Lactic Acid Bacteria–Gut-Microbiota-Mediated Intervention towards Inflammatory Bowel Disease
Abstract
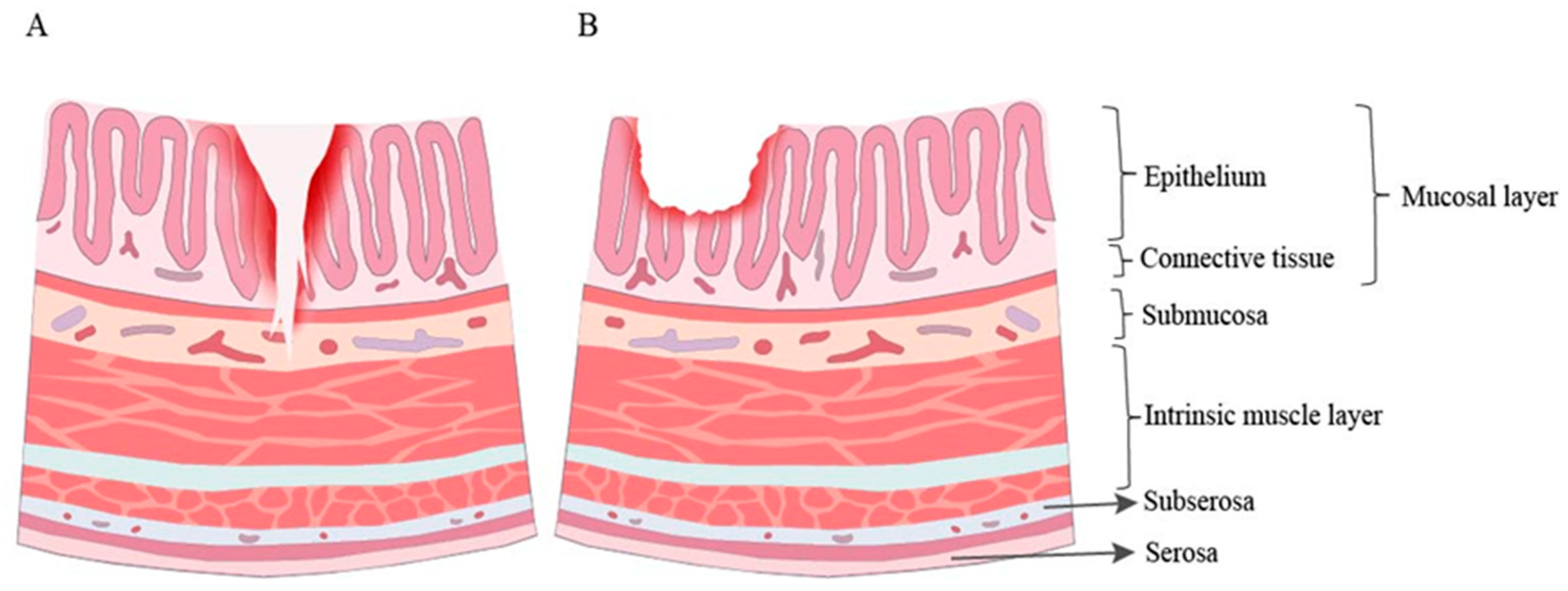
1. Introduction
2. Pathogenesis
2.1. Genetic Factors
2.2. Environmental Factors
2.3. Medication
2.4. Dietary Factors
3. The Role of LAB in Inflammatory Bowel Disease
3.1. The Role of LAB in UC
3.1.1. Improvement of Intestinal Barrier Function
3.1.2. Inhibition of Inflammatory Response
3.1.3. Regulation of Gut Microorganisms
3.2. The Role of LAB in CD
3.3. Distinctions in the Application of LAB for UC and CD
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Saez-Lara, M.J.; Gomez-Llorente, C.; Plaza-Diaz, J.; Gil, A. The Role of Probiotic Lactic Acid Bacteria and Bifidobacteria in the Prevention and Treatment of Inflammatory Bowel Disease and Other Related Diseases: A Systematic Review of Randomized Human Clinical Trials. BioMed Res. Int. 2015, 2015, 505878. [Google Scholar] [CrossRef]
- Flynn, S.; Eisenstein, S. Inflammatory Bowel Disease Presentation and Diagnosis. Surg. Clin. N. Am. 2019, 99, 1051–1062. [Google Scholar] [CrossRef] [PubMed]
- Raffals, L.E.; Saha, S.; Bewtra, M.; Norris, C.; Dobes, A.; Heller, C.; O’Charoen, S.; Fehlmann, T.; Sweeney, S.; Weaver, A.; et al. The Development and Initial Findings of a Study of a Prospective Adult Research Cohort with Inflammatory Bowel Disease (SPARC IBD). Inflamm. Bowel Dis. 2021, 28, 192–199. [Google Scholar] [CrossRef] [PubMed]
- Ungaro, R.; Mehandru, S.; Allen, P.B.; Peyrin-Biroulet, L.; Colombel, J.-F. Ulcerative Colitis. Lancet 2017, 389, 1756–1770. [Google Scholar] [CrossRef]
- Nakase, H.; Uchino, M.; Shinzaki, S.; Matsuura, M.; Matsuoka, K.; Kobayashi, T.; Saruta, M.; Hirai, F.; Hata, K.; Hiraoka, S.; et al. Evidence-Based Clinical Practice Guidelines for Inflammatory Bowel Disease 2020. J. Gastroenterol. 2021, 56, 489–526. [Google Scholar] [CrossRef]
- Greenfield, S.M.; Punchard, N.A.; Teare, J.P.; Thompson, R.P. The Mode of Action of the Aminosalicylates in Inflammatory Bowel Disease. Aliment. Pharmacol. Ther. 1993, 7, 369–383. [Google Scholar] [CrossRef] [PubMed]
- Wehkamp, J.; Götz, M.; Herrlinger, K.; Steurer, W.; Stange, E.F. Inflammatory Bowel Disease. Dtsch. Arztebl. Int. 2016, 113, 72–82. [Google Scholar] [CrossRef] [PubMed]
- Wehkamp, J.; Fellermann, K.; Herrlinger, K.R.; Bevins, C.L.; Stange, E.F. Mechanisms of Disease: Defensins in Gastrointestinal Diseases. Nat. Clin. Pract. Gastroenterol. Hepatol. 2005, 2, 406–415. [Google Scholar] [CrossRef]
- Duchmann, R.; May, E.; Heike, M.; Knolle, P.; Neurath, M.; Büschenfelde, K.-H.M.Z. T Cell Specificity and Cross Reactivity towards Enterobacteria, Bacteroides, Bifidobacterium, and Antigens from Resident Intestinal Flora in Humans. Gut 1999, 44, 812–818. [Google Scholar] [CrossRef]
- Burri, E.; Maillard, M.H.; Schoepfer, A.M.; Seibold, F.; Van Assche, G.; Rivière, P.; Laharie, D.; Manz, M.; Swiss IBDnet, an official working group of the Swiss Society of Gastroenterology. Treatment Algorithm for Mild and Moderate-to-Severe Ulcerative Colitis: An Update. Digestion 2020, 101 (Suppl. S1), 2–15. [Google Scholar] [CrossRef]
- Paramsothy, S.; Rosenstein, A.K.; Mehandru, S.; Colombel, J.-F. The Current State of the Art for Biological Therapies and New Small Molecules in Inflammatory Bowel Disease. Mucosal Immunol. 2018, 11, 1558–1570. [Google Scholar] [CrossRef]
- Sandborn, W.J.; Ghosh, S.; Panes, J.; Vranic, I.; Su, C.; Rousell, S.; Niezychowski, W.; Study A3921063 Investigators. Tofacitinib, an Oral Janus Kinase Inhibitor, in Active Ulcerative Colitis. N. Engl. J. Med. 2012, 367, 616–624. [Google Scholar] [CrossRef]
- Fontana, L.; Bermudez-Brito, M.; Plaza-Diaz, J.; Muñoz-Quezada, S.; Gil, A. Sources, Isolation, Characterisation and Evaluation of Probiotics. Br. J. Nutr. 2013, 109 (Suppl. S2), S35–S50. [Google Scholar] [CrossRef] [PubMed]
- Plaza-Diaz, J.; Ruiz-Ojeda, F.J.; Gil-Campos, M.; Gil, A. Mechanisms of Action of Probiotics. Adv. Nutr. 2019, 10, S49–S66. [Google Scholar] [CrossRef] [PubMed]
- Yan, F.; Polk, D.B. Probiotics and Immune Health. Curr. Opin. Gastroenterol. 2011, 27, 496–501. [Google Scholar] [CrossRef] [PubMed]
- Gourbeyre, P.; Denery, S.; Bodinier, M. Probiotics, Prebiotics, and Synbiotics: Impact on the Gut Immune System and Allergic Reactions. J. Leukoc. Biol. 2011, 89, 685–695. [Google Scholar] [CrossRef]
- Macpherson, A.J.; Harris, N.L. Interactions between Commensal Intestinal Bacteria and the Immune System. Nat. Rev. Immunol. 2004, 4, 478–485. [Google Scholar] [CrossRef]
