Segredo, M.P.d.F.; Batah, S.S.; Fabro, A.T.; Sciutto, E.; Fleury, A.; Hamamoto Filho, P.T.; Zanini, M.A. Oxidative Stress in the Murine Model of Extraparenchymal Neurocysticercosis. Microorganisms 2024, 12, 1860. https://doi.org/10.3390/microorganisms12091860
Generoso D, Martins TdC, Camacho CRC, Segredo MPdF, Batah SS, Fabro AT, Sciutto E, Fleury A, Hamamoto Filho PT, Zanini MA. Oxidative Stress in the Murine Model of Extraparenchymal Neurocysticercosis. Microorganisms. 2024; 12(9):1860. https://doi.org/10.3390/microorganisms12091860
Chicago/Turabian StyleGeneroso, Diego, Tatiane de Camargo Martins, Camila Renata Corrêa Camacho, Manuella Pacífico de Freitas Segredo, Sabrina Setembre Batah, Alexandre Todorovic Fabro, Edda Sciutto, Agnès Fleury, Pedro Tadao Hamamoto Filho, and Marco Antônio Zanini. 2024. "Oxidative Stress in the Murine Model of Extraparenchymal Neurocysticercosis" Microorganisms 12, no. 9: 1860. https://doi.org/10.3390/microorganisms12091860
APA StyleGeneroso, D., Martins, T. d. C., Camacho, C. R. C., Segredo, M. P. d. F., Batah, S. S., Fabro, A. T., Sciutto, E., Fleury, A., Hamamoto Filho, P. T., & Zanini, M. A. (2024). Oxidative Stress in the Murine Model of Extraparenchymal Neurocysticercosis. Microorganisms, 12(9), 1860. https://doi.org/10.3390/microorganisms12091860







