Supragingival Plaque Microbiomes in a Diverse South Florida Population
Abstract
:1. Introduction
2. Materials and Methods
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Peng, X.; Zhou, X.; Xu, X.; Li, Y.; Li, Y.; Li, J.; Su, X.; Huang, S.; Xu, J.; Liao, G. The Oral Microbiome Bank of China. Int. J. Oral Sci. 2018, 10, 16. [Google Scholar] [CrossRef]
- Deo, P.N.; Deshmukh, R. Oral microbiome: Unveiling the fundamentals. J. Oral Maxillofac. Pathol. 2019, 23, 122–128. [Google Scholar] [CrossRef] [PubMed]
- Peng, X.; Cheng, L.; You, Y.; Tang, C.; Ren, B.; Li, Y.; Xu, X.; Zhou, X. Oral microbiota in human systematic diseases. Int. J. Oral Sci. 2022, 14, 14. [Google Scholar] [CrossRef]
- Renson, A.; Jones, H.E.; Beghini, F.; Segata, N.; Zolnik, C.P.; Usyk, M.; Moody, T.U.; Thorpe, L.; Burk, R.; Waldron, L.; et al. Sociodemographic variation in the oral microbiome. Ann. Epidemiol. 2019, 35, 73–80.e72. [Google Scholar] [CrossRef]
- Liu, X.; Tong, X.; Zhu, J.; Tian, L.; Jie, Z.; Zou, Y.; Lin, X.; Liang, H.; Li, W.; Ju, Y.; et al. Metagenome-genome-wide association studies reveal human genetic impact on the oral microbiome. Cell Discov. 2021, 7, 117. [Google Scholar] [CrossRef] [PubMed]
- Stoy, S.; McMillan, A.; Ericsson, A.C.; Brooks, A.E. The effect of physical and psychological stress on the oral microbiome. Front. Psychol. 2023, 14, 1166168. [Google Scholar] [CrossRef]
- Santonocito, S.; Giudice, A.; Polizzi, A.; Troiano, G.; Merlo, E.M.; Sclafani, R.; Grosso, G.; Isola, G. A Cross-Talk between Diet and the Oral Microbiome: Balance of Nutrition on Inflammation and Immune System’s Response during Periodontitis. Nutrients 2022, 14, 2426. [Google Scholar] [CrossRef]
- Lloyd-Price, J.; Mahurkar, A.; Rahnavard, G.; Crabtree, J.; Orvis, J.; Hall, A.B.; Brady, A.; Creasy, H.H.; McCracken, C.; Giglio, M.G.; et al. Strains, functions and dynamics in the expanded Human Microbiome Project. Nature 2017, 550, 61–66. [Google Scholar] [CrossRef]
- Premaraj, T.S.; Vella, R.; Chung, J.; Lin, Q.; Hunter, P.; Underwood, K.; Premaraj, S.; Zhou, Y. Ethnic variation of oral microbiota in children. Sci. Rep. 2020, 10, 14788. [Google Scholar] [CrossRef]