- Mokoena, M.P. Lactic Acid Bacteria and Their Bacteriocins: Classification, Biosynthesis and Applications against Uropathogens: A Mini-Review. Molecules 2017, 22, 1255. [Google Scholar] [CrossRef]
- Hirayama, K.; Rafter, J. The Role of Probiotic Bacteria in Cancer Prevention. Microbes Infect. 2000, 2, 681–686. [Google Scholar] [CrossRef]
- Panwar, H.; Rashmi, H.M.; Batish, V.K.; Grover, S. Probiotics as Potential Biotherapeutics in the Management of Type 2 Diabetes—Prospects and Perspectives. Diabetes Metab. Res. Rev. 2013, 29, 103–112. [Google Scholar] [CrossRef]
- Abenavoli, L.; Scarpellini, E.; Colica, C.; Boccuto, L.; Salehi, B.; Sharifi-Rad, J.; Aiello, V.; Romano, B.; De Lorenzo, A.; Izzo, A.A.; et al. Gut Microbiota and Obesity: A Role for Probiotics. Nutrients 2019, 11, 2690. [Google Scholar] [CrossRef]
- Lee, N.-K.; Kim, S.-Y.; Han, K.J.; Eom, S.J.; Paik, H.-D. Probiotic Potential of Lactobacillus Strains with Anti-Allergic Effects from Kimchi for Yogurt Starters. LWT Food Sci. Technol. 2014, 58, 130–134. [Google Scholar] [CrossRef]
- Cristofori, F.; Dargenio, V.N.; Dargenio, C.; Miniello, V.L.; Barone, M.; Francavilla, R. Anti-Inflammatory and Immunomodulatory Effects of Probiotics in Gut Inflammation: A Door to the Body. Front. Immunol. 2021, 12, 578386. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.-Q.; Quan, K.-Y.; Feng, C.-J.; Zhang, T.; He, Q.-W.; Kwok, L.-Y.; Chen, Y.-F. The Lactobacillus Gasseri G098 Strain Mitigates Symptoms of DSS-Induced Inflammatory Bowel Disease in Mice. Nutrients 2022, 14, 3745. [Google Scholar] [CrossRef] [PubMed]
- Hevia, A.; López, P.; Suárez, A.; Jacquot, C.; Urdaci, M.C.; Margolles, A.; Sánchez, B. Association of Levels of Antibodies from Patients with Inflammatory Bowel Disease with Extracellular Proteins of Food and Probiotic Bacteria. BioMed Res. Int. 2014, 2014, 351204. [Google Scholar] [CrossRef]
- Scaldaferri, F.; Gerardi, V.; Lopetuso, L.R.; Del Zompo, F.; Mangiola, F.; Boškoski, I.; Bruno, G.; Petito, V.; Laterza, L.; Cammarota, G.; et al. Gut Microbial Flora, Prebiotics, and Probiotics in IBD: Their Current Usage and Utility. BioMed Res. Int. 2013, 2013, 435268. [Google Scholar] [CrossRef]
- Mirkov, M.U.; Verstockt, B.; Cleynen, I. Genetics of Inflammatory Bowel Disease: Beyond NOD2. Lancet Gastroenterol. Hepatol. 2017, 2, 224–234. [Google Scholar] [CrossRef]
- Huang, H.; Fang, M.; Jostins, L.; Mirkov, M.U.; Boucher, G.; Anderson, C.A.; Andersen, V.; Cleynen, I.; Cortes, A.; Crins, F.; et al. Fine-Mapping Inflammatory Bowel Disease Loci to Single-Variant Resolution. Nature 2017, 547, 173–178. [Google Scholar] [CrossRef]
- Peters, L.A.; Perrigoue, J.; Mortha, A.; Iuga, A.; Song, W.-M.; Neiman, E.M.; Llewellyn, S.R.; Di Narzo, A.; Kidd, B.A.; Telesco, S.E.; et al. A Functional Genomics Predictive Network Model Identifies Regulators of Inflammatory Bowel Disease. Nat. Genet. 2017, 49, 1437–1449. [Google Scholar] [CrossRef]
- Hugot, J.P.; Chamaillard, M.; Zouali, H.; Lesage, S.; Cézard, J.P.; Belaiche, J.; Almer, S.; Tysk, C.; O’Morain, C.A.; Gassull, M.; et al. Association of NOD2 Leucine-Rich Repeat Variants with Susceptibility to Crohn’s Disease. Nature 2001, 411, 599–603. [Google Scholar] [CrossRef]
- Radford-Smith, G.; Pandeya, N. Associations between NOD2/CARD15 Genotype and Phenotype in Crohn’s Disease—Are We There Yet? World J. Gastroenterol. 2006, 12, 7097–7103. [Google Scholar] [CrossRef]
- Ogura, Y.; Inohara, N.; Benito, A.; Chen, F.F.; Yamaoka, S.; Nunez, G. Nod2, a Nod1/Apaf-1 Family Member That Is Restricted to Monocytes and Activates NF-κB. J. Biol. Chem. 2001, 276, 4812–4818. [Google Scholar] [CrossRef] [PubMed]
- Gutierrez, O.; Pipaon, C.; Inohara, N.; Fontalba, A.; Ogura, Y.; Prosper, F.; Nunez, G.; Fernandez-Luna, J.L. Induction of Nod2 in Myelomonocytic and Intestinal Epithelial Cells via Nuclear Factor-κB Activation. J. Biol. Chem. 2002, 277, 41701–41705. [Google Scholar] [CrossRef]
- Rosenstiel, P.; Fantini, M.; Bräutigam, K.; Kühbacher, T.; Waetzig, G.H.; Seegert, D.; Schreiber, S. TNF-α and IFN-γ Regulate the Expression of the NOD2 (CARD15) Gene in Human Intestinal Epithelial Cells. Gastroenterology 2003, 124, 1001–1009. [Google Scholar] [CrossRef] [PubMed]
- Zanello, G.; Goethel, A.; Forster, K.; Geddes, K.; Philpott, D.J.; Croitoru, K. Nod2 Activates NF-kB in CD4+ T Cells but Its Expression Is Dispensable for T Cell-Induced Colitis. PLoS ONE 2013, 8, e82623. [Google Scholar] [CrossRef]
