Metabolites with Anti-Inflammatory Activities Isolated from the Mangrove Endophytic Fungus Dothiorella sp. ZJQQYZ-1
Abstract
1. Introduction
2. Materials and Methods
2.1. General Experimental Procedures
2.2. Fungal Material
2.3. Fermentation, Extraction and Isolation
2.4. Inhibitory Activity of NO Production Activity Assay
2.4.1. Cell Culture and Treatment
2.4.2. Cell Viability Assay
2.4.3. In Vitro Measurement of Nitric Oxide Production
2.4.4. Western Blotting
2.4.5. Statistical Analysis
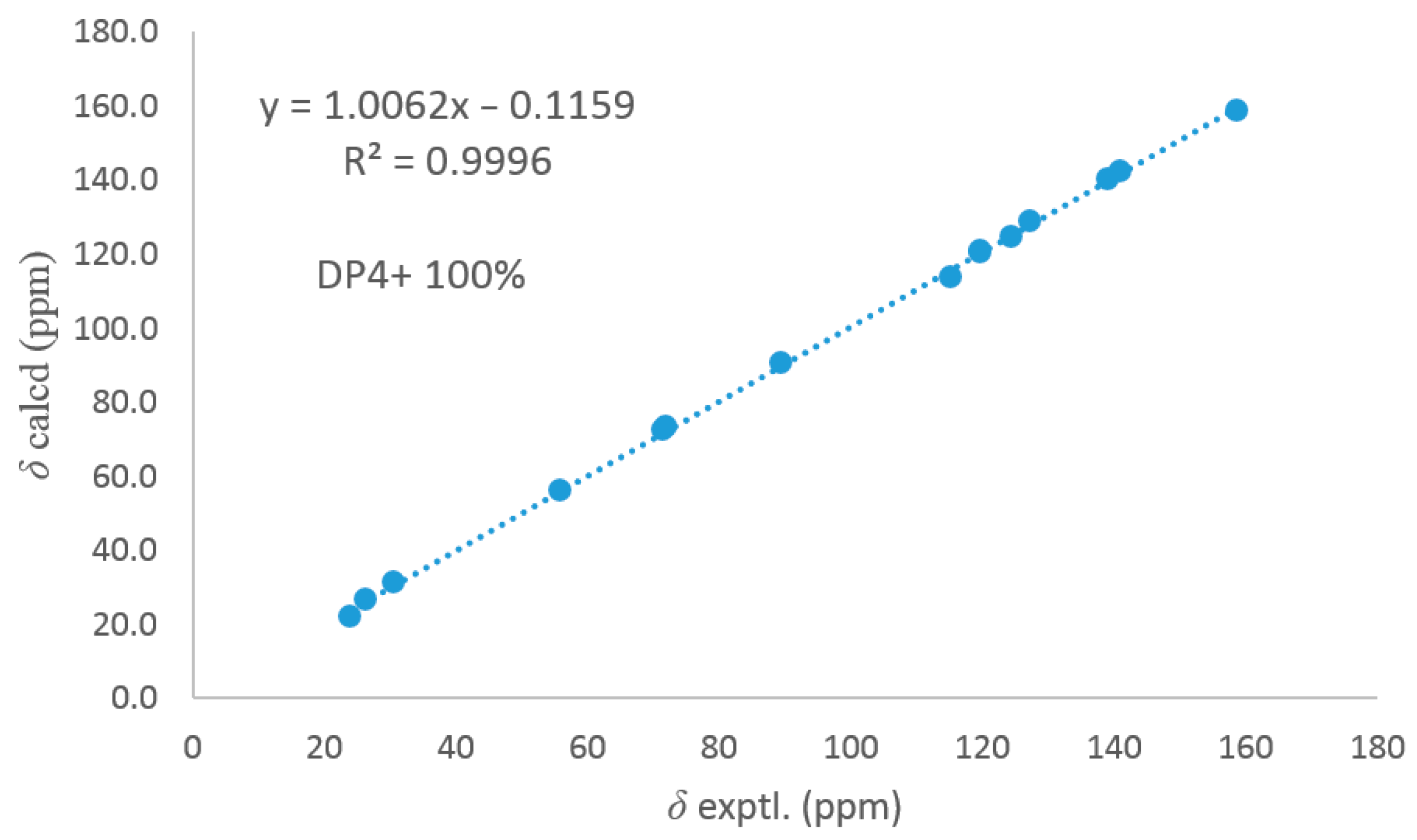
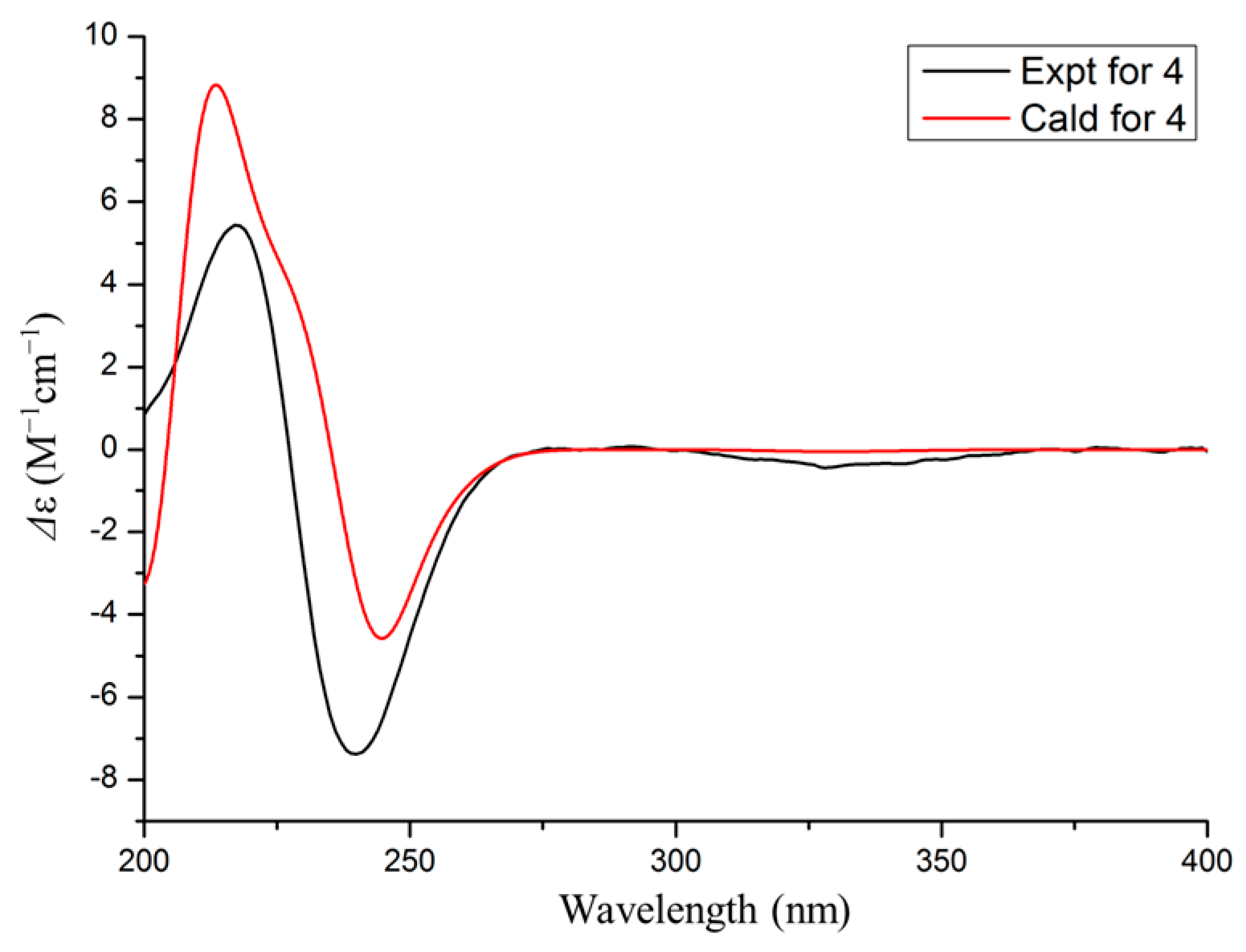
2.5. ECD Calculation and 13C NMR Calculation
3. Results
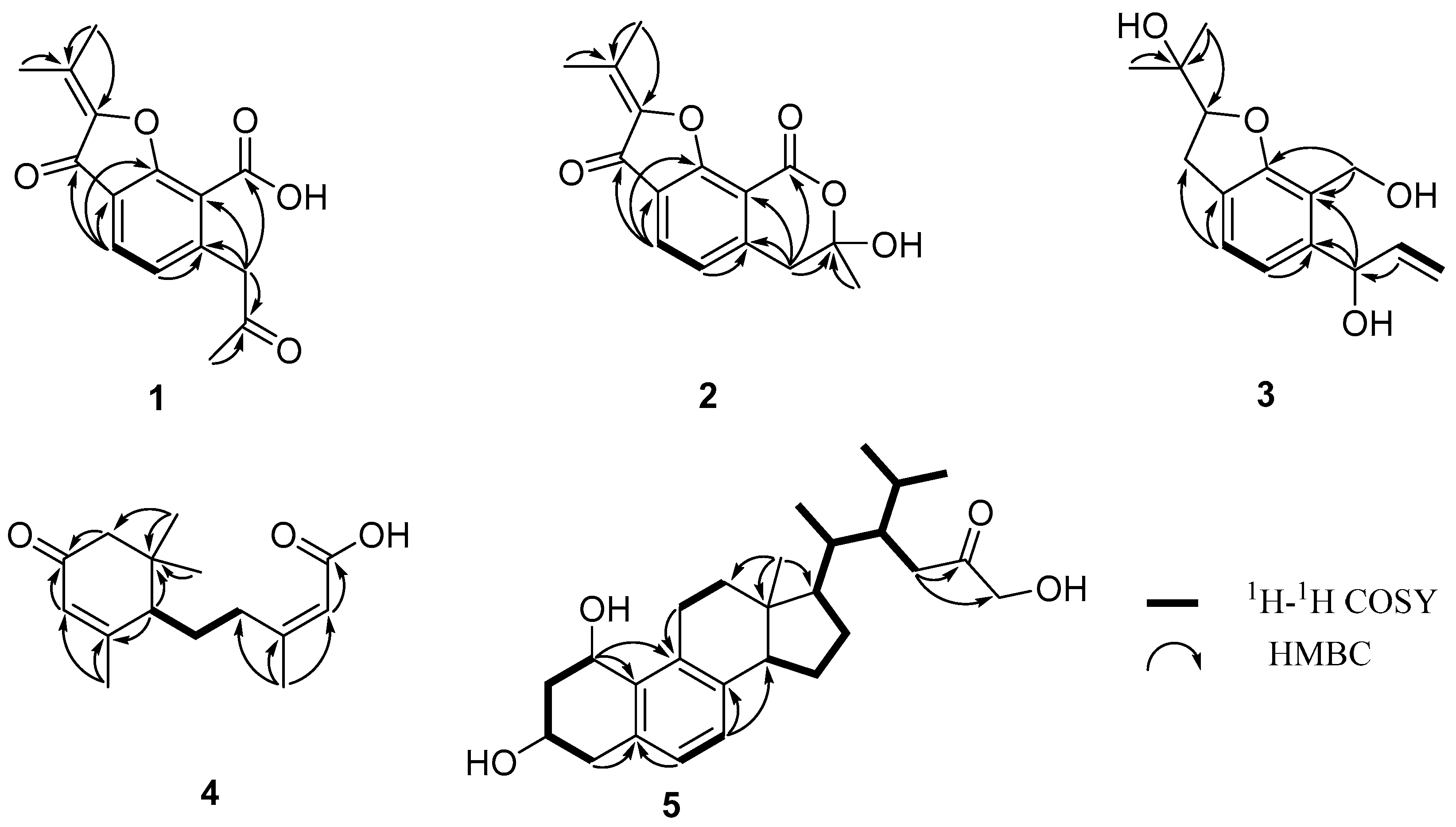
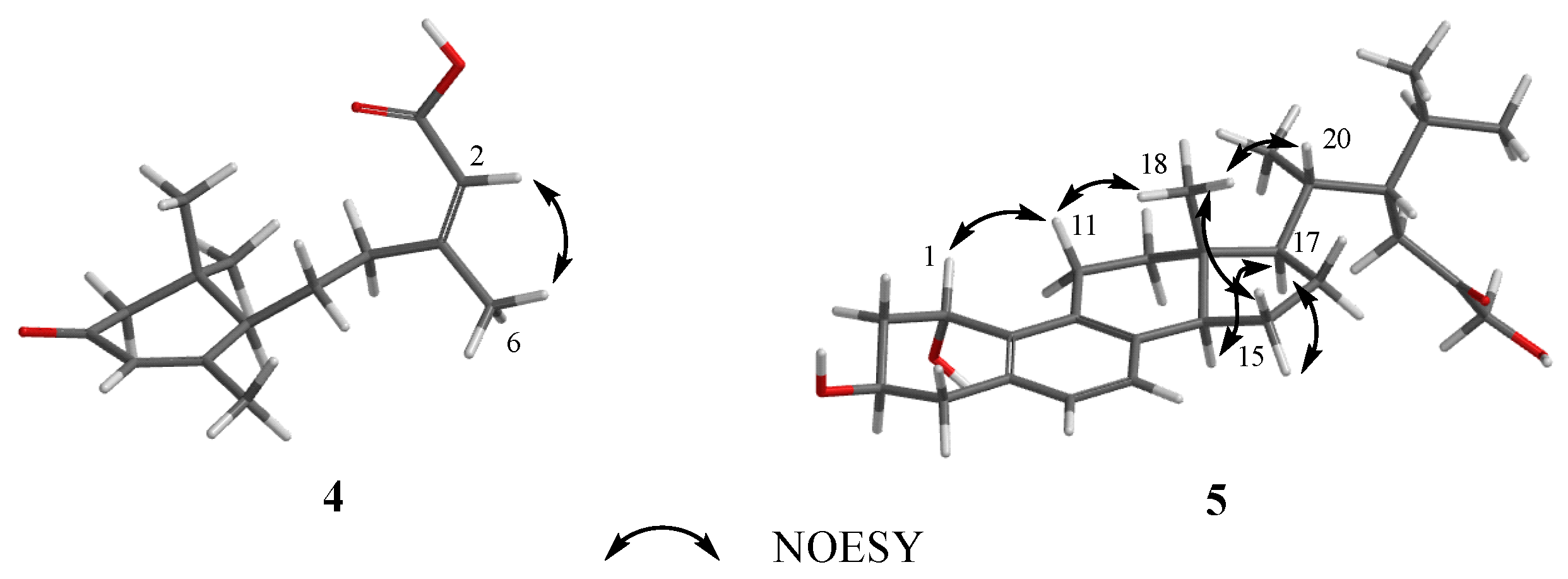
3.1. Structure Elucidation
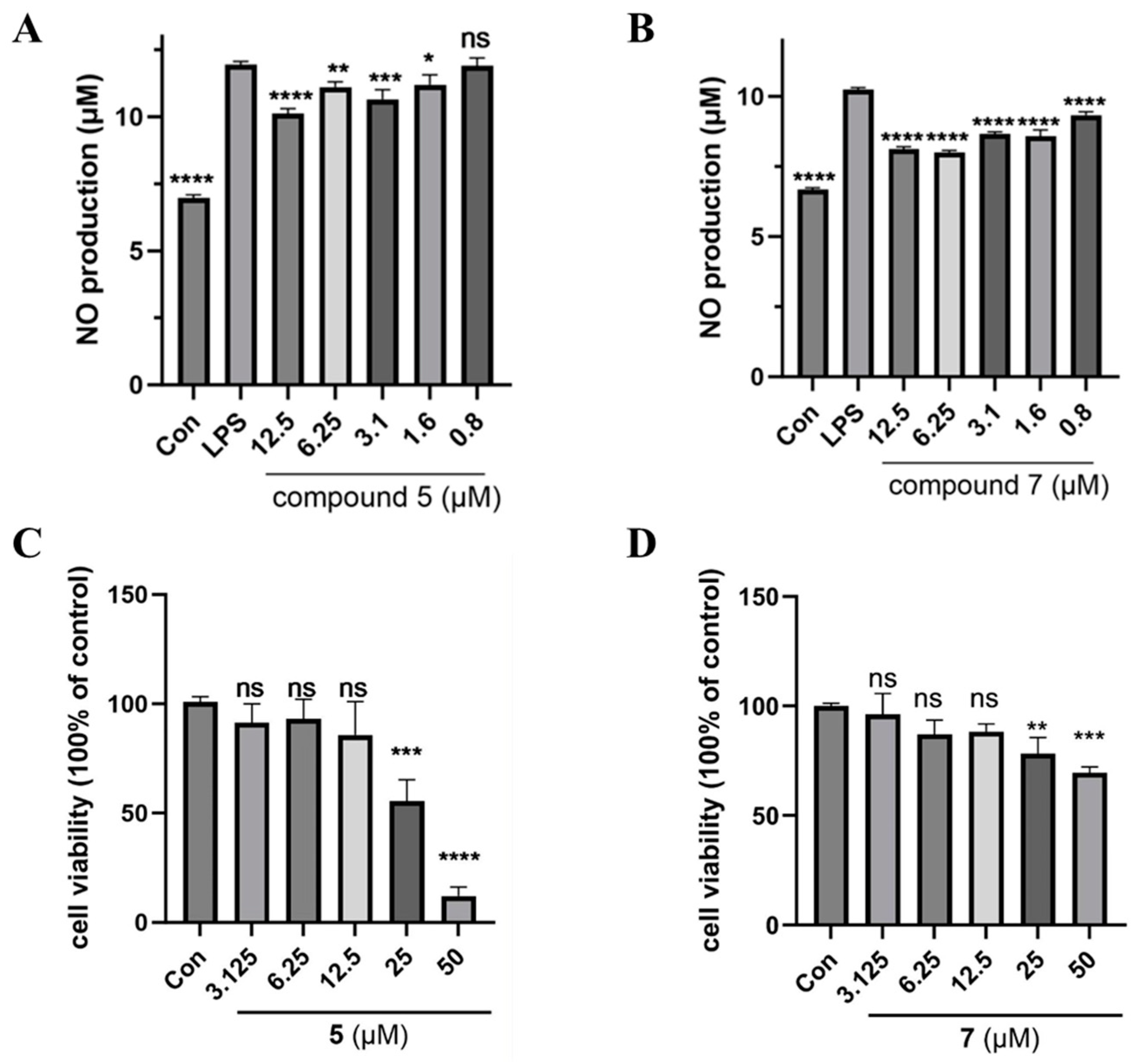
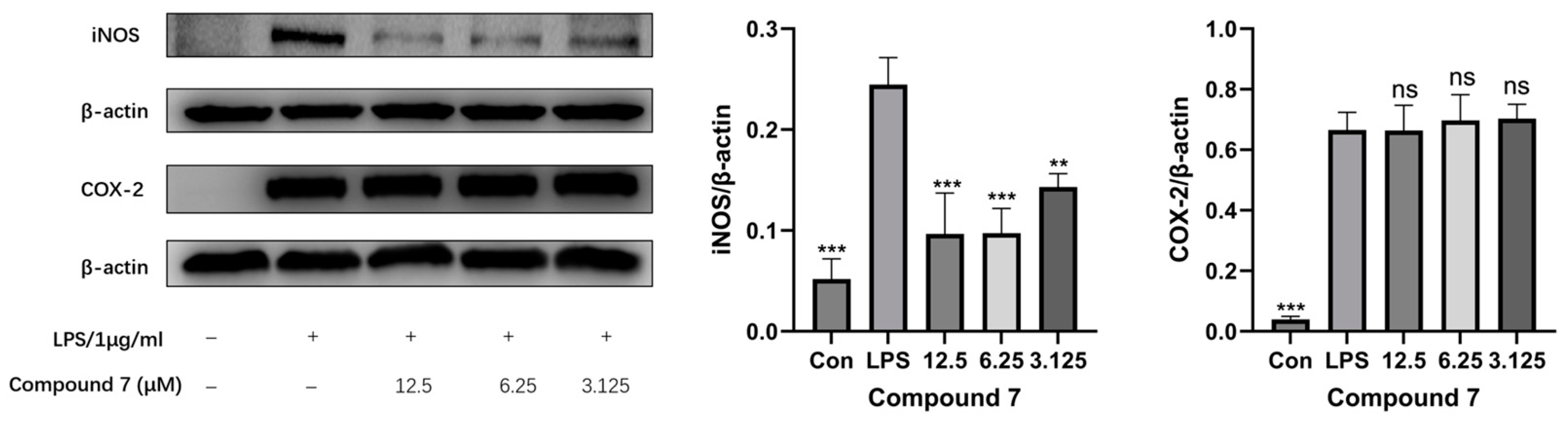
3.2. Anti-Inflammatory Screening of Extracts
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Farias, A.R.G.D.; Louangphan, J.; Afshari, N. Dothiorella saprophytica sp. nov.: A new Dothiorella sexual morph from Thailand. Phytotaxa 2023, 616, 149–159. [Google Scholar] [CrossRef]
- Du, X.P.; Su, W.J. Two New Polyketides from Mangrove Endophytic Fungus Dothiorella sp. Chem. Nat. Compd. 2014, 50, 214–216. [Google Scholar] [CrossRef]
- Dankyira, D.O.; Wang, B.; Cai, J.; Zeng, W.-N.; Huang, G.-L.; Zheng, C.-J. Antifungal Cytosporone Derivatives from the Mangrove-Derived Fungus Dothiorella sp. ML002. Chem. Nat. Compd. 2022, 58, 842–844. [Google Scholar] [CrossRef]
