How the Built Environment Shapes Children’s Microbiome: A Systematic Review
Abstract
1. Introduction
2. Methods
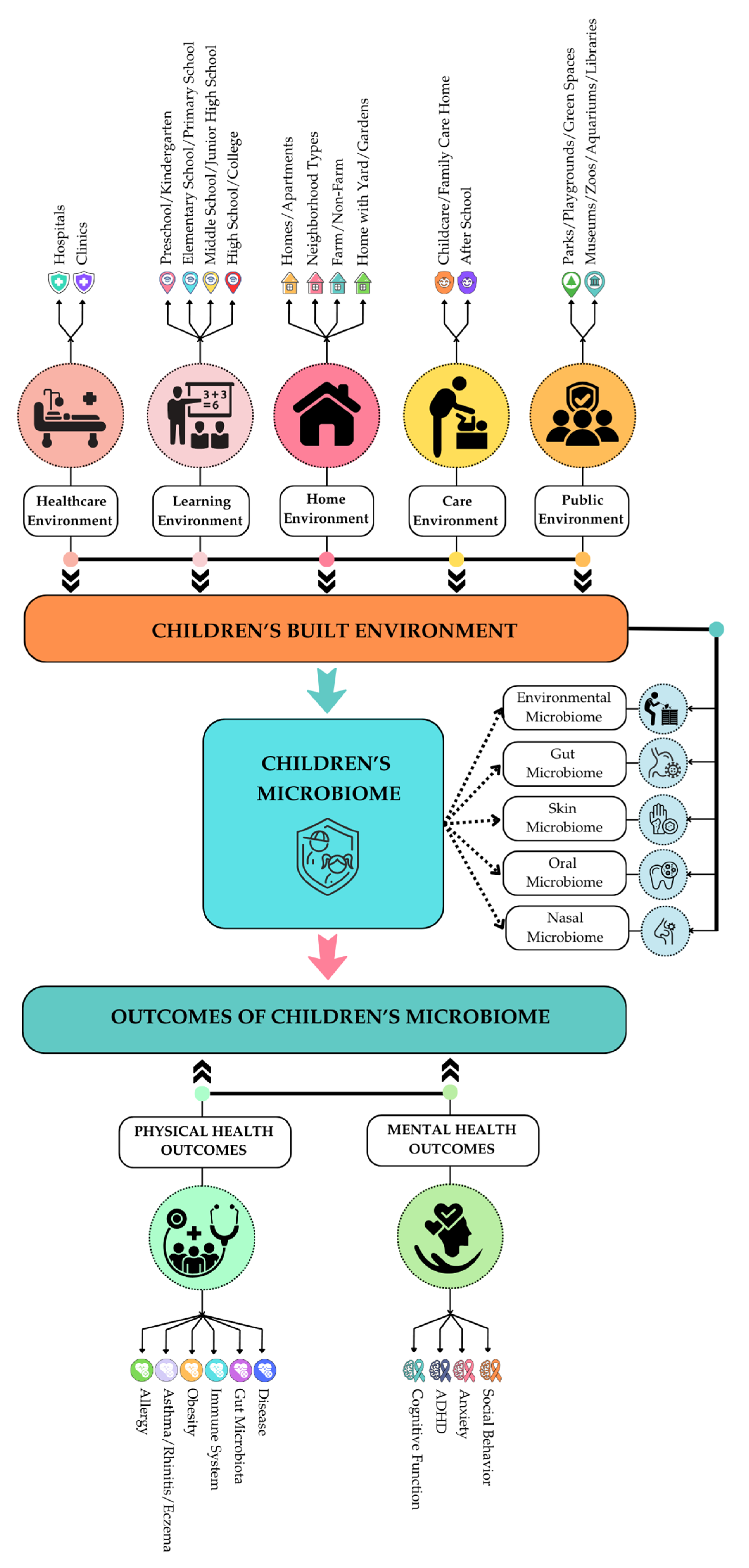
2.1. Defining Childhood BE and Its Factors Influencing Microbiome
2.2. Inclusion and Exclusion Criteria
2.3. Systematic Review Method
2.4. Prisma Diagram
3. Review Summary
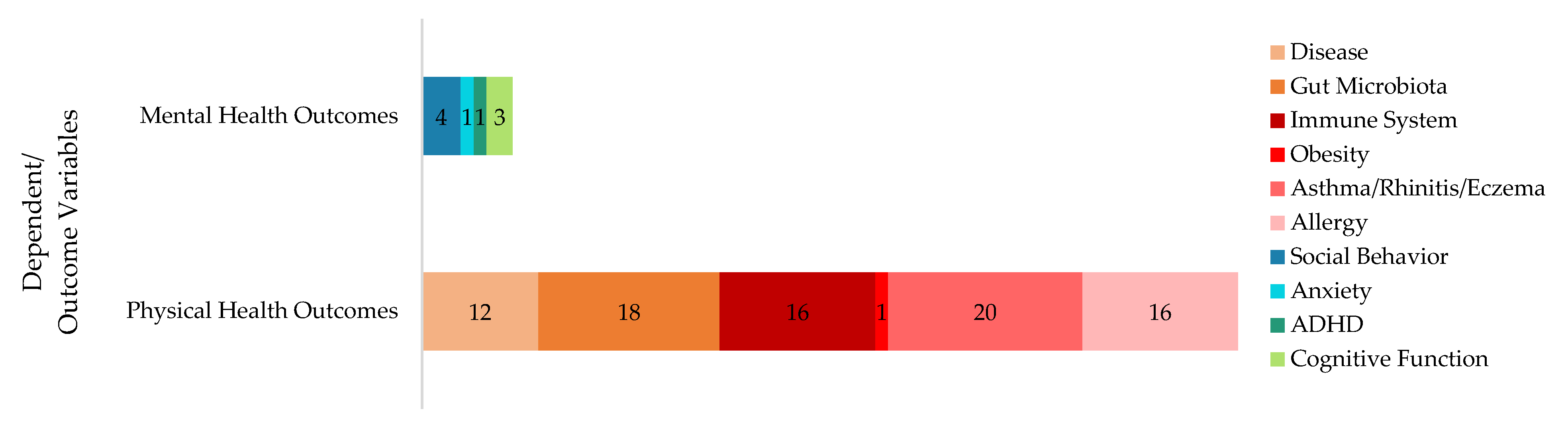
3.1. Research Questions, Dependent, and Independent Variables
3.2. Study Characteristics of the Reviewed Studies
3.3. Contextual Characteristics of the Reviewed Studies
3.4. Understanding Children’s Well-Being: The Role of the Microbiome
3.5. Microbiome Samples and Sampling Methods
3.5.1. Dust Samples
3.5.2. Fecal Samples
3.5.3. Skin and Saliva Samples
3.5.4. Air Samples
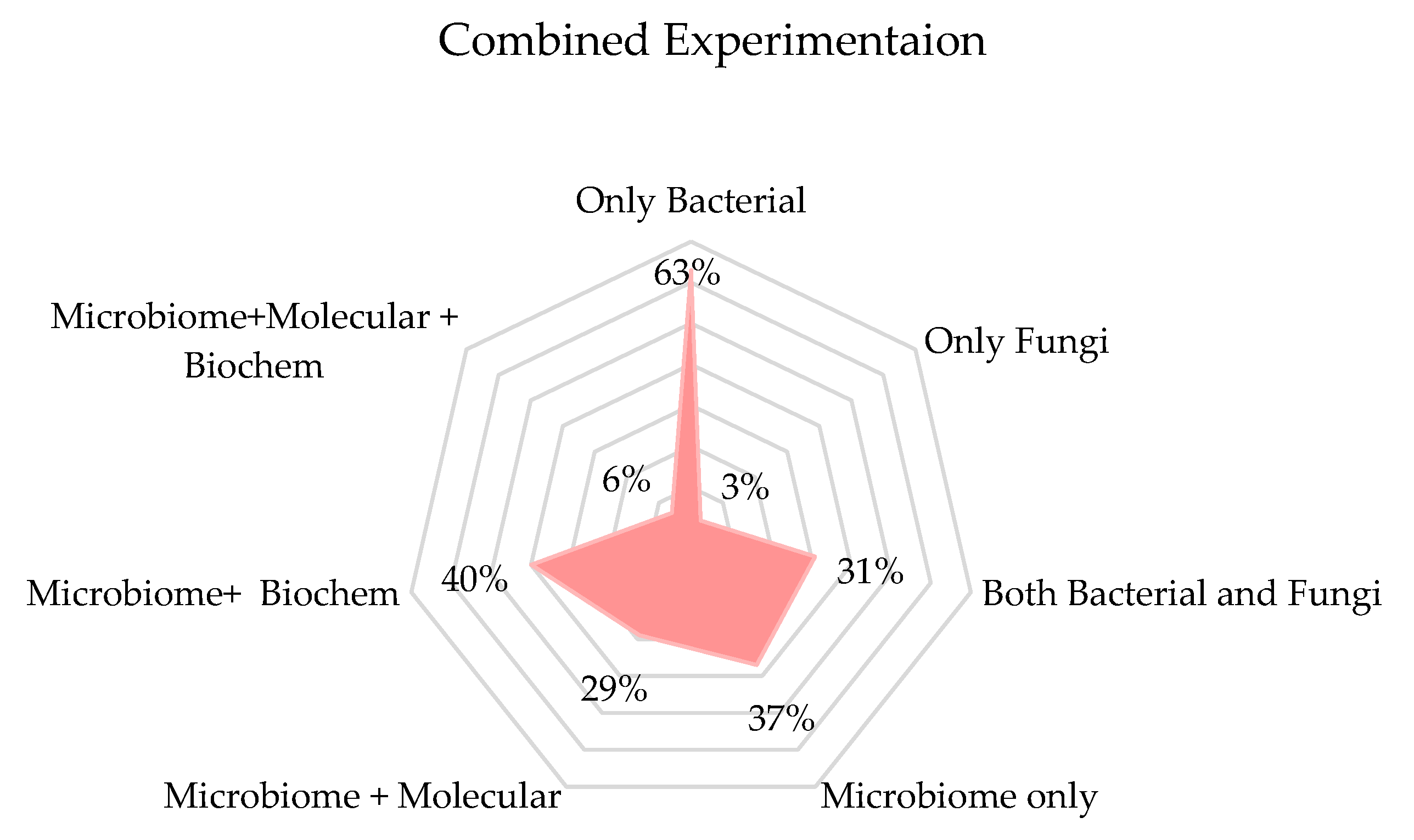
3.6. Biological Experiments
3.6.1. Molecular Analysis
3.6.2. Metabolomic Analysis
3.7. Bioinformatic and Metabolomic Data Analysis: Tools and Software
4. Discussion
4.1. An Emphasis on Children’s Exposure to Nature
4.2. Behavioral Outcomes of BE Microbiome
4.3. Rare Connection to BE Design
4.4. Was Multiple Sampling Necessary? Is Metabolomics Worth It?
4.5. Future Research Directions for Children’s Built Environment and Microbiome
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Appendix A
| ID | Paper Title | Citation |
| 1 | Environmental microbiome in the home and daycare settings during the COVID-19 pandemic, and potential risk of non-communicable disease in children | [33] |
| 2 | Alteration of oral microbiome composition in children living with pesticide-exposed farm workers | [38] |
| 3 | Association of indoor dust microbiota with cognitive function and behavior in preschool-aged children | [31] |
| 4 | Associations between environmental characteristics, high-resolution indoor microbiome, metabolome and allergic and non-allergic rhinitis symptoms for junior high school students | [32] |
| 5 | Diversity and compositional differences of the airborne microbiome in a biophilic indoor environment | [40] |
| 6 | Impact of environmental characteristics on children’s gut microbiota—A pilot study in assessing the role of indoor microbiome and metabolites | [37] |
| 7 | Indoor metabolites and chemicals outperform microbiome in classifying childhood asthma and allergic rhinitis | [47] |
| 8 | Optimising Early Childhood Educational Settings for Health Using Nature-Based Solutions: The Microbiome Aspect | [23] |
| 9 | Impact of the environment on the microbiome | [1] |
| 10 | Indoor microbiome, air pollutants and asthma, rhinitis and eczema in preschool children—A repeated cross-sectional study | [52] |
| 11 | Temporary establishment of bacteria from indoor plant leaves and soil on human skin | [54] |
| 12 | The bacterial community of childcare centers: potential implications for microbial dispersal and child exposure | [60] |
| 13 | Altered microbiomes in thirdhand smoke-exposed children and their home environments | [46] |
| 14 | Associations between the indoor microbiome, environmental characteristics and respiratory infections in junior high school students of Johor Bahru, Malaysia | [42] |
| 15 | Environmental Influences on the Human Gut Microbiota: A Longitudinal Pilot Study | [55] |
| 16 | Exposures to Semivolatile Organic Compounds in Indoor Environments and Associations with the Gut Microbiomes of Children | [51] |
| 17 | Long-term biodiversity intervention shapes health-associated commensal microbiota among urban day-care children | [53] |
| 18 | Spatiotemporal variation of the indoor mycobiome in daycare centers | [41] |
| 19 | Biodiversity intervention enhances immune regulation and health-associated commensal microbiota among daycare children | [59] |
| 20 | Environmental shaping of the bacterial and fungal community in infant bed dust and correlations with the airway microbiota | [61] |
| 21 | Impact of outdoor nature-related activities on gut microbiota, fecal serotonin, and perceived stress in preschool children: the Play&Grow randomized controlled trial | [35] |