- Philpott, D.J.; Sorbara, M.T.; Robertson, S.J.; Croitoru, K.; Girardin, S.E. NOD Proteins: Regulators of Inflammation in Health and Disease. Nat. Rev. Immunol. 2014, 14, 9–23. [Google Scholar] [CrossRef] [PubMed]
- Barnich, N.; Aguirre, J.E.; Reinecker, H.-C.; Xavier, R.; Podolsky, D.K. Membrane Recruitment of NOD2 in Intestinal Epithelial Cells Is Essential for Nuclear Factor–κB Activation in Muramyl Dipeptide Recognition. J. Cell Biol. 2005, 170, 21–26. [Google Scholar] [CrossRef] [PubMed]
- Aleksandrova, K.; Romero-Mosquera, B.; Hernandez, V. Diet, Gut Microbiome and Epigenetics: Emerging Links with Inflammatory Bowel Diseases and Prospects for Management and Prevention. Nutrients 2017, 9, 962. [Google Scholar] [CrossRef]
- Ventham, N.T.; Kennedy, N.A.; Adams, A.T.; Kalla, R.; Heath, S.; O’Leary, K.R.; Drummond, H.; IBD BIOM Consortium; IBD CHARACTER Consortium; Wilson, D.C.; et al. Integrative Epigenome-Wide Analysis Demonstrates That DNA Methylation May Mediate Genetic Risk in Inflammatory Bowel Disease. Nat. Commun. 2016, 7, 13507. [Google Scholar] [CrossRef]
- Ventham, N.T.; Kennedy, N.A.; Nimmo, E.R.; Satsangi, J. Beyond Gene Discovery in Inflammatory Bowel Disease: The Emerging Role of Epigenetics. Gastroenterology 2013, 145, 293–308. [Google Scholar] [CrossRef]
- Chapman, C.G.; Pekow, J. The Emerging Role of miRNAs in Inflammatory Bowel Disease: A Review. Ther. Adv. Gastroenterol. 2015, 8, 4–22. [Google Scholar] [CrossRef]
- Schönauen, K.; Le, N.; von Arnim, U.; Schulz, C.; Malfertheiner, P.; Link, A. Circulating and Fecal microRNAs as Biomarkers for Inflammatory Bowel Diseases. Inflamm. Bowel Dis. 2018, 24, 1547–1557. [Google Scholar] [CrossRef] [PubMed]
- Jostins, L.; Ripke, S.; Weersma, R.K.; Duerr, R.H.; McGovern, D.P.; Hui, K.Y.; Lee, J.C.; Schumm, L.P.; Sharma, Y.; Anderson, C.A.; et al. Host-Microbe Interactions Have Shaped the Genetic Architecture of Inflammatory Bowel Disease. Nature 2012, 491, 119–124. [Google Scholar] [CrossRef]
- Liu, J.Z.; van Sommeren, S.; Huang, H.; Ng, S.C.; Alberts, R.; Takahashi, A.; Ripke, S.; Lee, J.C.; Jostins, L.; Shah, T.; et al. Association Analyses Identify 38 Susceptibility Loci for Inflammatory Bowel Disease and Highlight Shared Genetic Risk across Populations. Nat. Genet. 2015, 47, 979–986. [Google Scholar] [CrossRef] [PubMed]
- Kamm, M.A. Rapid Changes in Epidemiology of Inflammatory Bowel Disease. Lancet 2017, 390, 2741–2742. [Google Scholar] [CrossRef] [PubMed]
- Ananthakrishnan, A.N.; McGinley, E.L.; Binion, D.G.; Saeian, K. Ambient Air Pollution Correlates with Hospitalizations for Inflammatory Bowel Disease: An Ecologic Analysis. Inflamm. Bowel Dis. 2011, 17, 1138–1145. [Google Scholar] [CrossRef]
- Opstelten, J.L.; Beelen, R.M.J.; Leenders, M.; Hoek, G.; Brunekreef, B.; van Schaik, F.D.M.; Siersema, P.D.; Eriksen, K.T.; Raaschou-Nielsen, O.; Tjønneland, A.; et al. Exposure to Ambient Air Pollution and the Risk of Inflammatory Bowel Disease: A European Nested Case-Control Study. Dig. Dis. Sci. 2016, 61, 2963–2971. [Google Scholar] [CrossRef]
- Rozich, J.J.; Holmer, A.; Singh, S. Effect of Lifestyle Factors on Outcomes in Patients with Inflammatory Bowel Diseases. Am. J. Gastroenterol. 2020, 115, 832–840. [Google Scholar] [CrossRef]
- Sokol, H.; Seksik, P.; Nion-Larmurier, I.; Vienne, A.; Beaugerie, L.; Cosnes, J. Current Smoking, Not Duration of Remission, Delays Crohn’s Disease Relapse Following Azathioprine Withdrawal. Inflamm. Bowel Dis. 2010, 16, 362–363. [Google Scholar] [CrossRef]
- Yan, S.; Ma, Z.; Jiao, M.; Wang, Y.; Li, A.; Ding, S. Effects of Smoking on Inflammatory Markers in a Healthy Population as Analyzed via the Gut Microbiota. Front. Cell. Infect. Microbiol. 2021, 11, 633242. [Google Scholar] [CrossRef] [PubMed]
- De Cruz, P.; Kamm, M.A.; Hamilton, A.L.; Ritchie, K.J.; Krejany, E.O.; Gorelik, A.; Liew, D.; Prideaux, L.; Lawrance, I.C.; Andrews, J.M.; et al. Crohn’s Disease Management after Intestinal Resection: A Randomised Trial. Lancet 2015, 385, 1406–1417. [Google Scholar] [CrossRef]
- Lynch, S.V.; Pedersen, O. The Human Intestinal Microbiome in Health and Disease. N. Engl. J. Med. 2016, 375, 2369–2379. [Google Scholar] [CrossRef] [PubMed]
- Kåhrström, C.T.; Pariente, N.; Weiss, U. Intestinal Microbiota in Health and Disease. Nature 2016, 535, 47. [Google Scholar] [CrossRef]
- Blaser, M.J. Antibiotic Use and Its Consequences for the Normal Microbiome. Science 2016, 352, 544–545. [Google Scholar] [CrossRef]
