Hypericin Suppresses SARS-CoV-2 Replication and Synergizes with Antivirals via Dual Targeting of RdRp and 3CLpro
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Culture
2.2. SARS-CoV-2 Isolates
2.3. Compounds
2.4. Viral Titration
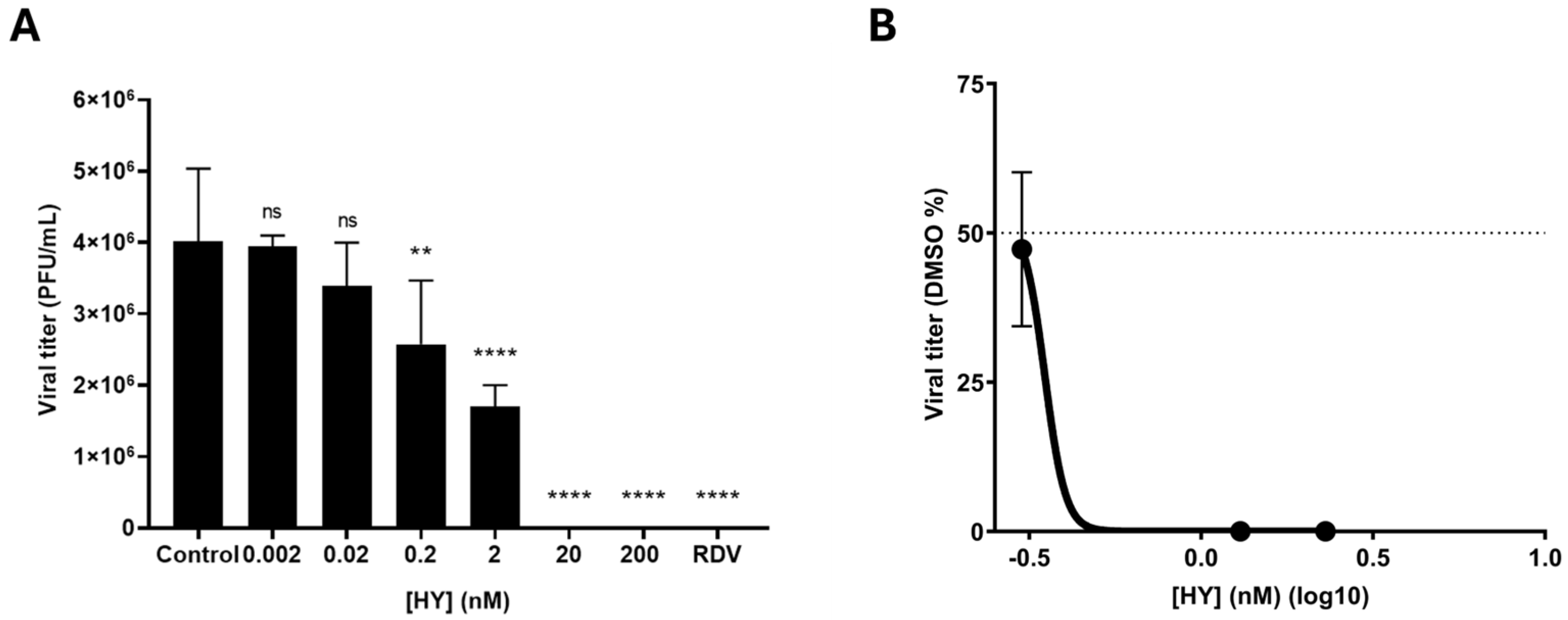
2.5. HY IC50 Determination In Vitro
2.6. Cytotoxicity Assays
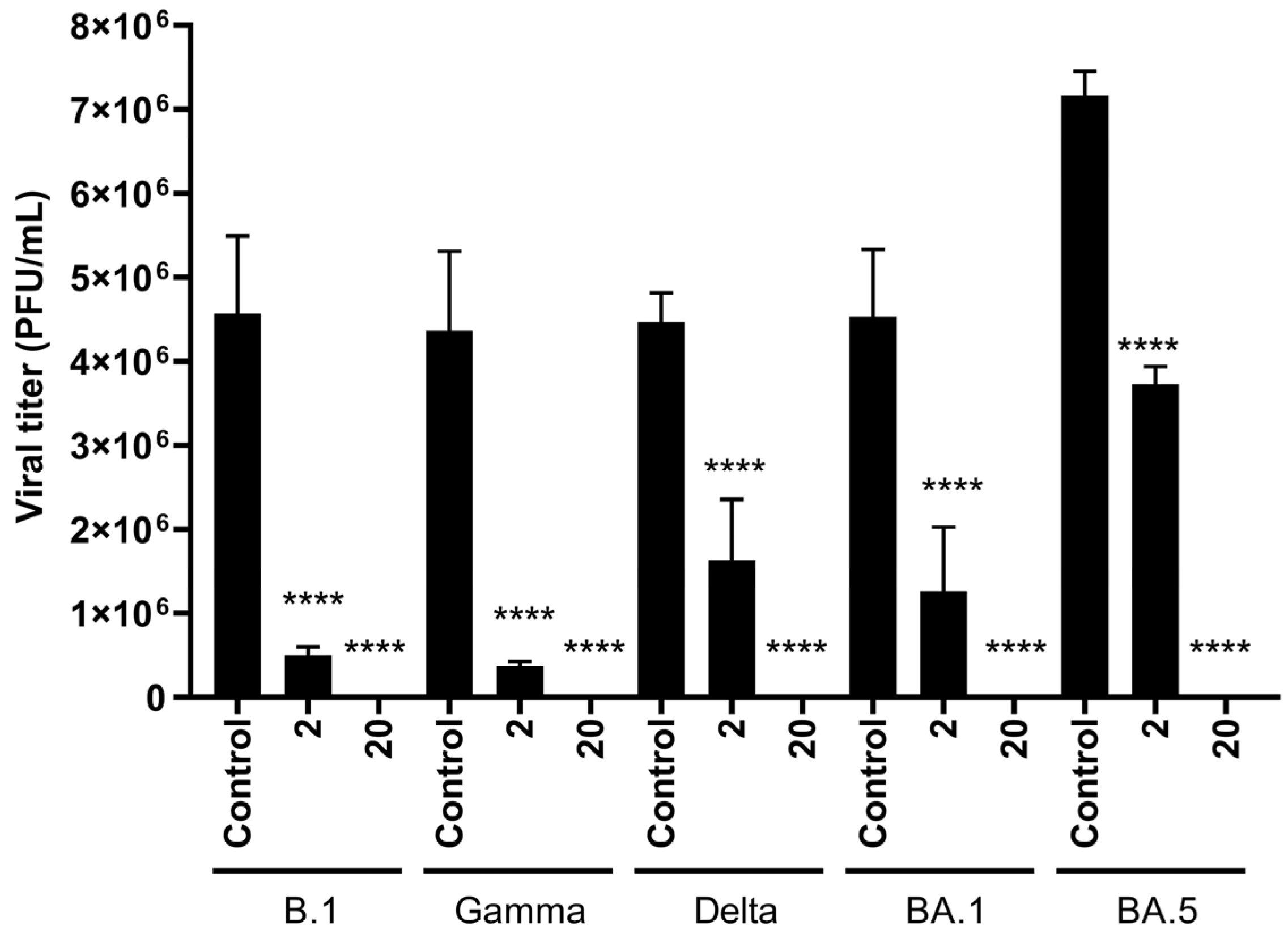
2.7. Evaluation of HY Antiviral Activity Against SARS-CoV-2 Variants
2.8. Determination of HY Pre- and Post-Infection Antiviral Effect
2.9. Drug Combination Assays
2.10. Evaluation of HY Antiviral Effect Against Influenza A(H1N1)pdm09 and DENV-2
2.11. SARS-CoV-2 3CLpro and RdRp Initial Structures
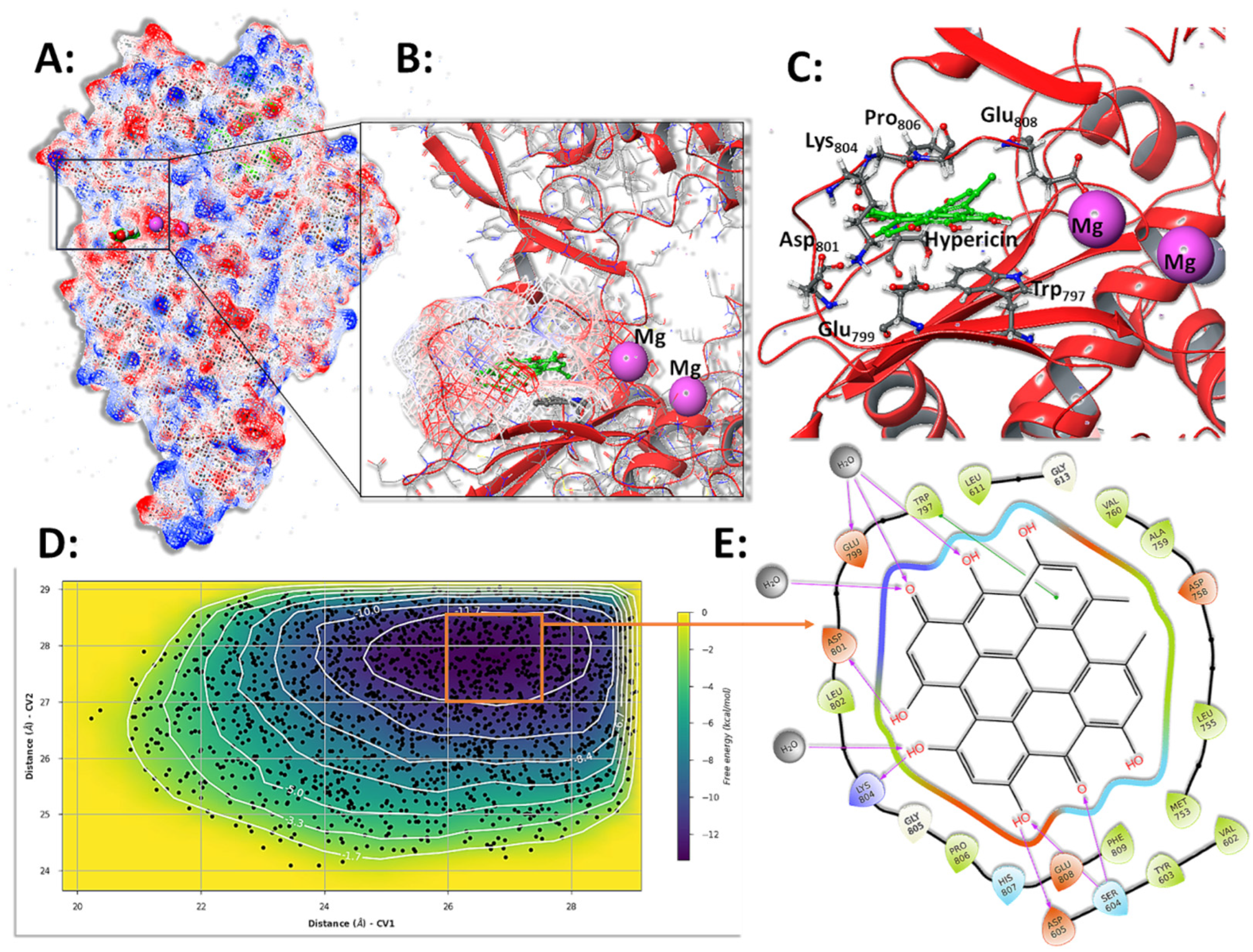
2.12. Molecular Simulation of the 3CLpro and RdRp in Complex with HY
2.13. Metadynamics Experiments
2.14. Bioinformatic Analysis of 3CLpro Conservation
2.15. Statistical Analysis
3. Results
3.1. HY Inhibits SARS-CoV-2 in a Concentration-Dependent Manner
3.2. HY Shows No Antiviral Activity Against RNA Viruses Such as Influenza A and DENV-2
3.3. HY Displays Broad-Spectrum Antiviral Activity Against SARS-CoV-2 Variants
3.4. HY Exhibits Post-Infection and Virucidal Activity Against SARS-CoV-2
3.5. Combination Therapy of HY with RDV and NMV Enhances Viral Inhibition
3.6. Molecular Simulations and Metadynamics of SARS-CoV-2 3CLpro in Complex with HY
3.7. Molecular Simulations and Metadynamics of SARS-CoV-2 RdRp in Complex with HY
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| MDPI | Multidisciplinary Digital Publishing Institute |
| DOAJ | Directory of open access journals |
| TLA | Three letter acronym |
| LD | Linear dichroism |
References
- Hu, B.; Guo, H.; Zhou, P.; Shi, Z.L. Characteristics of SARS-CoV-2 and COVID-19. Nat. Rev. Microbiol. 2020, 19, 141–154. [Google Scholar] [CrossRef]
- Lamers, M.M.; Haagmans, B.L. SARS-CoV-2 Pathogenesis. Nat. Rev. Microbiol. 2022, 20, 270–284. [Google Scholar] [CrossRef] [PubMed]
