Infectious Bronchitis Virus (IBV) in Vaccinated and Non-Vaccinated Broilers in Brazil: Surveillance and Persistence of Vaccine Viruses
Abstract
1. Introduction
2. Materials and Methods
2.1. Sampling
2.2. Extraction of Genetic Material and Molecular Detection of IBV
2.3. DNA Sequencing and Phylogenetic Analysis
3. Results
3.1. Prevalence of IBV in Vaccine and Non-Vaccine Samples
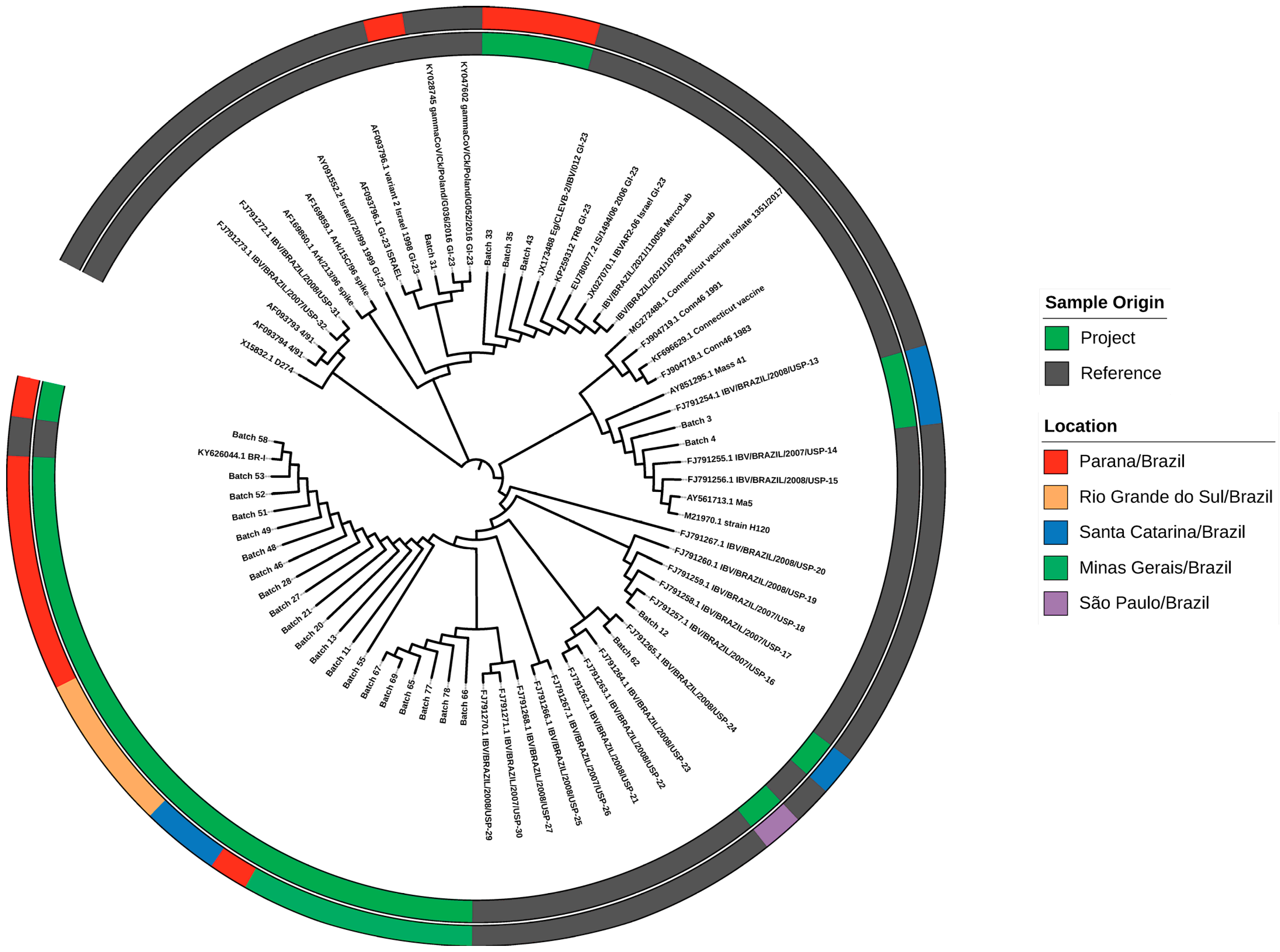
3.2. The S1 Glycoprotein Region Sequencing
3.3. Vaccine Persistence in Tracheal Samples
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| MDPI | Multidisciplinary Digital Publishing Institute |
| DOAJ | Directory of Open Access Journals |
| TLA | Three-letter acronym |
| LD | Linear dichroism |
References
- Yehia, N.; Salem, H.M.; Mahmmod, Y.; Said, D.; Samir, M.; Mawgod, S.A.; Sorour, H.K.; AbdelRahman, M.A.A.; Selim, S.; Saad, A.M.; et al. Common Viral and Bacterial Avian Respiratory Infections: An Updated Review. Poult. Sci. 2023, 102, 102553. [Google Scholar] [CrossRef]
- de Sjaak Wit, J.J.; Cook, J.K.A.; van der Heijden, H.M.J.F. Infectious Bronchitis Virus Variants: A Review of the History, Current Situation and Control Measures. Avian Pathol. 2011, 40, 223–235. [Google Scholar] [CrossRef]
- Molenaar, R.J.; Dijkman, R.; de Wit, J.J. Characterization of Infectious Bronchitis Virus D181, a New Serotype (GII-2). Avian Pathol. 2020, 49, 243–250. [Google Scholar] [CrossRef]
- Cavanagh, D. Coronavirus Avian Infectious Bronchitis Virus. Vet. Res. 2007, 38, 281–297. [Google Scholar] [CrossRef]
- Cavanagh, D.; Gelb, J.J. Infectious Bronchitis. In Diseases of Poultry; Saif, Y.M., Fadly, A.M., Glisson, J.R., McDougald, L.R., Nolan, L.K., Swayne, D.E., Eds.; Blackwell Publishing Ltd.: Hoboken, NJ, USA, 2008; pp. 117–135. [Google Scholar]
- de Wit, J.J.; Cook, J.K.A. Spotlight on Avian Coronaviruses. Avian Pathol. 2020, 49, 313–316. [Google Scholar] [CrossRef] [PubMed]
- Durães-Carvalho, R.; Caserta, L.C.; Barnabé, A.C.S.; Martini, M.C.; Simas, P.V.M.; Santos, M.M.B.; Salemi, M.; Arns, C.W. Phylogenetic and Phylogeographic Mapping of the Avian Coronavirus Spike Protein-Encoding Gene in Wild and Synanthropic Birds. Virus Res. 2015, 201, 101–112. [Google Scholar] [CrossRef] [PubMed]