| 22 | Indoor microbiome, environmental characteristics and asthma among junior high school students in Johor Bahru, Malaysia | [45] |
| 23 | Mechanisms of children’s soil exposure | [21] |
| 24 | Natural environments in the urban context and gut microbiota in infants | [62] |
| 25 | Transfer of environmental microbes to the skin and respiratory tract of humans after urban green space exposure | [63] |
| 26 | Yard vegetation is associated with gut microbiota composition | [36] |
| 27 | Early life home microbiome and hyperactivity/inattention in school-age children | [58] |
| 28 | Endocrine disruption and commensal bacteria alteration associated with gaseous and soil PAH contamination among daycare children | [48] |
| 29 | Evaluating the Effects of Farm Exposure on Infant Gut Microbiome | [34] |
| 30 | Farm-like indoor microbiota in non-farm homes protects children from asthma development | [56] |
| 31 | Short-term direct contact with soil and plant materials leads to an immediate increase in diversity of skin microbiota | [39] |
| 32 | Thinking bigger: How early-life environmental exposures shape the gut microbiome and influence the development of asthma and allergic disease | [24] |
| 33 | From Homes to Schools—The Impact of Ventilation and Cleaning on the Bacterial and Fungal Ecology of the Built Environment | [43] |
| 34 | Longitudinal development of the dust microbiome in a newly opened Norwegian kindergarten | [44] |
| 35 | Nature-oriented daycare diversifies skin microbiota in children—No robust association with allergies | [49] |
| 36 | The classroom microbiome and asthma morbidity in children attending 3 inner-city schools | [64] |
| 37 | Environmental and mucosal microbiota and their role in childhood asthma | [57] |
| 38 | Patterns in the skin microbiota differ in children and teenagers between rural and urban environments | [50] |
| 39 | Seasonal Dynamics of the Airborne Bacterial Community and Selected Viruses in a Children’s Daycare Center | [65] |
| 40 | The microbial environment and its influence on asthma prevention in early life | [22] |
| 41 | Metagenomic Insights into the Bioaerosols in the Indoor and Outdoor Environments of Childcare Facilities | [66] |
| 42 | Exposure to environmental microorganisms and childhood asthma | [7] |
References
- Chong, H.J.; D’amato, G.; Rosário Filho, N.A. Impact of the environment on the microbiome. J. Pediatr. 2022, 98, 32–37. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Yang, Z.; Hu, D.; Cao, L.; He, Q. Understanding building-occupant-microbiome interactions toward healthy built environments: A review. Front. Environ. Sci. Eng. 2021, 15, 65. [Google Scholar] [CrossRef] [PubMed]
- Gilbert, J.A.; Blaser, M.J.; Caporaso, J.G.; Jansson, J.K.; Lynch, S.V.; Knight, R. Current understanding of the human microbiome. Nat. Med. 2018, 24, 392–400. [Google Scholar] [CrossRef]
- Dominguez-Bello, M.G.; Godoy-Vitorino, F.; Knight, R.; Blaser, M.J. Role of the microbiome in human development. Gut 2019, 68, 1108–1114. [Google Scholar] [CrossRef]
- Robertson, R.C.; Manges, A.R.; Finlay, B.B.; Prendergast, A.J. The human microbiome and child growth–first 1000 days and beyond. Trends Microbiol. 2019, 27, 131–147. [Google Scholar] [CrossRef]
- Gaufin, T.; Tobin, N.H.; Aldrovandi, G.M. The importance of the microbiome in pediatrics and pediatric infectious diseases. Curr. Opin. Pediatr. 2018, 30, 117–124. [Google Scholar] [CrossRef]
- Ege, M.J.; Mayer, M.; Normand, A.-C.; Genuneit, J.; Cookson, W.O.; Braun-Fahrländer, C.; Heederik, D.; Piarroux, R.; von Mutius, E. Exposure to environmental microorganisms and childhood asthma. N. Engl. J. Med. 2011, 364, 701–709. [Google Scholar] [CrossRef] [PubMed]
- Yamazaki, Y.; Nakamura, Y.; Núñez, G. Role of the microbiota in skin immunity and atopic dermatitis. Allergol. Int. 2017, 66, 539–544. [Google Scholar] [CrossRef]
- Nance, C.L.; Deniskin, R.; Diaz, V.C.; Paul, M.; Anvari, S.; Anagnostou, A. The role of the microbiome in food allergy: A review. Children 2020, 7, 50. [Google Scholar] [CrossRef]
