The Microbiota–Human Health Axis
Abstract
:1. Introduction
2. Materials and Methods
3. The Multisystem Impact of Microbiota
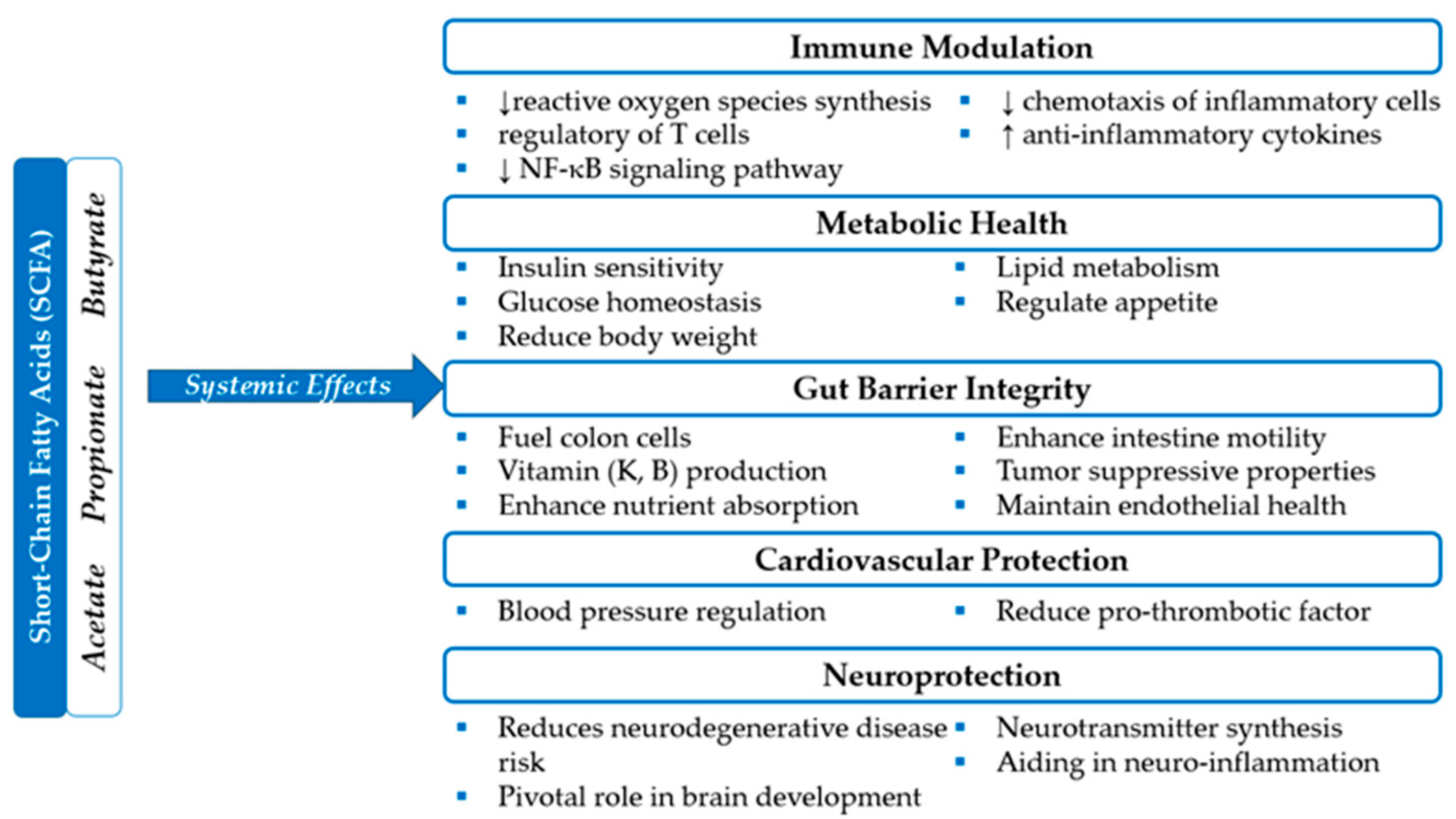
3.1. Digestive System and Metabolism
3.2. Integumentary System
3.3. Respiratory System
3.3.1. Upper Respiratory Tract Microbiota
3.3.2. Lower Respiratory Tract Microbiota
3.4. Urinary and Reproductive Systems
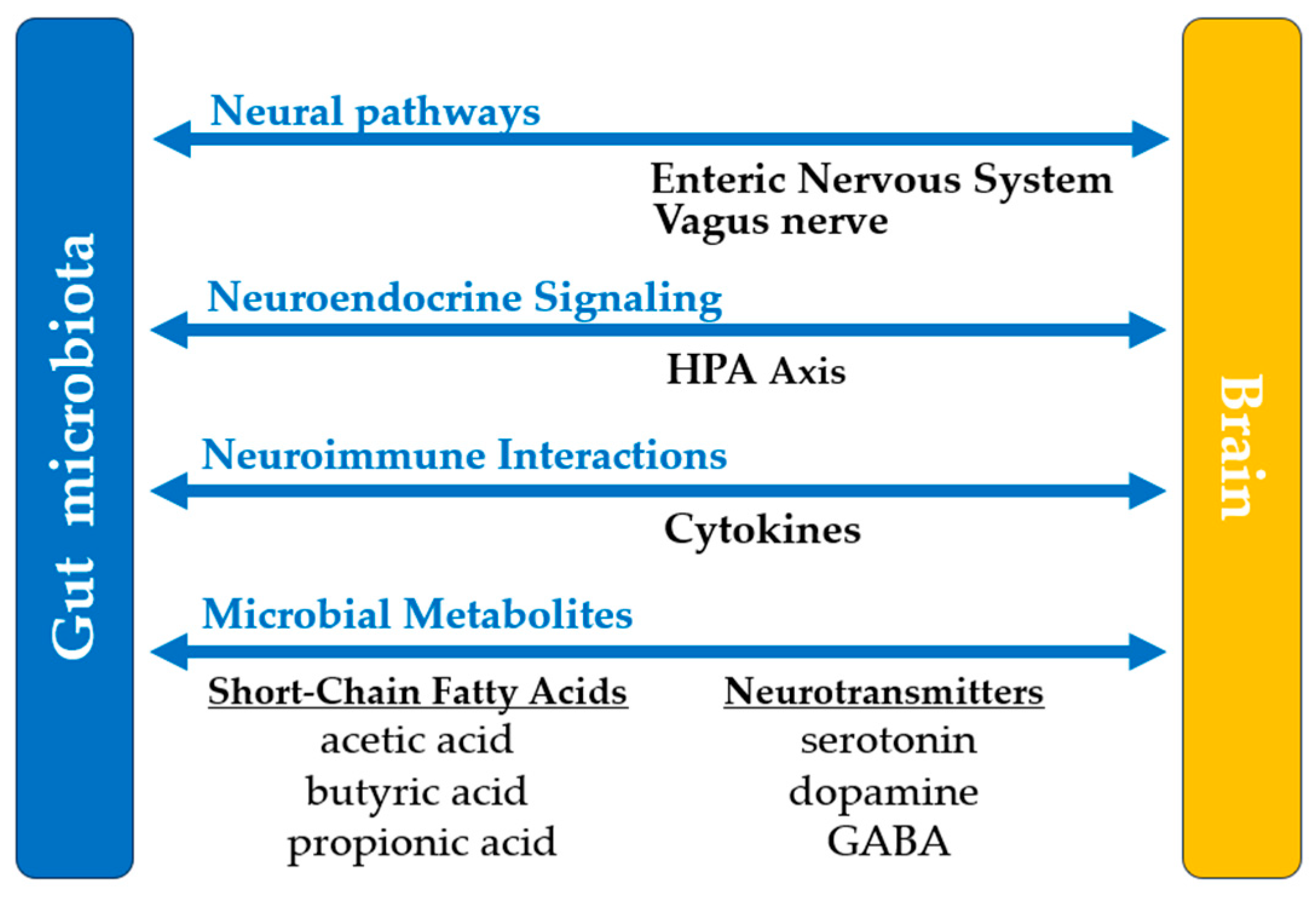
3.5. Central Nervous System
3.6. Cardiovascular System
3.7. Endocrine Function
3.8. Immune System
3.9. Microbiota—A New Frontier in Diagnostic and Treatment
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- FAQ. FAQ: Human Microbiome; American Society for Microbiology: Washington, DC, USA, 2013. Available online: https://www.ncbi.nlm.nih.gov/books/NBK562894/ (accessed on 15 March 2025).