- Carstens, E.B. Ratification Vote on Taxonomic Proposals to the International Committee on Taxonomy of Viruses (2009). Arch. Virol. 2010, 155, 133–146. [Google Scholar] [CrossRef] [PubMed]
- Rafique, S.; Jabeen, Z.; Pervaiz, T.; Rashid, F.; Luo, S.; Xie, L.; Xie, Z. Avian Infectious Bronchitis Virus (AIBV) Review by Continent. Front. Cell Infect. Microbiol. 2024, 14, 1325346. [Google Scholar] [CrossRef] [PubMed]
- Jordan, B. Vaccination against Infectious Bronchitis Virus: A Continuous Challenge. Vet. Microbiol. 2017, 206, 137–143. [Google Scholar] [CrossRef] [PubMed]
- Jackwood, M.W.; Clark, R.; Cheng, S.; Jordan, B.J. Protection Following Simultaneous Vaccination with Three or Four Different Attenuated Live Vaccine Types against Infectious Bronchitis Virus. Avian Pathol. 2020, 49, 335–341. [Google Scholar] [CrossRef] [PubMed]
- Valastro, V.; Holmes, E.C.; Britton, P.; Fusaro, A.; Jackwood, M.W.; Cattoli, G.; Monne, I. S1 Gene-Based Phylogeny of Infectious Bronchitis Virus: An Attempt to Harmonize Virus Classification. Infect. Genet. Evol. 2016, 39, 349–364. [Google Scholar] [CrossRef] [PubMed]
- Moreno, A.; Franzo, G.; Massi, P.; Tosi, G.; Blanco, A.; Antilles, N.; Biarnes, M.; Majó, N.; Nofrarías, M.; Dolz, R.; et al. A Novel Variant of the Infectious Bronchitis Virus Resulting from Recombination Events in Italy and Spain. Avian Pathol. 2017, 46, 28–35. [Google Scholar] [CrossRef]
- Britton, P.; Armesto, M.; Cavanagh, D.; Keep, S. Modification of the Avian Coronavirus Infectious Bronchitis Virus for Vaccine Development. Bioengineered 2012, 3, 114–119. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Khan, M.I. Use of Reverse Transcriptase-Polymerase Chain Reaction-Restriction Fragment Length Polymorphism to Examine the Interaction between Infectious Bronchitis Virus Strains Massachusetts 41 and JMK in Ovo. Avian Pathol. 2000, 29, 441–448. [Google Scholar] [CrossRef]
- Jia, W.; Karaca, K.; Parrish, C.R.; Naqi, S.A. A Novel Variant of Avian Infectious Bronchitis Virus Resulting from Recombination among Three Different Strains. Arch. Virol. 1995, 140, 259–271. [Google Scholar] [CrossRef] [PubMed]