- Koh, A.; De Vadder, F.; Kovatcheva-Datchary, P.; Bäckhed, F. From Dietary Fiber to Host Physiology: Short-Chain Fatty Acids as Key Bacterial Metabolites. Cell 2016, 165, 1332–1345. [Google Scholar] [CrossRef]
- Bhaskaran, N.; Quigley, C.; Paw, C.; Butala, S.; Schneider, E.; Pandiyan, P. Role of Short Chain Fatty Acids in Controlling Tregs and Immunopathology during Mucosal Infection. Front. Microbiol. 2018, 9, 1995. [Google Scholar] [CrossRef]
- Schilderink, R.; Verseijden, C.; Seppen, J.; Muncan, V.; Brink, G.R.v.D.; Lambers, T.T.; van Tol, E.A.; de Jonge, W.J. The SCFA Butyrate Stimulates the Epithelial Production of Retinoic Acid via Inhibition of Epithelial HDAC. Am. J. Physiol. Gastrointest. Liver Physiol. 2016, 310, G1138–G1146. [Google Scholar] [CrossRef] [PubMed]
- Topping, D.L.; Clifton, P.M. Short-Chain Fatty Acids and Human Colonic Function: Roles of Resistant Starch and Nonstarch Polysaccharides. Physiol. Rev. 2001, 81, 1031–1064. [Google Scholar] [CrossRef] [PubMed]
- Zimmermann, P.; Curtis, N. The Effect of Antibiotics on the Composition of the Intestinal Microbiota—A Systematic Review. J. Infect. 2019, 79, 471–489. [Google Scholar] [CrossRef]
- Stark, C.A.; Adamsson, I.; Edlund, C.; Sjösted, S.; Seensalu, R.; Wikström, B.; Nord, C.E. Effects of Omeprazole and Amoxycillin on the Human Oral and Gastrointestinal Microflora in Patients with Helicobacter Pylori Infection. J. Antimicrob. Chemother. 1996, 38, 927–939. [Google Scholar] [CrossRef]
- Floor, M.; van Akkeren, F.; Rozenberg-Arska, M.; Visser, M.; Kolsters, A.; Beumer, H.; Verhoef, J. Effect of Loracarbef and Amoxicillin on the Oropharyngeal and Intestinal Microflora of Patients with Bronchitis. Scand. J. Infect. Dis. 1994, 26, 191–197. [Google Scholar] [CrossRef]
- Brismar, B.; Edlund, C.; Nord, C.E. Impact of Cefpodoxime Proxetil and Amoxicillin on the Normal Oral and Intestinal Microflora. Eur. J. Clin. Microbiol. Infect. Dis. 1993, 12, 714–719. [Google Scholar] [CrossRef]
- Black, F.; Einarsson, K.; Lidbeck, A.; Orrhage, K.; Nord, C.E. Effect of Lactic Acid Producing Bacteria on the Human Intestinal Microflora during Ampicillin Treatment. Scand. J. Infect. Dis. 1991, 23, 247–254. [Google Scholar] [CrossRef] [PubMed]
- Edlund, C.; Alván, G.; Barkholt, L.; Vacheron, F.; Nord, C.E. Pharmacokinetics and Comparative Effects of Telithromycin (HMR 3647) and Clarithromycin on the Oropharyngeal and Intestinal Microflora. J. Antimicrob. Chemother. 2000, 46, 741–749. [Google Scholar] [CrossRef] [PubMed]
- Borzio, M.; Salerno, F.; Saudelli, M.; Galvagno, D.; Piantoni, L.; Fragiacomo, L. Efficacy of Oral Ciprofloxacin as Selective Intestinal Decontaminant in Cirrhosis. Ital. J. Gastroenterol. Hepatol. 1997, 29, 262–266. [Google Scholar] [PubMed]
- Edlund, C.; Beyer, G.; Hiemer-Bau, M.; Ziege, S.; Lode, H.; Nord, C.E. Comparative Effects of Moxifloxacin and Clarithromycin on the Normal Intestinal Microflora. Scand. J. Infect. Dis. 2000, 32, 81–85. [Google Scholar] [CrossRef]
- Pallav, K.; E Dowd, S.; Villafuerte, J.; Yang, X.; Kabbani, T.; Hansen, J.; Dennis, M.; A Leffler, D.; Newburg, D.S.; Kelly, C.P. Effects of Polysaccharopeptide from Trametes Versicolor and Amoxicillin on the Gut Microbiome of Healthy Volunteers: A Randomized Clinical Trial. Gut Microbes 2014, 5, 458–467. [Google Scholar] [CrossRef]
- Heinsen, F.-A.; Knecht, H.; Neulinger, S.C.; Schmitz, R.A.; Knecht, C.; Kühbacher, T.; Rosenstiel, P.C.; Schreiber, S.; Friedrichs, A.K.; Ott, S.J. Dynamic Changes of the Luminal and Mucosa-Associated Gut Microbiota during and after Antibiotic Therapy with Paromomycin. Gut Microbes 2015, 6, 243–254. [Google Scholar] [CrossRef]
- Zaura, E.; Brandt, B.W.; de Mattos, M.J.T.; Buijs, M.J.; Caspers, M.P.M.; Rashid, M.-U.; Weintraub, A.; Nord, C.E.; Savell, A.; Hu, Y.; et al. Same Exposure but Two Radically Different Responses to Antibiotics: Resilience of the Salivary Microbiome versus Long-Term Microbial Shifts in Feces. mBio 2015, 6, e01693-15. [Google Scholar] [CrossRef]
- Silveira, A.L.M.; Ferreira, A.V.M.; de Oliveira, M.C.; Rachid, M.A.; da Cunha Sousa, L.F.; Dos Santos Martins, F.; Gomes-Santos, A.C.; Vieira, A.T.; Teixeira, M.M. Preventive Rather than Therapeutic Treatment with High Fiber Diet Attenuates Clinical and Inflammatory Markers of Acute and Chronic DSS-Induced Colitis in Mice. Eur. J. Nutr. 2017, 56, 179–191. [Google Scholar] [CrossRef]
- Hou, J.K.; Abraham, B.; El-Serag, H. Dietary Intake and Risk of Developing Inflammatory Bowel Disease: A Systematic Review of the Literature. Am. J. Gastroenterol. 2011, 106, 563–573. [Google Scholar] [CrossRef]