- El-Sayed, A.; Aleya, L.; Kamel, M. Microbiota’s Role in Health and Diseases. Environ. Sci. Pollut. Res. Int. 2021, 28, 36967–36983. [Google Scholar] [CrossRef] [PubMed]
- Wen, L.; Duffy, A. Factors Influencing the Gut Microbiota, Inflammation, and Type 2 Diabetes. J. Nutr. 2017, 147, 1468S–1475S. [Google Scholar] [CrossRef] [PubMed]
- Gomaa, E.Z. Human Gut Microbiota/Microbiome in Health and Diseases: A Review. Antonie Van Leeuwenhoek 2020, 113, 2019–2040. [Google Scholar] [CrossRef]
- Man, W.H.; de Steenhuijsen Piters, W.A.; Bogaert, D. The Microbiota of the Respiratory Tract: Gatekeeper to Respiratory Health. Nat. Rev. Microbiol. 2017, 15, 259–270. [Google Scholar] [CrossRef] [PubMed]
- Lu, L.; Chen, X.; Liu, Y.; Yu, X. Gut Microbiota and Bone Metabolism. FASEB J. 2021, 35, e21740. [Google Scholar] [CrossRef]
- Portincasa, P.; Bonfrate, L.; Vacca, M.; De Angelis, M.; Farella, I.; Lanza, E.; Khalil, M.; Wang, D.Q.; Sperandio, M.; Di Ciaula, A. Gut Microbiota and Short Chain Fatty Acids: Implications in Glucose Homeostasis. Int. J. Mol. Sci. 2022, 23, 1105. [Google Scholar] [CrossRef]
- Jandhyala, S.M.; Talukdar, R.; Subramanyam, C.; Vuyyuru, H.; Sasikala, M.; Nageshwar Reddy, D. Role of the Normal Gut Microbiota. World J. Gastroenterol. 2015, 21, 8787–8803. [Google Scholar] [CrossRef]
- James, K.R.; Gomes, T.; Elmentaite, R.; Kumar, N.; Gulliver, E.L.; King, H.W.; Stares, M.D.; Bareham, B.R.; Ferdinand, J.R.; Petrova, V.N.; et al. Distinct Microbial and Immune Niches of the Human Colon. Nat. Immunol. 2020, 21, 343–353. [Google Scholar] [CrossRef]
- Koh, A.; De Vadder, F.; Kovatcheva-Datchary, P.; Bäckhed, F. From Dietary Fiber to Host Physiology: Short-Chain Fatty Acids as Key Bacterial Metabolites. Cell 2016, 165, 1332–1345. [Google Scholar] [CrossRef]
- Fusco, W.; Lorenzo, M.B.; Cintoni, M.; Porcari, S.; Rinninella, E.; Kaitsas, F.; Lener, E.; Mele, M.C.; Gasbarrini, A.; Collado, M.C.; et al. Short-Chain Fatty-Acid-Producing Bacteria: Key Components of the Human Gut Microbiota. Nutrients 2023, 15, 2211. [Google Scholar] [CrossRef]
- Hodgkinson, K.; El Abbar, F.; Dobranowski, P.; Manoogian, J.; Butcher, J.; Figeys, D.; Mack, D.; Stintzi, A. Butyrate’s role in human health and the current progress towards its clinical application to treat gastrointestinal disease. Clin. Nutr. 2023, 42, 61–75. [Google Scholar] [CrossRef] [PubMed]
- Louis, P.; Hold, G.L.; Flint, H.J. The Gut Microbiota, Bacterial Metabolites, and Colorectal Cancer. Nat. Rev. Microbiol. 2014, 12, 661–672. [Google Scholar] [CrossRef]
- Patrick, S. A tale of two habitats: Bacteroides fragilis, a lethal pathogen and resident in the human gastrointestinal microbiome. Microbiology 2022, 168, 001156. [Google Scholar] [CrossRef] [PubMed]
- Coletto, E.; Latousakis, D.; Pontifex, M.G.; Crost, E.H.; Vaux, L.; Perez Santamarina, E.; Goldson, A.; Brion, A.; Hajihosseini, M.K.; Vauzour, D.; et al. The role of the mucin-glycan foraging Ruminococcus gnavus in the communication between the gut and the brain. Gut Microbes 2022, 14, 2073784. [Google Scholar] [CrossRef]
- Collins, S.L.; Patterson, A.D. The Gut Microbiome: An Orchestrator of Xenobiotic Metabolism. Acta Pharm. Sin. B 2020, 10, 19–32. [Google Scholar] [CrossRef] [PubMed]
- Bartlett, A.; Kleiner, M. Dietary protein and the intestinal microbiota: An understudied relationship. iScience 2022, 25, 105313. [Google Scholar] [CrossRef]
- Tarracchini, C.; Lugli Gabriele, A.; Mancabelli, L.; van Sinderen, D.; Turroni, F.; Ventura, M.; Milani, C. Exploring the vitamin biosynthesis landscape of the human gut microbiota. mSystems 2024, 9, e00929-24. [Google Scholar] [CrossRef]
- Ríos-Covián, D.; Ruas-Madiedo, P.; Margolles, A.; Gueimonde, M.; de Los Reyes-Gavilán, C.G.; Salazar, N. Intestinal Short-Chain Fatty Acids and Their Link with Diet and Human Health. Front. Microbiol. 2016, 7, 185. [Google Scholar] [CrossRef]
- Ridlon, J.M.; Kang, D.J.; Hylemon, P.B. Bile salt biotransformations by human intestinal bacteria. J. Lipid Res. 2016, 47, 241–259. [Google Scholar] [CrossRef]
- Morales-Sánchez, A.; Fuentes-Pananá, E.M. Human Viruses and Cancer. Viruses 2014, 6, 4047–4049. [Google Scholar] [CrossRef]
- Brown, E.M.; Clardy, J.; Xavier, R.J. Gut microbiome lipid metabolism and its impact on host physiology. Cell Host Microbe 2023, 31, 173–186. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Chiang, J.Y.L. Bile acid signaling in metabolic disease and drug therapy. Pharmacol. Rev. 2014, 66, 948–983. [Google Scholar] [CrossRef] [PubMed]