- Scovino, A.M.; Dahab, E.C.; Vieira, G.F.; Freire-de-Lima, L.; Freire-de-Lima, C.G.; Morrot, A. SARS-CoV-2’s Variants of Concern: A Brief Characterization. Front. Immunol. 2022, 13, 834098. [Google Scholar] [CrossRef] [PubMed]
- Markov, P.V.; Ghafari, M.; Beer, M.; Lythgoe, K.; Simmonds, P.; Stilianakis, N.I.; Katzourakis, A. The Evolution of SARS-CoV-2. Nat. Rev. Microbiol. 2023, 21, 361–379. [Google Scholar] [CrossRef]
- Carabelli, A.M.; Peacock, T.P.; Thorne, L.G.; Harvey, W.T.; Hughes, J.; de Silva, T.I.; Peacock, S.J.; Barclay, W.S.; de Silva, T.I.; Towers, G.J.; et al. SARS-CoV-2 Variant Biology: Immune Escape, Transmission and Fitness. Nat. Rev. Microbiol. 2023, 21, 162–177. [Google Scholar] [CrossRef]
- Harvey, W.T.; Carabelli, A.M.; Jackson, B.; Gupta, R.K.; Thomson, E.C.; Harrison, E.M.; Ludden, C.; Reeve, R.; Rambaut, A.; Peacock, S.J.; et al. SARS-CoV-2 Variants, Spike Mutations and Immune Escape. Nat. Rev. Microbiol. 2021, 19, 409–424. [Google Scholar] [CrossRef]
- Ilmjärv, S.; Abdul, F.; Acosta-Gutiérrez, S.; Estarellas, C.; Galdadas, I.; Casimir, M.; Alessandrini, M.; Gervasio, F.L.; Krause, K.-H. Concurrent Mutations in RNA-Dependent RNA Polymerase and Spike Protein Emerged as the Epidemiologically Most Successful SARS-CoV-2 Variant. Sci. Rep. 2021, 11, 13705. [Google Scholar] [CrossRef]
- Ip, J.D.; Wing-Ho Chu, A.; Chan, W.-M.; Cheuk-Ying Leung, R.; Umer Abdullah, S.M.; Sun, Y.; To, K.K.-W. Global Prevalence of SARS-CoV-2 3CL Protease Mutations Associated with Nirmatrelvir or Ensitrelvir Resistance. eBioMedicine 2023, 91, 104559. [Google Scholar] [CrossRef]
- Muhar, B.K.; Nehira, J.; Malhotra, A.; Kotchoni, S.O. The Race for COVID-19 Vaccines: The Various Types and Their Strengths and Weaknesses. J. Pharm. Pract. 2023, 36, 953–966. [Google Scholar] [CrossRef]