- Yang, Y.; Zheng, W.; Cai, Q.; Shrubsole Martha, J.; Pei, Z.; Brucker, R.; Steinwandel, M.; Bordenstein Seth, R.; Li, Z.; Blot William, J.; et al. Racial Differences in the Oral Microbiome: Data from Low-Income Populations of African Ancestry and European Ancestry. mSystems 2019, 4, e00639-19. [Google Scholar] [CrossRef]
- Gupta, V.K.; Paul, S.; Dutta, C. Geography, Ethnicity or Subsistence-Specific Variations in Human Microbiome Composition and Diversity. Front. Microbiol. 2017, 8, 1162. [Google Scholar] [CrossRef] [PubMed]
- Ozga, A.T.; Sankaranarayanan, K.; Tito, R.Y.; Obregon-Tito, A.; Foster, M.; Tallbull, G.; Spicer, P.; Warinner, C.; Lewis, C.M., Jr. Oral microbiome diversity among Cheyenne and Arapaho individuals from Oklahoma. Am. J. Phys. Anthropol. 2016, 161, 321–327. [Google Scholar] [CrossRef] [PubMed]
- Handsley-Davis, M.; Skelly, E.; Johnson, N.W.; Kapellas, K.; Lalloo, R.; Kroon, J.; Weyrich, L.S. Biocultural Drivers of Salivary Microbiota in Australian Aboriginal and Torres Strait Islander Children. Front. Oral Health 2021, 2, 641328. [Google Scholar] [CrossRef] [PubMed]
- Contreras, M.; Costello, E.K.; Hidalgo, G.; Magris, M.; Knight, R.; Dominguez-Bello, M.G. The bacterial microbiota in the oral mucosa of rural Amerindians. Microbiology 2010, 156, 3282–3287. [Google Scholar] [CrossRef] [PubMed]
- Han, C.S.; Martin, M.A.; Dichosa, A.E.K.; Daughton, A.R.; Frietze, S.; Kaplan, H.; Gurven, M.D.; Alcock, J. Salivary microbiomes of indigenous Tsimane mothers and infants are distinct despite frequent premastication. PeerJ 2016, 4, e2660. [Google Scholar] [CrossRef]
- Rogers, G.B.; Ward, J.; Brown, A.; Wesselingh, S.L. Inclusivity and equity in human microbiome research. Lancet 2019, 393, 728–729. [Google Scholar] [CrossRef]
- Kaan, A.M.; Brandt, B.W.; Buijs, M.J.; Crielaard, W.; Keijser, B.J.; Zaura, E. Comparability of microbiota of swabbed and spit saliva. Eur. J. Oral Sci. 2022, 130, e12858. [Google Scholar] [CrossRef]
- Kazarina, A.; Kuzmicka, J.; Bortkevica, S.; Zayakin, P.; Kimsis, J.; Igumnova, V.; Sadovska, D.; Freimane, L.; Kivrane, A.; Namina, A.; et al. Oral microbiome variations related to ageing: Possible implications beyond oral health. Arch. Microbiol. 2023, 205, 116. [Google Scholar] [CrossRef]
- Lu, H.; Zou, P.; Zhang, Y.; Zhang, Q.; Chen, Z.; Chen, F. The sampling strategy of oral microbiome. iMeta 2022, 1, e23. [Google Scholar] [CrossRef]
