Symbiotic N2 Fixation, Leaf Photosynthesis, and Abiotic Stress Tolerance of Native Rhizobia Isolated from Soybean Nodules at Da, Upper West Region, Ghana
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Sites and Nodule Sample Collection
2.2. Rhizobial Isolation
2.3. Authentication of Rhizobial Isolates
2.4. Characterization of Rhizobial Isolates
2.5. Leaf Gas-Exchange Studies
2.6. Assessing Relative Symbiotic Effectiveness of Rhizobial Isolates
2.7. Shoot 15N/14N and 13C/12C Isotopic Analysis
2.8. Physiological Characterisation of Isolates
2.8.1. Assessing Temperature Tolerance
2.8.2. Measuring Drought Tolerance
2.8.3. Determining Salinity Tolerance
2.8.4. Assessing pH Tolerance
2.8.5. Acid–Alkali Production
2.9. Statistical Analysis
3. Results
3.1. Gas-Exchange Parameters
3.2. Plant Growth
3.3. Shoot C Concentration
3.4. Shoot δ13C and C:N Ratio
3.5. Nodulation Induced by Rhizobial Isolates
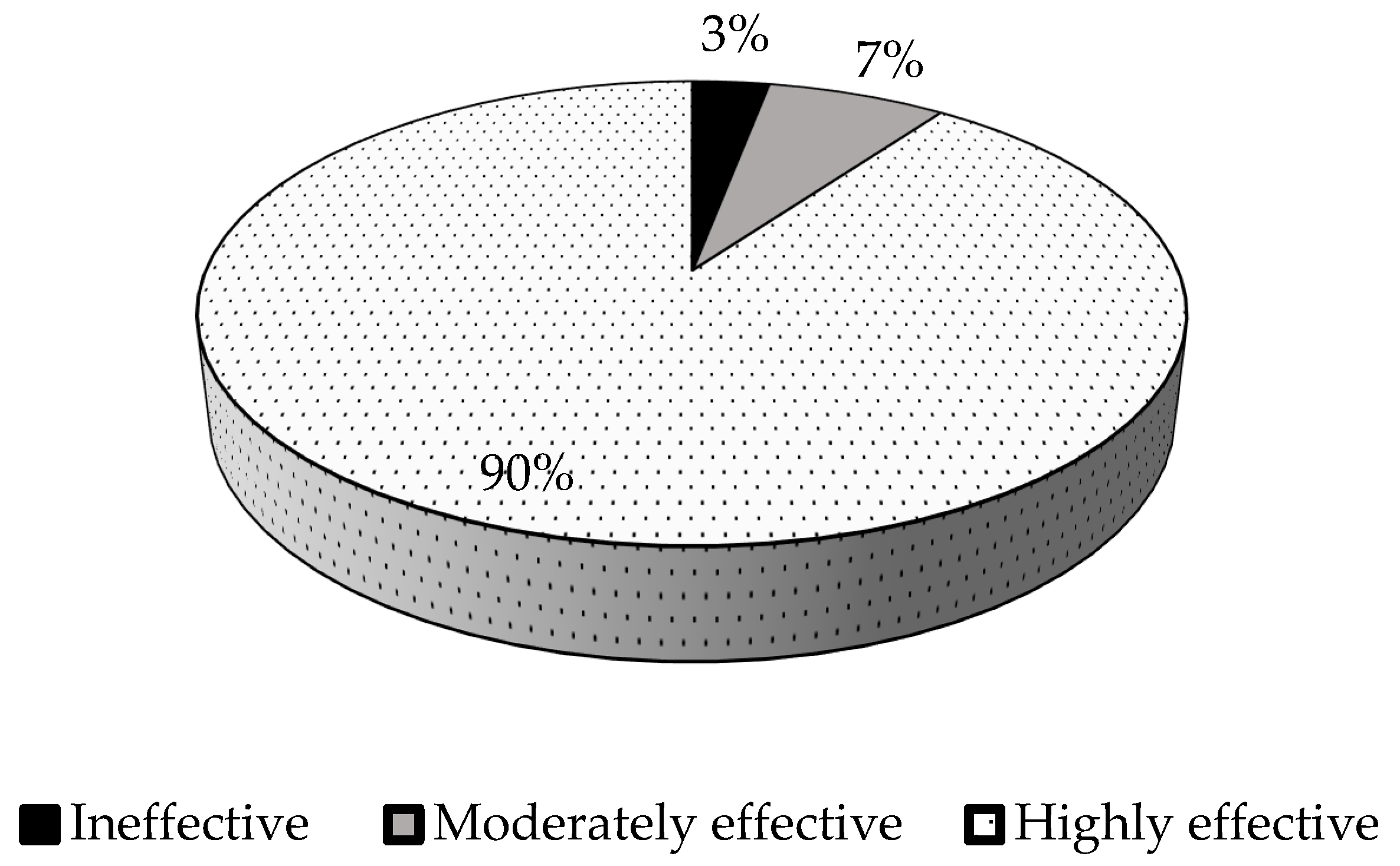
3.6. Relative Symbiotic Effectiveness of Rhizobial Isolates
3.7. Shoot N Concentration
3.8. Shoot δ15N and N-Fixed
3.9. Phenotypic Characterisation of the Rhizobial Isolates
3.10. Biochemical Characterisation of Rhizobial Isolates
3.10.1. Temperature Tolerance
3.10.2. Salinity Tolerance
3.10.3. Drought Tolerance
3.10.4. IAA Production
3.10.5. pH Tolerance
4. Discussion
4.1. Diversity of Soybean Rhizobial Isolates and Their Photosynthetic Performance
4.2. Plant Water-Use Efficiency and Strain Symbiotic Effectiveness
4.3. Plant Growth-Promoting Traits of the Rhizobial Isolates from Da
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| NaCl | Sodium Chloride |
| IAA | Indole acetic acid |
| PEG-6000 | Polyethylene glycol-6000 |
| BTB | Bromothymol blue |
| %RSE | Percent relative symbiotic effectiveness |
| O.D | Optical density |
| MDPI | Multidisciplinary Digital Publishing Institute |
| DOAJ | Directory of open access journals |
References
- Meena, I.; Meena, R.; Sharma, S.; Singh, D. Yield and Nutrient Uptake by Soybean as Influenced by Phosphorus and Sulphur Nutrition in Typic Haplustept. Madras Agric. J. 2015, 102, 1. [Google Scholar] [CrossRef]
- MacCarthy, D.S.; Traore, P.S.; Freduah, B.S.; Adiku, S.G.K.; Dodor, D.E.; Kumahor, S.K. Productivity of Soybean under Projected Climate Change in a Semi-Arid Region of West Africa: Sensitivity of Current Production System. Agronomy 2022, 12, 2614. [Google Scholar] [CrossRef]
- MoFA-SRID. Facts & Figures: Agriculture in Ghana, 2021; Statistics Research, and InformationDirectorate of Minister of Food and Agriculture: Accra, Ghana, 2022; pp. 1–143. [Google Scholar]
- FAOSTAT. Food and Agriculture Organization of the United Nations. Available online: https://www.fao.org/faostat/en/#data/QCL/visualize (accessed on 21 March 2024).