- Arora, T.; Vanslette, A.M.; Hjorth, S.A.; Bäckhed, F. Microbial regulation of enteroendocrine cells. Med 2021, 2, 553–570. [Google Scholar] [CrossRef]
- Zhou, Z.; Sun, B.; Yu, D.; Zhu, C. Gut Microbiota: An Important Player in Type 2 Diabetes Mellitus. Front. Cell. Infect. Microbiol. 2022, 12, 834485. [Google Scholar] [CrossRef]
- DeGruttola, A.K.; Low, D.; Mizoguchi, A.; Mizoguchi, E. Current Understanding of Dysbiosis in Disease in Human and Animal Models. Inflamm. Bowel Dis. 2016, 22, 1137–1150. [Google Scholar] [CrossRef]
- Zhang, K.; Zhang, Q.; Qiu, H.; Ma, Y.; Hou, N.; Zhang, J.; Kan, C.; Han, F.; Sun, X.; Shi, J. The complex link between the gut microbiome and obesity-associated metabolic disorders: Mechanisms and therapeutic opportunities. Heliyon 2024, 10, e37609. [Google Scholar] [CrossRef]
- Ebrahimzadeh Leylabadlo, H.; Sanaie, S.; Sadeghpour Heravi, F.; Ahmadian, Z.; Ghotaslou, R. From role of gut microbiota to microbial-based therapies in type 2-diabetes. Infect. Genet. Evol. 2020, 81, 104268. [Google Scholar] [CrossRef] [PubMed]
- Lopez, L.R.; Bleich, R.M.; Arthur, J.C. Microbiota Effects on Carcinogenesis: Initiation, Promotion, and Progression. Annu. Rev. Med. 2021, 72, 243–261. [Google Scholar] [CrossRef]
- Scheithauer, T.P.M.; Rampanelli, E.; Nieuwdorp, M.; Vallance, B.A.; Verchere, C.B.; van Raalte, D.H.; Herrema, H. Gut Microbiota as a Trigger for Metabolic Inflammation in Obesity and Type 2 Diabetes. Front. Immunol. 2020, 11, 571731. [Google Scholar] [CrossRef]
- American Society for Microbiology. Fecal Microbiota Transplants (FMT): Past, Present and Future. Available online: https://asm.org/articles/2024/february/fecal-microbiota-transplants-past-present-future (accessed on 15 March 2025).
- Reddi, S.; Senyshyn, L.; Ebadi, M.; Podlesny, D.; Minot, S.S.; Gooley, T.; Kabage, A.J.; Hill, G.R.; Lee, S.J.; Khoruts, A.; et al. Fecal microbiota transplantation to prevent acute graft-versus-host disease: Pre-planned interim analysis of donor effect. Nat. Commun. 2025, 16, 1034. [Google Scholar] [CrossRef]
- Drwiega, E.N.; Nascimento, D.C. A Comparison of Currently Available and Investigational Fecal Microbiota Transplant Products for Recurrent Clostridioides difficile Infection. Antibiotics 2024, 13, 436. [Google Scholar] [CrossRef] [PubMed]
- Carmona-Cruz, S.; Orozco-Covarrubias, L.; Sáez-de-Ocariz, M. The Human Skin Microbiome in Selected Cutaneous Diseases. Front. Cell. Infect. Microbiol. 2022, 12, 834135. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Qu, L.; Mijakovic, I.; Wei, Y. Advances in the human skin microbiota and its roles in cutaneous diseases. Microb. Cell Factories 2022, 21, 176. [Google Scholar] [CrossRef]
- Oh, J.; Byrd, A.L.; Park, M.; Kong, H.H.; Segre, J.A. Temporal Stability of the Human Skin Microbiome. Cell 2016, 165, 854–866. [Google Scholar] [CrossRef]
- Sinha, S.; Lin, G.; Ferenczi, K. The skin microbiome and the gut-skin axis. Clin. Dermatol. 2021, 39, 829–839. [Google Scholar] [CrossRef] [PubMed]
- Kengmo Tchoupa, A.; Kretschmer, D.; Schittek, B.; Peschel, A. The epidermal lipid barrier in microbiome-skin interaction. Trends Microbiol. 2023, 31, 723–734. [Google Scholar] [CrossRef]
- Lee, H.J.; Kim, M. Skin Barrier Function and the Microbiome. Int. J. Mol. Sci. 2022, 23, 13071. [Google Scholar] [CrossRef] [PubMed]
- Swaney, M.H.; Nelsen, A.; Sandstrom, S.; Kalan, L.R. Sweat and Sebum Preferences of the Human Skin Microbiota. Microbiol. Spectr. 2023, 11, e0418022. [Google Scholar] [CrossRef]
- Smythe, P.; Wilkinson, H.N. The Skin Microbiome: Current Landscape and Future Opportunities. Int. J. Mol. Sci. 2023, 24, 3950. [Google Scholar] [CrossRef]
- Lunjani, N.; Ahearn-Ford, S.; Dube, F.S.; Hlela, C.; O’Mahony, L. Mechanisms of microbe-immune system dialogue within the skin. Genes Immun. 2021, 22, 276–288. [Google Scholar] [CrossRef]
- Ying, S.; Zeng, D.N.; Chi, L.; Tan, Y.; Galzote, C.; Cardona, C.; Lax, S.; Gilbert, J.; Quan, Z.X. The Influence of Age and Gender on Skin-Associated Microbial Communities in Urban and Rural Human Populations. PLoS ONE 2015, 10, e0141842. [Google Scholar] [CrossRef] [PubMed]
- Boxberger, M.; Cenizo, V.; Cassir, N.; La Scola, B. Challenges in exploring and manipulating the human skin microbiome. Microbiome 2021, 9, 125. [Google Scholar] [CrossRef] [PubMed]
- Leung, M.H.Y.; Tong, X.; Lee, P.K.H. Indoor Microbiome and Airborne Pathogens. Compr. Biotechnol. 2019, 31, 96–106. [Google Scholar] [CrossRef]
- Hayleeyesus, S.F.; Manaye, A.M. Microbiological quality of indoor air in university libraries. Asian Pac. J. Trop. Biomed. 2014, 4 (Suppl. S1), S312–S317. [Google Scholar] [CrossRef]