- Jiang, H.; Cai, R.; Zang, Z.; Yang, W.; Wang, B.; Zhu, G.; Yuan, J.; She, Z. Azaphilone derivatives with anti-inflammatory activity from the mangrove endophytic fungus Penicillium scleroti-orum ZJHJJ-18. Bioorg. Chem. 2022, 122, 105721. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Feng, Z.; Zhang, W.; Xu, J. Evaluation of the antimicrobial and cytotoxic potential of endophytic fungi extracts from mangrove plants Rhi-zophora stylosa and R. mucronata. Sci. Rep. 2022, 12, 2733. [Google Scholar]
- Law, J.W.; Law, L.N.-S.; Letchumanan, V.; Tan, L.T.-H.; Wong, S.H.; Chan, K.-G.; Ab Mutalib, N.-S.; Lee, L.-H. Anticancer Drug Discovery from Microbial Sources: The Unique Mangrove Streptomycetes. Molecules 2020, 25, 5365. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.Y.; Tao, L.-Y.; Liang, Y.-J.; Chen, L.-M.; Mi, Y.-J.; Zheng, L.-S.; Wang, F.; She, Z.-G.; Lin, Y.-C.; To, K.K.W.; et al. Anthracenedione derivatives as anticancer agents isolated from secondary metabolites of the mangrove endo-phytic fungi. Mar. Drugs 2010, 8, 1469–1481. [Google Scholar] [CrossRef]
- Li, K.L.; Dai, Y.; She, J.L.; Zeng, Y.B.; Dai, H.F.; Ou, S.; Zhou, X.; Liu, Y. Bisabolanoic acid A, a new polychiral sesquiterpene with AChE inhibitory activity from a mangrove-derived fungus Colletotrichum sp. J. Asian Nat. Prod. Res. 2022, 24, 88–95. [Google Scholar] [CrossRef]
- Christophersen, C.; Crescente, O.; Frisvad, J.C.; Gram, L.; Nielsen, J.; Nielsen, P.H.; Rahbæk, L. Antibacterial activity of marine-derived fungi. Mycopathologia 1998, 143, 135–138. [Google Scholar] [CrossRef]
