Inhibitory Effect of Antimicrobial Peptides Bac7(17), PAsmr5-17 and PAβN on Bacterial Growth and Biofilm Formation of Multidrug-Resistant Acinetobacter baumannii
Abstract
1. Introduction
2. Materials and Methods
2.1. Bacterial Strains and Growth Conditions
2.2. Antimicrobial Susceptibility Testing (AST)
2.3. Whole-Genome Sequencing
2.4. Multilocus Sequence Typing, Antibiotic Resistance Genes and Biofilm-Associated Genes
2.5. Minimum Inhibitory Concentration (MIC) Assay
2.6. Biofilm Assay
- Non-biofilm producer: OD ≤ ODc
- Weak biofilm producer: ODc < OD ≤ 2× ODc
- Moderate biofilm producer: 2× ODc < OD ≤ 4× ODc
- Strong biofilm producer: 4× ODc < OD
2.7. Statistical Analysis
3. Results
3.1. Bacterial Strains
3.2. Antimicrobial Susceptibility Testing and AMR Genes
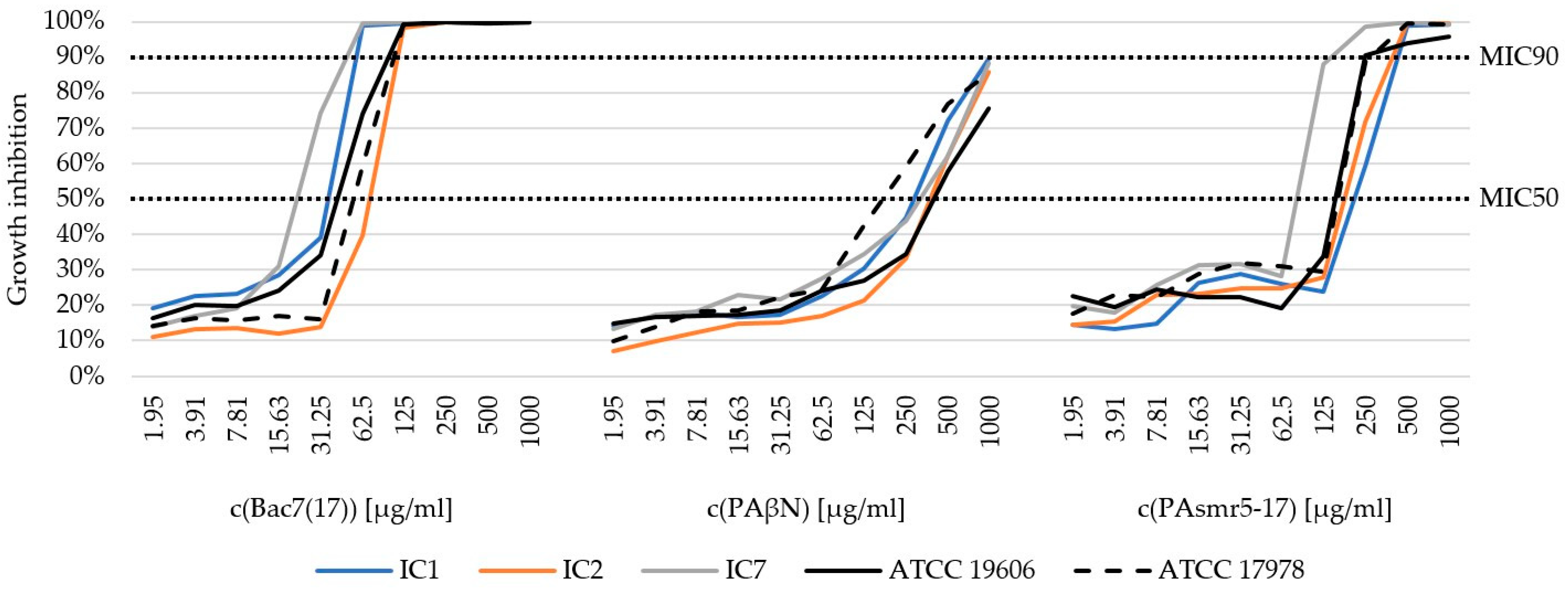
3.3. Minimum Inhibitory Concentration (MIC) of AMPs
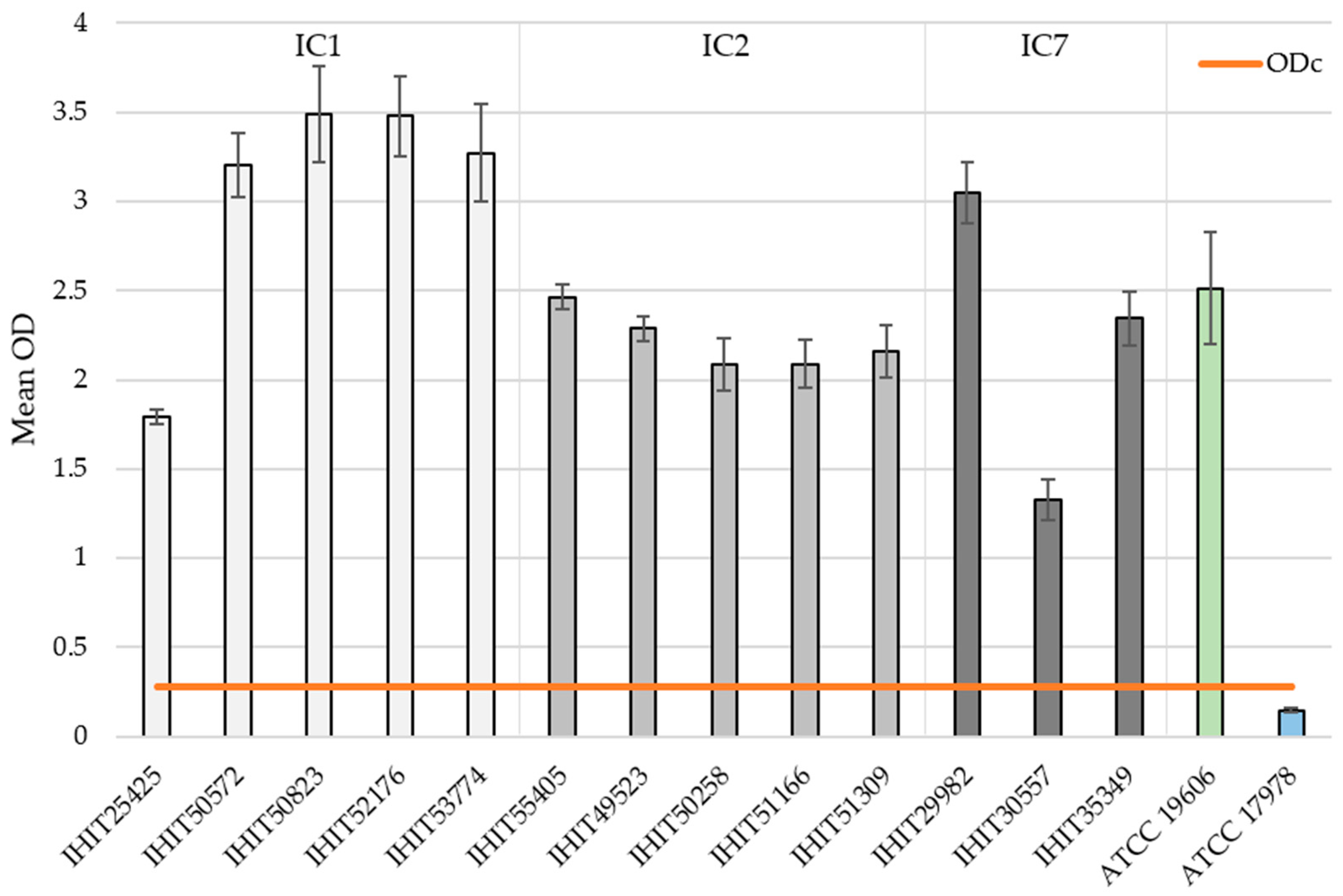
3.4. Biofilm Formation (BF) and Biofilm-Associated Genes (BAGs)
- Non-biofilm producer: OD ≤ 0.275
- Weak biofilm producer: 0.275 < OD ≤ 0.550
- Moderate biofilm producer: 0.550 < OD ≤ 1.100
- Strong biofilm producer: 1.100 < OD
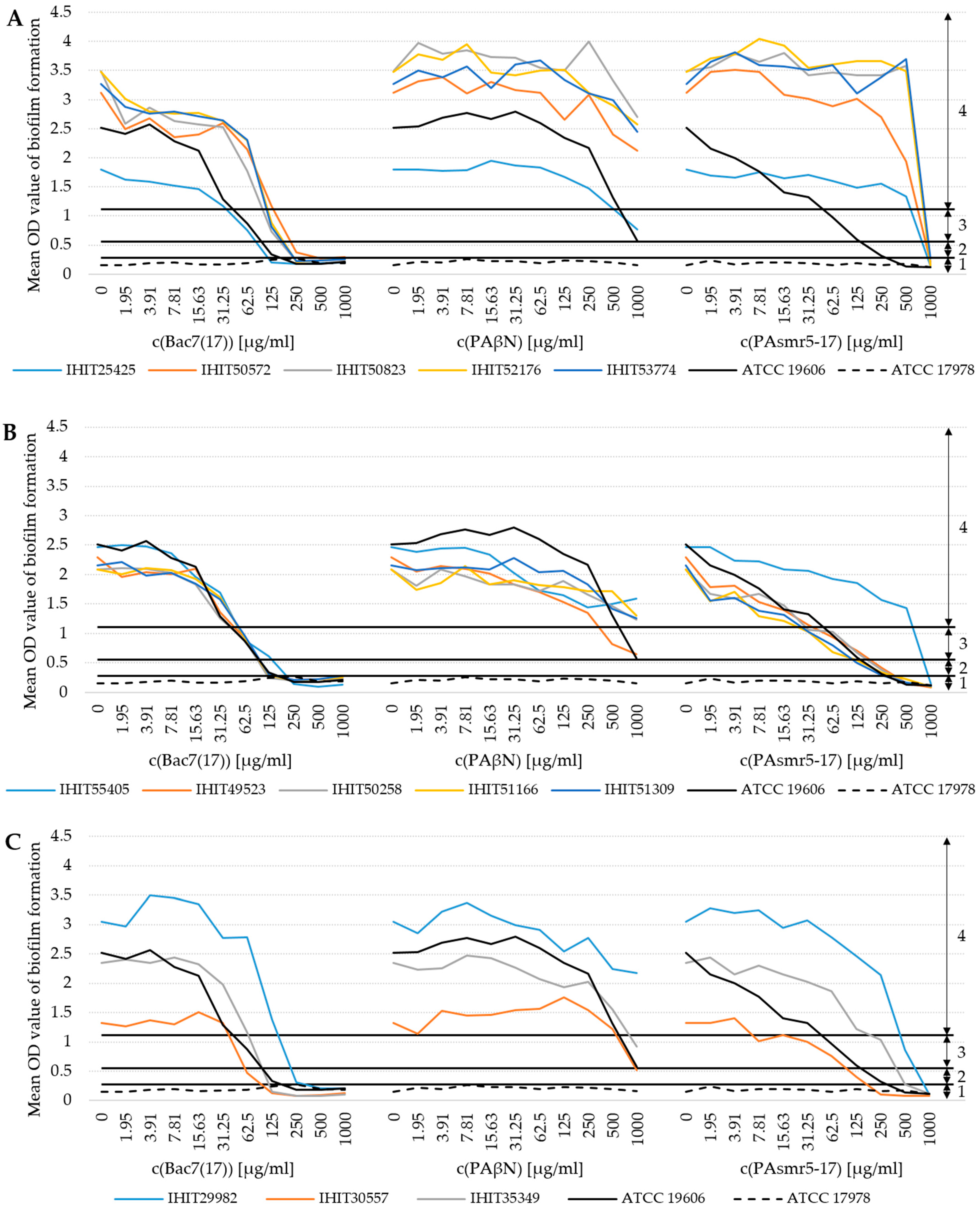
3.5. Influence of AMPs on Biofilm Formation (BF)
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Batt, C.A.; Tortorello, M.L. (Eds.) Encyclopedia of Food Microbiology, 2nd ed.; Elsevier: Amsterdam, The Netherlands, 2014; ISBN 9780123847300. [Google Scholar]
- Almasaudi, S.B. Acinetobacter spp. as nosocomial pathogens: Epidemiology and resistance features. Saudi J. Biol. Sci. 2018, 25, 586–596. [Google Scholar] [CrossRef] [PubMed]
- Antunes, L.C.S.; Visca, P.; Towner, K.J. Acinetobacter baumannii: Evolution of a global pathogen. Pathog. Dis. 2014, 71, 292–301. [Google Scholar] [CrossRef]
- Wareth, G.; Neubauer, H.; Sprague, L.D. Acinetobacter baumannii—A neglected pathogen in veterinary and environmental health in Germany. Vet. Res. Commun. 2019, 43, 1–6. [Google Scholar] [CrossRef]
- Rice, L.B. Federal funding for the study of antimicrobial resistance in nosocomial pathogens: No ESKAPE. J. Infect. Dis. 2008, 197, 1079–1081. [Google Scholar] [CrossRef]