- Flowers, L.; Grice, E.A. The Skin Microbiota: Balancing Risk and Reward. Cell Host Microbe 2020, 28, 190–200. [Google Scholar] [CrossRef]
- Gallo, R.L. S. epidermidis influence on host immunity: More than skin deep. Cell Host Microbe 2015, 17, 143–144. [Google Scholar] [CrossRef]
- Christensen, G.J.; Brüggemann, H. Bacterial skin commensals and their role as host guardians. Benef. Microbes 2014, 5, 201–215. [Google Scholar] [CrossRef]
- Severn, M.M.; Horswill, A.R. Staphylococcus epidermidis and its dual lifestyle in skin health and infection. Nat. Rev. Microbiol. 2023, 21, 97–111. [Google Scholar] [CrossRef]
- Gagliardi, A.; Totino, V.; Cacciotti, F.; Iebba, V.; Neroni, B.; Bonfiglio, G.; Trancassini, M.; Passariello, C.; Pantanella, F.; Schippa, S. Rebuilding the Gut Microbiota Ecosystem. Int. J. Environ. Res. Public Health 2018, 15, 1679. [Google Scholar] [CrossRef]
- Rozas, M.; Hart de Ruijter, A.; Fabrega, M.J.; Zorgani, A.; Guell, M.; Paetzold, B.; Brillet, F. From Dysbiosis to Healthy Skin: Major Contributions of Cutibacterium acnes to Skin Homeostasis. Microorganisms 2021, 9, 628. [Google Scholar] [CrossRef]
- Chen, Y.E.; Fischbach, M.A.; Belkaid, Y. Skin microbiota-host interactions. Nature 2018, 553, 427–436. [Google Scholar] [CrossRef] [PubMed]
- Jensen, M.G.; Svraka, L.; Baez, E.; Lund, M.; Poehlein, A.; Brüggemann, H. Species- and strain-level diversity of Corynebacteria isolated from human facial skin. BMC Microbiol. 2023, 23, 366. [Google Scholar] [CrossRef] [PubMed]
- Kanna, B.V.; Latha, R.; Kavitha, K.; Jeyakumari, D. Phenotypic Characterization of Malassezia spp. Isolated from Healthy Individuals. Res. J. Biotechnol. 2023, 18, 74–78. [Google Scholar] [CrossRef]
- Schmid, B.; Künstner, A.; Fähnrich, A.; Busch, H.; Glatz, M.; Bosshard, P.P. Longitudinal Characterization of the Fungal Skin Microbiota in Healthy Subjects Over a Period of 1 Year. J. Investig. Dermatol. 2022, 142, 2766–2772.e2768. [Google Scholar] [CrossRef]
- Silling, S.; Kreuter, A.; Gambichler, T.; Meyer, T.; Stockfleth, E.; Wieland, U. Epidemiology of Merkel Cell Polyomavirus Infection and Merkel Cell Carcinoma. Cancers 2022, 14, 6176. [Google Scholar] [CrossRef]
- Harper, A.; Vijayakumar, V.; Ouwehand, A.C.; ter Haar, J.; Obis, D.; Espadaler, J.; Binda, S.; Desiraju, S.; Day, R. Viral Infections, the Microbiome, and Probiotics. Front. Cell. Infect. Microbiol. 2021, 10, 596166. [Google Scholar] [CrossRef]
- Willmott, T.; Campbell, P.M.; Griffiths, C.E.M.; O’Connor, C.; Bell, M.; Watson, R.E.B.; McBain, A.J.; Langton, A.K. Behaviour and sun exposure in holidaymakers alters skin microbiota composition and diversity. Front. Aging 2023, 4, 1217635. [Google Scholar] [CrossRef]
- Junca, H.; Pieper, D.H.; Medina, E. The emerging potential ofmicrobiome transplantation on human health interventions. Comput. Struct. Biotechnol. J. 2022, 20, 615–627. [Google Scholar] [CrossRef]
- Romano-Bertrand, S.; Bourdier, A.; Aujoulat, F.; Michon, A.L.; Masnou, A.; Parer, S.; Marchandin, H.; Jumas-Bilak, E. Skin microbiota is the main reservoir of Roseomonas mucosa, an emerging opportunistic pathogen so far assumed to be environmental. Clin. Microbiol. Infect. 2016, 22, 737.e1–737.e7. [Google Scholar] [CrossRef]
- Li, R.; Li, J.; Zhou, X. Lung microbiome: New insights into the pathogenesis of respiratory diseases. Signal Transduct. Target. Ther. 2024, 9, 19. [Google Scholar] [CrossRef]
- Fabbrizzi, A.; Amedei, A.; Lavorini, F.; Renda, T.; Fontana, G. The Lung Microbiome: Clinical and Therapeutic Implications. Intern. Emerg. Med. 2019, 14, 1241–1250. [Google Scholar] [CrossRef] [PubMed]
- Natalini, J.G.; Singh, S.; Segal, L.N. The dynamic lung microbiome in health and disease. Nat. Rev. Microbiol. 2023, 21, 222–235. [Google Scholar] [CrossRef] [PubMed]
- Sommariva, M.; Le Noci, V.; Bianchi, F.; Camelliti, S.; Balsari, A.; Tagliabue, E.; Sfondrini, L. The lung microbiota: Role in maintaining pulmonary immune homeostasis and its implications in cancer development and therapy. Cell. Mol. Life Sci. 2020, 77, 2739–2749. [Google Scholar] [CrossRef] [PubMed]
- Santacroce, L.; Charitos, I.A.; Ballini, A.; Inchingolo, F.; Luperto, P.; De Nitto, E.; Topi, S. The Human Respiratory System and its Microbiome at a Glimpse. Biology 2020, 9, 318. [Google Scholar] [CrossRef]
- Prinzi, A. Normal Respiratory Microbiota in Health and Disease. Available online: https://asm.org/Articles/2020/February/Normal-Respiratory-Microbiota-in-Health-and-Diseas (accessed on 24 November 2024).