- Fritsch, J.; Garces, L.; Quintero, M.A.; Pignac-Kobinger, J.; Santander, A.M.; Fernández, I.; Ban, Y.J.; Kwon, D.; Phillips, M.C.; Knight, K.; et al. Low-Fat, High-Fiber Diet Reduces Markers of Inflammation and Dysbiosis and Improves Quality of Life in Patients with Ulcerative Colitis. Clin. Gastroenterol. Hepatol. 2021, 19, 1189–1199.e30. [Google Scholar] [CrossRef] [PubMed]
- Marton, L.T.; Goulart, R.d.A.; de Carvalho, A.C.A.; Barbalho, S.M. Omega Fatty Acids and Inflammatory Bowel Diseases: An Overview. Int. J. Mol. Sci. 2019, 20, 4851. [Google Scholar] [CrossRef]
- Scaioli, E.; Liverani, E.; Belluzzi, A. The Imbalance between N-6/n-3 Polyunsaturated Fatty Acids and Inflammatory Bowel Disease: A Comprehensive Review and Future Therapeutic Perspectives. Int. J. Mol. Sci. 2017, 18, 2619. [Google Scholar] [CrossRef] [PubMed]
- IBD in EPIC Study Investigators; Tjonneland, A.; Overvad, K.; Bergmann, M.M.; Nagel, G.; Linseisen, J.; Hallmans, G.; Palmqvist, R.; Sjodin, H.; Hagglund, G.; et al. Linoleic Acid, a Dietary n-6 Polyunsaturated Fatty Acid, and the Aetiology of Ulcerative Colitis: A Nested Case-Control Study within a European Prospective Cohort Study. Gut 2009, 58, 1606–1611. [Google Scholar] [CrossRef]
- Yao, J.; Wei, C.; Wang, J.-Y.; Zhang, R.; Li, Y.-X.; Wang, L.-S. Effect of Resveratrol on Treg/Th17 Signaling and Ulcerative Colitis Treatment in Mice. World J. Gastroenterol. 2015, 21, 6572–6581. [Google Scholar] [CrossRef] [PubMed]
- Lewis, J.D.; Abreu, M.T. Diet as a Trigger or Therapy for Inflammatory Bowel Diseases. Gastroenterology 2017, 152, 398–414.e6. [Google Scholar] [CrossRef]
- Castro, F.; de Souza, H.S.P. Dietary Composition and Effects in Inflammatory Bowel Disease. Nutrients 2019, 11, 1398. [Google Scholar] [CrossRef]
- Joossens, M.; Huys, G.; Cnockaert, M.; De Preter, V.; Verbeke, K.; Rutgeerts, P.; Vandamme, P.; Vermeire, S. Dysbiosis of the Faecal Microbiota in Patients with Crohn’s Disease and Their Unaffected Relatives. Gut 2011, 60, 631–637. [Google Scholar] [CrossRef]
- Chen, H.; Li, H.; Liu, Z. Interplay of Intestinal Microbiota and Mucosal Immunity in Inflammatory Bowel Disease: A Relationship of Frenemies. Ther. Adv. Gastroenterol. 2020, 13, 1756284820935188. [Google Scholar] [CrossRef]
- Petersen, L.M.; Bautista, E.J.; Nguyen, H.; Hanson, B.M.; Chen, L.; Lek, S.H.; Sodergren, E.; Weinstock, G.M. Community Characteristics of the Gut Microbiomes of Competitive Cyclists. Microbiome 2017, 5, 98. [Google Scholar] [CrossRef]
- Chelakkot, C.; Ghim, J.; Ryu, S.H. Mechanisms Regulating Intestinal Barrier Integrity and Its Pathological Implications. Exp. Mol. Med. 2018, 50, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Heiss, C.N.; Olofsson, L.E. Gut Microbiota-Dependent Modulation of Energy Metabolism. J. Innate Immun. 2018, 10, 163–171. [Google Scholar] [CrossRef] [PubMed]
- Reinhardt, C.; Bergentall, M.; Greiner, T.U.; Schaffner, F.; Ostergren-Lundén, G.; Petersen, L.C.; Ruf, W.; Bäckhed, F. Tissue Factor and PAR1 Promote Microbiota-Induced Intestinal Vascular Remodelling. Nature 2012, 483, 627–631. [Google Scholar] [CrossRef] [PubMed]
- Saadatzadeh, A.; Atyabi, F.; Fazeli, M.R.; Dinarvand, R.; Jamalifar, H.; Abdolghaffari, A.H.; Mahdaviani, P.; Mahbod, M.; Baeeri, M.; Baghaei, A.; et al. Biochemical and Pathological Evidences on the Benefit of a New Biodegradable Nanoparticles of Probiotic Extract in Murine Colitis. Fundam. Clin. Pharmacol. 2012, 26, 589–598. [Google Scholar] [CrossRef] [PubMed]
- Nakase, H.; Sato, N.; Mizuno, N.; Ikawa, Y. The Influence of Cytokines on the Complex Pathology of Ulcerative Colitis. Autoimmun. Rev. 2022, 21, 103017. [Google Scholar] [CrossRef]
- Johansson, M.E.V.; Gustafsson, J.K.; Holmén-Larsson, J.; Jabbar, K.S.; Xia, L.; Xu, H.; Ghishan, F.K.; Carvalho, F.A.; Gewirtz, A.T.; Sjövall, H.; et al. Bacteria Penetrate the Normally Impenetrable Inner Colon Mucus Layer in Both Murine Colitis Models and Patients with Ulcerative Colitis. Gut 2014, 63, 281–291. [Google Scholar] [CrossRef]
- Belo, G.A.; Cordeiro, B.F.; Oliveira, E.R.; Braga, M.P.; da Silva, S.H.; Costa, B.G.; Martins, F.d.S.; Jan, G.; Le Loir, Y.; Gala-García, A.; et al. SlpB Protein Enhances the Probiotic Potential of L. Lactis NCDO 2118 in Colitis Mice Model. Front. Pharmacol. 2021, 12, 755825. [Google Scholar] [CrossRef]
- Strugala, V.; Dettmar, P.W.; Pearson, J.P. Thickness and Continuity of the Adherent Colonic Mucus Barrier in Active and Quiescent Ulcerative Colitis and Crohn’s Disease. Int. J. Clin. Pract. 2008, 62, 762–769. [Google Scholar] [CrossRef]