- Nocera, F.P.; Attili, A.-R.; de Martino, L. Acinetobacter baumannii: Its Clinical Significance in Human and Veterinary Medicine. Pathogens 2021, 10, 127. [Google Scholar] [CrossRef] [PubMed]
- Müller, C.; Reuter, S.; Wille, J.; Xanthopoulou, K.; Stefanik, D.; Grundmann, H.; Higgins, P.G.; Seifert, H. A global view on carbapenem-resistant Acinetobacter baumannii. mBio 2023, 14, e0226023. [Google Scholar] [CrossRef]
- Karah, N.; Faille, N.; Grenier, F.; Abou-Fayad, A.; Higgins, P.G.; Al-Hassan, L.; Evans, B.A.; Poirel, L.; Bonnin, R.; Hammerum, A.M.; et al. Emergence of Acinetobacter baumannii International Clone 10 predominantly found in the Middle East. bioRxiv 2023. [Google Scholar] [CrossRef]
- Hansen, F.; Porsbo, L.J.; Frandsen, T.H.; Kaygisiz, A.N.S.; Roer, L.; Henius, A.E.; Holzknecht, B.J.; Søes, L.; Schønning, K.; Røder, B.L.; et al. Characterisation of carbapenemase-producing Acinetobacter baumannii isolates from danish patients 2014–2021: Detection of a new international clone—IC11. Int. J. Antimicrob. Agents 2023, 62, 106866. [Google Scholar] [CrossRef] [PubMed]
- Xu, A.; Li, M.; Hang, Y.; Zeng, L.; Zhang, X.; Hu, Y.; Guo, Q.; Wang, M. Multicenter retrospective genomic characterization of carbapenemase-producing Acinetobacter baumannii isolates from Jiangxi patients 2021–2022: Identification of a novel international clone, IC11. mSphere 2024, 9, e0027624. [Google Scholar] [CrossRef]
- Higgins, P.G.; Dammhayn, C.; Hackel, M.; Seifert, H. Global spread of carbapenem-resistant Acinetobacter baumannii. J. Antimicrob. Chemother. 2010, 65, 233–238. [Google Scholar] [CrossRef]
- Pour, N.K.; Dusane, D.H.; Dhakephalkar, P.K.; Zamin, F.R.; Zinjarde, S.S.; Chopade, B.A. Biofilm formation by Acinetobacter baumannii strains isolated from urinary tract infection and urinary catheters. FEMS Immunol. Med. Microbiol. 2011, 62, 328–338. [Google Scholar] [CrossRef] [PubMed]
- Müller, S.; Janssen, T.; Wieler, L.H. Multidrug resistant Acinetobacter baumannii in veterinary medicine—Emergence of an underestimated pathogen? Berl. Munch. Tierarztl. Wochenschr. 2014, 127, 435–446. [Google Scholar] [PubMed]
- Tomaras, A.P.; Dorsey, C.W.; Edelmann, R.E.; Actis, L.A. Attachment to and biofilm formation on abiotic surfaces by Acinetobacter baumannii: Involvement of a novel chaperone-usher pili assembly system. Microbiology 2003, 149, 3473–3484. [Google Scholar] [CrossRef]