- Kumpitsch, C.; Koskinen, K.; Schöpf, V.; Moissl-Eichinger, C. The microbiome of the upper respiratory tract in health and disease. BMC Biol. 2019, 17, 87. [Google Scholar] [CrossRef]
- Whiteside, S.A.; McGinniss, J.E.; Collman, R.G. The Lung Microbiome: Progress and promise. J. Clin. Investig. 2021, 131. [Google Scholar] [CrossRef]
- Chen, J.; Li, T.; Ye, C.; Zhong, J.; Huang, J.-D.; Ke, Y.; Sun, H. The Lung Microbiome: A new frontier for lung and brain disease. Int. J. Mol. Sci. 2023, 24, 2170. [Google Scholar] [CrossRef]
- Mathieu, E.; Escribano-Vazquez, U.; Descamps, D.; Cherbuy, C.; Langella, P.; Riffault, S.; Remot, A.; Thomas, M. Paradigms of lung microbiota functions in health and disease, particularly in asthma. Front. Physiol. 2018, 9, 1168. [Google Scholar] [CrossRef]
- Hérivaux, A.; Willis, J.R.; Mercier, T.; Lagrou, K.; Gonçalves, S.M.; Gonçales, R.A.; Maertens, J.; Carvalho, A.; Gabaldón, T.; Cunha, C. Lung microbiota predict invasive pulmonary aspergillosis and its outcome in immunocompromised patients. Thorax 2021, 77, 283–291. [Google Scholar] [CrossRef]
- Yagi, K.; Huffnagle, G.B.; Lukacs, N.W.; Asai, N. The Lung Microbiome during Health and Disease. Int. J. Mol. Sci. 2021, 22, 10872. [Google Scholar] [CrossRef]
- Huffnagle, G.B.; Dickson, R.P.; Lukacs, N.W. The Respiratory Tract Microbiome and Lung Inflammation: A Two-Way Street. Mucosal Immunol. 2017, 10, 299–306. [Google Scholar] [CrossRef] [PubMed]
- Dickson, R.P.; Erb-Downward, J.R.; Martinez, F.J.; Huffnagle, G.B. The Microbiome and the Respiratory Tract. Annu. Rev. Physiol. 2016, 78, 481–504. [Google Scholar] [CrossRef] [PubMed]
- Budden, K.F.; Gellatly, S.L.; Wood, D.L.; Cooper, M.A.; Morrison, M.; Hugenholtz, P.; Hansbro, P.M. Emerging pathogenic links between microbiota and the gut–lung axis. Nat. Rev. Microbiol. 2016, 15, 55–63. [Google Scholar] [CrossRef] [PubMed]
- Campbell, C.D.; Barnett, C.; Sulaiman, I.A. A clinicians’ review of the respiratory microbiome. Breathe 2022, 18, 210161. [Google Scholar] [CrossRef] [PubMed]
- King, A. Exploring the lung microbiome’s role in disease. Nature 2024. [Google Scholar] [CrossRef]
- Ruane, D.; Chorny, A.; Lee, H.; Faith, J.; Pandey, G.; Shan, M.; Simchoni, N.; Rahman, A.; Garg, A.; Weinstein, E.G.; et al. Microbiota Regulate the Ability of Lung Dendritic Cells to Induce IgA Class-Switch Recombination and Generate Protective Gastrointestinal Immune Responses. J. Exp. Med. 2016, 213, 53–73. [Google Scholar] [CrossRef]
- Khatiwada, S.; Subedi, A. Lung microbiome and coronavirus disease 2019 (COVID-19): Possible link and implications. Hum. Microbiome J. 2020, 17, 100073. [Google Scholar] [CrossRef]
- Mayo Clinic: The Gut-Lung Axis. Available online: https://www.mayoclinic.org/medical-professionals/pulmonary-medicine/news/the-gut-lung-axis-intestinal-microbiota-and-inflammatory-lung-disease/mqc-20483394 (accessed on 24 November 2024).
- Enaud, R.; Prevel, R.; Ciarlo, E.; Beaufils, F.; Wieërs, G.; Guery, B.; Delhaes, L. The gut-lung axis in health and respiratory diseases: A place for Inter-organ and inter-kingdom crosstalks. Front. Cell. Infect. Microbiol. 2020, 10, 9. [Google Scholar] [CrossRef]
- Dang, A.T.; Marsland, B.J. Microbes, metabolites, and the gut–lung axis. Mucosal Immunol. 2019, 12, 843–850. [Google Scholar] [CrossRef]
- Rastogi, S.; Mohanty, S.; Sharma, S.; Tripathi, P. Possible Role of Gut Microbes and Host’s Immune Response in Gut–Lung Homeostasis. Front. Immunol. 2022, 13, 954339. [Google Scholar] [CrossRef]
- Perez-Carrasco, V.; Soriano-Lerma, A.; Soriano, M.; Gutiérrez-Fernández, J.; Garcia-Salcedo, J.A. Urinary Microbiome: Yin and Yang of the Urinary Tract. Front. Cell. Infect. Microbiol. 2021, 11, 617002. [Google Scholar] [CrossRef] [PubMed]
- Pearce, M.M.; Hilt, E.E.; Rosenfeld, A.B.; Zilliox, M.J.; Thomas-White, K.; Fok, C.; Kliethermes, S.; Schreckenberger, P.C.; Brubaker, L.; Gai, X.; et al. The Female Urinary Microbiome: A Comparison of Women with and without Urgency Urinary Incontinence. mBio 2014, 5, e01283-14. [Google Scholar] [CrossRef] [PubMed]
- Robino, L.; Sauto, R.; Morales, C.; Navarro, N.; González, M.J.; Cruz, E.; Neffa, F.; Zeballos, J.; Scavone, P. Presence of intracellular bacterial communities in uroepithelial cells, a potential reservoir in symptomatic and non-symptomatic people. BMC Infect. Dis. 2024, 24, 590. [Google Scholar] [CrossRef] [PubMed]
- Modena, B.D.; Milam, R.; Harrison, F.; Cheeseman, J.A.; Abecassis, M.M.; Friedewald, J.J.; Kirk, A.D.; Salomon, D.R. Changes in Urinary Microbiome Populations Correlate in Kidney Transplants With Interstitial Fibrosis and Tubular Atrophy Documented in Early Surveillance Biopsies. Am. J. Transplant. 2017, 17, 712–723. [Google Scholar] [CrossRef]
- Onywera, H.; Williamson, A.-L.; Ponomarenko, J.; Meiring, T.L. The Penile Microbiota in Uncircumcised and Circumcised Men: Relationships With HIV and Human Papillomavirus Infections and Cervicovaginal Microbiota. Front. Med. 2020, 7, 383. [Google Scholar] [CrossRef]
- Meng, Y.; Sun, J.; Zhang, G. Vaginal microbiota transplantation is a truly opulent and promising edge: Fully grasp its potential. Front. Cell. Infect. Microbiol. 2024, 14, 1280636. [Google Scholar] [CrossRef]