- Sági, V.; Makra, N.; Csoszánszki, N.; Decmann, A.; Szabó, D.; Garami, M. The influence of the gut Microbiome in Paediatric Cancer Origin and Treatment. Antibiotics 2022, 11, 1521. [Google Scholar] [CrossRef] [PubMed]
- Mohammadkhah, A.I.; Simpson, E.B.; Patterson, S.G.; Ferguson, J.F. Development of the gut microbiome in children, and lifetime implications for obesity and cardiometabolic disease. Children 2018, 5, 160. [Google Scholar] [CrossRef] [PubMed]
- Kelsey, M.M.; Zaepfel, A.; Bjornstad, P.; Nadeau, K.J. Age-related consequences of childhood obesity. Gerontology 2014, 60, 222–228. [Google Scholar] [CrossRef]
- Hu, M.; Zhao, X.; Liu, Y.; Zhou, H.; You, Y.; Xue, Z. Complex interplay of gut microbiota between obesity and asthma in children. Front. Microbiol. 2023, 14, 1264356. [Google Scholar] [CrossRef]
- Ogden, C.L.; Carroll, M.D.; Fryar, C.D.; Flegal, K.M. Prevalence of Obesity Among Adults and Youth: United States, 2011–2014; CDC: Atlanta, GA, USA, 2015. [Google Scholar]
- Gunnell, D.J.; Frankel, S.J.; Nanchahal, K.; Peters, T.J.; Smith, G.D. Childhood obesity and adult cardiovascular mortality: A 57-y follow-up study based on the Boyd Orr cohort. Am. J. Clin. Nutr. 1998, 67, 1111–1118. [Google Scholar] [CrossRef] [PubMed]
- Weihrauch-Blüher, S.; Schwarz, P.; Klusmann, J.-H. Childhood obesity: Increased risk for cardiometabolic disease and cancer in adulthood. Metabolism 2019, 92, 147–152. [Google Scholar] [CrossRef]
- Liang, Y.; Hou, D.; Zhao, X.; Wang, L.; Hu, Y.; Liu, J.; Cheng, H.; Yang, P.; Shan, X.; Yan, Y. Childhood obesity affects adult metabolic syndrome and diabetes. Endocrine 2015, 50, 87–92. [Google Scholar] [CrossRef] [PubMed]
- Association, A.D. Economic costs of diabetes in the US in 2017. Diabetes Care 2018, 41, 917–928. [Google Scholar] [CrossRef]
- Perry, R.; Braileanu, G.; Palmer, T.; Stevens, P. The economic burden of pediatric asthma in the United States: Literature review of current evidence. Pharmacoeconomics 2019, 37, 155–167. [Google Scholar] [CrossRef]
- Barnett, S.B.L.; Nurmagambetov, T.A. Costs of asthma in the United States: 2002–2007. J. Allergy Clin. Immunol. 2011, 127, 145–152. [Google Scholar] [CrossRef]
- Schachter, A.E.; Gailey, A.; Egendorf, S.P.; Mielke, H.W. Mechanisms of children’s soil exposure. Curr. Probl. Pediatr. Adolesc. Health Care 2020, 50, 100742. [Google Scholar] [CrossRef]
- von Mutius, E. The microbial environment and its influence on asthma prevention in early life. J. Allergy Clin. Immunol. 2016, 137, 680–689. [Google Scholar] [CrossRef] [PubMed]
- Robinson, J.M.; Barrable, A. Optimising Early Childhood Educational Settings for Health Using Nature-Based Solutions: The Microbiome Aspect. Educ. Sci. 2023, 13, 211. [Google Scholar] [CrossRef]
- Sbihi, H.; Boutin, R.C.; Cutler, C.; Suen, M.; Finlay, B.B.; Turvey, S.E. Thinking bigger: How early-life environmental exposures shape the gut microbiome and influence the development of asthma and allergic disease. Allergy 2019, 74, 2103–2115. [Google Scholar] [CrossRef]
- Roof, K.; Oleru, N. Public health: Seattle and King County’s push for the built environment. J. Environ. Health 2008, 71, 24–27. [Google Scholar] [PubMed]
- Kaklauskas, A.; Gudauskas, R. Intelligent decision-support systems and the Internet of Things for the smart built environment. In Start-Up Creation; Elsevier: Amsterdam, The Netherlands, 2016; pp. 413–449. [Google Scholar]
- Kembel, S.W.; Meadow, J.F.; O’Connor, T.K.; Mhuireach, G.; Northcutt, D.; Kline, J.; Moriyama, M.; Brown, G.Z.; Bohannan, B.J.M.; Green, J.L. Architectural Design Drives the Biogeography of Indoor Bacterial Communities. PLoS ONE 2014, 9, e87093. [Google Scholar] [CrossRef]
- Mankiewicz Ledins, P.; Bhattacharya, C.; Dyson, A.; Hénaff, E. Growing indoor environmental infrastructure: Designing for microbial diversity with implications for pollutant metabolism and human health. Res. Dir. Biotechnol. Des. 2024, 2, e5. [Google Scholar] [CrossRef]
- Moher, D.; Shamseer, L.; Clarke, M.; Ghersi, D.; Liberati, A.; Petticrew, M.; Shekelle, P.; Stewart, L.A.; Group, P.-P. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst. Rev. 2015, 4, 349. [Google Scholar] [CrossRef] [PubMed]