- Wibowo, M.; Prachyawarakorn, V.; Aree, T.; Mahidol, C.; Ruchirawat, S.; Kittakoop, P. Cytotoxic sesquiterpenes from the endophytic fungus Pseudolagarobasidium acaciicola. Phytochemistry 2016, 122, 126–138. [Google Scholar] [CrossRef]
- Fu, G.; Wang, R.; Ding, J.; Qi, H.; Zhao, Z.; Chen, C.; Zhang, H.; Xue, Z.; Wang, J.; Wu, M. Micromonospora zhangzhouensis sp. nov., a Novel Actinobacterium Isolated from Mangrove Soil, Exerts a Cytotoxic Activity in vitro. Sci. Rep. 2020, 10, 3889. [Google Scholar] [CrossRef] [PubMed]
- Yan, Z.; Huang, C.; Guo, H.; Zheng, S.; He, J.; Lin, J.; Long, Y. Isobenzofuranone monomer and dimer derivatives from the mangrove endophytic fungus Epicoccum nigrum SCNU-F0002 possess α-glucosidase inhibitory and antioxidant activity. Bioorg. Chem. 2020, 94, 103407. [Google Scholar] [CrossRef]
- Liu, Y.; Xia, G.; Li, H.; Ma, L.; Ding, B.; Lu, Y.; He, L.; Xia, X.; She, Z. Vermistatin derivatives with α-glucosidase inhibitory activity from the mangrove endophytic fungus Penicillium sp. HN29-3B1. Planta Med. 2014, 80, 912–917. [Google Scholar] [CrossRef] [PubMed]
- Premanathan, M.; Arakaki, R.; Izumi, H.; Kathiresan, K.; Nakano, M.; Yamamoto, N.; Nakashima, H. Antiviral properties of a mangrove plant, Rhizophora apiculata Blume, against human immunodeficiency virus. Antivir. Res. 1999, 44, 113–122. [Google Scholar] [CrossRef]
- Dinarello, C.A. Anti-inflammatory Agents: Present and Future. Cell 2010, 140, 935–950. [Google Scholar] [CrossRef]
- Nguyen, T.-H.; Do, T.-H.; Tran, V.T.-H.; Nguyen, H.-A.; Pham, D.-V. Anti-inflammatory effect of a triterpenoid from Balanophora laxiflora: Results of bioactivity-guided isolation. Heliyon 2022, 8, e09070. [Google Scholar] [CrossRef]