- Rydland, H.T.; Friedman, J.; Stringhini, S.; Link, B.G.; Eikemo, T.A. The Radically Unequal Distribution of COVID-19 Vaccinations: A Predictable yet Avoidable Symptom of the Fundamental Causes of Inequality. Humanit. Soc. Sci. Commun. 2022, 9, 61. [Google Scholar] [CrossRef]
- Beladiya, J.; Kumar, A.; Vasava, Y.; Parmar, K.; Patel, D.; Patel, S.; Dholakia, S.; Sheth, D.; Boddu, S.H.S.; Patel, C. Safety and Efficacy of COVID-19 Vaccines: A Systematic Review and Meta-analysis of Controlled and Randomized Clinical Trials. Rev. Med. Virol. 2024, 34, e2507. [Google Scholar] [CrossRef]
- Islam, M.A. A Review of SARS-CoV-2 Variants and Vaccines: Viral Properties, Mutations, Vaccine Efficacy, and Safety. Infect. Med. 2023, 2, 247–261. [Google Scholar] [CrossRef]
- Li, G.; Hilgenfeld, R.; Whitley, R.; De Clercq, E. Therapeutic Strategies for COVID-19: Progress and Lessons Learned. Nat. Rev. Drug Discov. 2023, 22, 449–475. [Google Scholar] [CrossRef]
- Cao, X. COVID-19: Immunopathology and Its Implications for Therapy. Nat. Rev. Immunol. 2020, 20, 269–270. [Google Scholar] [CrossRef]
- Yuki, K.; Fujiogi, M.; Koutsogiannaki, S. COVID-19 Pathophysiology: A Review. Clin. Immunol. 2020, 215, 108427. [Google Scholar] [CrossRef]
- Russell, C.D.; Lone, N.I.; Baillie, J.K. Comorbidities, Multimorbidity and COVID-19. Nat. Med. 2023, 29, 334–343. [Google Scholar] [CrossRef] [PubMed]
- Mehta, P.; McAuley, D.F.; Brown, M.; Sanchez, E.; Tattersall, R.S.; Manson, J.J. COVID-19: Consider Cytokine Storm Syndromes and Immunosuppression. Lancet 2020, 395, 1033–1034. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Xie, X.; Tu, Z.; Fu, J.; Xu, D.; Zhou, Y. The Signal Pathways and Treatment of Cytokine Storm in COVID-19. Signal Transduct. Target. Ther. 2021, 6, 255. [Google Scholar] [CrossRef] [PubMed]
- Osuchowski, M.F.; Winkler, M.S.; Skirecki, T.; Cajander, S.; Shankar-Hari, M.; Lachmann, G.; Monneret, G.; Venet, F.; Bauer, M.; Brunkhorst, F.M.; et al. The COVID-19 Puzzle: Deciphering Pathophysiology and Phenotypes of a New Disease Entity. Lancet Respir. Med. 2021, 9, 622–642. [Google Scholar] [CrossRef]