- Nazir, M.; Al-Ansari, A.; Al-Khalifa, K.; Alhareky, M.; Gaffar, B.; Almas, K. Global Prevalence of Periodontal Disease and Lack of Its Surveillance. Sci. World J. 2020, 2020, 2146160. [Google Scholar] [CrossRef]
- Medicine, J.H. Periodontal Diseases. Available online: https://www.hopkinsmedicine.org/health/conditions-and-diseases/periodontal-diseases (accessed on 19 August 2024).
- Oliver, R.C.; Brown, L.J.; Löe, H. Periodontal diseases in the United States population. J. Periodontol. 1998, 69, 269–278. [Google Scholar] [CrossRef]
- Di Stefano, M.; Polizzi, A.; Santonocito, S.; Romano, A.; Lombardi, T.; Isola, G. Impact of Oral Microbiome in Periodontal Health and Periodontitis: A Critical Review on Prevention and Treatment. Int. J. Mol. Sci. 2022, 23, 5142. [Google Scholar] [CrossRef] [PubMed]
- Gasner, N.; Schure, R. Periodontal Disease; StatPearls Publishing: Treasure Island, FL, USA, 2023. [Google Scholar]
- Butera, A.; Gallo, S.; Maiorani, C.; Molino, D.; Chiesa, A.; Preda, C.; Esposito, F.; Scribante, A. Probiotic Alternative to Chlorhexidine in Periodontal Therapy: Evaluation of Clinical and Microbiological Parameters. Microorganisms 2020, 9, 69. [Google Scholar] [CrossRef]
- Scribante, A.; Gallo, S.; Pascadopoli, M.; Frani, M.; Butera, A. Ozonized gels vs chlorhexidine in non-surgical periodontal treatment: A randomized clinical trial. Oral Dis. 2023, 30, 3993–4000. [Google Scholar] [CrossRef] [PubMed]
- Alosaimi, B.; Hampson, L.; He, X.; Maranga, I.O.; Oliver, A.W.; Hampson, I.N. Increased prevalence of JC polyomavirus in cervical carcinomas from women infected with HIV. J. Med. Virol. 2014, 86, 672–677. [Google Scholar] [CrossRef] [PubMed]
- Eke, P.I.; Dye, B.A.; Wei, L.; Slade, G.D.; Thornton-Evans, G.O.; Borgnakke, W.S.; Taylor, G.W.; Page, R.C.; Beck, J.D.; Genco, R.J. Update on Prevalence of Periodontitis in Adults in the United States: NHANES 2009 to 2012. J. Periodontol. 2015, 86, 611–622. [Google Scholar] [CrossRef]
- Ortiz, A.P.; Acosta-Pagán, K.T.; Oramas-Sepúlveda, C.; Castañeda-Avila, M.A.; Vilanova-Cuevas, B.; Ramos-Cartagena, J.M.; Vivaldi, J.A.; Pérez-Santiago, J.; Pérez, C.M.; Godoy-Vitorino, F. Oral microbiota and periodontitis severity among Hispanic adults. Front. Cell. Infect. Microbiol. 2022, 12, 965159. [Google Scholar] [CrossRef]