- Scudellari, M. Cleaning up the hygiene hypothesis. Proc. Natl. Acad. Sci. USA 2017, 114, 1433–1436. [Google Scholar] [CrossRef]
- Dockx, Y.; Täubel, M.; Hogervorst, J.; Luyten, L.; Peusens, M.; Rasking, L.; Sleurs, H.; Witters, K.; Plusquin, M.; Valkonen, M.; et al. Association of indoor dust microbiota with cognitive function and behavior in preschool-aged children. Microbiome 2023, 11, 1. [Google Scholar] [CrossRef]
- Fu, X.; Du, B.; Meng, Y.; Li, Y.; Zhu, X.; Ou, Z.; Zhang, M.; Wen, H.; Ma’Pol, A.; Hashim, J.H.; et al. Associations between environmental characteristics, high-resolution indoor microbiome, metabolome and allergic and non-allergic rhinitis symptoms for junior high school students. Environ. Sci. Process. Impacts 2023, 25, 791–804. [Google Scholar] [CrossRef]
- McKay, J.A.; Crown, M.; Bashton, M.; Pearce, D.; Entwistle, J.A.; Sangal, V. Environmental microbiome in the home and daycare settings during the COVID-19 pandemic, and potential risk of non-communicable disease in children. Environ. Microbiol. Rep. 2024, 16, e13233. [Google Scholar] [CrossRef] [PubMed]
- Thorsen, J.; McCauley, K.; Fadrosh, D.; Lynch, K.; Barnes, K.L.; Bendixsen, C.G.; Seroogy, C.M.; Lynch, S.V.; Gern, J.E. Evaluating the Effects of Farm Exposure on Infant Gut Microbiome. J. Allergy Clin. Immunol. 2019, 143, AB299. [Google Scholar] [CrossRef]
- Sobko, T.; Liang, S.; Cheng, W.H.; Tun, H.M. Impact of outdoor nature-related activities on gut microbiota, fecal serotonin, and perceived stress in preschool children: The Play&Grow randomized controlled trial. Sci. Rep. 2020, 10, 21993. [Google Scholar]
- Parajuli, A.; Hui, N.; Puhakka, R.; Oikarinen, S.; Grönroos, M.; Selonen, V.A.; Siter, N.; Kramna, L.; Roslund, M.I.; Vari, H.K. Yard vegetation is associated with gut microbiota composition. Sci. Total Environ. 2020, 713, 136707. [Google Scholar] [CrossRef]
- Zhang, M.; Tang, H.; Chen, Y.; Chen, Z.; Xu, Y.; Fu, X.; Sun, Y.; Zhao, Z. Impact of environmental characteristics on children’s gut microbiota—A pilot study in assessing the role of indoor microbiome and metabolites. Environ. Res. 2023, 234, 116114. [Google Scholar] [CrossRef]
- Stanaway, I.B.; Wallace, J.C.; Hong, S.; Wilder, C.S.; Green, F.H.; Tsai, J.; Knight, M.; Workman, T.; Vigoren, E.M.; Smith, M.N. Alteration of oral microbiome composition in children living with pesticide-exposed farm workers. Int. J. Hyg. Environ. Health 2023, 248, 114090. [Google Scholar] [CrossRef]
- Grönroos, M.; Parajuli, A.; Laitinen, O.H.; Roslund, M.I.; Vari, H.K.; Hyöty, H.; Puhakka, R.; Sinkkonen, A. Short-term direct contact with soil and plant materials leads to an immediate increase in diversity of skin microbiota. MicrobiologyOpen 2019, 8, e00645. [Google Scholar] [CrossRef]
- Toyoda, A.; Shibata, Y.; Matsuo, Y.; Terada, K.; Sugimoto, H.; Higashi, K.; Mori, H.; Ikeuchi, A.; Ito, M.; Kurokawa, K.; et al. Diversity and compositional differences of the airborne microbiome in a biophilic indoor environment. Sci. Rep. 2023, 13, 8179. [Google Scholar] [CrossRef] [PubMed]
- Estensmo, E.L.F.; Morgado, L.; Maurice, S.; Martin-Sanchez, P.M.; Engh, I.B.; Mattsson, J.; Kauserud, H.; Skrede, I. Spatiotemporal variation of the indoor mycobiome in daycare centers. Microbiome 2021, 9, 220. [Google Scholar] [CrossRef]
- Fu, X.; Yuan, Q.; Zhu, X.; Li, Y.; Meng, Y.; Hashim, J.H.; Hashim, Z.; Ali, F.; Zheng, Y.W.; Lai, X.X.; et al. Associations between the indoor microbiome, environmental characteristics and respiratory infections in junior high school students of Johor Bahru, Malaysia. Environ. Sci. Process. Impacts 2021, 23, 1171–1181. [Google Scholar] [CrossRef]
- Kwam, S.E. From Homes to Schools—The Impact of Ventilation and Cleaning on the Bacterial and Fungal Ecology of the Built Environment. Ph.D. Thesis, Yale University, New Haven, CT, USA, 2018. [Google Scholar]
- Nygaard, A.B.; Charnock, C. Longitudinal development of the dust microbiome in a newly opened Norwegian kindergarten. Microbiome 2018, 6, 159. [Google Scholar] [CrossRef] [PubMed]