- Eze, E.C.; Chenia, H.Y.; El Zowalaty, M.E. Acinetobacter baumannii biofilms: Effects of physicochemical factors, virulence, antibiotic resistance determinants, gene regulation, and future antimicrobial treatments. Infect. Drug Resist. 2018, 11, 2277–2299. [Google Scholar] [CrossRef]
- Longo, F.; Vuotto, C.; Donelli, G. Biofilm formation in Acinetobacter baumannii. New Microbiol. 2014, 37, 119–127. [Google Scholar] [PubMed]
- Bardbari, A.M.; Arabestani, M.R.; Karami, M.; Keramat, F.; Alikhani, M.Y.; Bagheri, K.P. Correlation between ability of biofilm formation with their responsible genes and MDR patterns in clinical and environmental Acinetobacter baumannii isolates. Microb. Pathog. 2017, 108, 122–128. [Google Scholar] [CrossRef]
- Abdullahi, U.F.; Igwenagu, E.; Mu’azu, A.; Aliyu, S.; Umar, M.I. Intrigues of biofilm: A perspective in veterinary medicine. Vet World 2016, 9, 12–18. [Google Scholar] [CrossRef]
- Sarshar, M.; Behzadi, P.; Scribano, D.; Palamara, A.T.; Ambrosi, C. Acinetobacter baumannii: An Ancient Commensal with Weapons of a Pathogen. Pathogens 2021, 10, 387. [Google Scholar] [CrossRef]
- Choi, A.H.K.; Slamti, L.; Avci, F.Y.; Pier, G.B.; Maira-Litrán, T. The pgaABCD locus of Acinetobacter baumannii encodes the production of poly-beta-1-6-N-acetylglucosamine, which is critical for biofilm formation. J. Bacteriol. 2009, 191, 5953–5963. [Google Scholar] [CrossRef]
- Pakharukova, N.; Tuittila, M.; Paavilainen, S.; Malmi, H.; Parilova, O.; Teneberg, S.; Knight, S.D.; Zavialov, A.V. Structural basis for Acinetobacter baumannii biofilm formation. Proc. Natl. Acad. Sci. USA 2018, 115, 5558–5563. [Google Scholar] [CrossRef]
- Kim, H.-J.; Kim, N.-Y.; Ko, S.-Y.; Park, S.-Y.; Oh, M.-H.; Shin, M.-S.; Lee, Y.-C.; Lee, J.-C. Complementary Regulation of BfmRS Two-Component and AbaIR Quorum Sensing Systems to Express Virulence-Associated Genes in Acinetobacter baumannii. Int. J. Mol. Sci. 2022, 23, 13136. [Google Scholar] [CrossRef]
- Roy, S.; Chowdhury, G.; Mukhopadhyay, A.K.; Dutta, S.; Basu, S. Convergence of Biofilm Formation and Antibiotic Resistance in Acinetobacter baumannii Infection. Front. Med. 2022, 9, 793615. [Google Scholar] [CrossRef]