- Mitran, T.; Meena, R.S.; Lal, R.; Layek, J.; Kumar, S.; Datta, R. Role of soil phosphorus on legume production. In Legumes for Soil Health and Sustainable Management; Springer: Berlin/Heidelberg, Germany, 2018; pp. 487–510. [Google Scholar]
- Ayuba, J.; Jaiswal, S.K.; Mohammed, M.; Denwar, N.N.; Dakora, F.D. Adaptability to local conditions and phylogenetic differentiation of microsymbionts of TGx soybean genotypes in the semi-arid environments of Ghana and South Africa. Syst. Appl. Microbiol. 2021, 44, 126264. [Google Scholar] [CrossRef] [PubMed]
- Dlamini, S.T.; Jaiswal, S.K.; Mohammed, M.; Dakora, F.D. Studies of Phylogeny, Symbiotic Functioning and Ecological Traits of Indigenous Microsymbionts Nodulating Bambara Groundnut (Vigna subterranea L. Verdc) in Eswatini. Microb. Ecol. 2021, 82, 688–703. [Google Scholar] [CrossRef]
- Mwenda, G.M.; Hill, Y.J.; O’Hara, G.W.; Reeve, W.G.; Howieson, J.G.; Terpolilli, J.J. Competition in the Phaseolus vulgaris-Rhizobium symbiosis and the role of resident soil rhizobia in determining the outcomes of inoculation. Plant Soil 2023, 487, 61–77. [Google Scholar] [CrossRef]
- Kawaka, F.; Dida, M.M.; Opala, P.A.; Ombori, O.; Maingi, J.; Osoro, N.; Muthini, M.; Amoding, A.; Mukaminega, D.; Muoma, J. Symbiotic Efficiency of Native Rhizobia Nodulating Common Bean (Phaseolus vulgaris L.) in Soils of Western Kenya. Int. Sch. Res. Not. 2014, 2014, 258497. [Google Scholar] [CrossRef]
- Gyogluu, C.; Boahen, S.K.; Dakora, F.D. Response of promiscuous-nodulating soybean (Glycine max L. Merr.) genotypes to Bradyrhizobium inoculation at three field sites in Mozambique. Symbiosis 2016, 69, 81–88. [Google Scholar] [CrossRef]
- Samago, T.Y.; Anniye, E.W.; Dakora, F.D. Grain yield of common bean (Phaseolus vulgaris L.) varieties is markedly increased by rhizobial inoculation and phosphorus application in Ethiopia. Symbiosis 2018, 75, 245–255. [Google Scholar] [CrossRef]
- Samago, T.Y.; Dakora, F.D. Combined use of Rhizobium inoculation and low phosphorus application increased plant growth, root nodulation and grain yield of common bean (Phaseolus vulgaris) in Ethiopia. Front. Agric. Sci. Eng. 2025, 12, 104. [Google Scholar]
- Mohammed, M.; Mbah, G.C.; Sowley, E.N.K.; Dakora, F.D. Bradyrhizobium Inoculation of Field-Grown Kersting’s Groundnut [Macrotyloma geocarpum (Harms) Marechal & Baudet] Increased Grain Yield and N2 Fixation, Measured Using the Ureide, and 15N Natural Abundance Techniques. Front. Sustain. Food Syst. 2021, 5, 672247. [Google Scholar] [CrossRef]
- Mohammed, M.; Mbah, G.C.; Sowley, E.N.K.; Dakora, F.D. Cowpea Genotypic Variations in N2 Fixation, Water Use Efficiency (δ13C), and Grain Yield in Response to Bradyrhizobium Inoculation in the Field, Measured Using Xylem N Solutes, 15N, and 13C Natural Abundance. Front. Agron. 2022, 4, 764070. [Google Scholar] [CrossRef]
- Kumar, A.; Verma, J.P. Does plant—Microbe interaction confer stress tolerance in plants: A review? Microbiol. Res. 2018, 207, 41–52. [Google Scholar] [CrossRef] [PubMed]
- Sindhu, S.; Dahiya, A.; Gera, R.; Sindhu, S.S. Mitigation of abiotic stress in legume-nodulating rhizobia for sustainable crop production. Agric. Res. 2020, 9, 444–459. [Google Scholar] [CrossRef]
- Mendoza-Suárez, M.; Andersen, S.U.; Poole, P.S.; Sánchez-Cañizares, C. Competition, Nodule Occupancy, and Persistence of Inoculant Strains: Key Factors in the Rhizobium-Legume Symbioses. Front. Plant Sci. 2021, 12, 690567. [Google Scholar] [CrossRef]
- Bationo, A.; Fening, J.O.; Kwaw, A. Assessment of soil fertility status and integrated soil fertility management in Ghana. In Improving the Profitability, Sustainability and Efficiency of Nutrients Through Site Specific Fertilizer Recommendations in West Africa Agro-Ecosystems; Springer: Berlin/Heidelberg, Germany, 2018; Volume 1, pp. 93–138. [Google Scholar]
- Gopalakrishnan, S.; Sathya, A.; Vijayabharathi, R.; Varshney, R.K.; Gowda, C.L.; Krishnamurthy, L. Plant growth promoting rhizobia: Challenges and opportunities. 3 Biotech 2015, 5, 355–377. [Google Scholar] [CrossRef]
- Bouizgarne, B.; Oufdou, K.; Ouhdouch, Y. Actinorhizal and Rhizobial-Legume symbioses for alleviation. In Plant Microbes Symbiosis: Applied Facets; Springer: New Delhi, India, 2014. [Google Scholar]
- Rasanen, L.A.; Lindstrom, K. Effects of biotic and abiotic constraints on the symbiosis between rhizobia and the tropical leguminous trees Acacia and Prosopis. Indian J. Exp. Biol. 2003, 41, 1142–1159. [Google Scholar]
- Ali, Q.; Shabaan, M.; Ashraf, S.; Kamran, M.; Zulfiqar, U.; Ahmad, M.; Zahir, Z.A.; Sarwar, M.J.; Iqbal, R.; Ali, B.; et al. Comparative efficacy of different salt tolerant rhizobial inoculants in improving growth and productivity of Vigna radiata L. under salt stress. Sci. Rep. 2023, 13, 17442. [Google Scholar] [CrossRef]
- Khaitov, B.; Kurbonov, A.; Abdiev, A.; Adilov, M. Effect of chickpea in association with Rhizobium to crop productivity and soil fertility. Eurasian J. Soil Sci. 2016, 5, 105–112. [Google Scholar] [CrossRef]
- Bansal, M.; Kukreja, K.; Sunita, S.; Dudeja, S. Symbiotic effectivity of high temperature tolerant mungbean (Vigna radiata) rhizobia under different temperature conditions. Int. J. Curr. Microbiol. Appl. Sci. 2014, 3, 807–821. [Google Scholar]
- Yuan, K.; Reckling, M.; Ramirez, M.D.A.; Djedidi, S.; Fukuhara, I.; Ohyama, T.; Yokoyama, T.; Bellingrath-Kimura, S.D.; Halwani, M.; Egamberdieva, D. Characterization of rhizobia for the improvement of soybean cultivation at cold conditions in central Europe. Microbes Environ. 2020, 35, ME19124. [Google Scholar] [CrossRef]
- del-Canto, A.; Sanz-Saez, Á.; Sillero-Martínez, A.; Mintegi, E.; Lacuesta, M. Selected indigenous drought tolerant rhizobium strains as promising biostimulants for common bean in Northern Spain. Front. Plant Sci. 2023, 14, 1046397. [Google Scholar] [CrossRef] [PubMed]