- Fu, X.; Norbäck, D.; Yuan, Q.; Li, Y.; Zhu, X.; Hashim, J.H.; Hashim, Z.; Ali, F.; Zheng, Y.W.; Lai, X.X.; et al. Indoor microbiome, environmental characteristics and asthma among junior high school students in Johor Bahru, Malaysia. Environ. Int. 2020, 138, 105664. [Google Scholar] [CrossRef] [PubMed]
- Kelley, S.T.; Liu, W.; Quintana, P.J.E.; Hoh, E.; Dodder, N.G.; Mahabee-Gittens, E.M.; Padilla, S.; Ogden, S.; Frenzel, S.; Sisk-Hackworth, L.; et al. Altered microbiomes in thirdhand smoke-exposed children and their home environments. Pediatr. Res. 2021, 90, 1153–1160. [Google Scholar] [CrossRef]
- Sun, Y.; Tang, H.; Du, S.; Chen, Y.; Ou, Z.; Zhang, M.; Chen, Z.; Tang, Z.; Zhang, D.; Chen, T.; et al. Indoor metabolites and chemicals outperform microbiome in classifying childhood asthma and allergic rhinitis. Eco-Environ. Health 2023, 2, 208–218. [Google Scholar] [CrossRef]
- Roslund, M.I.; Rantala, S.; Oikarinen, S.; Puhakka, R.; Hui, N.; Parajuli, A.; Laitinen, O.H.; Hyöty, H.; Rantalainen, A.L.; Sinkkonen, A. Endocrine disruption and commensal bacteria alteration associated with gaseous and soil PAH contamination among daycare children. Environ. Int. 2019, 130, 104894. [Google Scholar] [CrossRef] [PubMed]
- Lehtimäki, J.; Laatikainen, T.; Karkman, A.; Von Hertzen, L.; Haahtela, T.; Hanski, I.; Ruokolainen, L. Nature-oriented daycare diversifies skin microbiota in children—No robust association with allergies. Pediatr. Allergy Immunol. 2018, 29, 318–321. [Google Scholar] [CrossRef]
- Lehtimäki, J.; Karkman, A.; Laatikainen, T.; Paalanen, L.; Von Hertzen, L.; Haahtela, T.; Hanski, I.; Ruokolainen, L. Patterns in the skin microbiota differ in children and teenagers between rural and urban environments. Sci. Rep. 2017, 7, 45651. [Google Scholar] [CrossRef]
- Gardner, C.M.; Hoffman, K.; Stapleton, H.M.; Gunsch, C.K. Exposures to Semivolatile Organic Compounds in Indoor Environments and Associations with the Gut Microbiomes of Children. Environ. Sci. Technol. Lett. 2021, 8, 73–79. [Google Scholar] [CrossRef]
- Sun, Y.; Meng, Y.; Ou, Z.; Li, Y.; Zhang, M.; Chen, Y.; Zhang, Z.; Chen, X.; Mu, P.; Norbäck, D.; et al. Indoor microbiome, air pollutants and asthma, rhinitis and eczema in preschool children—A repeated cross-sectional study. Environ. Int. 2022, 161, 107137. [Google Scholar] [CrossRef]
- Roslund, M.I.; Puhakka, R.; Nurminen, N.; Oikarinen, S.; Siter, N.; Grönroos, M.; Cinek, O.; Kramná, L.; Jumpponen, A.; Laitinen, O.H. Long-term biodiversity intervention shapes health-associated commensal microbiota among urban day-care children. Environ. Int. 2021, 157, 106811. [Google Scholar] [CrossRef]
- Mhuireach, G.Á.; Fahimipour, A.K.; Vandegrift, R.; Muscarella, M.E.; Hickey, R.; Bateman, A.C.; Van Den Wymelenberg, K.G.; Bohannan, B.J.M. Temporary establishment of bacteria from indoor plant leaves and soil on human skin. Environ. Microbiome 2022, 17, 61. [Google Scholar] [CrossRef] [PubMed]
- Brown, M.; Reeser, G.; Shinn, L.; Browning, M.; Schwingel, A.; Khan, N.; Holscher, H. Environmental Influences on the Human Gut Microbiota: A Longitudinal Pilot Study. Curr. Dev. Nutr. 2021, 5, 1151. [Google Scholar] [CrossRef]
- Kirjavainen, P.V.; Karvonen, A.M.; Adams, R.I.; Täubel, M.; Roponen, M.; Tuoresmäki, P.; Loss, G.; Jayaprakash, B.; Depner, M.; Ege, M.J. Farm-like indoor microbiota in non-farm homes protects children from asthma development. Nat. Med. 2019, 25, 1089–1095. [Google Scholar] [CrossRef]
- Birzele, L.T.; Depner, M.; Ege, M.J.; Engel, M.; Kublik, S.; Bernau, C.; Loss, G.J.; Genuneit, J.; Horak, E.; Schloter, M.; et al. Environmental and mucosal microbiota and their role in childhood asthma. Allergy 2017, 72, 109–119. [Google Scholar] [CrossRef] [PubMed]
- Casas, L.; Karvonen, A.M.; Kirjavainen, P.V.; Täubel, M.; Hyytiäinen, H.; Jayaprakash, B.; Lehmann, I.; Standl, M.; Pekkanen, J.; Heinrich, J. Early life home microbiome and hyperactivity/inattention in school-age children. Sci. Rep. 2019, 9, 17355. [Google Scholar] [CrossRef] [PubMed]