- Gautam, R.; Jachak, S.M. Recent developments in anti-inflammatory natural products. Med. Res. Rev. 2009, 29, 767–820. [Google Scholar] [CrossRef]
- Newman, D.J.; Cragg, G.M. Natural Products as Sources of New Drugs over the Nearly Four Decades from 01/1981 to 09/2019. J. Nat. Prod. 2020, 83, 770–803. [Google Scholar] [CrossRef] [PubMed]
- Bai, R.; Yao, C.; Zhong, Z.; Ge, J.; Bai, Z.; Ye, X.; Xie, T.; Xie, Y. Discovery of natural anti-inflammatory alkaloids: Potential leads for the drug discovery for the treatment of inflammation. Eur. J. Med. Chem. 2021, 213, 113165. [Google Scholar] [CrossRef]
- Ge, J.; Liu, Z.; Zhong, Z.; Wang, L.; Zhuo, X.; Li, J.; Jiang, X.; Ye, X.-Y.; Xie, T.; Bai, R. Natural terpenoids with anti-inflammatory activities: Potential leads for anti-inflammatory drug discovery. Bioorg. Chem. 2022, 124, 105817. [Google Scholar] [CrossRef]
- Passos, F.R.S.; Araújo-Filho, H.G.; Monteiro, B.S.; Shanmugam, S.; Araújo, A.A.d.S.; Almeida, J.R.G.d.S.; Thangaraj, P.; Júnior, L.J.Q.; Quintans, J.d.S.S. Anti-inflammatory and modulatory effects of steroidal saponins and sapogenins on cytokines: A review of pre-clinical research. Phytomedicine 2022, 96, 153842. [Google Scholar] [CrossRef] [PubMed]
- Yuan, D.; Li, C.; Huang, Q.; Fu, X.; Dong, H. Current advances in the anti-inflammatory effects and mechanisms of natural polysaccharides. Crit. Rev. Food Sci. Nutr. 2023, 63, 5890–5910. [Google Scholar]