- CBS. Expert on Florida Population Growth: “It’s the Highest Number It’s Ever Been”. CBS NEWS, 7 December 2023. [Google Scholar]
- DoH, F. Population Dashboard. Available online: https://www.flhealthcharts.gov/ChartsReports/rdPage.aspx?rdReport=PopAtlas.PopulationAtlasDASHBOARD (accessed on 10 June 2024).
- Bonefront, A. South Florida Adds Almost 30,000 New Residents, Census Data Shows. Sun Sentinel, 18 May 2023. [Google Scholar]
- NSU. NSU At a Glance. Available online: https://www.nova.edu/nsuflorida/index.html (accessed on 10 June 2024).
- D’Agostino, S.; Ferrara, E.; Valentini, G.; Stoica, S.A.; Dolci, M. Exploring Oral Microbiome in Healthy Infants and Children: A Systematic Review. Int. J. Environ. Res. Public Health 2022, 19, 11403. [Google Scholar] [CrossRef] [PubMed]
- BIOMCARE. Available online: https://biomcare.com/info/key-terms-in-microbiome-projects/#:~:text=While%20alpha%20diversity%20is%20a,different%20aspects%20of%20community%20heterogeneity (accessed on 20 August 2024).
- Segata, N.; Izard, J.; Waldron, L.; Gevers, D.; Miropolsky, L.; Garrett, W.S.; Huttenhower, C. Metagenomic biomarker discovery and explanation. Genome Biol. 2011, 12, R60. [Google Scholar] [CrossRef]
- Chapple, I.L.C.; Mealey, B.L.; Van Dyke, T.E.; Bartold, P.M.; Dommisch, H.; Eickholz, P.; Geisinger, M.L.; Genco, R.J.; Glogauer, M.; Goldstein, M.; et al. Periodontal health and gingival diseases and conditions on an intact and a reduced periodontium: Consensus report of workgroup 1 of the 2017 World Workshop on the Classification of Periodontal and Peri-Implant Diseases and Conditions. J. Periodontol. 2018, 89, S74–S84. [Google Scholar] [CrossRef]
- Caporaso, J.G.; Lauber, C.L.; Walters, W.A.; Berg-Lyons, D.; Lozupone, C.A.; Turnbaugh, P.J.; Fierer, N.; Knight, R. Global patterns of 16S rRNA diversity at a depth of millions of sequences per sample. Proc. Natl. Acad. Sci. USA 2011, 108 (Suppl. S1), 4516–4522. [Google Scholar] [CrossRef] [PubMed]
- Estaki, M.; Jiang, L.; Bokulich, N.A.; McDonald, D.; González, A.; Kosciolek, T.; Martino, C.; Zhu, Q.; Birmingham, A.; Vázquez-Baeza, Y.; et al. QIIME 2 Enables Comprehensive End-to-End Analysis of Diverse Microbiome Data and Comparative Studies with Publicly Available Data. Curr. Protoc. Bioinform. 2020, 70, e100. [Google Scholar] [CrossRef]