- Wong, C.C.M.; Zhang, L.; Wu, W.K.K.; Shen, J.; Chan, R.L.Y.; Lu, L.; Hu, W.; Li, M.X.; Li, L.F.; Ren, S.X.; et al. Cathelicidin-Encoding Lactococcus lactis Promotes Mucosal Repair in Murine Experimental Colitis. J. Gastroenterol. Hepatol. 2017, 32, 609–619. [Google Scholar] [CrossRef]
- Lin, Y.; Zheng, X.; Chen, J.; Luo, D.; Xie, J.; Su, Z.; Huang, X.; Yi, X.; Wei, L.; Cai, J.; et al. Protective Effect of Bruguiera gymnorrhiza (L.) Lam. Fruit on Dextran Sulfate Sodium-Induced Ulcerative Colitis in Mice: Role of Keap1/Nrf2 Pathway and Gut Microbiota. Front. Pharmacol. 2020, 10, 1602. [Google Scholar] [CrossRef]
- Hao, H.; Zhang, X.; Tong, L.; Liu, Q.; Liang, X.; Bu, Y.; Gong, P.; Liu, T.; Zhang, L.; Xia, Y.; et al. Effect of Extracellular Vesicles Derived from Lactobacillus Plantarum Q7 on Gut Microbiota and Ulcerative Colitis in Mice. Front. Immunol. 2021, 12, 777147. [Google Scholar] [CrossRef]
- El-Baz, A.M.; Khodir, A.E.; Adel El-Sokkary, M.M.; Shata, A. The Protective Effect of Lactobacillus versus 5-Aminosalicylic Acid in Ulcerative Colitis Model by Modulation of Gut Microbiota and Nrf2/Ho-1 Pathway. Life Sci. 2020, 256, 117927. [Google Scholar] [CrossRef] [PubMed]
- Dong, F.; Xiao, F.; Li, X.; Li, Y.; Wang, X.; Yu, G.; Zhang, T.; Wang, Y. Pediococcus pentosaceus CECT 8330 Protects DSS-Induced Colitis and Regulates the Intestinal Microbiota and Immune Responses in Mice. J. Transl. Med. 2022, 20, 33. [Google Scholar] [CrossRef]
- Wang, C.-S.-E.; Li, W.-B.; Wang, H.-Y.; Ma, Y.-M.; Zhao, X.-H.; Yang, H.; Qian, J.-M.; Li, J.-N. VSL#3 Can Prevent Ulcerative Colitis-Associated Carcinogenesis in Mice. World J. Gastroenterol. 2018, 24, 4254–4262. [Google Scholar] [CrossRef] [PubMed]
- Eeckhaut, V.; Machiels, K.; Perrier, C.; Romero, C.; Maes, S.; Flahou, B.; Steppe, M.; Haesebrouck, F.; Sas, B.; Ducatelle, R.; et al. Butyricicoccus pullicaecorum in Inflammatory Bowel Disease. Gut 2013, 62, 1745–1752. [Google Scholar] [CrossRef]
- Nishida, A.; Inoue, R.; Inatomi, O.; Bamba, S.; Naito, Y.; Andoh, A. Gut Microbiota in the Pathogenesis of Inflammatory Bowel Disease. Clin. J. Gastroenterol. 2018, 11, 1–10. [Google Scholar] [CrossRef]
- Liu, J.; Gao, Y.; Zhou, J.; Tang, X.; Wang, P.; Shen, L.; Chen, S. Changes in Serum Inflammatory Cytokine Levels and Intestinal Flora in a Self-Healing Dextran Sodium Sulfate-Induced Ulcerative Colitis Murine Model. Life Sci. 2020, 263, 118587. [Google Scholar] [CrossRef]
- Ma, C.; Sun, Z.; Zeng, B.; Huang, S.; Zhao, J.; Zhang, Y.; Su, X.; Xu, J.; Wei, H.; Zhang, H. Cow-to-Mouse Fecal Transplantations Suggest Intestinal Microbiome as One Cause of Mastitis. Microbiome 2018, 6, 200. [Google Scholar] [CrossRef]
- Azad, M.A.K.; Sarker, M.; Li, T.; Yin, J. Probiotic Species in the Modulation of Gut Microbiota: An Overview. BioMed Res. Int. 2018, 2018, 9478630. [Google Scholar] [CrossRef]
- Islam, S.U. Clinical Uses of Probiotics. Medicine 2016, 95, e2658. [Google Scholar] [CrossRef]
- Gao, P.; Ma, C.; Sun, Z.; Wang, L.; Huang, S.; Su, X.; Xu, J.; Zhang, H. Feed-Additive Probiotics Accelerate yet Antibiotics Delay Intestinal Microbiota Maturation in Broiler Chicken. Microbiome 2017, 5, 91. [Google Scholar] [CrossRef] [PubMed]
- Olekhnovich, E.I.; Batotsyrenova, E.G.; Yunes, R.A.; Kashuro, V.A.; Poluektova, E.U.; Veselovsky, V.A.; Ilina, E.N.; Danilenko, V.N.; Klimina, K.M. The Effects of Levilactobacillus Brevis on the Physiological Parameters and Gut Microbiota Composition of Rats Subjected to Desynchronosis. Microb. Cell Factories 2021, 20, 226. [Google Scholar] [CrossRef]
- Deng, M.; Wu, X.; Duan, X.; Xu, J.; Yang, X.; Sheng, X.; Lou, P.; Shao, C.; Lv, C.; Yu, Z. Lactobacillus Paracasei L9 Improves Colitis by Expanding Butyrate-Producing Bacteria That Inhibit the IL-6/STAT3 Signaling Pathway. Food Funct. 2021, 12, 10700–10713. [Google Scholar] [CrossRef] [PubMed]
- Jang, Y.J.; Kim, W.-K.; Han, D.H.; Lee, K.; Ko, G. Lactobacillus Fermentum Species Ameliorate Dextran Sulfate Sodium-Induced Colitis by Regulating the Immune Response and Altering Gut Microbiota. Gut Microbes 2019, 10, 696–711. [Google Scholar] [CrossRef] [PubMed]
- Kostic, A.D.; Xavier, R.J.; Gevers, D. The Microbiome in Inflammatory Bowel Disease: Current Status and the Future Ahead. Gastroenterology 2014, 146, 1489–1499. [Google Scholar] [CrossRef]