- Avelar Ferreira, P.A.; Bomfeti, C.A.; Lima Soares, B.; de Souza Moreira, F.M. Efficient nitrogen-fixing Rhizobium strains isolated from amazonian soils are highly tolerant to acidity and aluminium. World J. Microbiol. Biotechnol. 2012, 28, 1947–1959. [Google Scholar] [CrossRef] [PubMed]
- Ibny, F.Y.; Jaiswal, S.K.; Mohammed, M.; Dakora, F.D. Symbiotic effectiveness and ecologically adaptive traits of native rhizobial symbionts of Bambara groundnut (Vigna subterranea L. Verdc.) in Africa and their relationship with phylogeny. Sci. Rep. 2019, 9, 12666. [Google Scholar]
- Mbah, G.C.; Mohammed, M.; Jaiswal, S.K.; Dakora, F.D. Phylogenetic Relationship, Symbiotic Effectiveness, and Biochemical Traits of Native Rhizobial Symbionts of Cowpea (Vigna unguiculata L. Walp) in South African Soil. J. Soil Sci. Plant Nutr. 2022, 22, 2235–2254. [Google Scholar] [CrossRef]
- Jaiswal, S.K.; Dakora, F.D. Maximizing Photosynthesis and Plant Growth in African Legumes Through Rhizobial Partnerships: The Road Behind and Ahead. Microorganisms 2025, 13, 581. [Google Scholar] [CrossRef]
- Mohammed, M.; Jaiswal, S.K.; Sowley, E.N.; Ahiabor, B.D.; Dakora, F.D. Symbiotic N2 fixation and grain yield of endangered Kersting’s groundnut landraces in response to soil and plant associated Bradyrhizobium inoculation to promote ecological resource-use efficiency. Front. Microbiol. 2018, 9, 2105. [Google Scholar] [CrossRef]
- Somasegaran, P.; Hoben, H.J. Handbook for Rhizobia: Methods in Legume-Rhizobium Technology; Springer Science & Business Media: Berlin, Germany, 2012. [Google Scholar]
- Vincent, J.M. A Manual for the Practical Study of the Root-Nodule Bacteria; Wiley Online Library: Hoboken, NJ, USA, 1970. [Google Scholar]
- Pongslip, N. Phenotypic and Genotypic Diversity of Rhizobia; Bentham Science Publishers: Sharjah, United Arab Emirates, 2012. [Google Scholar]
- Makoi, J.H.J.R.; Chimphango, S.B.M.; Dakora, F.D. Photosynthesis, water-use efficiency and δ13C of five cowpea genotypes grown in mixed culture and at different densities with sorghum. Photosynthetica 2010, 48, 143–155. [Google Scholar] [CrossRef]
- Rai, V.; Sanagala, R.; Sinilal, B.; Yadav, S.; Sarkar, A.K.; Dantu, P.K.; Jain, A. Iron availability affects phosphate deficiency-mediated responses, and evidence of cross-talk with auxin and zinc in Arabidopsis. Plant Cell Physiol. 2015, 56, 1107–1123. [Google Scholar] [CrossRef]
- Kebede, E.; Amsalu, B.; Argaw, A.; Tamiru, S. Symbiotic effectiveness of cowpea (Vigna unguiculata (L.) Walp.) nodulating rhizobia isolated from soils of major cowpea producing areas in Ethiopia. Cogent Food Agric. 2020, 6, 1763648. [Google Scholar] [CrossRef]
- Purcino, H.; Festin, P.; Elkan, G. Identification of effective strains of Bradyrhizobium for Arachis pintoi. Trop. Agric. 2000, 77, 226–231. [Google Scholar]
- Ngwenya, Z.D.; Dakora, F.D. Symbiotic Functioning and Photosynthetic Rates Induced by Rhizobia Associated with Jack Bean (Canavalia ensiformis L.) Nodulation in Eswatini. Microorganisms 2023, 11, 2786. [Google Scholar] [CrossRef] [PubMed]
- Mariotti, A.; Germon, J.; Hubert, P.; Kaiser, P.; Letolle, R.; Tardieux, A.; Tardieux, P. Experimental determination of nitrogen kinetic isotope fractionation: Some principles; illustration for the denitrification and nitrification processes. Plant Soil 1981, 62, 413–430. [Google Scholar] [CrossRef]
- Farquhar, G.D.; Ehleringer, J.R.; Hubick, K.T. Carbon isotope discrimination and photosynthesis. Annu. Rev. Plant Biol. 1989, 40, 503–537. [Google Scholar] [CrossRef]
- Pausch, R.C.; Mulchi, C.L.; Lee, E.H.; Forseth, I.N.; Slaughter, L.H. Use of 13C and 15N isotopes to investigate O3 effects on C and N metabolism in soybeans. Part I. C fixation and translocation. Agric. Ecosyst. Environ. 1996, 59, 69–80. [Google Scholar] [CrossRef]
- Mohammed, M.A.; Chernet, M.T.; Tuji, F.A. Phenotypic, stress tolerance, and plant growth promoting characteristics of rhizobial isolates of grass pea. Int. Microbiol. 2020, 23, 607–618. [Google Scholar] [CrossRef]
- Susilowati, A.; Puspita, A.; Yunus, A. Drought resistant of bacteria producing exopolysaccharide and IAA in rhizosphere of soybean plant (Glycine max) in Wonogiri Regency Central Java Indonesia. IOP Conf. Ser. Earth Environ. Sci. 2018, 142, 012058. [Google Scholar]
- Guerrero-Castro, J.; Lozano, L.; Sohlenkamp, C. Dissecting the acid stress response of Rhizobium tropici CIAT 899. Front. Microbiol. 2018, 9, 846. [Google Scholar] [CrossRef]
- Rejili, M.; Mahdhi, M.; Fterich, A.; Dhaoui, S.; Guefrachi, I.; Abdeddayem, R.; Mars, M. Symbiotic nitrogen fixation of wild legumes in Tunisia: Soil fertility dynamics, field nodulation and nodules effectiveness. Agric. Ecosyst. Environ. 2012, 157, 60–69. [Google Scholar] [CrossRef]
- Grönemeyer, J.L.; Kulkarni, A.; Berkelmann, D.; Hurek, T.; Reinhold-Hurek, B. Rhizobia indigenous to the Okavango region in Sub-Saharan Africa: Diversity, adaptations, and host specificity. Appl. Environ. Microbiol. 2014, 80, 7244–7257. [Google Scholar] [CrossRef]
- Ngwenya, Z.D.; Mohammed, M.; Jaiswal, S.K.; Dakora, F.D. Phylogenetic relationships among Bradyrhizobium species nodulating groundnut (Arachis hypogea L.), jack bean (Canavalia ensiformis L.) and soybean (Glycine max Merr.) in Eswatini. Sci. Rep. 2022, 12, 10629. [Google Scholar] [CrossRef]
- Gyogluu, C.; Mohammed, M.; Jaiswal, S.K.; Kyei-Boahen, S.; Dakora, F.D. Assessing host range, symbiotic effectiveness, and photosynthetic rates induced by native soybean rhizobia isolated from Mozambican and South African soils. Symbiosis 2018, 75, 257–266. [Google Scholar] [CrossRef]
- Mohammed, M.; Jaiswal, S.K.; Dakora, F.D. Distribution and correlation between phylogeny and functional traits of cowpea (Vigna unguiculata L. Walp.)-nodulating microsymbionts from Ghana and South Africa. Sci. Rep. 2018, 8, 18006. [Google Scholar] [CrossRef] [PubMed]