- Chacón, J.L.; Chacón, R.D.; Sánchez-Llatas, C.J.; Morín, J.G.; Astolfi-Ferreira, C.S.; Piantino Ferreira, A.J. Antigenic and Molecular Characterization of Isolates of the Brazilian Genotype BR-I (GI-11) of Infectious Bronchitis Virus Supports Its Recognition as BR-I Serotype. Avian Pathol. 2023, 52, 323–338. [Google Scholar] [CrossRef]
- Fraga, A.P.; Balestrin, E.; Ikuta, N.; Fonseca, A.S.K.; Spilki, F.R.; Canal, C.W.; Lunge, V.R. Emergence of a New Genotype of Avian Infectious Bronchitis Virus in Brazil. Avian Dis. 2013, 57, 225–232. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Mendoza-González, L.; Marandino, A.; Panzera, Y.; Tomás, G.; Williman, J.; Techera, C.; Gayosso-Vázquez, A.; Ramírez-Andoney, V.; Alonso-Morales, R.; Realpe-Quintero, M.; et al. Research Note: High Genetic Diversity of Infectious Bronchitis Virus from Mexico. Poult. Sci. 2022, 101, 102076. [Google Scholar] [CrossRef]
- Abdel-Sabour, M.A.; Rohaim, M.A.; Salman, O.J.A.; Abodalal, S.E.; Mohammad, F.F.; Madkour, M.S.; Abdel-Wanis, N.A.; Munir, M. Immunogenicity and Efficacy of a Bivalent Vaccine against Infectious Bronchitis Virus. Comp. Immunol. Microbiol. Infect. Dis. 2021, 77, 101670. [Google Scholar] [CrossRef]
- Bhuiyan, M.d.S.A.; Amin, Z.; Rodrigues, K.F.; Saallah, S.; Shaarani, S.M.d.; Sarker, S.; Siddiquee, S. Infectious Bronchitis Virus (Gammacoronavirus) in Poultry Farming: Vaccination, Immune Response and Measures for Mitigation. Vet. Sci. 2021, 8, 273. [Google Scholar] [CrossRef] [PubMed]
- Bande, F.; Arshad, S.S.; Hair Bejo, M.; Moeini, H.; Omar, A.R. Progress and Challenges toward the Development of Vaccines against Avian Infectious Bronchitis. J. Immunol. Res. 2015, 2015, 424860. [Google Scholar] [CrossRef] [PubMed]
- de Fraga, A.P.; Gräf, T.; Pereira, C.S.; Ikuta, N.; Fonseca, A.S.K.; Lunge, V.R. Phylodynamic Analysis and Molecular Diversity of the Avian Infectious Bronchitis Virus of Chickens in Brazil. Infect. Genet. Evol. 2018, 61, 77–83. [Google Scholar] [CrossRef] [PubMed]
- Ikuta, N.; Kipper, D.; de Freitas, D.S.S.; Fonseca, A.S.K.; Lunge, V.R. Evolution and Epidemic Spread of the Avian Infectious Bronchitis Virus (IBV) GI-23 in Brazil. Viruses 2023, 15, 1229. [Google Scholar] [CrossRef]