- Lee, C.-R.; Lee, J.H.; Park, M.; Park, K.S.; Bae, I.K.; Kim, Y.B.; Cha, C.-J.; Jeong, B.C.; Lee, S.H. Biology of Acinetobacter baumannii: Pathogenesis, Antibiotic Resistance Mechanisms, and Prospective Treatment Options. Front. Cell. Infect. Microbiol. 2017, 7, 55. [Google Scholar] [CrossRef] [PubMed]
- Isler, B.; Doi, Y.; Bonomo, R.A.; Paterson, D.L. New Treatment Options against Carbapenem-Resistant Acinetobacter baumannii Infections. Antimicrob. Agents Chemother. 2019, 63, e01110-18. [Google Scholar] [CrossRef] [PubMed]
- Giguère, S.; Prescott, J.F.; Dowling, P.M. (Eds.) Antimicrobial Therapy in Veterinary Medicine, 5th ed.; Wiley-Blackwell: Ames, IA, USA, 2013; ISBN 9781118675014. [Google Scholar]
- Wong, D.; Nielsen, T.B.; Bonomo, R.A.; Pantapalangkoor, P.; Luna, B.; Spellberg, B. Clinical and Pathophysiological Overview of Acinetobacter Infections: A Century of Challenges. Clin. Microbiol. Rev. 2017, 30, 409–447. [Google Scholar] [CrossRef] [PubMed]
- European Medicines Agency. Categorisation of Antibiotics in the European Union. Available online: https://www.ema.europa.eu/en/news/categorisation-antibiotics-used-animals-promotes-responsible-use-protect-public-and-animal-health (accessed on 8 April 2024).
- van der Kolk, J.H.; Endimiani, A.; Graubner, C.; Gerber, V.; Perreten, V. Acinetobacter in veterinary medicine, with an emphasis on Acinetobacter baumannii. J. Glob. Antimicrob. Resist. 2019, 16, 59–71. [Google Scholar] [CrossRef]
- Schwabe, U.; Paffrath, D. Menge verordneter Antibiotika in Deutschland nach den wichtigsten Substanzgruppen in den Jahren von 2008 bis 2021. Available online: https://de.statista.com/statistik/daten/studie/371902/umfrage/menge-verordneter-antibiotika-in-deutschland-nach-den-wichtigsten-substanzgruppen/ (accessed on 1 February 2024).
- Bundesamt für Verbraucherschutz und Lebensmittelsicherheit. Abgegebene Menge Antibiotika in der Veterinärmedizin in Deutschland nach Wirkstoffklassen in den Jahren 2015 bis 2022 (in Tonnen) [Graph]. Available online: https://de.statista.com/statistik/daten/studie/371869/umfrage/abgegebene-menge-antibiotika-in-der-veterinaermedizin-in-deutschland-nach-wirkstoffklassen/ (accessed on 1 February 2024).