- Callahan, B.J.; McMurdie, P.J.; Rosen, M.J.; Han, A.W.; Johnson, A.J.A.; Holmes, S.P. DADA2: High-resolution sample inference from Illumina amplicon data. Nat. Methods 2016, 13, 581–583. [Google Scholar] [CrossRef]
- Quast, C.; Pruesse, E.; Yilmaz, P.; Gerken, J.; Schweer, T.; Yarza, P.; Peplies, J.; Glöckner, F.O. The SILVA ribosomal RNA gene database project: Improved data processing and web-based tools. Nucleic Acids Res. 2013, 41, D590–D596. [Google Scholar] [CrossRef]
- Willis, J.R.; Gabaldón, T. The Human Oral Microbiome in Health and Disease: From Sequences to Ecosystems. Microorganisms 2020, 8, 308. [Google Scholar] [CrossRef] [PubMed]
- Jandhyala, S.M.; Talukdar, R.; Subramanyam, C.; Vuyyuru, H.; Sasikala, M.; Nageshwar Reddy, D. Role of the normal gut microbiota. World J. Gastroenterol. 2015, 21, 8787–8803. [Google Scholar] [CrossRef] [PubMed]
- Dewhirst, F.E.; Chen, T.; Izard, J.; Paster, B.J.; Tanner, A.C.R.; Yu, W.-H.; Lakshmanan, A.; Wade, W.G. The Human Oral Microbiome. J. Bacteriol. 2010, 192, 5002–5017. [Google Scholar] [CrossRef]
- Zaura, E.; Keijser, B.J.; Huse, S.M.; Crielaard, W. Defining the healthy “core microbiome” of oral microbial communities. BMC Microbiol. 2009, 9, 259. [Google Scholar] [CrossRef]
- Nearing, J.T.; Comeau, A.M.; Langille, M.G.I. Identifying biases and their potential solutions in human microbiome studies. Microbiome 2021, 9, 113. [Google Scholar] [CrossRef]
- Li, W.; Ma, Z. FBA Ecological Guild: Trio of Firmicutes-Bacteroidetes Alliance against Actinobacteria in Human Oral Microbiome. Sci. Rep. 2020, 10, 287. [Google Scholar] [CrossRef]
- Brennan, C.A.; Garrett, W.S. Fusobacterium nucleatum-symbiont, opportunist and oncobacterium. Nat. Rev. Microbiol. 2019, 17, 156–166. [Google Scholar] [CrossRef] [PubMed]
- Shin, N.-R.; Whon, T.W.; Bae, J.-W. Proteobacteria: Microbial signature of dysbiosis in gut microbiota. Trends Biotechnol. 2015, 33, 496–503. [Google Scholar] [CrossRef] [PubMed]
- Abranches, J.; Zeng, L.; Kajfasz, J.K.; Palmer, S.R.; Chakraborty, B.; Wen, Z.T.; Richards, V.P.; Brady, L.J.; Lemos, J.A. Biology of Oral Streptococci. Microbiol. Spectr. 2018, 6. [Google Scholar] [CrossRef] [PubMed]