- Roslund, M.I.; Puhakka, R.; Grönroos, M.; Nurminen, N.; Oikarinen, S.; Gazali, A.M.; Cinek, O.; Kramná, L.; Siter, N.; Vari, H.K.; et al. Biodiversity intervention enhances immune regulation and health-associated commensal microbiota among daycare children. Sci. Adv. 2020, 6, eaba2578. [Google Scholar] [CrossRef]
- Beasley, D.; Monsur, M.; Hu, J.; Dunn, R.; Madden, A. The bacterial community of childcare centers: Potential implications for microbial dispersal and child exposure. Environ. Microbiome 2022, 17, 8. [Google Scholar] [CrossRef]
- Gupta, S.; Hjelmsø, M.H.; Lehtimäki, J.; Li, X.; Mortensen, M.S.; Russel, J.; Trivedi, U.; Rasmussen, M.A.; Stokholm, J.; Bisgaard, H.; et al. Environmental shaping of the bacterial and fungal community in infant bed dust and correlations with the airway microbiota. Microbiome 2020, 8, 115. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, C.C.; Gascon, M.; Osornio-Vargas, A.R.; Shier, C.; Guttman, D.S.; Becker, A.B.; Azad, M.B.; Sears, M.R.; Lefebvre, D.L.; Moraes, T.J.; et al. Natural environments in the urban context and gut microbiota in infants. Environ. Int. 2020, 142, 105881. [Google Scholar] [CrossRef]
- Selway, C.A.; Mills, J.G.; Weinstein, P.; Skelly, C.; Yadav, S.; Lowe, A.; Breed, M.F.; Weyrich, L.S. Transfer of environmental microbes to the skin and respiratory tract of humans after urban green space exposure. Environ. Int. 2020, 145, 106084. [Google Scholar] [CrossRef]
- Lai, P.S.; Kolde, R.; Franzosa, E.A.; Gaffin, J.M.; Baxi, S.N.; Sheehan, W.J.; Gold, D.R.; Gevers, D.; Xavier, R.J.; Phipatanakul, W. The classroom microbiome and asthma morbidity in children attending 3 inner-city schools. J. Allergy Clin. Immunol. 2018, 141, 2311–2313. [Google Scholar] [CrossRef]
- Prussin, A.J.; Vikram, A.; Bibby, K.J.; Marr, L.C. Seasonal Dynamics of the Airborne Bacterial Community and Selected Viruses in a Children’s Daycare Center. PLoS ONE 2016, 11, e0151004. [Google Scholar] [CrossRef]
- Shin, S.-K.; Kim, J.; Ha, S.-M.; Oh, H.-S.; Chun, J.; Sohn, J.; Yi, H. Metagenomic Insights into the Bioaerosols in the Indoor and Outdoor Environments of Childcare Facilities. PLoS ONE 2015, 10, e0126960. [Google Scholar] [CrossRef]




| Inclusion Criteria | Exclusion Criteria |
|---|---|
| 1. Articles published from 2010 to 2024 | 1. Unavailable full text |
| 2. English language | 2. Focus on children with Autism Spectrum Disorder (ASD) |
| 3. Focus on the built environment | 3. Not related to the human factor and/or occupant factor |
| 4. Focus on children | 4. Study with adults |
| Children | Child * OR Early child * OR Preschool * OR Kid OR Kindergarten OR Young child * OR School-aged child * OR Youth |
| Microbiome | Microbial OR Microbiology OR Microorganism * OR Microbe * |
| Built Environment | Outdoor OR Nature * OR Veg * OR Indoor OR Surface OR Childcare OR Home OR Land * OR Physical * OR Environment * OR Soil OR Air OR Water OR Bio * OR Rural OR Urban OR Build * OR Expos * OR Vent * |
| STUDY TOPIC & PAPER ID | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | 17 | 18 | 19 | 20 | 21 | 22 | 23 | 24 | 25 | 26 | 27 | 28 | 29 | 30 | 31 | 32 | 33 | 34 | 35 | 36 | 37 | 38 | 39 | 40 | 41 | 42 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1. Impact of Environmental Exposure on Human Microbiome | ||||||||||||||||||||||||||||||||||||||||||
| What is the variation between microbial diversity of taxa between home and daycare environments? | ||||||||||||||||||||||||||||||||||||||||||
| How does pesticide exposure influence the oral microbiome composition of the children of farmworkers? | ||||||||||||||||||||||||||||||||||||||||||
| How do indoor microbiome and metabolites influence the diversity of the gut microbiota in children?? | ||||||||||||||||||||||||||||||||||||||||||
| How do environmental exposures, both indoor and outdoor, influence the microbiome composition in children? | ||||||||||||||||||||||||||||||||||||||||||
| How does the living environment (rural vs. urban) influence the composition and diversity of skin microbiota? | ||||||||||||||||||||||||||||||||||||||||||