- Tacconelli, E.; Carrara, E.; Savoldi, A.; Harbarth, S.; Mendelson, M.; Monnet, D.L.; Pulcini, C.; Kahlmeter, G.; Kluytmans, J.; Carmeli, Y.; et al. Discovery, research, and development of new antibiotics: The WHO priority list of antibiotic-resistant bacteria and tuberculosis. Lancet Infect. Dis. 2018, 18, 318–327. [Google Scholar] [CrossRef]
- Jesudason, T. WHO publishes updated list of bacterial priority pathogens. Lancet Microbe 2024, 5, 100940. [Google Scholar] [CrossRef]
- Mulukutla, A.; Shreshtha, R.; Kumar Deb, V.; Chatterjee, P.; Jain, U.; Chauhan, N. Recent advances in antimicrobial peptide-based therapy. Bioorg. Chem. 2024, 145, 107151. [Google Scholar] [CrossRef]
- Bahar, A.A.; Ren, D. Antimicrobial peptides. Pharmaceuticals 2013, 6, 1543–1575. [Google Scholar] [CrossRef]
- Huan, Y.; Kong, Q.; Mou, H.; Yi, H. Antimicrobial Peptides: Classification, Design, Application and Research Progress in Multiple Fields. Front. Microbiol. 2020, 11, 582779. [Google Scholar] [CrossRef]
- Neshani, A.; Sedighian, H.; Mirhosseini, S.A.; Ghazvini, K.; Zare, H.; Jahangiri, A. Antimicrobial peptides as a promising treatment option against Acinetobacter baumannii infections. Microb. Pathog. 2020, 146, 104238. [Google Scholar] [CrossRef]
- Phoenix, D.A.; Dennison, S.R.; Harris, F. (Eds.) Antimicrobial Peptides, 1st ed.; Wiley-VHC: Weinheim, Germany, 2013; ISBN 978-3-527-65288-4. [Google Scholar]
- Jaśkiewicz, M.; Neubauer, D.; Kazor, K.; Bartoszewska, S.; Kamysz, W. Antimicrobial Activity of Selected Antimicrobial Peptides Against Planktonic Culture and Biofilm of Acinetobacter baumannii. Probiotics Antimicrob. Proteins 2019, 11, 317–324. [Google Scholar] [CrossRef] [PubMed]
- Lewies, A.; Du Plessis, L.H.; Wentzel, J.F. Antimicrobial Peptides: The Achilles’ Heel of Antibiotic Resistance? Probiotics Antimicrob. Proteins 2019, 11, 370–381. [Google Scholar] [CrossRef] [PubMed]
- Benincasa, M.; Scocchi, M.; Podda, E.; Skerlavaj, B.; Dolzani, L.; Gennaro, R. Antimicrobial activity of Bac7 fragments against drug-resistant clinical isolates. Peptides 2004, 25, 2055–2061. [Google Scholar] [CrossRef]
- Müller, D. Identifizierung, Charakterisierung und Untersuchungen der Struktur-Wirkungs-Beziehung Sowie des Mode of Action Eines Neuen Antimikrobiellen Peptids aus Hirudo Verbana. Ph.D. Thesis, Philipps-Universität Marburg, Marburg, Germany, 2019. [Google Scholar]
- Jamshidi, S.; Sutton, J.M.; Rahman, K.M. Computational Study Reveals the Molecular Mechanism of the Interaction between the Efflux Inhibitor PAβN and the AdeB Transporter from Acinetobacter baumannii. ACS Omega 2017, 2, 3002–3016. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, C.J.; Stone, T.A.; Deber, C.M. Peptide-Based Efflux Pump Inhibitors of the Small Multidrug Resistance Protein from Pseudomonas aeruginosa. Antimicrob. Agents Chemother. 2019, 63, e00730-19. [Google Scholar] [CrossRef]
- Magiorakos, A.-P.; Srinivasan, A.; Carey, R.B.; Carmeli, Y.; Falagas, M.E.; Giske, C.G.; Harbarth, S.; Hindler, J.F.; Kahlmeter, G.; Olsson-Liljequist, B.; et al. Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: An international expert proposal for interim standard definitions for acquired resistance. Clin. Microbiol. Infect. 2012, 18, 268–281. [Google Scholar] [CrossRef]
- Klotz, P.; Jacobmeyer, L.; Stamm, I.; Leidner, U.; Pfeifer, Y.; Semmler, T.; Prenger-Berninghoff, E.; Ewers, C. Carbapenem-resistant Acinetobacter baumannii ST294 harbouring the OXA-72 carbapenemase from a captive grey parrot. J. Antimicrob. Chemother. 2018, 73, 1098–1100. [Google Scholar] [CrossRef]
- Clinical and Laboratory Standards Institute. Performance Standards for Antimicrobial Susceptibility Testing, 33rd ed.; Clinical and Laboratory Standards Institute: Berwyn, PA, USA, 2023. [Google Scholar]
- European Committee on Antimicrobial Susceptibility Testing. Breakpoint Tables for Interpretation of MICs and Zone Diameters, Version 13.0. 2023. Available online: http://www.eucast.org (accessed on 8 April 2024).