- Toussi, S.S.; Hammond, J.L.; Gerstenberger, B.S.; Anderson, A.S. Therapeutics for COVID-19. Nat. Microbiol. 2023, 8, 771–786. [Google Scholar] [CrossRef]
- Yuan, Y.; Jiao, B.; Qu, L.; Yang, D.; Liu, R. The Development of COVID-19 Treatment. Front. Immunol. 2023, 14, 1125246. [Google Scholar] [CrossRef] [PubMed]
- van de Veerdonk, F.L.; Giamarellos-Bourboulis, E.; Pickkers, P.; Derde, L.; Leavis, H.; van Crevel, R.; Engel, J.J.; Wiersinga, W.J.; Vlaar, A.P.J.; Shankar-Hari, M.; et al. A Guide to Immunotherapy for COVID-19. Nat. Med. 2022, 28, 39–50. [Google Scholar] [CrossRef] [PubMed]
- V’kovski, P.; Kratzel, A.; Steiner, S.; Stalder, H.; Thiel, V. Coronavirus Biology and Replication: Implications for SARS-CoV-2. Nat. Rev. Microbiol. 2021, 19, 155–170. [Google Scholar] [CrossRef]
- Steiner, S.; Kratzel, A.; Barut, G.T.; Lang, R.M.; Aguiar Moreira, E.; Thomann, L.; Kelly, J.N.; Thiel, V. SARS-CoV-2 Biology and Host Interactions. Nat. Rev. Microbiol. 2024, 22, 206–225. [Google Scholar] [CrossRef]
- Vangeel, L.; Chiu, W.; De Jonghe, S.; Maes, P.; Slechten, B.; Raymenants, J.; André, E.; Leyssen, P.; Neyts, J.; Jochmans, D. Remdesivir, Molnupiravir and Nirmatrelvir Remain Active against SARS-CoV-2 Omicron and Other Variants of Concern. Antivir. Res. 2022, 198, 105252. [Google Scholar] [CrossRef] [PubMed]
- Ford, E.S.; Simmons, W.; Karmarkar, E.N.; Yoke, L.H.; Braimah, A.B.; Orozco, J.J.; Ghiuzeli, C.M.; Barnhill, S.; Sack, C.L.; Benditt, J.O.; et al. Successful Treatment of Prolonged, Severe Coronavirus Disease 2019 Lower Respiratory Tract Disease in a B Cell Acute Lymphoblastic Leukemia Patient With an Extended Course of Remdesivir and Nirmatrelvir/Ritonavir. Clin. Infect. Dis. 2023, 76, 926–929. [Google Scholar] [CrossRef]
- Murakami, N.; Hayden, R.; Hills, T.; Al-Samkari, H.; Casey, J.; Del Sorbo, L.; Lawler, P.R.; Sise, M.E.; Leaf, D.E. Therapeutic Advances in COVID-19. Nat. Rev. Nephrol. 2023, 19, 38–52. [Google Scholar] [CrossRef]