- Osei, O.; Abaidoo, R.C.; Ahiabor, B.D.K.; Boddey, R.M.; Rouws, L.F.M. Bacteria related to Bradyrhizobium yuanmingense from Ghana are effective groundnut micro-symbionts. Appl. Soil Ecol. 2018, 127, 41–50. [Google Scholar] [CrossRef] [PubMed]
- Belane, A.K.; Dakora, F.D. Assessing the relationship between photosynthetic C accumulation and symbiotic N nutrition in leaves of field-grown nodulated cowpea (Vigna unguiculata L. Walp.) genotypes. Photosynthetica 2015, 53, 562–571. [Google Scholar] [CrossRef]
- Parvin, S.; Uddin, S.; Tausz-Posch, S.; Armstrong, R.; Tausz, M. Carbon sink strength of nodules but not other organs modulates photosynthesis of faba bean (Vicia faba) grown under elevated [CO2] and different water supply. New Phytol. 2020, 227, 132–145. [Google Scholar] [CrossRef]
- Phillips, D.A.; Joseph, C.M.; Yang, G.-P.; Martínez-Romero, E.; Sanborn, J.R.; Volpin, H. Identification of lumichrome as a Sinorhizobium enhancer of alfalfa root respiration and shoot growth. Proc. Natl. Acad. Sci. USA 1999, 96, 12275–12280. [Google Scholar] [CrossRef]
- Matiru, V.; Dakora, F. Xylem transport and shoot accumulation of lumichrome, a newly recognized rhizobial signal, alters root respiration, stomatal conductance, leaf transpiration and photosynthetic rates in legumes and cereals. New Phytol. 2005, 165, 847–855. [Google Scholar] [CrossRef]
- Matiru, V.N.; Dakora, F.D. The rhizosphere signal molecule lumichrome alters seedling development in both legumes and cereals. New Phytol. 2005, 166, 439–444. [Google Scholar] [CrossRef]
- Dakora, F.D.; Matiru, V.; Kanu, A.S. Rhizosphere ecology of lumichrome and riboflavin, two bacterial signal molecules eliciting developmental changes in plants. Front. Plant Sci. 2015, 6, 146621. [Google Scholar] [CrossRef]
- Bhargava, Y.; Murthy, J.; Kumar, T.R.; Rao, M.N. Phenotypic, stress tolerance and plant growth promoting characteristics of rhizobial isolates from selected wild legumes of semiarid region, Tirupati, India. Adv. Microbiol. 2016, 6, 1–12. [Google Scholar] [CrossRef]
- Mhadhbi, H.; Chihaoui, S.; Mhamdi, R.; Mnasri, B.; Jebara, M. A highly osmotolerant rhizobial strain confers a better tolerance of nitrogen fixation and enhances protective activities to nodules of Phaseolus vulgaris under drought stress. Afr. J. Biotechnol. 2011, 10, 4555–4563. [Google Scholar]
- Li, D.; Gao, Y. Hydrogen Ion Concentration Index of Culture Media. In Quality Management in the Assisted Reproduction Laboratory; Springer Nature: Singapore, 2024; pp. 37–47. [Google Scholar]
- Oliveira, A.N.d.; Oliveira, L.A.d.; Andrade, J.S. Production and some properties of crude alkaline proteases of indigenous Central Amazonian rhizobia strains. Braz. Arch. Biol. Technol. 2010, 53, 1185–1195. [Google Scholar] [CrossRef]
- Adhikari, D.; Kaneto, M.; Itoh, K.; Suyama, K.; Pokharel, B.B.; Gaihre, Y.K. Genetic diversity of soybean-nodulating rhizobia in Nepal in relation to climate and soil properties. Plant Soil 2012, 357, 131–145. [Google Scholar] [CrossRef]
- Yan, J.; Han, X.Z.; Ji, Z.J.; Li, Y.; Wang, E.T.; Xie, Z.H.; Chen, W.F. Abundance and diversity of soybean-nodulating rhizobia in black soil are impacted by land use and crop management. Appl. Environ. Microbiol. 2014, 80, 5394–5402. [Google Scholar] [CrossRef] [PubMed]


| Isolates | A | Gs | Ci | E | Ci/Ca | WUEi |
|---|---|---|---|---|---|---|
| µmol (CO2) m−2s−1 | Mol (H2O) m−2s−1 | µmol (CO2) molair−1 | Mol (H2O) m−2s−1 | µmol (CO2) mol−1(H2O) | ||
| TUTGMGH1 | 11.34 ± 0.76 d–l | 0.16 ± 0.01 j–m | 257.38 ± 1.38 fg | 5.41 ± 0.07 ij | 0.65 ± 0.01 i–j | 70.36 ± 2.43 bc |
| TUTGMGH2 | 12.48 ± 0.40 fg | 0.26 ± 0.01 d–f | 285.89 ± 3.89 a–d | 7.81 ± 0.21 d–h | 0.74 ± 0.01 a–e | 48.30 ± 2.11 d–g |
| TUTGMGH3 | 11.59 ± 0.01 g–k | 0.19 ± 0.02 h–j | 277.25 ± 5.98 a–f | 6.12 ± 0.44 g–j | 0.72 ± 0.01 a–i | 63.80 ± 7.59 b–e |
| TUTGMGH4 | 14.81 ± 0.07 bc | 0.33 ± 0.0001 b | 285.99 ± 0.27 a–d | 9.09 ± 0.01 b–d | 0.75 ± 0.001 abc | 44.54 ± 0.13 h–j |
| TUTGMGH5 | 10.62 ± 0.12 j–m | 0.18 ± 0.03 h–l | 261.91 ± 21.91 d–g | 9.84 ± 2.46 cd | 0.67 ± 0.06 g–j | 65.69 ± 16.51 b–d |
| TUTGMGH6 | 11.81 ± 0.01 g–i | 0.30 ± 0.03 c | 290.18 ± 0.66 ab | 7.97 ± 0.001 c–g | 0.76 ± 0.003 ab | 40.49 ± 3.49 ij |
| TUTGMGH7 | 11.26 ± 0.02 h–m | 0.27 ± 0.003 cd | 290.69 ± 3.60 ab | 8.29 ± 0.004 b–f | 0.75 ± 0.01 a–c | 41.29 ± 0.54 ij |
| TUTGMGH8 | 16.16 ± 0.63 a | 0.39 ± 0.03 a | 285.08 ± 0.25 a–e | 12.62 ± 0.01 a | 0.76 ± 0.003 ab | 41.06 ± 1.20 ij |
| TUTGMGH9 | 13.05 ± 0.10 ef | 0.21 ± 0.01 g–i | 266.68 ± 5.76 b–h | 7.40 ± 0.04 c–h | 0.70 ± 0.01 b–j | 62.08 ± 2.23 b–f |
| TUTGMGH10 | 12.44 ± 0.31 fg | 0.24 ± 0.01 e–g | 271.05 ± 1.85 a–f | 8.12 ± 0.35 c–f | 0.71 ± 0.01 a–i | 53.08 ± 1.38 b–i |
| TUTGMGH11 | 16.18 ± 0.01 a | 0.39 ± 0.001 a | 286.22 ± 0.18 a–d | 10.11 ± 0.01 b | 0.76 ± 0.001 ab | 41.57 ± 0.11 ij |
| TUTGMGH12 | 9.03 ± 0.73 o | 0.14 ± 0.002 lm | 260.12 ± 9.90 e–g | 6.01 ± 0.06 h–j | 0.67 ± 0.02 f–j | 62.60 ± 5.87 b–f |
| TUTGMGH13 | 11.78 ± 0.74 g–j | 0.23 ± 0.01 e–g | 274.35 ± 1.67 a–g | 8.74 ± 0.10 b–e | 0.70 ± 0.001 a–i | 50.58 ± 0.47 e–j |
| TUTGMGH14 | 10.94 ± 0.00 h–m | 0.27 ± 0.001 c–e | 283.46 ± 7.89 a–e | 8.38 ± 1.22 b–f | 0.74 ± 0.02 a–d | 40.83 ± 0.16 ij |
| TUTGMGH15 | 9.25 ± 0.04 no | 0.13 ± 0.01 mn | 243.343 ± 10.34 gh | 7.19 ± 1.25 d–i | 0.63 ± 0.02 j | 71.59 ± 6.07 b |
| TUTGMGH16 | 10.15 ± 0.08 mn | 0.17 ± 0.003 i–l | 263.78 ± 3.02 c–g | 7.09 ± 0.13 e–i | 0.69 ± 0.01 c–j | 58.43 ± 1.51 c–g |
| TUTGMGH17 | 10.96 ± 0.38 h–m | 0.25 ± 0004 d–f | 288.84 ± 3.23 a–c | 8.41 ± 0.03 b–f | 0.76 ± 0.01 ab | 43.13 ± 1.53 ij |
| TUTGMGH18 | 7.76 ± 0.08 p | 0.15 ± 0.0001 k–m | 281.28 ± 0.91 a–f | 7.24 ± 0.002 c–i | 0.71 ± 0.002 a–i | 51.77 ± 0.57–i |