- Bortolaia, V.; Kaas, R.S.; Ruppe, E.; Roberts, M.C.; Schwarz, S.; Cattoir, V.; Philippon, A.; Allesoe, R.L.; Rebelo, A.R.; Florensa, A.F.; et al. ResFinder 4.0 for predictions of phenotypes from genotypes. J. Antimicrob. Chemother. 2020, 75, 3491–3500. [Google Scholar] [CrossRef]
- Clausen, P.T.L.C.; Aarestrup, F.M.; Lund, O. Rapid and precise alignment of raw reads against redundant databases with KMA. BMC Bioinform. 2018, 19, 307. [Google Scholar] [CrossRef]
- Camacho, C.; Coulouris, G.; Avagyan, V.; Ma, N.; Papadopoulos, J.; Bealer, K.; Madden, T.L. BLAST+: Architecture and applications. BMC Bioinform. 2009, 10, 421. [Google Scholar] [CrossRef]
- Feng, Y.; Zou, S.; Chen, H.; Yu, Y.; Ruan, Z. BacWGSTdb 2.0: A one-stop repository for bacterial whole-genome sequence typing and source tracking. Nucleic Acids Res. 2021, 49, 644–650. [Google Scholar] [CrossRef] [PubMed]
- Ruan, Z.; Feng, Y. BacWGSTdb, a database for genotyping and source tracking bacterial pathogens. Nucleic Acids Res. 2016, 44, D682–D687. [Google Scholar] [CrossRef]
- Ruan, Z.; Yu, Y.; Feng, Y. The global dissemination of bacterial infections necessitates the study of reverse genomic epidemiology. Brief. Bioinform. 2020, 21, 741–750. [Google Scholar] [CrossRef] [PubMed]
- Larsen, M.V.; Cosentino, S.; Rasmussen, S.; Friis, C.; Hasman, H.; Marvig, R.L.; Jelsbak, L.; Sicheritz-Pontén, T.; Ussery, D.W.; Aarestrup, F.M.; et al. Multilocus sequence typing of total-genome-sequenced bacteria. J. Clin. Microbiol. 2012, 50, 1355–1361. [Google Scholar] [CrossRef]
- Center for Genomic Epidemiology. MLST. Available online: https://cge.food.dtu.dk/services/MLST/ (accessed on 8 September 2022).
- Stillger, L.; Viau, L.; Kamm, L.; Holtmann, D.; Müller, D. Optimization of antimicrobial peptides for the application against biocorrosive bacteria. Appl. Microbiol. Biotechnol. 2023, 107, 4041–4049. [Google Scholar] [CrossRef] [PubMed]
- O’Toole, G.A. Microtiter Dish Biofilm Formation Assay. J. Vis. Exp. 2011, 47, 2437–2438. [Google Scholar] [CrossRef]
- Qi, L.; Li, H.; Zhang, C.; Liang, B.; Li, J.; Wang, L.; Du, X.; Liu, X.; Qiu, S.; Song, H. Relationship between Antibiotic Resistance, Biofilm Formation, and Biofilm-Specific Resistance in Acinetobacter baumannii. Front. Microbiol. 2016, 7, 483. [Google Scholar] [CrossRef]
- Fursova, N.K.; Fursov, M.V.; Astashkin, E.I.; Fursova, A.D.; Novikova, T.S.; Kislichkina, A.A.; Sizova, A.A.; Fedyukina, G.N.; Savin, I.A.; Ershova, O.N. Multidrug-Resistant and Extensively Drug-Resistant Acinetobacter baumannii Causing Nosocomial Meningitis in the Neurological Intensive Care Unit. Microorganisms 2023, 11, 2020. [Google Scholar] [CrossRef]
- Stepanović, S.; Vuković, D.; Hola, V.; Di Bonaventura, G.; Djukić, S.; Cirković, I.; Ruzicka, F. Quantification of biofilm in microtiter plates: Overview of testing conditions and practical recommendations for assessment of biofilm production by Staphylococci. APMIS 2007, 115, 891–899. [Google Scholar] [CrossRef] [PubMed]
- Soto, S.M. Role of efflux pumps in the antibiotic resistance of bacteria embedded in a biofilm. Virulence 2013, 4, 223–229. [Google Scholar] [CrossRef]
- Rühl-Teichner, J.; Jacobmeyer, L.; Leidner, U.; Semmler, T.; Ewers, C. Genomic Diversity, Antimicrobial Susceptibility, and Biofilm Formation of Clinical Acinetobacter baumannii Isolates from Horses. Microorganisms 2023, 11, 556. [Google Scholar] [CrossRef]
- Jacobmeyer, L.; Semmler, T.; Stamm, I.; Ewers, C. Genomic Analysis of Acinetobacter baumannii Isolates Carrying OXA-23 and OXA-58 Genes from Animals Reveals ST1 and ST25 as Major Clonal Lineages. Antibiotics 2022, 11, 1045. [Google Scholar] [CrossRef]
- Shelenkov, A.; Akimkin, V.; Mikhaylova, Y. International Clones of High Risk of Acinetobacter baumannii-Definitions, History, Properties and Perspectives. Microorganisms 2023, 11, 2115. [Google Scholar] [CrossRef] [PubMed]