- Trevisol, I.M.; Caron, L.; Mores, M.A.Z.; Voss-Rech, D.; da Silva Zani, G.; Back, A.; Marchesi, J.A.P.; Esteves, P.A. Pathogenicity of GI-23 Avian Infectious Bronchitis Virus Strain Isolated in Brazil. Viruses 2023, 15, 1200. [Google Scholar] [CrossRef]
- Lisowska, A.; Sajewicz-Krukowska, J.; Fusaro, A.; Pikula, A.; Domanska-Blicharz, K. First Characterization of a Middle-East GI-23 Lineage (Var2-like) of Infectious Bronchitis Virus in Europe. Virus Res. 2017, 242, 43–48. [Google Scholar] [CrossRef] [PubMed]
- ABPA (Brazilian Animal Protein Association). Anual Report 2024; ABPA (Brazilian Animal Protein Association): Sao Paulo, Brazil, 2024. [Google Scholar]
- Jones, R.C.; Worthington, K.J.; Gough, R.E. Detection of the Italy O2 Strain of Infectious Bronchitis Virus in the UK. Vet. Rec. 2005, 156, 260. [Google Scholar] [PubMed]
- Worthington, K.J.; Currie, R.J.W.; Jones, R.C. A reverse transcriptase-polymerase chain reaction survey of infectious bronchitis virus genotypes in Western Europe from 2002 to 2006. Avian Pathol. 2008, 37, 247–257. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Stecher, G.; Li, M.; Knyaz, C.; Tamura, K. MEGA X: Molecular evolutionary genetics analysis across computing platforms. Mol. Biol. Evol. 2018, 35, 1547–1549. [Google Scholar] [CrossRef] [PubMed]
- Chacón, R.D.; Astolfi-Ferreira, C.S.; Chacón, J.L.; Nuñez, L.F.N.; De la Torre, D.I.; Piantino Ferreira, A.J. A Seminested RT-PCR for Molecular Genotyping of the Brazilian BR-I Infectious Bronchitis Virus Strain (GI-11). Mol. Cell Probes 2019, 47, 101426. [Google Scholar] [CrossRef] [PubMed]
- Jackwood, M.W. Review of Infectious Bronchitis Virus Around the World. Avian Dis. 2012, 56, 634–641. [Google Scholar] [CrossRef]
- Ikuta, N.; Fonseca, A.S.K.; Fernando, F.S.; Filho, T.F.; Martins, N.R.d.S.; Lunge, V.R. Emergence and Molecular Characterization of the Avian Infectious Bronchitis Virus GI-23 in Commercial Broiler Farms from South America. Transbound. Emerg. Dis. 2022, 69, 3167–3172. [Google Scholar] [CrossRef]
- Saraiva, G.L.; Santos, M.R.; Pereira, C.G.; Vidigal, P.M.P.; Fietto, J.L.R.; de Oliveira Mendes, T.A.; Bressan, G.C.; Soares-Martins, J.A.P.; de Almeida, M.R.; Silva-Júnior, A. Evaluation of the Genetic Variability Found in Brazilian Commercial Vaccines for Infectious Bronchitis Virus. Virus Genes 2018, 54, 77–85. [Google Scholar] [CrossRef] [PubMed]