- Chen, Y.; Yang, W.C.; Long, Y.H.; She, Z.G. Metabolites with anti-inflammatory and a-glucosidase inhibitory activities from the mangrove endophytic fungus Phoma sp. SYSU-SK-7. Tetrahedron Lett. 2020, 61, 152578. [Google Scholar] [CrossRef]
- Lu, Z.Y.; Wang, Y.; Miao, C.; Liu, P.; Hong, K.; Zhu, W.M. Sesquiterpenoids and Benzofuranoids from the Marine-Derived Fungus Aspergillus ustus 094102. J. Nat. Prod. 2009, 72, 1761–1767. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Sun, D.; Xiong, L.; Zhang, Z.; Li, Y.; Liu, K.; Li, H.; Chen, L. Phenolics and terpenoids with good anti-inflammatory activity from the fruits of Amomum villosum and the anti-inflammatory mechanism of active diterpene. Bioorg. Chem. 2024, 145, 107190. [Google Scholar] [CrossRef]
- Wang, Q.; Wu, Z.; Li, C.; Qin, G.; Hu, X.; Guo, P.; Ding, A.; Xu, W.; Wang, W.; Xuan, L. Haperforatones A-M, thirteen undescribed limonoids from Harrisonia perforata with anti-inflammatory activity. Bioorg. Chem. 2024, 151, 107631. [Google Scholar] [CrossRef]
- Chen, Y.; Yang, W.C.; She, Z.G. Didymorenloids A and B, two polycyclic cyclopenta[b]fluorene-type alkaloids with anti-hepatoma activity from the mangrove endophytic fungus Didymella sp. CYSK-4. Org. Chem. Front. 2024, 11, 1706. [Google Scholar] [CrossRef]
- Autschbach, J. Computing chiroptical properties with first-principles theoretical methods: Background and illustrative examples. Chirality 2009, 21, E116–E152. [Google Scholar] [CrossRef]
- Pescitelli, G.; Kurtán, T.; Flörke, U.; Krohn, K. Absolute structural elucidation of natural products—A focus on quantum-mechanical calculations of solid-state CD spectra. Chirality 2009, 21, E181–E201. [Google Scholar] [CrossRef]