- Lapaquette, P.; Glasser, A.-L.; Huett, A.; Xavier, R.J.; Darfeuille-Michaud, A. Crohn’s Disease-Associated Adherent-Invasive E. Coli Are Selectively Favoured by Impaired Autophagy to Replicate Intracellularly. Cell. Microbiol. 2010, 12, 99–113. [Google Scholar] [CrossRef]
- Darfeuille-Michaud, A.; Boudeau, J.; Bulois, P.; Neut, C.; Glasser, A.-L.; Barnich, N.; Bringer, M.-A.; Swidsinski, A.; Beaugerie, L.; Colombel, J.-F. High Prevalence of Adherent-Invasive Escherichia Coli Associated with Ileal Mucosa in Crohn’s Disease. Gastroenterology 2004, 127, 412–421. [Google Scholar] [CrossRef]
- Basson, A.R.; Lam, M.; Cominelli, F. Complementary and Alternative Medicine (CAM) and Next-Generation CAM (NG-CAM) Strategies for Therapeutic Gut Microbiota Modulation in Inflammatory Bowel Disease. Gastroenterol. Clin. N. Am. 2017, 46, 689–729. [Google Scholar] [CrossRef]
- Oka, A.; Sartor, R.B. Microbial-Based and Microbial-Targeted Therapies for Inflammatory Bowel Diseases. Dig. Dis. Sci. 2020, 65, 757–788. [Google Scholar] [CrossRef]
- Notararigo, S.; Varela, E.; Otal, A.; Cristobo, I.; Antolín, M.; Guarner, F.; Prieto, A.; López, P. Evaluation of an O2-Substituted (1–3)-β-D-Glucan, Produced by Pediococcus parvulus 2.6, in Ex Vivo Models of Crohn’s Disease. Front. Microbiol. 2021, 12, 621280. [Google Scholar] [CrossRef]
- LeBlanc, J.G.; del Carmen, S.; Miyoshi, A.; Azevedo, V.; Sesma, F.; Langella, P.; Bermúdez-Humarán, L.G.; Watterlot, L.; Perdigon, G.; LeBlanc, A.d.M.d. Use of Superoxide Dismutase and Catalase Producing Lactic Acid Bacteria in TNBS Induced Crohn’s Disease in Mice. J. Biotechnol. 2011, 151, 287–293. [Google Scholar] [CrossRef] [PubMed]
- Yoshimatsu, Y.; Yamada, A.; Furukawa, R.; Sono, K.; Osamura, A.; Nakamura, K.; Aoki, H.; Tsuda, Y.; Hosoe, N.; Takada, N.; et al. Effectiveness of Probiotic Therapy for the Prevention of Relapse in Patients with Inactive Ulcerative Colitis. World J. Gastroenterol. 2015, 21, 5985–5994. [Google Scholar] [CrossRef]
- Palumbo, V.D.; Romeo, M.; Gammazza, A.M.; Carini, F.; Damiani, P.; Damiano, G.; Buscemi, S.; Lo Monte, A.I.; Gerges-Geagea, A.; Jurjus, A.; et al. The Long-Term Effects of Probiotics in the Therapy of Ulcerative Colitis: A Clinical Study. Biomed. Pap. 2016, 160, 372–377. [Google Scholar] [CrossRef]
- Curciarello, R.; Canziani, K.E.; Salto, I.; Barbiera Romero, E.; Rocca, A.; Doldan, I.; Peton, E.; Brayer, S.; Sambuelli, A.M.; Goncalves, S.; et al. Probiotic Lactobacilli Isolated from Kefir Promote Down-Regulation of Inflammatory Lamina Propria T Cells from Patients with Active IBD. Front. Pharmacol. 2021, 12, 658026. [Google Scholar] [CrossRef] [PubMed]
- Tanihiro, R.; Yuki, M.; Sakano, K.; Sasai, M.; Sawada, D.; Ebihara, S.; Hirota, T. Effects of Heat-Treated Lactobacillus helveticus CP790-Fermented Milk on Gastrointestinal Health in Healthy Adults: A Randomized Double-Blind Placebo-Controlled Trial. Nutrients 2024, 16, 2191. [Google Scholar] [CrossRef] [PubMed]
- Fedorak, R.N.; Feagan, B.G.; Hotte, N.; Leddin, D.; Dieleman, L.A.; Petrunia, D.M.; Enns, R.; Bitton, A.; Chiba, N.; Paré, P.; et al. The Probiotic VSL#3 Has Anti-Inflammatory Effects and Could Reduce Endoscopic Recurrence after Surgery for Crohn’s Disease. Clin. Gastroenterol. Hepatol. 2015, 13, 928–935.e2. [Google Scholar] [CrossRef]
- Shen, M.; Shi, Y.; Ge, Z.; Qian, J. Effects of Mesalamine Combined with Live Combined Bifidobacterium, Lactobacillus and Enterococcus Capsules on Intestinal Mucosa Barrier Function and Intestinal Microbiota in Mildly Active Crohn’s Disease Patients. J. Investig. Surg. 2024, 37, 2297565. [Google Scholar] [CrossRef]
- Uronis, J.M.; Arthur, J.C.; Keku, T.; Fodor, A.; Carroll, I.M.; Cruz, M.L.; Appleyard, C.B.; Jobin, C. Gut Microbial Diversity Is Reduced by the Probiotic VSL#3 and Correlates with Decreased TNBS-Induced Colitis. Inflamm. Bowel Dis. 2011, 17, 289–297. [Google Scholar] [CrossRef]
- Dou, X.; Qiao, L.; Chang, J.; Yan, S.; Song, X.; Chen, Y.; Xu, Q.; Xu, C. Lactobacillus Casei ATCC 393 and It’s Metabolites Alleviate Dextran Sulphate Sodium-Induced Ulcerative Colitis in Mice through the NLRP3-(Caspase-1)/IL-1β Pathway. Food Funct. 2021, 12, 12022–12035. [Google Scholar] [CrossRef]
- Wang, W.; Chen, L.; Zhou, R.; Wang, X.; Song, L.; Huang, S.; Wang, G.; Xia, B. Increased Proportions of Bifidobacterium and the Lactobacillus Group and Loss of Butyrate-Producing Bacteria in Inflammatory Bowel Disease. J. Clin. Microbiol. 2014, 52, 398–406. [Google Scholar] [CrossRef]