- Flageul, A.; Allée, C.; Courtillon, C.; Béven, V.; Quenault, H.; Blanchard, Y.; Amelot, M.; Courtois, D.; De Wit, S.; Eterradossi, N.; et al. Infectious Bronchitis Coronavirus: Genome Evolution in Vaccinated and Non-Vaccinated SPF Chickens. Viruses 2022, 14, 1392. [Google Scholar] [CrossRef]
- Guzmán, M.; Hidalgo, H. Live Attenuated Infectious Bronchitis Virus Vaccines in Poultry: Modifying Local Viral Populations Dynamics. Animals 2020, 10, 2058. [Google Scholar] [CrossRef]
- Jackwood, M.W.; de Wit, S. Infectious Bronchitis. In Diseases of Poultry; Wiley: Hoboken, NJ, USA, 2013; pp. 139–159. [Google Scholar]
- Lisowska, A.; Pikuła, A.; Opolska, J.; Jasik, A.; Kycko, A.; Domańska-Blicharz, K. Virulence Properties of GI-23 Infectious Bronchitis Virus Isolated in Poland and Efficacy of Different Vaccination Strategies. Pathogens 2021, 10, 522. [Google Scholar] [CrossRef] [PubMed]
- Legnardi, M.; Franzo, G.; Koutoulis, K.C.; Wiśniewski, M.; Catelli, E.; Tucciarone, C.M.; Cecchinato, M. Vaccine or Field Strains: The Jigsaw Pattern of Infectious Bronchitis Virus Molecular Epidemiology in Poland. Poult. Sci. 2019, 98, 6388–6392. [Google Scholar] [CrossRef] [PubMed]
- Rohaim, M.A.; El Naggar, R.F.; Abdelsabour, M.A.; Mohamed, M.H.A.; El-Sabagh, I.M.; Munir, M. Evolutionary Analysis of Infectious Bronchitis Virus Reveals Marked Genetic Diversity and Recombination Events. Genes 2020, 11, 605. [Google Scholar] [CrossRef]
- Villanueva-Pérez, D.; Tataje-Lavanda, L.; Montalván-Avalos, A.; Paredes-Inofuente, D.; Montoya-Ortiz, S.; Isasi-Rivas, G.; Fernández, M.F.; Fernández-Sánchez, M.; Fernández-Díaz, M. Detection and Molecular Characterization of GI-1 and GI-23 Avian Infectious Bronchitis Virus in Broilers Indicate the Emergence of New Genotypes in Bolivia. Viruses 2024, 16, 1463. [Google Scholar] [CrossRef] [PubMed]
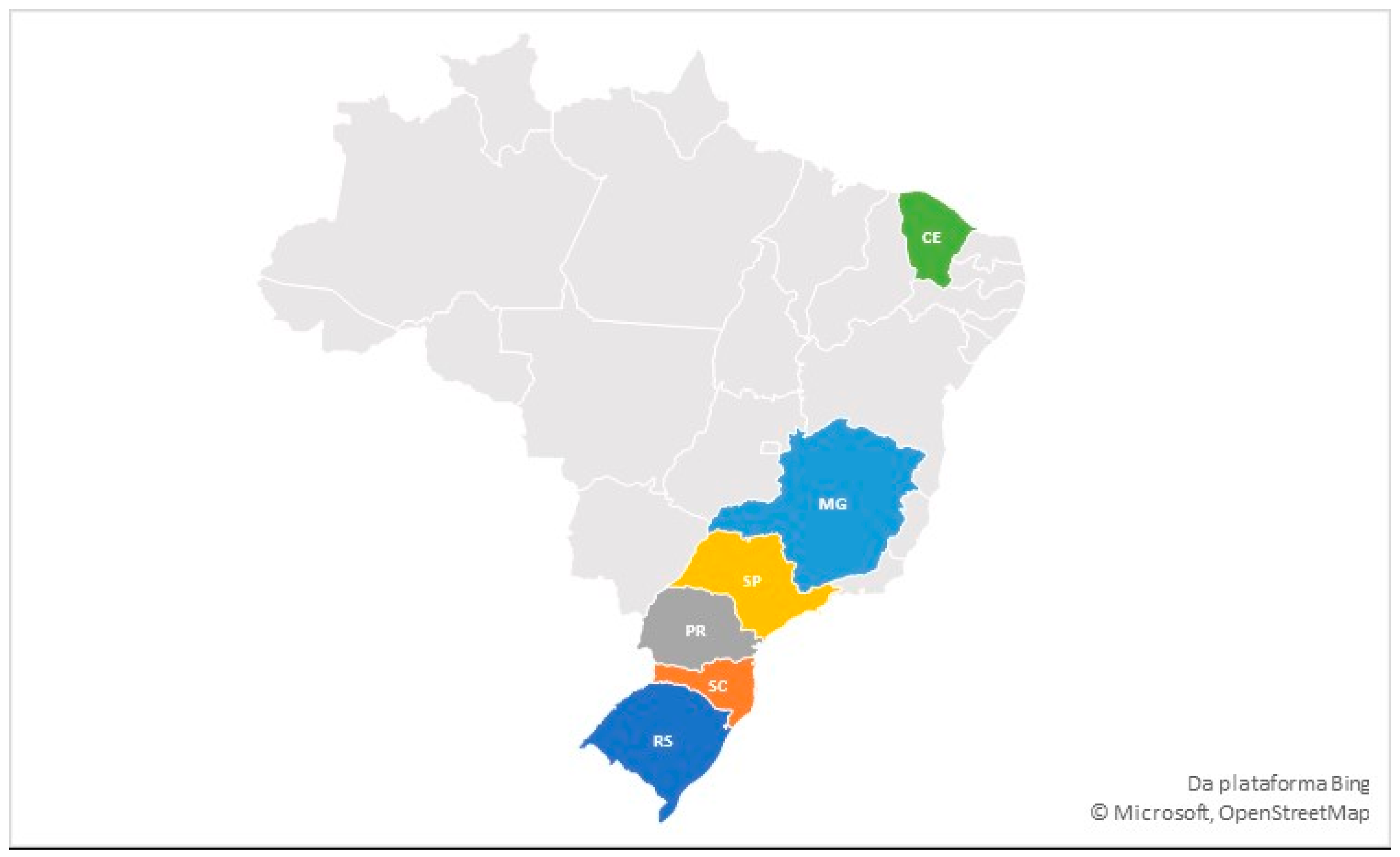
| State | Vaccination Program | Vaccination Date | Location and Vaccination Route |
|---|---|---|---|
| Santa Catarina | GI-1 + GI-11 | First day of life | Spray in the hatchery |
| Rio Grande do Sul | GI-1 + GI-11 | ||
| Paraná | GI-1 + GI-11 | ||
| Minas Gerais | Unvaccinated birds for IBV | ||