- Stadler, E.; Burgess, M.T.; Schlub, T.E.; Khan, S.R.; Chai, K.L.; McQuilten, Z.K.; Wood, E.M.; Polizzotto, M.N.; Kent, S.J.; Cromer, D.; et al. Monoclonal Antibody Levels and Protection from COVID-19. Nat. Commun. 2023, 14, 4545. [Google Scholar] [CrossRef]
- Iketani, S.; Mohri, H.; Culbertson, B.; Hong, S.J.; Duan, Y.; Luck, M.I.; Annavajhala, M.K.; Guo, Y.; Sheng, Z.; Uhlemann, A.C.; et al. Multiple Pathways for SARS-CoV-2 Resistance to Nirmatrelvir. Nature 2023, 613, 558–564. [Google Scholar] [CrossRef]
- Stevens, L.J.; Pruijssers, A.J.; Lee, H.W.; Gordon, C.J.; Tchesnokov, E.P.; Gribble, J.; George, A.S.; Hughes, T.M.; Lu, X.; Li, J.; et al. Mutations in the SARS-CoV-2 RNA-Dependent RNA Polymerase Confer Resistance to Remdesivir by Distinct Mechanisms. Sci. Transl. Med. 2022, 14, eabo0718. [Google Scholar] [CrossRef]
- Nooruzzaman, M.; Johnson, K.E.E.; Rani, R.; Finkelsztein, E.J.; Caserta, L.C.; Kodiyanplakkal, R.P.; Wang, W.; Hsu, J.; Salpietro, M.T.; Banakis, S.; et al. Emergence of Transmissible SARS-CoV-2 Variants with Decreased Sensitivity to Antivirals in Immunocompromised Patients with Persistent Infections. Nat. Commun. 2024, 15, 7999. [Google Scholar] [CrossRef] [PubMed]
- Lenard, J.; Rabson, A.; Vanderoef, R. Photodynamic Inactivation of Infectivity of Human Immunodeficiency Virus and Other Enveloped Viruses Using Hypericin and Rose Bengal: Inhibition of Fusion and Syncytia Formation. Proc. Natl. Acad. Sci. USA 1993, 90, 158–162. [Google Scholar] [CrossRef]
- Jacobson, J.M.; Feinman, L.; Liebes, L.; Ostrow, N.; Koslowski, V.; Tobia, A.; Cabana, B.E.; Lee, D.H.; Spritzler, J.; Prince, A.M. Pharmacokinetics, Safety, and Antiviral Effects of Hypericin, a Derivative of St. John’s Wort Plant, in Patients with Chronic Hepatitis C Virus Infection. Antimicrob. Agents Chemother. 2001, 45, 517–524. [Google Scholar] [CrossRef]