- Wen, S.T.; Fan, W.L.; Guo, H.X.; Huang, C.Y.; Yan, Z.Y.; Long, Y.H. Two new secondary metabolites from the mangrove endophytic fungus Pleosporales sp. SK7. Nat. Prod. Res. 2020, 34, 2919–2925. [Google Scholar] [CrossRef]
- Wang, L.; Jiang, Y.; Yaseen, A.; Li, F.; Chen, B.; Shen, X.F.; Zheng, C.; Zhang, G.; Wang, M. Steroidal alkaloids from the bulbs of Fritillaria pallidiflora Schrenk and their anti-inflammatory activity—ScienceDirect. Bioorg. Chem. 2021, 112, 104845. [Google Scholar] [CrossRef] [PubMed]
- Cai, P.; Smith, D. Cunningham, B. Phycomysterols and Other Sterols from the Fungus Phycomyces blakesleeanus. J. Nat. Prod. 1998, 61, 791–795. [Google Scholar] [CrossRef] [PubMed]
- Qin, X.D.; Liu, J.K. Natural Aromatic Steroids as Potential Molecular Fossils from the Fruiting Bodies of the Ascomycete Daldinia concentrica. J. Nat. Prod. 2004, 67, 2133–2135. [Google Scholar] [CrossRef]
- Li, G.; Kusari, S.; Kusari, P.; Kayser, O.; Spiteller, M. Endophytic Diaporthe sp. LG23 Produces a Potent Antibacterial Tetracyclic Triterpenoid. J. Nat. Prod. 2015, 78, 2128–2132. [Google Scholar] [CrossRef] [PubMed]


| 1 | 2 | 3 | 4 | ||||||
|---|---|---|---|---|---|---|---|---|---|
| No. | δH a (J in Hz) | δC a | δH b (J in Hz) | δC b | δH c (J in Hz) | δC c | No. | δH c (J in Hz) | δC c |
| 2 | 144.5 | 145.2 | 4.58, t (8.9) | 89.4 | 1 | 170.3 | |||
| 3α | 182.3 | 182.5 | 3.10, dd (9.5, 15.7) | 30.5 | 2 | 5.71, s | 115.3 | ||