| São Paulo | GI-1 | ||
| Ceará | GI-1 |
| State | Age of Collection (Days Old) | Total Samples | Number of Positive Samples—IBV | Suspected IBV Strains Identified Through RT-PCR |
|---|---|---|---|---|
| Rio Grande do Sul | 16–26 | 15 | 14 | (07) GI-11 (01) GI-11, GI-23 (05) GI-1, GI-11 (01) GI-1, GI-11, GI-23 |
| Santa Catarina | 17–25 | 15 | 14 | (02) GI-1 (02) GI-11 (10) GI-1, GI-11 |
| Paraná | 20–32 | 30 | 25 | (11) GI-11 (03) GI-1, GI-11 (06) GI-11, GI-23 (02) GI-1, GI-11, GI-23 (03) GI-1, GI-23 |
| São Paulo | 21–27 | 10 | 5 | (01) Untyped (01) GI-11 (02) GI-1, GI-11 (01) GI-1 |
| Minas Gerais | 13–20 | 10 | 10 | (07) Untyped (03) GI-11 |
| Ceará | 24–28 | 20 | 14 | (04) Untyped (01) GI-1, GI-11 (09) GI-1 |
| State | Batch Number | Age (Days) | Vaccine | IBV Genotype |
|---|---|---|---|---|
| Santa Catarina | 1 | 17 | Infectious bronchitis—GI-1 (live) + GI-11 (live) | GI-1 and GI-11 |
| 2 | 17 | GI-1 and GI-11 | ||
| 3 | 19 | GI-1 | ||
| 4 | 25 | GI-1 | ||
| 5 | 25 | GI-1 and GI-11 | ||
| 6 | 20 | GI-1 and GI-11 | ||
| 7 | 18 | GI-1 and GI-11 | ||
| 8 | 18 | GI-1 and GI-11 | ||
| 9 | 17 | GI-1 and GI-11 | ||
| 10 | 21 | GI-1 and GI-11 | ||
| 11 | 22 | GI-11 | ||
| 13 | 19 | GI-11 | ||
| 14 | 18 | GI-1 and GI-11 | ||
| 15 | 17 | GI-1 and GI-11 | ||
| Rio Grande do Sul | 16 | 24 | Infectious bronchitis—GI-1 (live) + GI-11 (live) | GI-11 and GI-23 |
| 17 | 23 | GI-11 | ||
| 18 | 26 | GI-1 and GI-11 | ||
| 19 | 27 | GI-1, GI-11, and GI-23 | ||
| 20 | 21 | GI-11 | ||
| 21 | 20/21 | GI-11 | ||
| 22 | 24 | GI-1 and GI-11 | ||
| 23 | 24 | GI-1 and GI-11 | ||
| 24 | 16 | GI-1 and GI-11 | ||
| 25 | 22 | GI-11 | ||
| 26 | 22 | GI-1 and GI-11 | ||
| 27 | 21 | GI-11 | ||
| 28 | 21 | GI-11 | ||
| 29 | 22 | GI-11 | ||
| Paraná | 32 | 32 | Infectious bronchitis—GI-1 (live) + GI-11 (live) Infectious bronchitis—GI-1 (live) + GI-11 (live) | GI-1 and GI-23 |
| 34 | 25 | GI-1 and GI-23 | ||
| 36 | 21 | GI-11 and GI-23 | ||
| 37 | 24 | GI-11 and GI-23 | ||
| 38 | 24 | GI-11 and GI-23 | ||
| 39 | 22 | GI-1, GI-11, and GI-23 | ||
| 41 | 31 | GI-1, GI-11, and GI-23 | ||
| 42 | 28 | GI-1 and GI-23 | ||
| 44 | 32 | GI-11 and GI-23 | ||
| 45 | 27 | GI-11 and GI-23 | ||
| 46 | 22 | GI-11 | ||
| 47 | 23 | GI-11 | ||