- Wright, M.L.; Fettweis, J.M.; Eaves, L.J.; Silberg, J.L.; Neale, M.C.; Serrano, M.G.; Jimenez, N.R.; Prom-Wormley, E.; Girerd, P.H.; Borzelleca, J.F.; et al. Vaginal microbiome Lactobacillus crispatus is heritable among European American women. Commun. Biol. 2021, 4, 872. [Google Scholar] [CrossRef]
- Pohl, H.G.; Groah, S.L.; Pérez-Losada, M.; Ljungberg, I.; Sprague, B.M.; Chandal, N.; Caldovic, L.; Hsieh, M. The Urine Microbiome of Healthy Men and Women Differs by Urine Collection Method. Int. Neurourol. J. 2020, 24, 41–51. [Google Scholar] [CrossRef]
- Ng, Q.X.; Peters, C.; Venkatanarayanan, N.; Goh, Y.Y.; Ho, C.Y.X.; Yeo, W.-S. Use of Lactobacillus spp. to prevent recurrent urinary tract infections in females. Med. Hypotheses 2018, 114, 49–54. [Google Scholar] [CrossRef]
- Ghartey, J.P.; Smith, B.C.; Chen, Z.; Buckley, N.; Lo, Y.; Ratner, A.J.; Herold, B.C.; Burk, R.D. Lactobacillus crispatus dominant vaginal microbiome is associated with inhibitory activity of female genital tract secretions against Escherichia coli. PLoS ONE 2014, 9, e96659. [Google Scholar] [CrossRef]
- Liu, X.; Si, S.; Huang, L.; Zhang, M.; Chen, W.; Wang, L.; Yu, Y. Vaginal flora during pregnancy and subsequent risk of preterm birth or prelabor rupture of membranes: A nested case–control study from China. BMC Pregnancy Childbirth 2023, 23, 244. [Google Scholar] [CrossRef]
- Dong, Y.-H.; Fu, Z.; Zhang, N.-N.; Shao, J.-Y.; Shen, J.; Yang, E.; Sun, S.-Y.; Zhao, Z.-M.; Xiao, A.; Liu, C.-J.; et al. Urogenital tract and rectal microbiota composition and its influence on reproductive outcomes in infertile patients. Front. Microbiol. 2023, 14, 1051437. [Google Scholar] [CrossRef] [PubMed]
- Souza, S.V.; Monteiro, P.B.; Moura, G.A.; Santos, N.O.; Fontanezi, C.T.B.; Gomes, I.A.; Teixeira, C.A. Vaginal microbioma and the presence of Lactobacillus spp. as interferences in female fertility: A review system. JBRA Assist. Reprod. 2023, 27, 496–506. [Google Scholar] [CrossRef]
- El-Sayed, M.M.; Mohak, S.; Gala, D.; Fabian, R.; Peterfi, Z.; Fabian, Z. The Role of the Intestinal Microbiome in Multiple Sclerosis-Lessons to Be Learned from Hippocrates. Biology 2023, 12, 1463. [Google Scholar] [CrossRef] [PubMed]
- Zancan, V.; Nasello, M.; Bigi, R.; Reniè, R.; Buscarinu, M.C.; Mechelli, R.; Ristori, G.; Salvetti, M.; Bellucci, G. Gut Microbiota Composition Is Causally Linked to Multiple Sclerosis: A Mendelian Randomization Analysis. Microorganisms 2024, 12, 1476. [Google Scholar] [CrossRef] [PubMed]
- Morais, L.H.; Schreiber, H.L.; Mazmanian, S.K. The gut microbiota–brain axis in behaviour and brain disorders. Nat. Rev. Microbiol. 2021, 19, 241–255. [Google Scholar] [CrossRef]
- Scheperjans, F.; Aho, V.; Pereira, P.A.B.; Koskinen, K.; Paulin, L.; Pekkonen, E.; Haapaniemi, E.; Kaakkola, S.; Eerola-Rautio, J.; Pohja, M.; et al. Gut microbiota are related to Parkinson’s disease and clinical phenotype. Mov. Disord. 2015, 30, 350–358. [Google Scholar] [CrossRef]
- Kappéter, Á.; Sipos, D.; Varga, A.; Vigvári, S.; Halda-Kiss, B.; Péterfi, Z. Migraine as a Disease Associated with Dysbiosis and Possible Therapy with Fecal Microbiota Transplantation. Microorganisms 2023, 11, 2083. [Google Scholar] [CrossRef]
- Doroszkiewicz, J.; Groblewska, M.; Mroczko, B. The Role of Gut Microbiota and Gut–Brain Interplay in Selected Diseases of the Central Nervous System. Int. J. Mol. Sci. 2021, 22, 10028. [Google Scholar] [CrossRef]
- Cekanaviciute, E.; Yoo, B.B.; Runia, T.F.; Debelius, J.W.; Singh, S.; Nelson, C.A.; Kanner, R.; Bencosme, Y.; Lee, Y.K.; Hauser, S.L.; et al. Gut bacteria from multiple sclerosis patients modulate human T cells and exacerbate symptoms in mouse models. Proc. Natl. Acad. Sci. USA 2017, 114, 10713–10718. [Google Scholar] [CrossRef]
- Xue, L.J.; Yang, X.-Z.; Tong, Q.; Shen, P.; Ma, S.-J.; Wu, S.-N.; Zheng, J.-L.; Wang, H.-G. Fecal microbiota transplantation therapy for Parkinson’s disease: A preliminary study. Medicine 2020, 99, e22035. [Google Scholar] [CrossRef]
- Akbari, E.; Asemi, Z.; Daneshvar Kakhaki, R.; Bahmani, F.; Kouchaki, E.; Tamtaji, O.R.; Taghizadeh, M. Effect of Probiotic Supplementation on Cognitive Function and Metabolic Status in Alzheimer’s Disease. Front. Aging Neurosci. 2016, 8, 256. [Google Scholar] [CrossRef]
- V, D.A.; Sarnataro, D. Probiotics, prebiotics and their role in Alzheimer’s disease. Neural Regen Res 2021, 16, 1768–1769. [Google Scholar] [CrossRef]
- Westfall, S.; Pasinetti, G.M. The Gut Microbiota Links Dietary Polyphenols With Management of Psychiatric Mood Disorders. Front Neurosci 2019, 13, 1196. [Google Scholar] [CrossRef] [PubMed]
- Clemente-Suárez, V.J.; Redondo-Flórez, L.; Rubio-Zarapuz, A.; Martín-Rodríguez, A.; Tornero-Aguilera, J.F. Microbiota Implications in Endocrine-Related Diseases: From Development to Novel Therapeutic Approaches. Biomedicines 2024, 12, 221. [Google Scholar] [CrossRef] [PubMed]
- Lymperopoulos, A.; Suster, M.S.; Borges, J.I. Short-Chain Fatty Acid Receptors and Cardiovascular Function. Int. J. Mol. Sci. 2022, 23, 3303. [Google Scholar] [CrossRef]
- Yu, F.; Zong, B.; Ji, L.; Sun, P.; Jia, D.; Wang, R. Free Fatty Acids and Free Fatty Acid Receptors: Role in Regulating Arterial Function. Int. J. Mol. Sci. 2024, 25, 7853. [Google Scholar] [CrossRef]