| TUTGMGH19 | 11.44 ± 0.36 g–l | 0.28 ± 0.003 cd | 293.69 ± 2.53 a | 8.67 ± 0.32 b–e | 0.77 ± 0.02 a | 41.01 ± 0.85 ij |
| TUTGMGH20 | 7.86 ± 0.02 p | 0.21 ± 0.01 k–n | 294.82 ± 3.90 a | 7.84 ± 0.06 d–h | 0.76 ± 0.01 ab | 37.63 ± 1.68 j |
| TUTGMGH21 | 10.40 ± 0.29 lm | 0.15 ± 0.002 k–m | 230.75 ± 16.97 h | 6.12 ± 0.002 g–j | 0.61 ± 0.04 e–j | 70.88 ± 2.75 bc |
| TUTGMGH22 | 15.71 ± 0.35 ab | 0.30 ± 0.0014 c | 272.51 ± 2.23 a–g | 7.79 ± 0.11 d–h | 0.71 ± 0.07 a–i | 52.62 ± 0.54 e–i |
| TUTGMGH23 | 12.04 ± 0.29 f–h | 0.25 ± 0.02 d–f | 276.83 ± 6.81 a–f | 8.34 ± 0.02 b–f | 0.76 ± 0.004 ab | 47.90 ± 2.15 g–j |
| TUTGMGH24 | 13.57 ± 0.42 de | 0.21 ± 0.002 gh | 262.06 ± 4.01 b–g | 7.91 ± 0.16–h | 0.67 ± 0.01 f–j | 63.60 ± 1.42 b–e |
| TUTGMGH25 | 13.07 ± 0.19 ef | 0.18 ± 0.002 h–k | 265.45 ± 1.68 b–g | 6.49 ± 0.04 f–j | 0.66 ± 0.01 h–j | 71.43 ± 1.26 b |
| TUTGMGH26 | 10.58 ± 0.19 j–l | 0.11 ± 0.001 n | 209.91 ± 3.81 i | 4.80 ± 0.03 jk | 0.53 ± 0.010 j | 97.09 ± 2.40 a |
| TUTGMGH27 | 11.46 ± 0.01 g–l | 0.20 ± 0.001 g–i | 245.10 ± 20.48 gh | 7.40 ± 0.54 d–h | 0.64 ± 0.06 j | 56.32 ± 0.23 d–h |
| TUTGMGH28 | 11.403 ± 0.08 g–l | 0.22 ± 0.002 fg | 279.53 ± 0.97 a–f | 7.18 ± 0.05 d–i | 0.73 ± 0.002 a–h | 50.81 ± 0.70 f–i |
| TUTGMGH29 | 10.67 ± 0.17 i–m | 0.23 ± 0.001 fg | 279.44 ± 0.07 a–f | 8.24 ± 0.50 b–f | 0.74 ± 0.02 a–f | 46.63 ± 0.63 d–j |
| TUTGMGH30 | 14.74 ± 0.71 bc | 0.20 ± 0.002 g–i | 259.90 ± 6.45 e–g | 7.79 ± 0.47 d–h | 0.64 ± 0.02 j | 72.20 ± 3.08 b |
| TUTGMGH31 | 11.49 ± 0.28 g–l | 0.18 ± 0.001 h–l | 265.91 ± 8.97 b–g | 7.68 ± 0.57 d–h | 0.68 ± 0.02 d–j | 64.15 ± 0.68 b–e |
| Bradyrhizobium strain WB74 | 15.59 ± 0.03 bc | 0.37 ± 0.001 a | 279.293 ± 0.24 a–f | 12.10 ± 0.01 a | 0.75 ± 0.001 a–d | 42.39 ± 0.18 ij |
| Uninoculated | 2.64 ± 0.26 q | 0.07 ± 0.0002 o | 293.67 ± 0.69 a | 3.58 ± 0.01 i | 0.76 ± 0.019 ab | 40.43 ± 4.08 ij |
| 5 mM KNO3 | 14.47 ± 0.53 cd | 0.21 ± 0.001 g–i | 256.56 ± 4.31 fg | 8.81 ± 0.29 b–e | 0.72 ± 0.002 a–i | 70.34 ± 2.60 bc |
| F-statistics | 59.32 ** | 46.96 ** | 6.66 *** | 9.72 *** | 6.99 *** | 12.68 ** |
| Isolates | Shoot Dry Matter | Root Dry Matter | Total Biomass | C Concentration | C Content | δ13C | C:N Ratio |
|---|---|---|---|---|---|---|---|
| g Plant−1 | g Plant−1 | g Plant−1 | % | g Plant−1 | ‰ | g·g−1 | |
| TUTGMGH1 | 1.72 ± 0.09 a–d | 0.64 ± 0.03 b–e | 2.36 ± 0.13 bc | 43.30 ± 0.05 f–h | 74.48 ± 4.05 ab | −27.52 ± 0.01 e–i | 18.31 ± 0.04 c–g |
| TUTGMGH2 | 1.44 ± 0.03 c–h | 0.50 ± 0.05 c–g | 1.94 ± 0.07 c–k | 43.27 ± 0.03 f–i | 62.16 ± 1.22 b–k | −27.95 ± 0.02 mn | 17.99 ± 0.03 c–i |
| TUTGMGH3 | 1.74 ± 0.09 a–c | 0.51 ± 0.01 c–g | 2.25 ± 0.11 c–f | 43.42 ± 0.28 d–g | 75.58 ± 4.29 bc | −27.51 ± 0.01 d–h | 18.59 ± 0.18 c–g |
| TUTGMGH4 | 1.32 ± 0.23 e–l | 0.37 ± 0.01 f–h | 1.69 ± 0.26 h–l | 42.99 ± 0.13 ij | 56.83 ± 10.89 d–m | −27.65 ± 0.06 h–k | 17.40 ± 0.02 e–l |
| TUTGMGH5 | 1.22 ± 0.05 h–l | 0.35 ± 0.01 gh | 1.57 ± 0.05 g–k | 43.37 ± 0.06 e–g | 52.92 ± 2.07 h–m | −28.11 ± 0.03 no | 17.33 ± 0.11 e–l |
| TUTGMGH6 | 1.49 ± 0.07 c–j | 0.85 ± 0.18 b | 2.33 ± 0.19 b–d | 43.81 ± 0.09 bc | 65.14 ± 3.01 b–j | −27.74 ± 0.03 j–l | 17.55 ± 0.27 d–l |
| TUTGMGH7 | 1.54 ± 0.01 b–i | 0.50 ± 0.04–g | 2.03 ± 0.03 c–h | 43.72 ± 0.03 b–d | 67.19 ± 0.35 c–g | −28.07 ± 0.01 no | 15.57 ± 0.10 l |
| TUTGMGH8 | 1.27 ± 0.14 f–l | 0.45 ± 0.02 d–h | 1.59 ± 0.05 d–j | 42.82 ± 0.10 jk | 54.37 ± 6.13 g–m | −28.13 ± 0.06 o | 16.54 ± 0.45 g–l |
| TUTGMGH9 | 1.61 ± 0.19 b–f | 0.62 ± 0.01 b–e | 2.23 ± 0.19 b–e | 43.27 ± 0.09 f–i | 69.64 ± 8.01 b–e | −27.19 ± 0.04 a | 16.69 ± 0.10 f–l |
| TUTGMGH10 | 1.08 ± 0.02 l | 0.39 ± 0.01 e–h | 1.47 ± 0.01 l–n | 42.76 ± 0.016 jk | 46.04 ± 0.86 lm | −27.67 ± 0.03 h–l | 16.17 ± 0.10 h–m |
| TUTGMGH11 | 1.53 ± 0.05 b–i | 0.37 ± 0.02 f–h | 1.90 ± 0.04 d–k | 43.69 ± 0.11 b–d | 66.85 ± 2.11 c–g | −27.25 ± 0.02 ab | 17.07 ± 0.28 e–l |
| TUTGMGH12 | 1.38 ± 0.03 d–k | 0.57 ± 0.02 c–g | 1.95 ± 0.05 c–k | 43.44 ± 0.02 d–g | 59.81 ± 1.40 i–m | −27.76 ± 0.07 j–l | 19.54 ± 0.24 cd |
| TUTGMGH13 | 1.21 ± 0.08 i–l | 0.52 ± 0.002 c–g | 1.73 ± 0.08 g–l | 42.86 ± 0.011 j | 51.86 ± 3.23 g–k | −27.69 ± 0.01 i–l | 21.54 ± 0.07 b |
| TUTGMGH14 | 0.45 ± 0.05 mn | 0.23 ± 0.01 h | 0.68 ± 0.05 n | 43.66 ± 0.11 b–e | 19.44 ± 2.42 mn | −27.60 ± 0.05 g–j | 19.86 ± 1.04 bc |
| TUTGMGH15 | 1.23 ± 0.09 g–l | 0.61 ± 0.003 c–f | 1.84 ± 0.09 e–l | 43.45 ± 0.09 d–g | 53.43 ± 3.96 h–l | −27.38 ± 0.09 b–f | 19.87 ± 0.17 bc |
| TUTGMGH16 | 1.54 ± 0.03 b–i | 0.61 ± 0.01 c–f | 2.15 ± 0.04 c–g | 43.01 ± 0.13 h–j | 66.24 ± 1.60 b–i | −27.85 ± 0.01 lm | 16.04 ± 0.09 i–l |
| TUTGMGH17 | 1.57 ± 0.01 b–h | 0.67 ± 0.08 b–d | 1.90 ± 0.40 c–f | 43.56 ± 0.01 c–f | 68.25 ± 0.28 b–g | −27.40 ± 0.02 b–f | 18.78 ± 0.36 bcd |
| TUTGMGH18 | 0.72 ± 0.01 m | 0.42 ± 0.05 d–h | 1.14 ± 0.04 n | 42.53 ± 0.35 kl | 30.48 ± 0.38 no | −27.97 ± 0.05 m–o | 18.67 ± 0.37 c–f |
| TUTGMGH19 | 1.56 ± 0.07 b–i | 0.60 ± 0.01 c–f | 2.16 ± 0.06 c–h | 43.32 ± 0.07 fg | 67.43 ± 2.83 b–h | −27.35 ± 0.02 a–e | 17.68 ± 2.09 d–k |
| TUTGMGH20 | 1.62 ± 0.04 b–f | 0.61 ± 0.03 c–f | 2.23 ± 0.07 c–f | 43.81 ± 0.03 bc | 70.97 ± 1.74 a–d | −27.82 ± 0.03 k–m | 15.68 ± 0.68 kl |
| TUTGMGH21 | 1.99 ± 0.10 a | 0.72 ± 0.02 bc | 2.70 ± 0.12 ab | 42.77 ± 0.03 jk | 84.98 ± 4.38 a | −27.37 ± 0.03 b–f | 17.05 ± 0.10 e–l |
| TUTGMGH22 | 1.17 ± 0.09 j–l | 0.64 ± 0.09 b–e | 1.80 ± 0.15 f–l | 41.88 ± 0.06 m | 48.87 ± 3.73 j–l | −27.45 ± 0.05 c–g | 17.18 ± 0.04 e–l |
| TUTGMGH23 | 1.55 ± 0.10 c–g | 0.52 ± 0.07 c–g | 2.07 ± 0.15 c–h | 43.35 ± 0.15 e–g | 70.75 ± 2.02 a–d | −28.44 ± 0.04 q | 16.03 ± 1.29 i–l |
| TUTGMGH24 | 1.61 ± 0.02 b–f | 0.51 ± 0.12 c–g | 2.11 ± 0.11 c–i | 44.42 ± 0.09 a | 71.36 ± 0.84 a–d | −27.24 ± 0.01 ab | 18.61 ± 0.36 e–g |
| TUTGMGH25 | 1.30 ± 0.03 e–k | 0.61 ± 0.05 c–f | 1.91 ± 0.02 d–k | 42.43 ± 0.05 l | 55.02 ± 1.38 e–l | −28.29 ± 0.04 p | 15.91 ± 0.50 j–l |