- Pu, X.Y.; Liang, J.P.; Wang, X.H.; Xu, T.; Hua, L.Y.; Shang, R.F.; Liu, Y.; Xing, Y.M. Anti-Influenza A Virus Effect of Hypericum Perforatum L. Extract. Virol. Sin. 2009, 24, 19–27. [Google Scholar] [CrossRef]
- Shih, C.M.; Wu, C.H.; Wu, W.J.; Hsiao, Y.M.; Ko, J.L. Hypericin Inhibits Hepatitis C Virus Replication via Deacetylation and Down-Regulation of Heme Oxygenase-1. Phytomedicine 2018, 46, 193–198. [Google Scholar] [CrossRef]
- Chen, H.; Muhammad, I.; Zhang, Y.; Ren, Y.; Zhang, R.; Huang, X.; Diao, L.; Liu, H.; Li, X.; Sun, X.; et al. Antiviral Activity against Infectious Bronchitis Virus and Bioactive Components of Hypericum perforatum L. Front. Pharmacol. 2019, 10, 470683. [Google Scholar] [CrossRef]
- Rook, A.H.; Wood, G.S.; Duvic, M.; Vonderheid, E.C.; Tobia, A.; Cabana, B. A Phase II Placebo-Controlled Study of Photodynamic Therapy with Topical Hypericin and Visible Light Irradiation in the Treatment of Cutaneous T-Cell Lymphoma and Psoriasis. J. Am. Acad. Dermatol. 2010, 63, 984–990. [Google Scholar] [CrossRef]
- Dong, X.; Zeng, Y.; Zhang, Z.; Fu, J.; You, L.; He, Y.; Hao, Y.; Gu, Z.; Yu, Z.; Qu, C.; et al. Hypericin-Mediated Photodynamic Therapy for the Treatment of Cancer: A Review. J. Pharm. Pharmacol. 2021, 73, 425–436. [Google Scholar] [CrossRef]
- Matos, A.d.R.; Caetano, B.C.; de Almeida Filho, J.L.; Martins, J.S.C.d.C.; de Oliveira, M.G.P.; Sousa, T.d.C.; Horta, M.A.P.; Siqueira, M.M.; Fernandez, J.H. Identification of Hypericin as a Candidate Repurposed Therapeutic Agent for COVID-19 and Its Potential Anti-SARS-CoV-2 Activity. Front. Microbiol. 2022, 13, 828984. [Google Scholar] [CrossRef]
- Mohamed, F.F.; Anhlan, D.; Schöfbänker, M.; Schreiber, A.; Classen, N.; Hensel, A.; Hempel, G.; Scholz, W.; Kühn, J.; Hrincius, E.R.; et al. Hypericum Perforatum and Its Ingredients Hypericin and Pseudohypericin Demonstrate an Antiviral Activity against SARS-CoV-2. Pharmaceuticals 2022, 15, 530. [Google Scholar] [CrossRef]