- Robles-Vera, I.; Toral, M.; de la Visitación, N.; Aguilera-Sánchez, N.; Redondo, J.M.; Duarte, J. Protective Effects of Short-Chain Fatty Acids on Endothelial Dysfunction Induced by Angiotensin II. Front. Physiol 2020, 11, 277. [Google Scholar] [CrossRef]
- Roy, R.; Wilcox, J.; Webb, A.J.; O’Gallagher, K. Dysfunctional and Dysregulated Nitric Oxide Synthases in Cardiovascular Disease: Mechanisms and Therapeutic Potential. Int. J. Mol. Sci. 2023, 24, 15200. [Google Scholar] [CrossRef]
- Shanmugham, M.; Bellanger, S.; Leo, C.H. Gut-Derived Metabolite, Trimethylamine-N-oxide (TMAO) in Cardio-Metabolic Diseases: Detection, Mechanism, and Potential Therapeutics. Pharmaceuticals 2023, 16, 504. [Google Scholar] [CrossRef]
- Pluznick, J.L. Gut microbiota in renal physiology: Focus on short-chain fatty acids and their receptors. Kidney Int. 2016, 90, 1191–1198. [Google Scholar] [CrossRef] [PubMed]
- Canyelles, M.; Borràs, C.; Rotllan, N.; Tondo, M.; Escolà-Gil, J.C.; Blanco-Vaca, F. Gut Microbiota-Derived TMAO: A Causal Factor Promoting Atherosclerotic Cardiovascular Disease? Int. J. Mol. Sci. 2023, 24, 1940. [Google Scholar] [CrossRef]
- Wang, L.; Hu, J. Unraveling the gut microbiota’s role in salt-sensitive hypertension: Current evidences and future directions. Front. Cardiovasc. Med. 2024, 11, 1410623. [Google Scholar] [CrossRef]
- Christiansen, C.B.; Gabe, M.B.N.; Svendsen, B.; Dragsted, L.O.; Rosenkilde, M.M.; Holst, J.J. The impact of short-chain fatty acids on GLP-1 and PYY secretion from the isolated perfused rat colon. Am. J. Physiol. Gastrointest. Liver Physiol. 2018, 315, G53–G65. [Google Scholar] [CrossRef]
- Zhang, Y.; Meng, X.; Ma, Z.; Sun, Z.; Wang, Z. Effects of Probiotic Supplementation on Nutrient Intake, Ghrelin, and Adiponectin Concentrations in Diabetic Hemodialysis Patients. Altern. Ther. Health Med. 2023, 29, 36–42. [Google Scholar]
- Hamamah, S.; Hajnal, A.; Covasa, M. Influence of Bariatric Surgery on Gut Microbiota Composition and Its Implication on Brain and Peripheral Targets. Nutrients 2024, 16, 1071. [Google Scholar] [CrossRef] [PubMed]
- Rusch, J.A.; Layden, B.T.; Dugas, L.R. Signalling cognition: The gut microbiota and hypothalamic-pituitary-adrenal axis. Front. Endocrinol. 2023, 14, 1130689. [Google Scholar] [CrossRef]
- Vagnerová, K.; Vodička, M.; Hermanová, P.; Ergang, P.; Šrůtková, D.; Klusoňová, P.; Balounová, K.; Hudcovic, T.; Pácha, J. Interactions between gut microbiota and acute restraint stress in peripheral structures of the hypothalamic–pituitary–adrenal axis and the intestine of male mice. Front. Immunol. 2019, 10, 2655. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Chen, D.; Zhao, C.; Jiang, L.; Mao, S.; Song, C.; Gao, F. Decreased abundance of Akkermansia after adrenocorticotropic hormone therapy in patients with West syndrome. BMC Microbiol. 2021, 21, 126. [Google Scholar] [CrossRef]
- Shulhai, A.-M.; Rotondo, R.; Petraroli, M.; Patianna, V.; Predieri, B.; Iughetti, L.; Esposito, S.; Street, M.E. The Role of Nutrition on Thyroid Function. Nutrients 2024, 16, 2496. [Google Scholar] [CrossRef]
- Hu, S.; Ding, Q.; Zhang, W.; Kang, M.; Ma, J.; Zhao, L. Gut microbial beta-glucuronidase: A vital regulator in female estrogen metabolism. Gut Microbes 2023, 15, 2156157. [Google Scholar] [CrossRef] [PubMed]
- Sui, Y.; Wu, J.; Chen, J. The role of gut microbial β-glucuronidase in estrogen reactivation and breast cancer. Front. Cell Dev. Biol. 2021, 9, 631552. [Google Scholar] [CrossRef]
- Choden, T.; Cohen, N.A. The gut microbiome and the immune system. Explor. Med. 2022, 3, 219–233. [Google Scholar] [CrossRef]
- Zheng, D.; Liwinski, T.; Elinav, E. Interaction between microbiota and immunity in health and disease. Cell Res. 2020, 30, 492–506. [Google Scholar] [CrossRef] [PubMed]
- Al-Fakhrany, O.M.; Elekhnawy, E. Next-generation probiotics: The upcoming biotherapeutics. Mol. Biol. Rep. 2024, 51, 505. [Google Scholar] [CrossRef] [PubMed]
- Wastyk, H.C.; Fragiadakis, G.K.; Perelman, D.; Dahan, D.; Merrill, B.D.; Yu, F.B.; Topf, M.; Gonzalez, C.G.; Van Treuren, W.; Han, S.; et al. Gut-microbiota-targeted diets modulate human immune status. Cell 2021, 184, 4137–4153.e4114. [Google Scholar] [CrossRef]
- Tian, H.; Wang, X.; Fang, Z.; Li, L.; Wu, C.; Bi, D.; Li, N.; Chen, Q.; Qin, H. Fecal microbiota transplantation in clinical practice: Present controversies and future prospects. hLife 2024, 2, 269–283. [Google Scholar] [CrossRef]
| Skin Region | Predominant Microorganisms | |
|---|---|---|
| Oily Zones | Cutibacterium acnes Malassezia spp. | Staphylococcus spp. Corynebacterium spp. |
| Moist Zones | Staphylococcus spp. Corynebacterium spp. | Micrococcaceae spp. |
| Dry Zones | Staphylococcus epidermidis Micrococcus spp. Proteobacteria spp. | Bacteroidetes spp. Actinobacteria spp. |
| Palms and Soles | Corynebacterium spp. Staphylococcus spp. Malassezia spp. Aspergillus spp. | Cryptococcus spp. Rhodotorula spp. Epicoccum spp. |
| Scalp | Malassezia spp. Cutibacterium spp. | Demodex spp. |
| Perianal Area | Enterococcus spp. Escherichia coli | Bacteroides spp. |
| System | Microbiota–System Relationship |
|---|---|