| TUTGMGH26 | 0.60 ± 0.32 mn | 0.60 ± 0.23 c–f | 1.20 ± 0.44 mn | 43.21 ± 0.12 g–i | 26.00 ± 13.95 mn | −27.33 ± 0.02 a–c | 16.87 ± 0.58 e–l |
| TUTGMGH27 | 1.48 ± 0.15 c–j | 0.53 ± 0.02 c–g | 2.00 ± 0.14 c–j | 42.84 ± 0.04 j | 63.25 ± 6.52 b–j | −27.34 ± 0.01 a–d | 16.86 ± 0.58 e–l |
| TUTGMGH28 | 1.54 ± 0.15 b–i | 0.58 ± 0.09 c–g | 2.12 ± 0.20 c–h | 43.90 ± 0.03 b | 67.76 ± 6.50 b–h | −27.54 ± 0.01 f–i | 17.55 ± 0.29 d–l |
| TUTGMGH29 | 1.48 ± 0.02 c–k | 0.45 ± 0.01 d–h | 1.93 ± 0.01 c–k | 44.30 ± 0.02 a | 65.56 ± 0.74 b–h | −27.74 ± 0.01 j–l | 17.54 ± 0.67 d–l |
| TUTGMGH30 | 1.58 ± 0.09 b–f | 0.72 ± 0.05 bc | 2.30 ± 0.04 b–d | 43.83 ± 0.01 bc | 69.23 ± 3.79 b–f | −27.66 ± 0.10 h–k | 17.84 ± 0.13 d–j |
| TUTGMGH31 | 1.13 ± 0.04 kl | 0.41 ± 0.12–h | 1.54 ± 0.07 k–m | 42.84 ± 0.04 j | 48.26 ± 1.83 k–m | −27.34 ± 0.11 a–d | 16.86 ± 0.58 e–l |
| Bradyrhizobium strain WB74 | 1.07 ± 0.001 l | 0.57 ± 0.04 c–g | 1.64 ± 0004 i–m | 43.88 ± 0.01 b | 46.95 ± 0.02 l | −29.55 ± 0.06 r | 21.59 ± 0.08 b |
| Uninoculated | 0.33 ± 0.01 n | 0.22 ± 0.07 h | 0.54 ± 0.07 o | 40.15 ± 0.02 n | 13.12 ± 0.30 n | −27.77 ± 0.09 j–l | 18.15 ± 0.98 c–i |
| 5 mM KNO3 | 1.85 ± 0.07 ab | 1.13 ± 0.08 a | 2.97 ± 0.07 a | 41.77 ± 0.01 m | 77.14 ± 2.78 ab | −27.75 ± 0.01 j–l | 23.59 ± 0.1.71 a |
| F-statistics | 13.50 ** | 5.74 *** | 15.74 ** | 68.00 ** | 13.18 ** | 70.00 ** | 8.90 *** |
| Isolates | Nodule Number | Nodule Fresh Weight | Relative Symbiotic Effectiveness | N Concentration | N Content | δ15N | N-Fixed |
|---|---|---|---|---|---|---|---|
| per Plant | g Plant−1 | % | % | g Plant−1 | ‰ | g Plant−1 | |
| TUTGMGH1 | 19 ± 0.88 h–j | 0.64 ± 0.01 a | 161 ± 8.63 ab | 2.36 ± 0.01 f–i | 4.07 ± 0.23 b–e | −2.02 ± 0.02 f–i | 3.82 ± 0.23 b–e |
| TUTGMGH2 | 10 ± 0.58 k | 0.52 ± 0.003 a–c | 134 ± 2.55 b–i | 2.41 ± 0.02 d–i | 3.46 ± 0.06 c–h | −2.08 ± 0.05 f–k | 3.21 ± 0.06 c–h |
| TUTGMGH3 | 37 ± 1.15 ab | 0.44 ± 0.02 c–f | 163 ± 8.63 ab | 2.25 ± 0.02 g–i | 3.91 ± 0.19 b–f | −1.76 ± 0.05 d | 3.66 ± 0.19 b–f |
| TUTGMGH4 | 30 ± 1.15 b–e | 0.61 ± 0.01 ab | 124 ± 24.07 d–j | 2.53 ± 0.03 b–h | 3.36 ± 0.70 c–h | −2.15 ± 0.05 g–l | 3.11 ± 0.70 c–h |
| TUTGMGH5 | 25 ± 6.35 e–i | 0.34 ± 0.002 f–i | 114 ± 4.32 f–j | 2.52 ± 0.03 b–h | 3.07 ± 0.14 f–h | −1.94 ± 0.02 d–g | 2.82 ± 0.14 f–h |
| TUTGMGH6 | 18 ± 0.58 h–j | 0.52 ± 0.001 bc | 139 ± 6.14 b–h | 2.60 ± 0.01 b–f | 3.87 ± 0.18 b–f | −1.95 ± 0.02 d–g | 3.62 ± 0.18 b–f |
| TUTGMGH7 | 20 ± 6.69 g–j | 0.42 ± 0.02 c–g | 144 ± 0.82 b–g | 2.72 ± 0.03 a–c | 4.18 ± 0.03 a–d | −2.28 ± 0.01 j–l | 3.93 ± 0.03 a–d |
| TUTGMGH8 | 24 ± 1.73–j | 0.44 ± 0.01 c–f | 119 ± 1.49 e–j | 2.61 ± 0.07 b–f | 3.31 ± 0.40 d–h | −2.35 ± 0.01 l | 3.06 ± 0.40 d–h |
| TUTGMGH9 | 35 ± 5.77 a–c | 0.51 ± 0.10 b–d | 150 ± 17.59 b–e | 2.59 ± 0.02 b–f | 4.16 ± 0.45 a–d | −2.17 ± 0.02 g–l | 3.91 ± 0.45 a–d |
| TUTGMGH10 | 16 ± 0.58 jk | 0.43 ± 0.01 c–f | 101 ± 1.89 j | 2.61 ± 0.04 b–f | 2.81 ± 0.05 gh | −1.76 ± 0.02 d | 2.56 ± 0.05 gh |
| TUTGMGH11 | 28 ± 0.88 c–g | 0.43 ± 0.003 c–f | 143 ± 4.32 b–g | 2.59 ± 0.06 b–f | 3.96 ± 0.15 b–f | −2.31 ± 0.03 kl | 3.71 ± 0.15 b–f |
| TUTGMGH12 | 34 ± 0.33 bd | 0.56 ± 0.01 ab | 129 ± 2.97 c–j | 2.27 ± 0.05 g–i | 3.13 ± 0.11 e–h | −2.18 ± 0.01 g–l | 2.88 ± 0.11 e–h |
| TUTGMGH13 | 30 ± 1.15 b–e | 0.40 ± 0.03 d–h | 133 ± 7.01 g–j | 2.33 ± 0.08 f–i | 2.79 ± 0.07 gh | −2.13 ± 0.05 g–l | 2.54 ± 0.07 gh |
| TUTGMGH14 | 24 ± 2.03 e–j | 0.36 ± 0.04 f–h | 42 ± 5.13 k | 2.23 ± 0.21 hi | 0.99 ± 0.16 ij | −1.26 ± 0.03 c | 0.75 ± 0.16 j |
| TUTGMGH15 | 26 ± 6.43 d–h | 0.40 ± 0.03 d–h | 115 ± 8.63 f–j | 2.28 ± 0.03 g–i | 2.81 ± 0.25 gh | −2.16 ± 0.02 g–l | 2.56 ± 0.25 gh |
| TUTGMGH16 | 20 ± 0.58 g–j | 0.43 ± 0.10 c–f | 144 ± 3.24 b–g | 2.70 ± 0.01 a–d | 4.16 ± 0.10 a–d | −2.64 ± 0.28 m | 3.91 ± 0.10 a–d |
| TUTGMGH17 | 25 ± 3.06 e–g | 0.43 ± 0.01 c–f | 146 ± 0.627 b–g | 2.23 ± 0.02 g–i | 3.50 ± 0.02 c–h | −2.11 ± 0.05 g–l | 3.25 ± 0.02 c–h |
| TUTGMGH18 | 20 ± 3.76 g–j | 0.19 ± 0.01 jk | 67 ± 0.82 j | 2.17 ± 0.11 i | 1.56 ± 0.01 i | −2.14 ± 0.01 g–l | 1.31 ± 0.10 i |
| TUTGMGH19 | 22 ± 0.58 e–j | 0.55 ± 0.03 ab | 145 ± 6.21 b–g | 2.78 ± 0.06 ab | 4.33 ± 0.24 a–c | −2.01 ± 0.03 e–h | 4.08 ± 0.24 a–c |
| TUTGMGH20 | 43 ± 1.45 a | 0.38 ± 0.01 e–h | 151 ± 3.77 b–e | 2.41 ± 0.11 d–i | 3.90 ± 0.15 b–f | −2.29 ± 0.04 j–l | 3.65 ± 0.15 b–f |
| TUTGMGH21 | 27 ± 0.33 c–h | 0.50 ± 0.01 b–e | 186 ± 9.51 a | 2.53 ± 0.03 b–g | 5.02 ± 0.23 a | −2.23 ± 0.03 h–l | 4.77 ± 0.23 a |
| TUTGGH22 | 20 ± 0.88 g–j | 0.39 ± 0.04 e–i | 109 ± 8.24 h–j | 2.34 ± 0.10 f–i | 2.71 ± 0.14 gh | −2.23 ± 0.01 h–l | 2.46 ± 0.14 gh |
| TUTGMGH23 | 23 ± 0.33 e–j | 0.51 ± 0.01 b–e | 153 ± 4.59 b–d | 2.92 ± 0.04 a | 4.77 ± 0.12 ab | −2.65 ± 0.24 m | 4.53 ± 0.12 ab |
| TUTGMGH24 | 21 ± 0.33 f–j | 0.38 ± 0.03 d–h | 150 ± 1.89 b–e | 2.39 ± 0.04 f–i | 3.84 ± 0.10 b–f | −1.26 ± 0.03 c | 3.59 ± 0.10 b–f |
| TUTGMGH25 | 35 ± 1.53 ab | 0.58 ± 0.01 ab | 121 ± 2.97 b–j | 2.69 ± 0.07 a–e | 3.49 ± 0.01 c–h | −2.79 ± 0.04 m | 3.24 ± 0.01 c–h |
| TUTGMGH26 | 16 ± 0.88 i–k | 0.18 ± 0.05 k | 56 ± 29.93 k | 2.46 ± 0.16 c–i | 1.57 ± 0.92 i | −2.05 ± 0.001 f–j | 1.32 ± 0.92 i |
| TUTGMGH27 | 16 ± 1.73 jk | 0.24 ± 0.03 i–k | 138 ± 14.30 b–i | 2.37 ± 0.16 f–i | 3.47 ± 0.35 c–h | −2.08 ± 0.03 f–k | 3.22 ± 0.35 c–h |
| TUTGMGH28 | 29 ± 1.15 b–f | 0.31 ± 0.01 g–i | 144 ± 13.75 b–g | 2.36 ± 0.15 f–i | 3.64 ± 0.41 c–g | −1.86 ± 0.05 d–f | 3.39 ± 0.41 c–g |
| TUTGMGH29 | 20 ± 0.33 g–j | 0.43 ± 0.002 c–f | 138 ± 1.62 b–i | 2.43 ± 0.20 c–i | 3.60 ± 0.29 c–g | −2.10 ± 0.05 f–k | 3.35 ± 0.29 c–g |
| TUTGMGH30 | 21 ± 1.45 f–g | 0.40 ± 0.08 d–h | 147 ± 8.09 b–f | 2.46 ± 0.02 c–i | 3.88 ± 0.20 b–f | −1.79 ± 0.02 de | 3.63 ± 0.20 b–f |
| TUTGMGH31 | 16 ± 0.58 jk | 0.31 ± 0.01 g–i | 105 ± 4.05 ij | 2.37 ± 0.16 f–i | 2.68 ± 0.29 gh | −2.08 ± 0.04 f–k | 2.43 ± 0.29 gh |