- Delcanale, P.; Uriati, E.; Mariangeli, M.; Mussini, A.; Moreno, A.; Lelli, D.; Cavanna, L.; Bianchini, P.; Diaspro, A.; Abbruzzetti, S.; et al. The Interaction of Hypericin with SARS-CoV-2 Reveals a Multimodal Antiviral Activity. ACS Appl. Mater. Interfaces 2022, 14, 14025–14032. [Google Scholar] [CrossRef] [PubMed]
- Rosa, R.B.; Dantas, W.M.; do Nascimento, J.C.F.; da Silva, M.V.; de Oliveira, R.N.; Pena, L.J. In Vitro and In Vivo Models for Studying SARS-CoV-2, the Etiological Agent Responsible for COVID-19 Pandemic. Viruses 2021, 13, 379. [Google Scholar] [CrossRef]
- Barreto-vieira, D.F.; Alexandre, M.; Garcia, C.C.; Miranda, M.D.; Matos, R.; Caetano, B.C.; Resende, P.C.; Motta, F.C.; Siqueira, M.M.; Girard-dias, W.; et al. Morphology and Morphogenesis of SARS-CoV-2 in Vero-E6 Cells. Mem. Inst. Oswaldo Cruz 2021, 116, e200443. [Google Scholar] [CrossRef]
- Amorim, M.T.; Hernández, L.H.A.; Naveca, F.G.; Essashika Prazeres, I.T.; Wanzeller, A.L.M.; Silva, E.V.P.d.; Casseb, L.M.N.; Silva, F.S.d.; da Silva, S.P.; Nunes, B.T.D.; et al. Emergence of a New Strain of DENV-2 in South America: Introduction of the Cosmopolitan Genotype through the Brazilian-Peruvian Border. Trop. Med. Infect. Dis. 2023, 8, 325. [Google Scholar] [CrossRef] [PubMed]
- WHO. Information for the Molecular Detection of Influenza Viruses. It Is Strongly Recommended That All Un-Subtypeable Influenza A Specimens of Human Origin, That May Represent Either Seasonal Viruses Displaying Significant Genetic/Antigenic Drift or Zoonotic Viruses/Events with Pandemic Potential, Should Be Immediately Sent for Detailed Characterization to One of the Six WHO Collaborating Centres (WHO CC) 1 for Reference & Research on Influenza. 2021. Available online: https://cdn.who.int/media/docs/default-source/influenza/molecular-detention-of-influenza-viruses/protocols_influenza_virus_detection_feb_2021.pdf (accessed on 17 April 2025).