- Wieczorek, P.; Sacha, P.; Hauschild, T.; Zórawski, M.; Krawczyk, M.; Tryniszewska, E. Multidrug resistant Acinetobacter baumannii--the role of AdeABC (RND family) efflux pump in resistance to antibiotics. Folia Histochem Cytobiol 2008, 46, 257–267. [Google Scholar] [CrossRef] [PubMed]
- Loehfelm, T.W.; Luke, N.R.; Campagnari, A.A. Identification and characterization of an Acinetobacter baumannii biofilm-associated protein. J. Bacteriol. 2008, 190, 1036–1044. [Google Scholar] [CrossRef]
- Balducci, E.; Papi, F.; Capialbi, D.E.; Del Bino, L. Polysaccharides’ Structures and Functions in Biofilm Architecture of Antimicrobial-Resistant (AMR) Pathogens. Int. J. Mol. Sci. 2023, 24, 4030. [Google Scholar] [CrossRef]
- He, X.; Lu, F.; Yuan, F.; Jiang, D.; Zhao, P.; Zhu, J.; Cheng, H.; Cao, J.; Lu, G. Biofilm Formation Caused by Clinical Acinetobacter baumannii Isolates Is Associated with Overexpression of the AdeFGH Efflux Pump. Antimicrob. Agents Chemother. 2015, 59, 4817–4825. [Google Scholar] [CrossRef]
- Richmond, G.E.; Evans, L.P.; Anderson, M.J.; Wand, M.E.; Bonney, L.C.; Ivens, A.; Chua, K.L.; Webber, M.A.; Sutton, J.M.; Peterson, M.L.; et al. The Acinetobacter baumannii Two-Component System AdeRS Regulates Genes Required for Multidrug Efflux, Biofilm Formation, and Virulence in a Strain-Specific Manner. mBio 2016, 7, e00430-16. [Google Scholar] [CrossRef]
- Chen, L.; Li, H.; Wen, H.; Zhao, B.; Niu, Y.; Mo, Q.; Wu, Y. Biofilm formation in Acinetobacter baumannii was inhibited by PAβN while it had no association with antibiotic resistance. Microbiologyopen 2020, 9, e1063. [Google Scholar] [CrossRef] [PubMed]
| Isolate ID | Original Name | Host | Source | Date of Isolation | Country | IC | STPa | Acquired OXA 1 | Intrinsic OXA |
|---|---|---|---|---|---|---|---|---|---|
| IHIT25425 | 22/09 | Human | Trachea | 30.06.2009 | DE | 1 | 1 | 23PL | 69 |
| IHIT50572 | VB939187.1 | Cat | Urine | 22.12.2022 | DE | 1 | 1 | 58PL | 69 |
| IHIT50823 | VB949552.2 | Dog | Wound | 16.01.2023 | DE | 1 | 1 | 58PL | 69 |
| IHIT52176 | VB948535 | Dog | Wound | 20.04.2023 | DE | 1 | 1 | 58PL | 69 |
| IHIT53774 | VB952338 | Dog | Urine | 03.02.2023 | DE | 1 | 1 | 58PL | 69 |
| IHIT55405 | 34/15-1 | Human | Unknown | 21.12.2023 | BG | 2 | 2 | 23PL | 66 |
| IHIT49523 | 2214/22-1 | Cat | Abscess eye | 12.09.2022 | DE | 2 | 2 | 23PL | 66 |
| IHIT50258 | 2814/22 | Dog | CVC | 23.11.2022 | DE | 2 | 2 | 23PL | 66 |
| IHIT51166 | 237/23 | Dog | Skin suture | 08.02.2023 | DE | 2 | 2 | 23PL | 66 |
| IHIT51309 | 314/23-1 | Dog | Wound | 22.02.2023 | DE | 2 | 2 | 23PL | 66 |
| IHIT29982 | BF135647 | Dog | Urine | 27.07.2015 | FR | 7 | 25 | 23CH | 64 |
| IHIT30557 | BF136700 | Dog | Urine | 09.12.2015 | FR | 7 | 25 | 23CH | 64 |
| IHIT35349 | SG1998 | Human | GIT | 06.07.2014 | DE | 7 | 25 | 23CH | 64 |
| Antibiotic Class | Phenotypic Resistance | Genotypic Resistance | |||
|---|---|---|---|---|---|
| Antibiotic | Abbr. | Resistant Isolates (n) | AMR Genes | Positive Isolates (n) | |
| β-Lactams | Ampicillin * | AMP | 13 | ||
| Amoxicillin–Clavulanate * | AMC | 13 | blaADC-like | 13 | |
| Cefalexin * | CEX | 13 | blaOXA-23 | 9 | |
| Cefotaxime | CTX | 13 | blaOXA-58 | 4 | |
| Ceftazidime | CAZ | 7 | blaOXA-64 | 3 | |
| Cefepime | FEP | 13 | blaOXA-66 | 5 | |
| Imipenem | IPM | 13 | blaOXA-69 | 5 | |
| Meropenem | MEM | 13 | blaPER-7 | 1 | |
| Piperacillin | PIP | 13 | blaTEM-1D | 4 | |
| Piperacillin–Tazobactam | TZP | 13 | |||
| Aminoglycosides | aadA1 | 6 | |||
| aac(3)-Ia | 9 | ||||
| aac(3)-IIa | 3 | ||||
| Gentamicin | GEN | 13 | aac(6′)-Ian | 3 | |
| Amikacin | AMK | 5 | aac(6′)-Ip | 3 | |
| Tobramycin | TOB | 5 | aph(3′)-Ia | 4 | |
| aph(3′)-VIa | 2 | ||||
| aph(3″)-Ib | 7 | ||||
| aph(6)-Id | 7 | ||||
| Phenicols | ABUW_0982 | 13 | |||
| Chloramphenicol * | CHL | 13 | catA1 | 5 | |
| craA | 5 | ||||
| Sulfonamides/ Trimethoprims | Trimethoprim–Sulfamethoxazole | SXT | 13 | sul1 | 11 |
| sul2 | 7 | ||||
| Tetracyclines | Tetracycline | TET | 13 | tet(A) | 5 |
| tet(B) | 7 | ||||
| Fluoroquinolones | Ciprofloxacin | CIP | 13 | none ** | − |
| Enrofloxacin | ENR | 13 | |||
| Marbofloxacin | MAR | 13 | |||