| 2. Influence of Nature and Biodiversity on Microbial Diversity | ||||||||||||||||||||||||||||||||||||||||||
| How does the composition of the airborne microbiome in indoor environments influenced by biophilic design? | ||||||||||||||||||||||||||||||||||||||||||
| How do nature-based solutions influence microbiome diversity in early childhood educational settings? | ||||||||||||||||||||||||||||||||||||||||||
| How does exposure to biodiversity-enriched/ natural environments affect children’s microbiota? | ||||||||||||||||||||||||||||||||||||||||||
| What are the impacts of outdoor nature-related activities on gut microbiota and psychological health of children? | ||||||||||||||||||||||||||||||||||||||||||
| How does direct contact with soil/plant materials altering skin microbiota? | ||||||||||||||||||||||||||||||||||||||||||
| 3. Microbial Transfer & Exposure Mechanisms | ||||||||||||||||||||||||||||||||||||||||||
| Can indoor plant leaves and soil bacteria temporarily establish themselves on human skin after physical contact? | ||||||||||||||||||||||||||||||||||||||||||
| How does the microbial composition vary between different surfaces and locations within childcare centers? | ||||||||||||||||||||||||||||||||||||||||||
| How do children’s behaviors and soil pollution contribute to their exposure to soil contaminants? | ||||||||||||||||||||||||||||||||||||||||||
| How does exposure to urban green spaces affect the transfer and diversity of environmental microbes to human skin? | ||||||||||||||||||||||||||||||||||||||||||
| 4. Microbiome-Health Relationships (Respiratory, Immune, Allergies, Asthma) | ||||||||||||||||||||||||||||||||||||||||||
| How are microbiome variations associated with allergic and non-allergic rhinitis symptoms among students? | ||||||||||||||||||||||||||||||||||||||||||
| How do the profiles of indoor metabolites and chemicals differ between homes with/without asthma or allergic rhinitis? | ||||||||||||||||||||||||||||||||||||||||||
| How does exposure to thirdhand smoke (THS) alter the microbiomes of children and their home environments? | ||||||||||||||||||||||||||||||||||||||||||
| How do indoor microbiome composition and environmental factors influence the respiratory microbiota/asthma/allergy? | ||||||||||||||||||||||||||||||||||||||||||
| How does SVOC exposure affect gut microbiome and dysbiosis? | ||||||||||||||||||||||||||||||||||||||||||
| How does the Indoor microbiome diversity influence asthma/ hyperactivity/ inattention symptoms? | ||||||||||||||||||||||||||||||||||||||||||
| How does exposure to PAHs affect the commensal microbiota of children and disrupt endocrine signaling pathways? | ||||||||||||||||||||||||||||||||||||||||||
| How does exposure to farm environments influencing microbiome and asthma risks? | ||||||||||||||||||||||||||||||||||||||||||
| 5. Indoor Environmental Factors and Microbial Communities | ||||||||||||||||||||||||||||||||||||||||||
| How does the diversity of fungal microbiota in indoor dust relate to cognitive and behavioral outcomes in children? | ||||||||||||||||||||||||||||||||||||||||||
| How do indoor emissions and outdoor ventilation contribute to the microbial communities? | ||||||||||||||||||||||||||||||||||||||||||
| How does the microbial community composition in the dust of a newly opened kindergarten change over time? | ||||||||||||||||||||||||||||||||||||||||||
| How do human occupancy and seasonal changes influence airborne microbiota in daycare centers? | ||||||||||||||||||||||||||||||||||||||||||
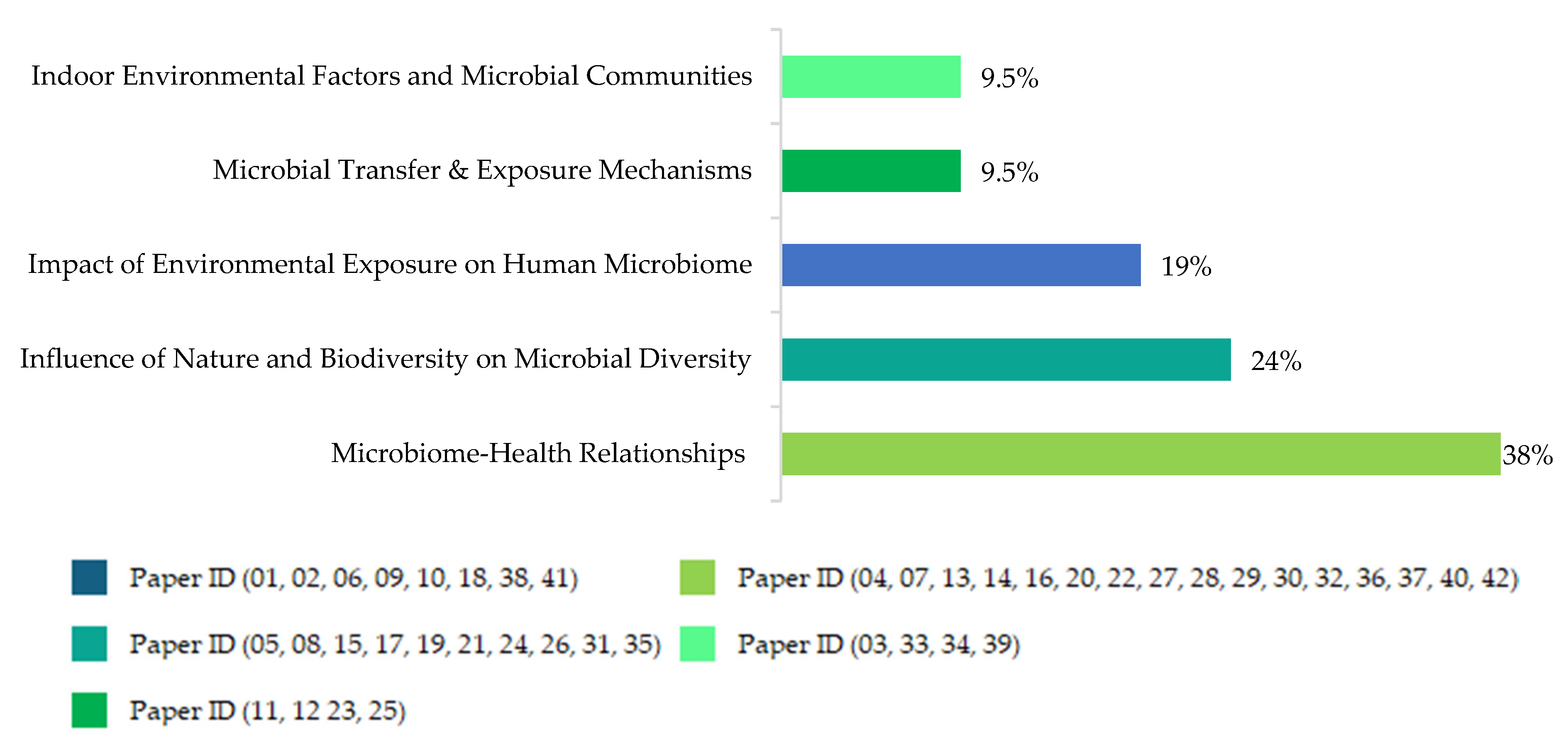
 , 2. Influence of Nature and Biodiversity on Microbial Diversity =
, 2. Influence of Nature and Biodiversity on Microbial Diversity =  , 3. Microbial Transfer & Exposure Mechanisms =
, 3. Microbial Transfer & Exposure Mechanisms =  , 4. Microbiome-Health Relationships (Respiratory, Immune, Allergies, Asthma) =
, 4. Microbiome-Health Relationships (Respiratory, Immune, Allergies, Asthma) =  , 5. Indoor Environmental Factors and Microbial Communities =
, 5. Indoor Environmental Factors and Microbial Communities =  .