| 1. Digestive System | Provides a protective barrier against pathogens. Supports digestion (polysaccharides and proteins). Enhances gut barrier integrity and reduces inflammation. Produces vitamins B-complex and K. Metabolizes dietary fibers Regulates glucose and lipid metabolism. Detoxifies xenobiotics and binds heavy metals. Influences systemic immune responses through modulation of gut-associated lymphoid tissue. |
| 2. Integumentary System | Provides a protective barrier against pathogens. Regulates skin pH and supports lipid production. Stimulates skin regeneration and wound healing. Adapts to environmental factors. Modulates immune responses to maintain local homeostasis. |
| 3. Respiratory System | Prevents colonization by respiratory pathogens. Modulates immune responses via alveolar macrophages and regulatory T cells. Supports mucosal barrier integrity and facilitates mucociliary clearance. Influences respiratory health through the gut–lung axis, producing SCFAs that modulate lung immunity. Contributes to IgA production, enhancing mucosal defenses. |
| 4. Urinary System | Maintains urinary tract health by balancing microbial populations and preventing infections. Modulates local pH. Shares microbial communities with the reproductive system, supporting urogenital health. |
| 5. Reproductive System | Regulates vaginal pH. Supports healthy pregnancy by modulating local immune responses. Protects against sexually transmitted infections through microbial competition and lactic acid production. Enhances fertility. Interacts with the urinary microbiota to maintain overall urogenital health. |
| 6. Central Nervous System | Facilitates gut–brain communication via the vagus nerve. Produces neurotransmitters, influencing mood and cognition. Modulates stress responses. Reduces neuroinflammation. Promotes neuroprotection. Influences the progression and management of neurodegenerative diseases. |
| 7. Cardiovascular System | Regulates blood pressure. Modulates lipid metabolism. Reduces systemic inflammation. Protects vascular integrity. Prevents thrombosis. Produces TMA, which is linked to cardiovascular health. Supports endothelial function and vascular tone. |
| 8. Endocrine System | Regulating appetite and glucose metabolism. Modulates bile acid metabolism, influencing energy expenditure and lipid homeostasis. Affects cortisol production and stress adaptation. Supports thyroid hormone synthesis. Regulates estrogen levels. |
| 9. Immune system | Influences the maturation and differentiation of T and B cells. Regulates the production of immunoglobulins. Balancs pro-inflammatory and anti-inflammatory responses. Modulates Treg activity and systemic inflammation. Promoting local immune tolerance. Reinforces the intestinal barrier. Prevents chronic inflammation and autoimmune diseases. |
| System | Relevant Microorganisms | Products/Biomarkers |
|---|---|---|
| 1. Digestive System | Faecalibacterium prausnitzii Akkermansia muciniphila Roseburia spp. Bacteroides spp. Lactobacillus spp. Eggerthella lenta Firmicutes spp. Firmicutes/Bacteroidetes ratio | SCFAs mucin lipopolysaccharides GLP-1 PYY |
| 2. Integumentary System | Cutibacterium acnes Staphylococcus epidermidis Staphylococcus aureus Corynebacterium jeikeium Malassezia spp. Candida spp. Merkel cell polyomavirus | free fatty acids AMPs lactic acid |
| 3. Respiratory System | Prevotella spp. Streptococcus spp. Veillonella spp. Haemophilus influenzae Moraxella catarrhalis | IL-10 IL-6 IL-8 |
| 4. Urinary and Reproductive System | Lactobacillus crispatus Lactobacillus gasseri Gardnerella vaginalis Corynebacterium spp. Escherichia coli | |
| 5. Central Nervous System | Lactobacillus plantarum Bifidobacterium spp. | SCFAs PYY serotonin dopamine GABA |
| 6. Cardiovascular System | Eubacterium coprostanoligenes Lactobacillus spp. Bifidobacterium spp. Ruminococcus spp. | SCFAs TMA arachidonic acid |
| 7. Endocrine System | Akkermansia muciniphila Bacteroides spp. Bifidobacterium spp. | SCFAs GLP-1 PYY β-glucuronidase leptin cholecystokinin |
| 8. Immune System | Bacteroides fragilis Lactobacillus spp. Bifidobacterium spp. Faecalibacterium prausnitzii | SCFAs IL-10 AMPs |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
John, H.T.; Thomas, T.C.; Chukwuebuka, E.C.; Ali, A.B.; Anass, R.; Tefera, Y.Y.; Babu, B.; Negrut, N.; Ferician, A.; Marian, P. The Microbiota–Human Health Axis. Microorganisms 2025, 13, 948. https://doi.org/10.3390/microorganisms13040948
John HT, Thomas TC, Chukwuebuka EC, Ali AB, Anass R, Tefera YY, Babu B, Negrut N, Ferician A, Marian P. The Microbiota–Human Health Axis. Microorganisms. 2025; 13(4):948. https://doi.org/10.3390/microorganisms13040948
Chicago/Turabian StyleJohn, Harrie Toms, Treesa Clare Thomas, Ezenwa Collins Chukwuebuka, Ali Bacar Ali, Reggani Anass, Yididiya Yilma Tefera, Bency Babu, Nicoleta Negrut, Anca Ferician, and Paula Marian. 2025. "The Microbiota–Human Health Axis" Microorganisms 13, no. 4: 948. https://doi.org/10.3390/microorganisms13040948
APA StyleJohn, H. T., Thomas, T. C., Chukwuebuka, E. C., Ali, A. B., Anass, R., Tefera, Y. Y., Babu, B., Negrut, N., Ferician, A., & Marian, P. (2025). The Microbiota–Human Health Axis. Microorganisms, 13(4), 948. https://doi.org/10.3390/microorganisms13040948