- Giacomini Jonathan, J.; Torres-Morales, J.; Dewhirst Floyd, E.; Borisy Gary, G.; Mark Welch Jessica, L. Site Specialization of Human Oral Veillonella Species. Microbiol. Spectr. 2023, 11, e0404222. [Google Scholar] [CrossRef]
- Könönen, E.; Fteita, D.; Gursoy, U.K.; Gursoy, M. Prevotella species as oral residents and infectious agents with potential impact on systemic conditions. J. Oral Microbiol. 2022, 14, 2079814. [Google Scholar] [CrossRef]
- Shigeishi, H.; Hamada, N.; Kaneyasu, Y.; Niitani, Y.; Takemoto, T.; Ohta, K. Prevalence of oral Capnocytophaga species and their association with dental plaque accumulation and periodontal inflammation in middle-aged and older people. Biomed. Rep. 2024, 20, 99. [Google Scholar] [CrossRef]
- Cakur, B.; Yıldız, M.; Dane, S.; Zorba, Y.O. The effect of right or left handedness on caries experience and oral hygiene. J. Neurosci. Rural Pract. 2011, 2, 40–42. [Google Scholar] [CrossRef]
- Tezel, A.; Canakçi, V.; Ciçek, Y.; Demir, T. Evaluation of gingival recession in left- and right-handed adults. Int. J. Neurosci. 2001, 110, 135–146. [Google Scholar] [CrossRef]
- Shang, Q.; Gao, Y.; Qin, T.; Wang, S.; Shi, Y.; Chen, T. Interaction of Oral and Toothbrush Microbiota Affects Oral Cavity Health. Front. Cell. Infect. Microbiol. 2020, 10, 17. [Google Scholar] [CrossRef]
- Sakalauskienė, Z.; Vehkalahti, M.M.; Murtomaa, H.; Mačiulskienė, V. Factors related to gender differences in toothbrushing among Lithuanian middle-aged university employees. Medicina 2011, 47, 180–186. [Google Scholar] [CrossRef]
- Liu, X.; Tong, X.; Jie, Z.; Zhu, J.; Tian, L.; Sun, Q.; Ju, Y.; Zou, L.; Lu, H.; Qiu, X.; et al. Sex differences in the oral microbiome, host traits, and their causal relationships. iScience 2023, 26, 105839. [Google Scholar] [CrossRef] [PubMed]
- Giordano-Kelhoffer, B.; Lorca, C.; March Llanes, J.; Rábano, A.; Del Ser, T.; Serra, A.; Gallart-Palau, X. Oral Microbiota, Its Equilibrium and Implications in the Pathophysiology of Human Diseases: A Systematic Review. Biomedicines 2022, 10, 1803. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.J.; Jiang, T.; Ma, X.X.; Hu, X.J.; Huang, J.B.; Cui, L.T.; Cui, J.; Yao, X.H.; Shi, Y.L.; Li, J.; et al. Relationships between Diurnal Changes of Tongue Coating Microbiota and Intestinal Microbiota. Front. Cell. Infect. Microbiol. 2022, 12, 813790. [Google Scholar] [CrossRef] [PubMed]