| Bradyrhizbium strain WB74 | 30 ± 0.33 b–e | 0.29 ± 0.01 h–j | 100 ± 0.00 j | 2.40 ± 0.16 e–i | 2.57 ± 0.17 h | −2.26 ± 0.04 i–l | 2.32 ± 0.17 h |
| Uninoculated | NA | NA | NA | 1.13 ± 0.05 k | 0.37 ± 0.02 j | +1.55 ± 0.01 b | NA |
| 5 mM KNO3 | NA | NA | NA | 1.39 ± 0.02 j | 2.57 ± 0.12 g | +2.16 ± 0.06 a | NA |
| F-statistics | 8.05 *** | 9.95 *** | 9.90 *** | 14.97 ** | 12.96 ** | 194.76 ** | 12.96 ** |
| Isolates | Temperature °C | Salinity (NaCl) % | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 25 | 28 | 30 | 37 | 40 | 45 | 0.01 | 0.50 | 1 | 2 | 3 | 4 | 5 | |
| TUTGMGH1 | ++ | +++ | +++ | ++ | ++ | ++ | +++ | ++ | − | − | − | − | − |
| TUTGMGH2 | ++ | +++ | ++ | ++ | +++ | ++ | +++ | + | + | + | + | + | − |
| TUTGMGH3 | + | + | + | + | + | + | +++ | +++ | +++ | ++ | ++ | ++ | ++ |
| TUTGMGH4 | + | + | ++ | + | + | + | +++ | +++ | ++ | +++ | +++ | − | − |
| TUTGMGH5 | +++ | +++ | +++ | ++ | − | − | +++ | − | − | − | − | − | − |
| TUTGMGH6 | +++ | ++ | +++ | +++ | ++ | ++ | +++ | + | + | + | + | + | + |
| TUTGMGH7 | ++ | ++ | +++ | +++ | ++ | ++ | +++ | +++ | ++ | ++ | ++ | ++ | ++ |
| TUTGMGH8 | + | ++ | ++ | + | + | + | +++ | +++ | +++ | +++ | +++ | +++ | +++ |
| TUTGMGH9 | ++ | +++ | +++ | ++ | − | − | +++ | +++ | +++ | ++ | ++ | ++ | ++ |
| TUTGMGH10 | ++ | +++ | +++ | + | +++ | ++ | +++ | +++ | +++ | +++ | +++ | − | − |
| TUTGMGH11 | ++ | +++ | ++ | +++ | ++ | ++ | +++ | − | − | − | − | − | − |
| TUTGMGH12 | +++ | ++ | ++ | + | + | + | +++ | +++ | +++ | ++ | + | + | − |
| TUTGMGH13 | + | ++ | ++ | + | + | ++ | +++ | +++ | +++ | +++ | +++ | ++ | + |
| TUTGMGH14 | ++ | ++ | +++ | ++ | ++ | ++ | +++ | ++ | + | + | + | + | − |
| TUTGMGH15 | ++ | +++ | + | + | + | ++ | +++ | +++ | ++ | ++ | ++ | ++ | + |
| TUTGMGH16 | +++ | +++ | +++ | +++ | +++ | + | +++ | − | − | − | − | − | − |
| TUTGMGH17 | +++ | +++ | +++ | +++ | +++ | +++ | +++ | + | − | − | − | − | − |
| TUTGMGH18 | + | ++ | +++ | +++ | + | + | +++ | ++ | ++ | ++ | ++ | + | + |
| TUTGMGH19 | ++ | +++ | +++ | ++ | ++ | ++ | +++ | ++ | ++ | ++ | ++ | + | + |
| TUTGMGH20 | + | ++ | +++ | +++ | ++ | + | +++ | +++ | ++ | ++ | ++ | + | + |
| TUTGMGH21 | ++ | ++ | +++ | +++ | +++ | ++ | +++ | +++ | ++ | ++ | ++ | + | + |
| TUTGMGH22 | +++ | +++ | +++ | ++ | +++ | ++ | +++ | +++ | +++ | +++ | +++ | ++ | ++ |
| TUTGMGH23 | ++ | ++ | ++ | + | + | + | +++ | +++ | ++ | ++ | ++ | + | + |
| TUTGMGH24 | + | ++ | ++ | + | ++ | ++ | +++ | ++ | − | − | − | − | − |
| TUTGMGH25 | + | +++ | +++ | +++ | ++ | ++ | +++ | + | − | − | − | − | − |
| TUTGMGH26 | + | ++ | +++ | +++ | +++ | ++ | +++ | +++ | ++ | ++ | ++ | ++ | − |
| TUTGMGH27 | + | ++ | +++ | +++ | +++ | ++ | +++ | +++ | +++ | +++ | ++ | ++ | ++ |
| TUTGMGH28 | + | +++ | +++ | ++ | − | − | +++ | +++ | +++ | +++ | ++ | ++ | − |
| TUTGMGH29 | + | +++ | + | + | ++ | + | +++ | ++ | ++ | + | + | + | + |
| TUTGMGH30 | + | +++ | ++ | + | + | + | +++ | +++ | ++ | ++ | + | + | + |
| TUTGMGH31 | + | ++ | +++ | ++ | +++ | ++ | +++ | +++ | +++ | +++ | ++ | ++ | ++ |
| Isolates | Drought | IAA | |||
|---|---|---|---|---|---|
| Control | 5% | 15% | 30% | (µg mL−1) | |
| TUTGMGH1 | 0.170 ± 0.012 i–k | 0.204 ± 0.003 f–h | 0.084 ± 0.001 lm | 0.078 ± 0.0003 e–h | 8.59 ± 0.03 d |
| TUTGMGH2 | 0.333 ± 0.006 k–m | 0.142 ± 0.001 | 0.088 ± 0.005 j–l | 0.065 ± 0.006 gh | 6.52 ± 0.15 f |
| TUTGMGH3 | 0.262 ± 0.001 h–j | 0.258 ± 0.002 de | 0.176 ± 0.004 b | 0.069 ± 0.002 fgh | 9.92 ± 0.10 b |
| TUTGMGH4 | 0.330 ± 0.036 d–g | 0.210 ± 0.001 fg | 0.077 ± 0.001 mn | 0.075 ± 0.0003 e–h | 8.59 ± 0.47 d |
| TUTGMGH5 | 0.238 ± 0.023 ij | 0.103 ± 0.001 k–n | 0.144 ± 0.003 e | 0.083 ± 0.002 d–h | 9.39 ± 0.16 bc |
| TUTGMGH6 | 0.634 ± 0.048 b | 0.256 ± 0.005 de | 0.114 ± 0.001 h | 0.083 ± 0.002 d–h | 8.74 ± 0.054 cd |
| TUTGMGH7 | 0.225 ± 0.003 i–k | 0.131 ± 0.001 i–m | 0.110 ± 0.002 h | 0.084 ± 0.001 d–h | 8.49 ± 0.15 de |
| TUTGMGH8 | 0.282 ± 0.0196 f–i | 0.145 ± 0.005 | 0.135 ± 0.006 ef | 0.086 ± 0.002 d–f | 9.85 ± 0.01 b |
| TUTGMGH9 | 0.526 ± 0.015 c | 0.523 ± 0.003 a | 0.157 ± 0.009 d | 0.067 ± 0.0003 f–h | 8.30 ± 0.13 de |
| TUTGMGH10 | 0.277 ± 0.003 f–i | 0.377 ± 0.067 c | 0.171 ± 0.003 bc | 0.154 ± 0.026 b | 4.52 ± 0.03 g |
| TUTGMGH11 | 0.137 ± 0.002 mn | 0.099 ± 0.001 l–n | 0.085 ± 0.002 k–m | 0.106 ± 00.004 c | 8.65 ± 0.11 d |
| TUTGMGH12 | 0.639 ± 0.019 a | 0.424 ± 0.002 b | 0.243 ± 0.001 a | 0.164 ± 0.001 a | 8.11 ± 0.64 de |
| TUTGMGH13 | 0.338 ± 0.007 d–f | 0.175 ± 0.0003 f–k | 0.165 ± 0.002 cd | 0.065 ± 0.002 h | 7.75 ± 0.10 e |
| TUTGMGH14 | 0.105 ± 0.002 n | 0.093 ± 0.0003 mn | 0.057 ± 0.0003 p | 0.089 ± 0.0003 c–e | 4.01 ± 0.07 gh |
| TUTGMGH15 | 0.307 ± 0.003 e–h | 0.082 ± 0.001 n | 0.072 ± 0.001 no | 0.085 ± 0.0003 d–g | 7.75 ± 0.05 e |
| TUTGMGH16 | 0.447 ± 0.003 d | 0.218 ± 0.0003 ef | 0.164 ± 0.001 cd | 0.098 ± 0.0003 cd | 11.37 ± 0.23 a |
| TUTGMGH17 | 0.350 ± 0.002 d–f | 0.172 ± 0.010 f–k | 0.106 ± 0.0003 hi | 0.041 ± 0.001 i | 8.49 ± 0.04 de |
| TUTGMGH18 | 0.203 ± 0.042 j–l | 0.096 ± 0.002 l–n | 0.127 ± 0.001 fg | 0.069 ± 0.001 f–h | 4.16 ± 0.37 gh |
| TUTGMGH19 | 0.223 ± 0.007 i–k | 0.461 ± 0.038 b | 0.124 ± 0.003 g | 0.142 ± 0.001 b | 4.69 ± 0.35 g |
| TUTGMGH20 | 0.314 ± 0.005 e–h | 0.128 ± 0.0003 i–n | 0.097 ± 0.001 ij | 0.075 ± 0.001 e–h | 3.00 ± 0.14 ij |
| TUTGMGH21 | 0.270 ± 0.061 g–j | 0.167 ± 0.006 g–j | 0.166 ± 0.005 cd | 0.101 ± 0.0123 cd | 6.35 ± 0.03 f |
| TUTGMGH22 | 0.266 ± 0.002 g–j | 0.086 ± 0.003 mn | 0.05 ± 0.001 q | 0.067 ± 0.0003 f–h | 8.06 ± 0.69 de |
| TUTGMGH23 | 0.265 ± 0.013 h–j | 0.176 ± 0.004 f–j | 0.081 ± 0.001 lm | 0.085 ± 0.00 d–1 | 4.48 ± 0.29 g |
| TUTGMGH24 | 0.156 ± 0.011 l–n | 0.127 ± 0.006 j–n | 0.090 ± 0.0003 j–l | 0.091 ± 0.003 c–e | 1.21 ± 0.14 k |
| TUTGMGH25 | 0.228 ± 0.012 i–k | 0.128 ± 0.001 i–n | 0.139 ± 0.003 e | 0.078 ± 0.001 e–h | 2.76 ± 0.04 jk |