| Polymyxine | Colistin | COL | 0 | none | − |
| Bac7(17) | PAβN | PAsmr5-17 | |||||
|---|---|---|---|---|---|---|---|
| Isolate | IC | MIC50 | MIC90 | MIC50 | MIC90 | MIC50 | MIC90 |
| IHIT25425 | 1 | * 31.25 | 62.5 | 250 | >1000 | * 125 | 250 |
| IHIT50572 | 1 | 31.25 | 62.5 | 250 | 1000 | 250 | 500 |
| IHIT50823 | 1 | 31.25 | 62.5 | 500 | 1000 | 250 | 500 |
| IHIT52176 | 1 | 31.25 | 62.5 | 250 | 1000 | 250 | 500 |
| IHIT53774 | 1 | * 31.25 | 62.5 | 500 | >1000 | 250 | 500 |
| IHIT55405 | 2 | 62.5 | 125 | 500 | >1000 | 250 | 500 |
| IHIT49523 | 2 | * 62.5 | 125 | 500 | >1000 | 250 | 500 |
| IHIT50258 | 2 | * 62.5 | 125 | 250 | >1000 | * 125 | 250 |
| IHIT51166 | 2 | * 62.5 | 125 | 500 | 1000 | 250 | 500 |
| IHIT51309 | 2 | * 62.5 | 125 | 500 | >1000 | 250 | 500 |
| IHIT29982 | 7 | 31.25 | 62.5 | 500 | >1000 | * 62.5 | 125 |
| IHIT30557 | 7 | 15.63 | 62.5 | 250 | 1000 | 62.5 | 125 |
| IHIT35349 | 7 | 31.25 | 62.5 | 500 | >1000 | 125 | 250 |
| ATCC 19606T | - | 62.5 | 125 | 500 | >1000 | * 125 | 250 |
| ATCC 17978 | - | 62.5 | 125 | 250 | >1000 | * 125 | 500 |
| Isolate | IC | BF | SD | Classification |
|---|---|---|---|---|
| IHIT25425 | 1 | 1.792 | ±0.042 | strong |
| IHIT50572 | 1 | 3.113 | ±0.179 | strong |
| IHIT50823 | 1 | 3.491 | ±0.269 | strong |
| IHIT52176 | 1 | 3.480 | ±0.224 | strong |
| IHIT53774 | 1 | 3.273 | ±0.275 | strong |
| IHIT55405 | 2 | 2.463 | ±0.069 | strong |
| IHIT49523 | 2 | 2.289 | ±0.070 | strong |
| IHIT50258 | 2 | 2.086 | ±0.150 | strong |
| IHIT51166 | 2 | 2.090 | ±0.134 | strong |
| IHIT51309 | 2 | 2.158 | ±0.148 | strong |
| IHIT29982 | 7 | 3.047 | ±0.173 | strong |
| IHIT30557 | 7 | 1.325 | ±0.114 | strong |
| IHIT35349 | 7 | 2.344 | ±0.150 | strong |
| ATCC 19606T | - | 2.514 | ±0.316 | strong |
| ATCC 17978 | - | 0.242 | ±0.011 | none |
| Isolate | IC | Biofilm-Associated Genes (BAGs) | |||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| abaI | abaR | bap | blp1 | blp2 | bfmR | bfmS | csuA | csuA/B | csuB | csuC | csuD | csuE | ompA | pgaA | pgaB | pgaC | pgaD | ||
| IHIT25425 | 1 | ||||||||||||||||||
| IHIT50572 | 1 | ||||||||||||||||||
| IHIT50823 | 1 | ||||||||||||||||||
| IHIT52176 | 1 | ||||||||||||||||||
| IHIT53774 | 1 | ||||||||||||||||||
| IHIT55405 | 2 | ||||||||||||||||||
| IHIT49523 | 2 | ||||||||||||||||||
| IHIT50258 | 2 | ||||||||||||||||||
| IHIT51166 | 2 | ||||||||||||||||||
| IHIT51309 | 2 | ||||||||||||||||||
| IHIT29982 | 7 | ||||||||||||||||||
| IHIT30557 | 7 | ||||||||||||||||||
| IHIT35349 | 7 | ||||||||||||||||||
| ATCC 19606T | nt | ||||||||||||||||||
| ATCC 17978 | nt | ||||||||||||||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rühl-Teichner, J.; Müller, D.; Stamm, I.; Göttig, S.; Leidner, U.; Semmler, T.; Ewers, C. Inhibitory Effect of Antimicrobial Peptides Bac7(17), PAsmr5-17 and PAβN on Bacterial Growth and Biofilm Formation of Multidrug-Resistant Acinetobacter baumannii. Microorganisms 2025, 13, 639. https://doi.org/10.3390/microorganisms13030639
Rühl-Teichner J, Müller D, Stamm I, Göttig S, Leidner U, Semmler T, Ewers C. Inhibitory Effect of Antimicrobial Peptides Bac7(17), PAsmr5-17 and PAβN on Bacterial Growth and Biofilm Formation of Multidrug-Resistant Acinetobacter baumannii. Microorganisms. 2025; 13(3):639. https://doi.org/10.3390/microorganisms13030639
Chicago/Turabian StyleRühl-Teichner, Johanna, Daniela Müller, Ivonne Stamm, Stephan Göttig, Ursula Leidner, Torsten Semmler, and Christa Ewers. 2025. "Inhibitory Effect of Antimicrobial Peptides Bac7(17), PAsmr5-17 and PAβN on Bacterial Growth and Biofilm Formation of Multidrug-Resistant Acinetobacter baumannii" Microorganisms 13, no. 3: 639. https://doi.org/10.3390/microorganisms13030639
APA StyleRühl-Teichner, J., Müller, D., Stamm, I., Göttig, S., Leidner, U., Semmler, T., & Ewers, C. (2025). Inhibitory Effect of Antimicrobial Peptides Bac7(17), PAsmr5-17 and PAβN on Bacterial Growth and Biofilm Formation of Multidrug-Resistant Acinetobacter baumannii. Microorganisms, 13(3), 639. https://doi.org/10.3390/microorganisms13030639