| 3β | 3.20, dd (8.4, 15.7) | 3 | 161.8 | ||||||
| 3a | 123.0 | 123.9 | 127.2 | 4 | 2.26, m | 40.7 | |||
| 4 | 7.77, d (7.8) | 126.2 | 7.88, d (7.8) | 129.5 | 7.05, d (7.5) | 124.4 | 5α | 1.58, m | 28.0 |
| 5 | 7.11, d (7.8) | 127.1 | 7.09, d (7.8) | 122.4 | 6.84, d (7.5) | 119.8 | 5β | 1.88, m | |
| 5a | 118.4 | 109.9 | 140.9 | 6 | 2.18, s | 19.2 | |||
| 6 | 4.16, s | 49.1 | 3.26, d (16.9) | 39.4 | 5.39, s | 72.0 | 1′ | 1.89, m | 50.6 |
| 3.37, d (16.9) | 2′ | 36.3 | |||||||
| 7 | 204.7 | 104.9 | 6.12, m | 139.0 | 3′α | 2.07, d (17.4) | 47.0 | ||
| 9α | 166.0 | 163.9 | 4.65, d (12.0) | 55.8 | 3′β | 2.37, d (17.4) | |||
| 9β | 4.78, d (12.0) | 4′ | 199.1 | ||||||
| 9a | 145.2 | 147.6 | 119.7 | 5′ | 5.86, s | 125.4 | |||
| 9b | 162.3 | 161.0 | 158.7 | 6′ | 164.5 | ||||
| 10α | 2.18, s | 30.3 | 1.70, s | 22.0 | 5.26, d (10.5) | 115.2 | 7′ | 2.0, s | 24.6 |
| 10β | 5.37, d (9.1) | 8′ | 1.08, s | 27.1 | |||||
| 11 | 133.5 | 135.8 | 71.6 | 9’ | 1.04, s | 28.7 | |||
| 12 | 2.32, s | 17.3 | 2.37, s | 17.0 | 1.15, s | 23.9 | |||
| 13 | 2.09, s | 20.3 | 2.22, s | 20.0 | 1.36, s | 26.5 | |||
| 5 | 5 | ||||
|---|---|---|---|---|---|
| No. | δH (J in Hz) | δC | No. | δH (J in Hz) | δC |
| 1 | 5.04, m | 65.2 | 14 | 2.68, dd (8.0, 11.3) | 51.5 |
| 2α | 1.74, m | 41.4 | 15α | 2.08, m | 23.6 |
| 2β | 2.29, m | 15β | 1.56, m | ||
| 3 | 4.26, m | 62.5 | 16α | 2.15, m | 28.5 |
| 4α | 3.11, dd (4.8, 15.3) | 38.9 | 16β | 1.49, m | |
| 4β | 2.59, dd (10.8, 15.3) | 17 | 1.42, m | 53.1 | |
| 5 | 133.3 | 18 | 0.60, s | 10.6 | |
| 6 | 6.92, d (7.9) | 125.4 | 20 | 1.88, m | 36.1 |