| TUTGMGH26 | 0.258 ± 0.009 h–j | 0.161 ± 0.005 h–j | 0.096 ± 0.001 j | 0.090 ± 0.001 c–e | 0.98 ± 0.11 l |
| TUTGMGH27 | 0.235 ± 0.008 ij | 0.159 ± 0.003 h–j | 0.162 ± 0.001 cd | 0.074 ± 0.002 e–h | 3.02 ± 0.22 ij |
| TUTGMGH28 | 0.388 ± 0.002 de | 0.159 ± 0.003 h–j | 0.162 ± 0.001 cd | 0.074 ± 0.002 e–h | 2.14 ± 0.02 k |
| TUTGMGH29 | 0.269 ± 0.001 g–j | 0.105 ± 0.0003 k–n | 0.067 ± 0.001 o | 0.044 ± 0.004 i | 4.12 ± 0.07 gh |
| TUTGMGH30 | 0.227 ± 0.003 i–k | 0.282 ± 0.003 d | 0.094 ± 0.001 jk | 0.089 ± 0.001 c–e | 3.59 ± 0.26 hi |
| TUTGMGH31 | 0.257 ± 0.009 h– | 0.160 ± 0.004 h–j | 0.096 ± 0.001 j | 0.091 ± 0.001 c–e | 0.98 ± 0.11 l |
| F-statistics | 41.294 ** | 62.881 ** | 207.34 ** | 22.857 ** | 135.02 ** |
| Isolates | 4 | 5 | 6 | 7 | 8.5 | pH Indicator (BTB) |
|---|---|---|---|---|---|---|
| TUTGHGM1 | 0.184 ± 0.002 k–m | 0.466 ± 0.008 b | 0.287 ± 0.079 de | 0.178 ± 0.006 l | 0.341 ± 0.055 d–h | Blue |
| TUTGHGM2 | 0.208 ± 0.008 h–m | 0.151 ± 0.008 k | 0.169 ± 0.004 m–o | 0.250 ± 0.005 jk | 0.355 ± 0.004 d–g | Yellow |
| TUTGHGM3 | 0.514 ± 0.019 b | 0.452 ± 0.005 b | 0.277 ± 0.009 d–f | 0.245 ± 0.004 jk | 0.284 ± 0.001 g–j | Yellow |
| TUTGHGM4 | 0.479 ± 0.062 b | 0.349 ± 0.005 c | 0.440 ± 0.009 b | 0.429 ± 0.004 bc | 0.475 ± 0.060 b | Blue |
| TUTGHGM5 | 0.158 ± 0.008 m–o | 0.240 ± 0.002 fg | 0.158 ± 0.002 no | 0.237 ± 0.003 jk | 0.258 ± 0.010 g–j | Blue |
| TUTGHGM6 | 0.230 ± 0.015 h–l | 0.260 ± 0.026 ef | 0.242 ± 0.011 e–j | 0.238 ± 0.007 jk | 0.417 ± 0.062 b–d | Yellow |
| TUTGHGM7 | 0.181 ± 0.002 lm | 0.153 ± 0.002 k | 0.242 ± 0.0002 e–j | 0.222 ± 0.003 j–l | 0.242 ± 0.009 h–j | Blue |
| TUTGHGM8 | 0.125 ± 0.005 no | 0.279 ± 0.002 e | 0.179 ± 0.004 l–o | 0.416 ± 0.006 b–d | 0.385 ± 0.063 b–f | Yellow |
| TUTGHGM9 | 0.585 ± 0.004 a | 0.697 ± 0.008 a | 0.556 ± 0.013 a | 0.504 ± 0.003 a | 0.388 ± 0.044 b–f | Yellow |
| TUTGHGM10 | 0.284 ± 0.045 fg | 0.334 ± 0.005 cd | 0.164 ± 0.007 no | 0.421 ± 0.101 b–d | 0.462 ± 0.0002 bc | Blue |
| TUTGHGM11 | 0.217 ± 0.004 h–l | 0.199 ± 0.006 hi | 0.231 ± 0.004 f–k | 0.271 ± 0.005 h–j | 0.220 ± 0.006 j | Blue |
| TUTGHGM12 | 0.423 ± 0.001 c | 0.326 ± 0.006 cd | 0.279 ± 0.002 d–f | 0.383 ± 0.009 c–e | 0.576 ± 0.005 a | Blue |
| TUTGHGM13 | 0.260 ± 0.001 gh | 0.321 ± 0.005 d | 0.204 ± 0.010 h–n | 0.209 ± 0.007 kl | 0.320 ± 0.004 d–j | Yellow |
| TUTGHGM14 | 0.256 ± 0.001 g–i | 0.182 ± 0.015 j | 0.183 ± 0.002 k–o | 0.318 ± 0.010 f–h | 0.301 ± 0.012 e–j | Blue |
| TUTGHGM15 | 0.328 ± 0.010 ef | 0.233 ± 0.006 gh | 0.256 ± 0.010 e–h | 0.447 ± 0.08 b | 0.240 ± 0.006 h–j | Yellow |
| TUTGHGM16 | 0.186 ± 0.010 j–m | 0.156 ± 0.002 k | 0.251 ± 0.003 e–i | 0.341 ± 0.090 e–g | 0.327 ± 0.019 d–i | Yellow |
| TUTGHGM17 | 0.330 ± 0.001 ef | 0.062 ± 0.002 o | 0.223 ± 0.003 j–l | 0.335 ± 0.010 e–g | 0.459 ± 0.060 bc | Blue |
| TUTGHGM18 | 0.128 ± 0.004 no | 0.186 ± 0.001 ij | 0.073 ± 0.0004 p | 0.214 ± 0.002 j–l | 0.397 ± 0.059 b–e | Yellow |
| TUTGHGM19 | 0.351 ± 0.016 de | 0.329 ± 0.001 cd | 0.376 ± 0.004 c | 0.372 ± 0.007 d–f | 0.311 ± 0.031 e–j | Blue |
| TUTGHGM20 | 0.341 ± 0.005 de | 0.209 ± 0.004 hi | 0.273 ± 0.002 e–g | 0.229 ± 0.002 j–l | 0.362 ± 0.010 c–f | Blue |
| TUTGHGM21 | 0.190 ± 0.020 j–m | 0.126 ± 0.007 lm | 0.170 ± 0.010 m–o | 0.174 ± 0.007 l | 0.402 ± 0.013 b–d | Blue |
| TUTGHGM22 | 0.240 ± 0.006 g–k | 0.095 ± 0.002 n | 0.144 ± 0.001 o | 0.264 ± 0.003 i–k | 0.268 ± 0.015 g–j | Blue |
| TUTGHGM23 | 0.318 ± 0.005 ef | 0.225 ± 0.003 gh | 0.199 ± 0.001 i–n | 0.251 ± 0.005 jk | 0.230 ± 0.004 ij | Yellow |
| TUTGHGM24 | 0.175 ± 0.012 l–n | 0.134 ± 0.007 kl | 0.195 ± 0.006 j–o | 0.411 ± 0.001 b–d | 0.380 ± 0.005 b–f | Blue |
| TUTGHGM25 | 0.192 ± 0.006 j–m | 0.143 ± 0.015 kl | 0.154 ± 0.005 no | 0.329 ± 0.002 e–g | 0.223 ± 0.006 ij | Blue |
| TUTGHGM26 | 0.213 ± 0.004 h–l | 0.154 ± 0.008 k | 0.206 ± 0.004 h–n | 0.216 ± 0.007 j–l | 0.320 ± 0.015 e–j | Yellow |
| TUTGHGM27 | 0.113 ± 0.002 o | 0.109 ± 0.008 mn | 0.218 ± 0.0004 h–m | 0.225 ± 0.003 j–l | 0.461 ± 0.014 bc | Blue |
| TUTGHGM28 | 0.384 ± 0.00001 cd | 0.232 ± 0.010 gh | 0.292 ± 0.002 de | 0.224 ± 0.007 j–l | 0.285 ± 0.018 f–j | Blue |
| TUTGHGM29 | 0.204 ± 0.003 i–m | 0.153 ± 0.005 k | 0.187 ± 0.008 k–o | 0.225 ± 0.006 j–l | 0.302 ± 0.032 e–j | Yellow |
| TUTGHGM30 | 0.240 ± 0.020 g–j | 0.248 ± 0.007 fg | 0.326 ± 0.005 d | 0.311 ± 0.003 ghi | 0.420 ± 0.005 b–d | Yellow |
| TUTGHGM31 | 0.123 ± 0.002 no | 0.111 ± 0.008 mn | 0.216 ± 0.0004 h–m | 0.215 ± 0.003 j–l | 0.461 ± 0.014 bc | Yellow |
| F-statistics | 50.01 ** | 268.21 ** | 36.60 ** | 26.49 ** | 8.23 *** | N/A |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mataboge, M.T.; Mohammed, M.; Dakora, F.D. Symbiotic N2 Fixation, Leaf Photosynthesis, and Abiotic Stress Tolerance of Native Rhizobia Isolated from Soybean Nodules at Da, Upper West Region, Ghana. Microorganisms 2025, 13, 876. https://doi.org/10.3390/microorganisms13040876
Mataboge MT, Mohammed M, Dakora FD. Symbiotic N2 Fixation, Leaf Photosynthesis, and Abiotic Stress Tolerance of Native Rhizobia Isolated from Soybean Nodules at Da, Upper West Region, Ghana. Microorganisms. 2025; 13(4):876. https://doi.org/10.3390/microorganisms13040876
Chicago/Turabian StyleMataboge, Mmatladi Tesia, Mustapha Mohammed, and Felix Dapare Dakora. 2025. "Symbiotic N2 Fixation, Leaf Photosynthesis, and Abiotic Stress Tolerance of Native Rhizobia Isolated from Soybean Nodules at Da, Upper West Region, Ghana" Microorganisms 13, no. 4: 876. https://doi.org/10.3390/microorganisms13040876
APA StyleMataboge, M. T., Mohammed, M., & Dakora, F. D. (2025). Symbiotic N2 Fixation, Leaf Photosynthesis, and Abiotic Stress Tolerance of Native Rhizobia Isolated from Soybean Nodules at Da, Upper West Region, Ghana. Microorganisms, 13(4), 876. https://doi.org/10.3390/microorganisms13040876