- Yatsunenko, T.; Rey, F.E.; Manary, M.J.; Trehan, I.; Dominguez-Bello, M.G.; Contreras, M.; Magris, M.; Hidalgo, G.; Baldassano, R.N.; Anokhin, A.P.; et al. Human gut microbiome viewed across age and geography. Nature 2012, 486, 222–227. [Google Scholar] [CrossRef] [PubMed]
- Freire, M.; Moustafa, A.; Harkins, D.M.; Torralba, M.G.; Zhang, Y.; Leong, P.; Saffery, R.; Bockmann, M.; Kuelbs, C.; Hughes, T.; et al. Longitudinal Study of Oral Microbiome Variation in Twins. Sci. Rep. 2020, 10, 7954. [Google Scholar] [CrossRef]
- Lawal, F.J.; Baer, S.L. Capnocytophaga gingivalis Bacteremia After Upper Gastrointestinal Bleeding in Immunocompromised Patient. J. Investig. Med. High Impact Case Rep. 2021, 9, 23247096211020672. [Google Scholar] [CrossRef]
- Idate, U.; Bhat, K.; Kotrashetti, V.; Kugaji, M.; Kumbar, V. Molecular identification of Capnocytophaga species from the oral cavity of patients with chronic periodontitis and healthy individuals. J. Oral Maxillofac. Pathol. 2020, 24, 397. [Google Scholar] [CrossRef]
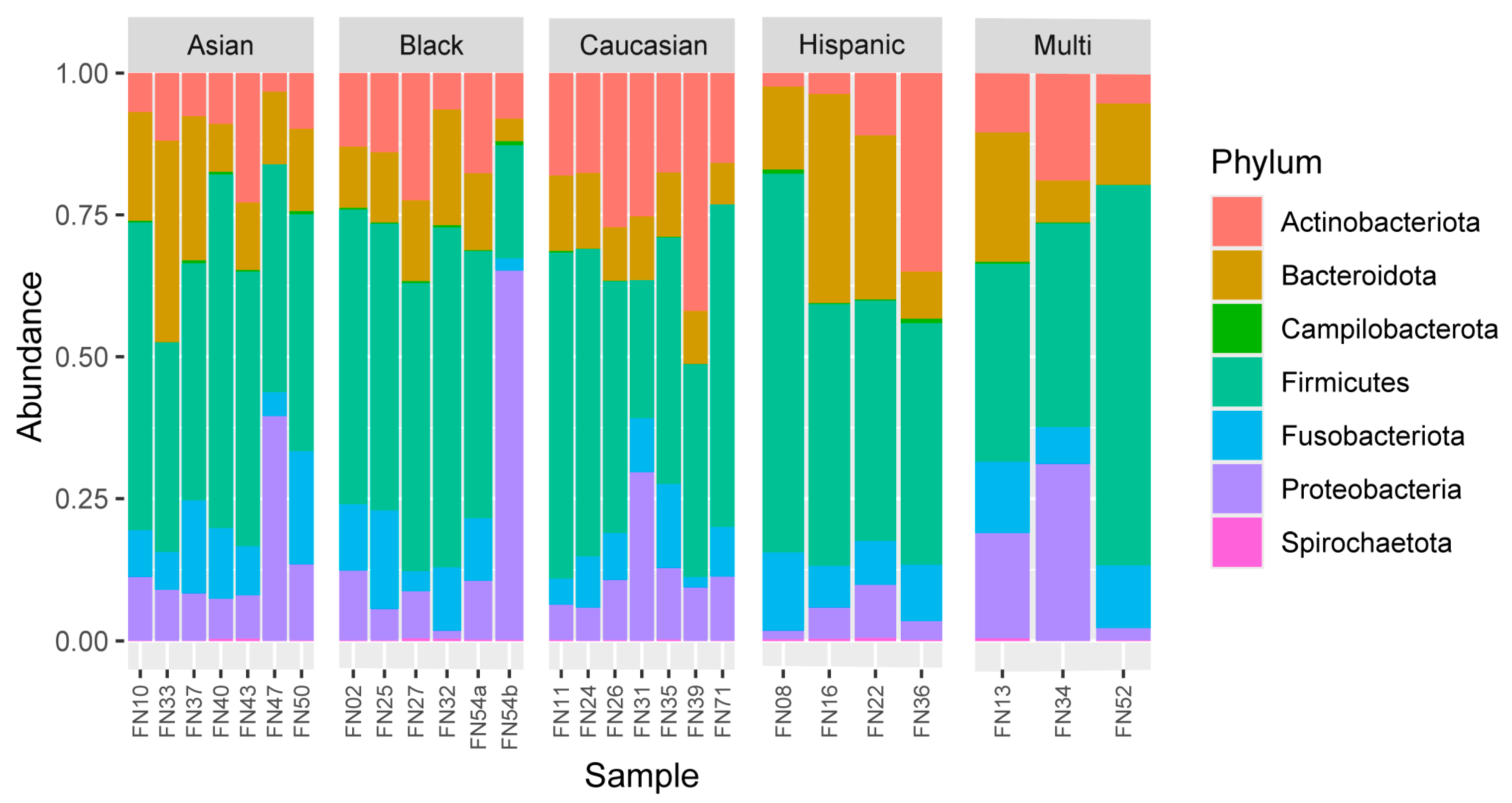




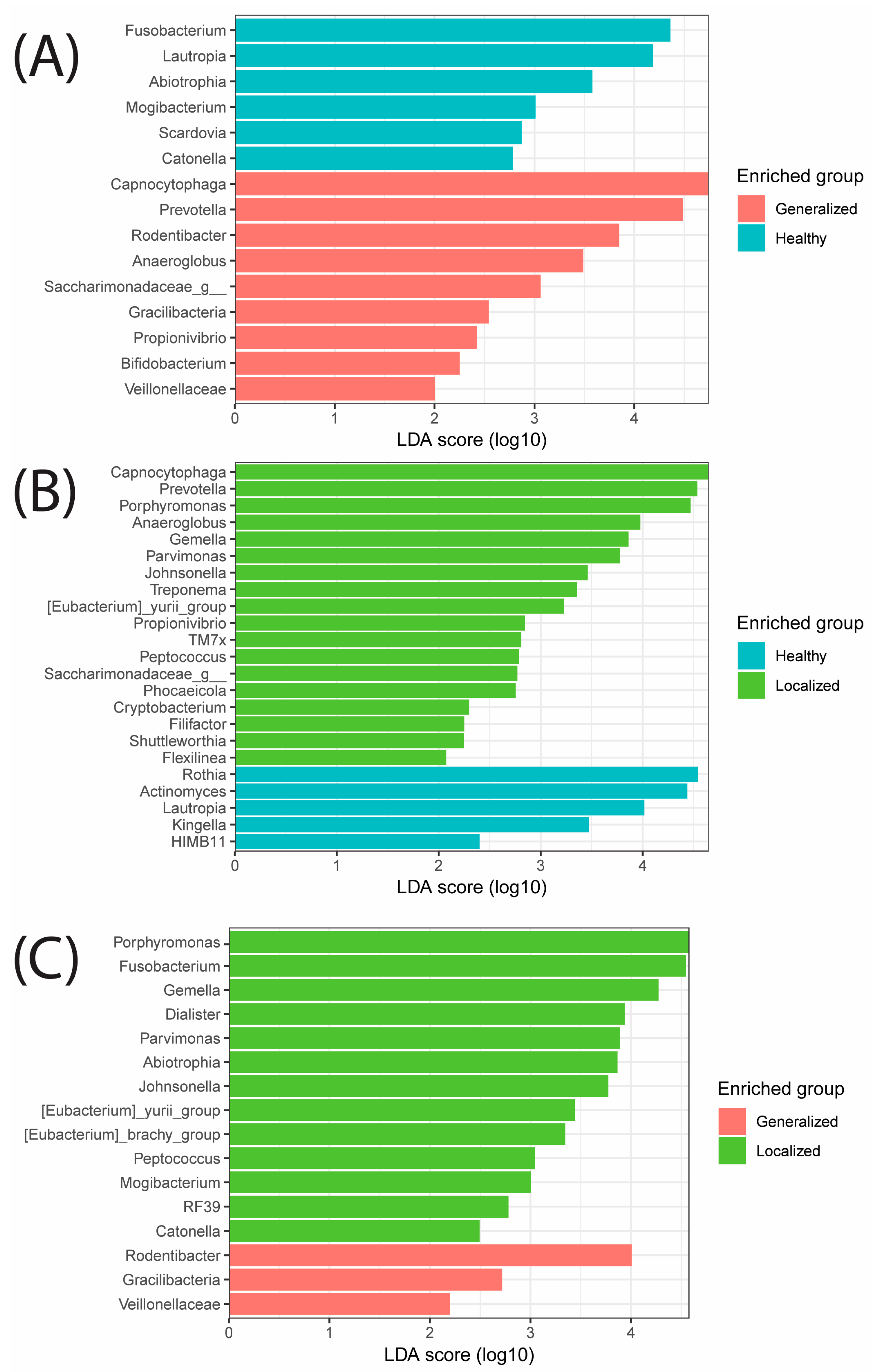
| Sample | Age Range | Age | Ethnicity | Sex | BMI_range | Height (in) | Weight (lbs) | BMI | Health State |
|---|---|---|---|---|---|---|---|---|---|
| FN02 | <22 | 20 | Black | M | overweight | 77 | 199 | 34.15 | Healthy |
| FN08 | <22 | 27 | Hispanic | M | overweight | 71 | 185 | 25.8 | Localized |
| FN10 | 23–27 | 27 | Asian | F | normal | 61 | 100 | 18.89 | Localized |
| FN11 | <22 | 20 | Caucasian | M | overweight | 71 | 180 | 25.1 | Healthy |
| FN13 | <22 | 24 | Multi (Caucasian, Asian) | M | overweight | 68 | 167 | 25.39 | Healthy |
| FN16 | <22 | 22 | Hispanic | M | obese | 72 | 250 | 33.9 | Localized |
| FN22 | 33–38 | 20 | Hispanic | M | overweight | 69 | 190 | 28.06 | Localized |
| FN24 | 28–32 | 21 | Caucasian | F | normal | 65 | 139 | 23.13 | Generalized |
| FN25 | <22 | 38 | Black | M | obese | 76 | 250 | 30.43 | Healthy |
| FN26 | 33–38 | 30 | Caucasian | F | obese | 48 | 145 | 44.24 | Healthy |
| FN27 | 33–38 | 18 | Black | F | normal | 60 | 125 | 24.41 | Healthy |