- Bourreille, A.; Cadiot, G.; Le Dreau, G.; Laharie, D.; Beaugerie, L.; Dupas, J.-L.; Marteau, P.; Rampal, P.; Moyse, D.; Saleh, A.; et al. Saccharomyces Boulardii Does Not Prevent Relapse of Crohn’s Disease. Clin. Gastroenterol. Hepatol. 2013, 11, 982–987. [Google Scholar] [CrossRef]
- Steed, H.; Macfarlane, G.T.; Blackett, K.L.; Bahrami, B.; Reynolds, N.; Walsh, S.V.; Cummings, J.H.; Macfarlane, S. Clinical Trial: The Microbiological and Immunological Effects of Synbiotic Consumption—A Randomized Double-Blind Placebo-Controlled Study in Active Crohn’s Disease. Aliment. Pharmacol. Ther. 2010, 32, 872–883. [Google Scholar] [CrossRef] [PubMed]
- Dong, J.; Teng, G.; Wei, T.; Gao, W.; Wang, H. Methodological Quality Assessment of Meta-Analyses and Systematic Reviews of Probiotics in Inflammatory Bowel Disease and Pouchitis. PLoS ONE 2016, 11, e0168785. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Tang, S.; Baker, S.S.; Arijs, I.; Liu, W.; Alkhouri, R.; Lan, P.; Baker, R.D.; Tang, Z.; Ji, G.; et al. Difference in Pathomechanism between Crohn’s Disease and Ulcerative Colitis Revealed by Colon Transcriptome. Inflamm. Bowel Dis. 2019, 25, 722–731. [Google Scholar] [CrossRef] [PubMed]



| Disease | Probiotic Types and Dosage | Benefits | Reference |
|---|---|---|---|
| UC | 9 Bio-Three tablets (2 mg Streptococcus faecalis T-110, 10 mg Clostridium butyricum TO-A, 10 mg Bacillus enterocolitica TO-A) daily for 12 months | Probiotics effective in maintaining clinical remission in resting UC patients | [113] |
| A single daily oral dose of mesalazine 1200 mg and a probiotic mixture (consisting of Lactobacillus salivarius, Lactobacillus acidophilus, and Bifidobacterium bifidum BGN4) twice daily for 2 years | The combination therapy group showed better improvement compared to the control group (1200 mg of oral mesalazine daily), and the beneficial effects of probiotics were evident even after two years of treatment | [114] | |
| After 48 h of incubation under aerobic conditions, Lactobacillus kefiri CIDCA 8348 was resuspended in PBS at a concentration of approximately 1–2 × 108 CFU/mL | L. kefiri reduced the secretion of TNF-α, IL-6, IFN-γ, and IL-13 in patients. It also induced an increase in the frequency of CD4FOXP3 lamina propria T cells and an increase in IL-10 levels | [115] | |
| 100 mL of Lactobacillus helveticus CP790-fermented milk (1 × 1010 CFU/100 mL) per day for 4 weeks | Ingestion of Lactobacillus helveticus CP790 reduced the abundance of Desulfovibrio vulnificus and improved constipation symptoms in subjects, as well as improving overall mood and depression in healthy individuals | [116] | |
| CD | VSL#3 sachets (one sachet containing 450 billion live bacteria twice daily) for 90 days, followed by open-label VSL#3 (one sachet twice daily) for up to 12 months | While there was no statistical difference in endoscopic recurrence rates at day 90 between VSL#3 and placebo, lower mucosal cytokine levels and reduced recurrence rates were observed with long-term VSL#3 use | [117] |
| Daily oral mesalazine enteric-coated tablets (1 g/day, 3 times/day) and probiotic combination live capsules (Bacteroides vulgatus ≥ 1.0 × 106 CFU, Lactobacillus acidophilus ≥ 1.0 × 106 CFU, and Enterococcus faecalis ≥ 1.0 × 106 CFU) for 4 weeks | Compared with the control group, the observation group had higher numbers of Lactobacillus acidophilus and Bifidobacterium Longum, higher levels of serum IL-10, higher levels of peripheral blood CD4+ and CD4+/CD8+, and a higher total clinical effectiveness rate than the control group | [118] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, D.; Liu, Z.; Fan, X.; Zhao, T.; Wen, D.; Huang, X.; Li, B. Lactic Acid Bacteria–Gut-Microbiota-Mediated Intervention towards Inflammatory Bowel Disease. Microorganisms 2024, 12, 1864. https://doi.org/10.3390/microorganisms12091864
Li D, Liu Z, Fan X, Zhao T, Wen D, Huang X, Li B. Lactic Acid Bacteria–Gut-Microbiota-Mediated Intervention towards Inflammatory Bowel Disease. Microorganisms. 2024; 12(9):1864. https://doi.org/10.3390/microorganisms12091864
Chicago/Turabian StyleLi, Diantong, Zhenjiang Liu, Xueni Fan, Tingting Zhao, Dongxu Wen, Xiaodan Huang, and Bin Li. 2024. "Lactic Acid Bacteria–Gut-Microbiota-Mediated Intervention towards Inflammatory Bowel Disease" Microorganisms 12, no. 9: 1864. https://doi.org/10.3390/microorganisms12091864
APA StyleLi, D., Liu, Z., Fan, X., Zhao, T., Wen, D., Huang, X., & Li, B. (2024). Lactic Acid Bacteria–Gut-Microbiota-Mediated Intervention towards Inflammatory Bowel Disease. Microorganisms, 12(9), 1864. https://doi.org/10.3390/microorganisms12091864