| 48 | 23 | GI-11 | ||
| 49 | 25 | GI-11 | ||
| 50 | 25 | GI-1 and GI-11 | ||
| 51 | 25 | GI-11 | ||
| 52 | 25 | GI-11 | ||
| 53 | 25 | GI-11 | ||
| 54 | 20 | GI-1 and GI-11 | ||
| 55 | 24 | GI-11 | ||
| 56 | 20 | GI-11 and GI-23 | ||
| 57 | 25 | GI-11 | ||
| 58 | 25 | GI-11 | ||
| 59 | 24 | GI-1, GI-11 | ||
| 60 | 24 | GI-11 | ||
| São Paulo | 62 | 26 | Infectious bronchitis—GI-1 (live) | GI-11 |
| 71 | 23 | GI-1 and GI-11 | ||
| 72 | 27 | GI-1 and GI-11 | ||
| 73 | 23 | GI-1 | ||
| Ceará | 81 | 24 | Infectious bronchitis—GI-1 (live) | GI-1 |
| 84 | 24 | GI-1 and GI-11 | ||
| 85 | 24 | GI-1 | ||
| 87 | 24 | GI-1 | ||
| 89 | 24 | GI-1 | ||
| 91 | 24 | GI-1 | ||
| 92 | 24 | GI-1 | ||
| 94 | 24 | GI-1 | ||
| 95 | 24 | GI-1 | ||
| 98 | 24 | GI-1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Salles, G.B.C.; Pilati, G.V.T.; Savi, B.P.; Dahmer, M.; Muniz, E.C.; Vogt, J.R.; Lima Neto, A.J.d.; Fongaro, G. Infectious Bronchitis Virus (IBV) in Vaccinated and Non-Vaccinated Broilers in Brazil: Surveillance and Persistence of Vaccine Viruses. Microorganisms 2025, 13, 521. https://doi.org/10.3390/microorganisms13030521
Salles GBC, Pilati GVT, Savi BP, Dahmer M, Muniz EC, Vogt JR, Lima Neto AJd, Fongaro G. Infectious Bronchitis Virus (IBV) in Vaccinated and Non-Vaccinated Broilers in Brazil: Surveillance and Persistence of Vaccine Viruses. Microorganisms. 2025; 13(3):521. https://doi.org/10.3390/microorganisms13030521
Chicago/Turabian StyleSalles, Gleidson Biasi Carvalho, Giulia Von Tönnemann Pilati, Beatriz Pereira Savi, Mariane Dahmer, Eduardo Correa Muniz, Josias Rodrigo Vogt, Antonio José de Lima Neto, and Gislaine Fongaro. 2025. "Infectious Bronchitis Virus (IBV) in Vaccinated and Non-Vaccinated Broilers in Brazil: Surveillance and Persistence of Vaccine Viruses" Microorganisms 13, no. 3: 521. https://doi.org/10.3390/microorganisms13030521
APA StyleSalles, G. B. C., Pilati, G. V. T., Savi, B. P., Dahmer, M., Muniz, E. C., Vogt, J. R., Lima Neto, A. J. d., & Fongaro, G. (2025). Infectious Bronchitis Virus (IBV) in Vaccinated and Non-Vaccinated Broilers in Brazil: Surveillance and Persistence of Vaccine Viruses. Microorganisms, 13(3), 521. https://doi.org/10.3390/microorganisms13030521