.THEMATIC STREAMS OF RESEARCH QUESTIONS AND THEIR ASSOCIATED PAPER IDs | INDEPENDENT VARIABLES | DEPENDENT VARIABLES | ||||||||||||||||||||||||||||||||||||||||||
| Environmental contexts: | Microbial diversity and composition: | Environmental exposure: | Human Activities and Behaviors: | Temporal/spatial dynamics: | Health and disease outcomes: | Microbial diversity and composition: | Cognitive and behavioral outcomes: | |||||||||||||||||||||||||||||||||||||
| Home vs. daycare | Farm vs. non-farm | Nature-oriented vs. conventional daycare | Urban vs. rural environment | Indoor vs. outdoor environments | Nature-based solutions/ Biodiversity intervention | Indoor plants (leaves and soil) | Gardening families vs non-gardening families | Bacterial/fungal/ microbial communities | Dust microbiota | Surface-specific microbiota | Semivolatile organic compounds (SVOCs) | Pesticides (e.g., azinphos-methyl) | Polycyclic aromatic hydrocarbons (PAHs) | Outdoor/indoor air pollutants (e.g., NO2) | Indoor relative humidity | Urban green | THS exposure | Soil pollution | Dietary habits | Occupational Status | Pet exposure | Personal hygiene practices | Children’s behaviors | Seasonal changes | Spatiotemporal variation | Occupant density/ Room usage | Ventilation rates | Time and duration of exposure | Allergic and non-allergic rhinitis (AR/NAR) | Asthma, Eczema, Respiratory infections | Immune health | Psychological well-being | Antibiotic resistance genes (ARGs) | Gut, Oral, Skin, Nasal, airway microbiota | Indoor and outdoor microbial community | Dysbiosis (unhealthy microbial balance) | Non-pathogenic environmental mycobacteria | Human-associated microbial taxa | Fungal/ Bacterial community composition | Cognitive function | Hyperactivity/inattention | Behavioral outcomes in children | Psychobehavioral development | |
| Impact of Environmental Exposure on Human Microbiome (01, 02, 06, 09, 10, 18, 38, 41) | ||||||||||||||||||||||||||||||||||||||||||||
| Influence of Nature and Biodiversity on Microbial Diversity (05, 08, 15, 17, 19, 21, 24, 26, 31, 35) | ||||||||||||||||||||||||||||||||||||||||||||
| Microbial Transfer & Exposure Mechanisms (11, 12 23, 25) | ||||||||||||||||||||||||||||||||||||||||||||
| Microbiome-Health Relationships (Respiratory, Immune, Allergies, Asthma) (04, 07, 13, 14, 16, 20, 22, 27, 28, 29, 30, 32, 36, 37, 40, 42) | ||||||||||||||||||||||||||||||||||||||||||||
| Indoor Environmental Factors and Microbial Communities (03, 33, 34, 39) | ||||||||||||||||||||||||||||||||||||||||||||
 , 2. Influence of Nature and Biodiversity on Microbial Diversity =
, 2. Influence of Nature and Biodiversity on Microbial Diversity =  , 3. Microbial Transfer & Exposure Mechanisms =
, 3. Microbial Transfer & Exposure Mechanisms =  , 4. Microbiome-Health Relationships (Respiratory, Immune, Allergies, Asthma) =
, 4. Microbiome-Health Relationships (Respiratory, Immune, Allergies, Asthma) =  , 5. Indoor Environmental Factors and Microbial Communities =
, 5. Indoor Environmental Factors and Microbial Communities =  .
.| Combined Sample | Sample Types | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Dust | Feces | Soil | Nose | Skin | Mouth | Ear | Urine | Blood | Air | Leaf | Potting soil | |
| Type 01 | ||||||||||||
| Type 02 | ||||||||||||
| Type 03 | ||||||||||||
| Type 04 | ||||||||||||
| Type 05 | ||||||||||||
| Type 06 | ||||||||||||
| Type 07 | ||||||||||||
| Type 08 | ||||||||||||
| Type 09 | ||||||||||||
| Type 10 | ||||||||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Samira, R.; Monsur, M.; Trina, N.A. How the Built Environment Shapes Children’s Microbiome: A Systematic Review. Microorganisms 2025, 13, 950. https://doi.org/10.3390/microorganisms13040950
Samira R, Monsur M, Trina NA. How the Built Environment Shapes Children’s Microbiome: A Systematic Review. Microorganisms. 2025; 13(4):950. https://doi.org/10.3390/microorganisms13040950
Chicago/Turabian StyleSamira, Rozalynne, Muntazar Monsur, and Nazia Afrin Trina. 2025. "How the Built Environment Shapes Children’s Microbiome: A Systematic Review" Microorganisms 13, no. 4: 950. https://doi.org/10.3390/microorganisms13040950
APA StyleSamira, R., Monsur, M., & Trina, N. A. (2025). How the Built Environment Shapes Children’s Microbiome: A Systematic Review. Microorganisms, 13(4), 950. https://doi.org/10.3390/microorganisms13040950