| 7 | 6.90, d (7.9) | 126.3 | 21 | 0.93, d (6.7) | 20.0 |
| 8 | 138.2 | 22 | 2.02, m | 41.3 | |
| 9 | 135.5 | 23 | 1.55, m | 30.9 | |
| 10 | 132.3 | 24 | 0.95, d (6.6) | 12.7 | |
| 11a | 2.94, m | 23.1 | 25 | 0.81, d (6.6) | 20.7 |
| 11β | 3.17, dd (7.2, 18.0) | 26α | 2.40, m | 36.8 | |
| 12α | 2.19, m | 36.8 | 27 | 211.0 | |
| 12β | 1.75, m | 28 | 4.27, m | 67.4 | |
| 13 | 42.0 | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Z.; Zhu, J.; Mu, R.; Wang, C.; Sun, Y.; Qian, B.; Li, N.; Chen, Y. Metabolites with Anti-Inflammatory Activities Isolated from the Mangrove Endophytic Fungus Dothiorella sp. ZJQQYZ-1. Microorganisms 2025, 13, 890. https://doi.org/10.3390/microorganisms13040890
Li Z, Zhu J, Mu R, Wang C, Sun Y, Qian B, Li N, Chen Y. Metabolites with Anti-Inflammatory Activities Isolated from the Mangrove Endophytic Fungus Dothiorella sp. ZJQQYZ-1. Microorganisms. 2025; 13(4):890. https://doi.org/10.3390/microorganisms13040890
Chicago/Turabian StyleLi, Zhaokun, Junhao Zhu, Ruxue Mu, Chenxi Wang, Yuru Sun, Bingbing Qian, Ning Li, and Yan Chen. 2025. "Metabolites with Anti-Inflammatory Activities Isolated from the Mangrove Endophytic Fungus Dothiorella sp. ZJQQYZ-1" Microorganisms 13, no. 4: 890. https://doi.org/10.3390/microorganisms13040890
APA StyleLi, Z., Zhu, J., Mu, R., Wang, C., Sun, Y., Qian, B., Li, N., & Chen, Y. (2025). Metabolites with Anti-Inflammatory Activities Isolated from the Mangrove Endophytic Fungus Dothiorella sp. ZJQQYZ-1. Microorganisms, 13(4), 890. https://doi.org/10.3390/microorganisms13040890