| FN31 | 28–32 | 35 | Caucasian | M | normal | 73 | 155 | 20.45 | Healthy |
| FN32 | 28–32 | 33 | Black | F | normal | 67 | 135 | 21.14 | Localized |
| FN33 | <22 | 30 | Asian | M | normal | 70 | 134 | 19.22 | Localized |
| FN34 | 33-38 | 29 | Multi (Caucasian, Hispanic) | M | normal | 70 | 170 | 24.39 | Healthy |
| FN35 | 28–32 | 22 | Caucasian | M | normal | 69 | 145 | 21.41 | Healthy |
| FN36 | 28–32 | 37 | Hispanic | F | overweight | 64 | 164 | 28.15 | Healthy |
| FN37 | <22 | 29 | Asian | M | normal | 73 | 160 | 21.11 | Generalized |
| FN39 | 33–38 | 31 | Caucasian | F | normal | 68 | 140 | 21.28 | Healthy |
| FN40 | <22 | 20 | Asian | M | normal | 64 | 135 | 24.03 | Healthy |
| FN43 | <22 | 33 | Asian | M | normal | 67 | 130 | 20.36 | Healthy |
| FN47 | 23–27 | 19 | Asian | M | normal | 71 | 160 | 22.31 | Healthy |
| FN50 | 23–27 | 19 | Asian | F | normal | 64 | 135 | 23.17 | Healthy |
| FN52 | 23–27 | 25 | Multi | M | overweight | 67 | 230 | 36.02 | Healthy |
| FN54a | 23–27 | 26 | Black | M | obese | 72 | 400 | 54.24 | Healthy |
| FN54b | 23–27 | 26 | Black | M | obese | 72 | 400 | 54.24 | Healthy |
| FN71 | 23–27 | 23 | Caucasian | F | normal | 66 | 125 | 20.17 | Healthy |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Demehri, S.; Vardar, S.; Godoy, C.; Lopez, J.V.; Samuel, P.; Kawai, T.; Ozga, A.T. Supragingival Plaque Microbiomes in a Diverse South Florida Population. Microorganisms 2024, 12, 1921. https://doi.org/10.3390/microorganisms12091921
Demehri S, Vardar S, Godoy C, Lopez JV, Samuel P, Kawai T, Ozga AT. Supragingival Plaque Microbiomes in a Diverse South Florida Population. Microorganisms. 2024; 12(9):1921. https://doi.org/10.3390/microorganisms12091921
Chicago/Turabian StyleDemehri, Sharlene, Saynur Vardar, Cristina Godoy, Jose V. Lopez, Paisley Samuel, Toshihisa Kawai, and Andrew T. Ozga. 2024. "Supragingival Plaque Microbiomes in a Diverse South Florida Population" Microorganisms 12, no. 9: 1921. https://doi.org/10.3390/microorganisms12091921
APA StyleDemehri, S., Vardar, S., Godoy, C., Lopez, J. V., Samuel, P., Kawai, T., & Ozga, A. T. (2024). Supragingival Plaque Microbiomes in a Diverse South Florida Population. Microorganisms, 12(9), 1921. https://doi.org/10.3390/microorganisms12091921