- Hatada, R.; Okuwaki, K.; Mochizuki, Y.; Handa, Y.; Fukuzawa, K.; Komeiji, Y.; Okiyama, Y.; Tanaka, S. Fragment Molecular Orbital Based Interaction Analyses on COVID-19 Main Protease - Inhibitor N3 Complex (PDB ID: 6LU7). J. Chem. Inf. Model. 2020, 60, 3593–3602. [Google Scholar] [CrossRef]
- Yin, W.; Mao, C.; Luan, X.; Shen, D.D.; Shen, Q.; Su, H.; Wang, X.; Zhou, F.; Zhao, W.; Gao, M.; et al. Structural Basis for Inhibition of the RNA-Dependent RNA Polymerase from SARS-CoV-2 by Remdesivir. Science 2020, 368, 1499–1504. [Google Scholar] [CrossRef] [PubMed]
- Filho, J.L.d.A.; del Real Tamariz, A.; Fernandez, J.H. AutoModel: A Client-Server Tool for Intuitive and Interactive Homology Modeling of Protein-Ligand Complexes. In Advances in Bioinformatics and Computational Biology; Springer: Cham, Switzerland, 2018; pp. 78–89. [Google Scholar] [CrossRef]
- Zielkiewicz, J. Structural Properties of Water: Comparison of the SPC, SPCE, TIP4P, and TIP5P Models of Water. J. Chem. Phys. 2005, 123, 104501. [Google Scholar] [CrossRef]
- Bergdorf, M.; Baxter, S.; Rendleman, C.A.; Shaw, D.E. Desmond/GPU Performance as of October 2015; TR--2015-01; D. E. Shaw Research: New York, NY, USA, 2015; pp. 1–10. [Google Scholar]
- Hoover, W.G. Canonical Dynamics: Equilibrium Phase-Space Distributions. Phys. Rev. A 1985, 31, 1695. [Google Scholar] [CrossRef]
- Martyna, G.J.; Tobias, D.J.; Klein, M.L. Constant Pressure Molecular Dynamics Algorithms. J. Chem. Phys. 1994, 101, 4177–4189. [Google Scholar] [CrossRef]
- Predescu, C.; Lerer, A.K.; Lippert, R.A.; Towles, B.; Grossman, J.P.; Dirks, R.M.; Shaw, D.E. The u -Series: A Separable Decomposition for Electrostatics Computation with Improved Accuracy. J. Chem. Phys. 2020, 152, 84113. [Google Scholar] [CrossRef]
- Chen, C.; Nadeau, S.; Yared, M.; Voinov, P.; Xie, N.; Roemer, C.; Stadler, T. CoV-Spectrum: Analysis of Globally Shared SARS-CoV-2 Data to Identify and Characterize New Variants. Bioinformatics 2022, 38, 1735–1737. [Google Scholar] [CrossRef]
- Barlow, P.; van Schalkwyk, M.C.; McKee, M.; Labonté, R.; Stuckler, D. COVID-19 and the Collapse of Global Trade: Building an Effective Public Health Response. Lancet Planet. Health 2021, 5, e102–e107. [Google Scholar] [CrossRef]
- Tang, J.; Colacino, J.M.; Larsen, S.H.; Spitzer, W. Virucidal Activity of Hypericin against Enveloped and Non-Enveloped DNA and RNA Viruses. Antivir. Res. 1990, 13, 313–325. [Google Scholar] [CrossRef] [PubMed]
- Kocanova, S.; Hornakova, T.; Hritz, J.; Jancura, D.; Chorvat, D.; Mateasik, A.; Ulicny, J.; Refregiers, M.; Maurizot, J.; Miskovsky, P. Characterization of the Interaction of Hypericin with Protein Kinase C in U-87 MG Human Glioma Cells. Photochem. Photobiol. 2006, 82, 720–728. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Sarma, S.; Katiyar, S.P.; Das, M.; Bhardwaj, R.; Sundar, D.; Dubey, V.K. Probing the Molecular Mechanism of Hypericin-Induced Parasite Death Provides Insight into the Role of Spermidine beyond Redox Metabolism in Leishmania Donovani. Antimicrob. Agents Chemother. 2015, 59, 15–24. [Google Scholar] [CrossRef]
- Aoki-Utsubo, C.; Chen, M.; Hotta, H. Time-of-Addition and Temperature-Shift Assays to Determine Particular Step(s) in the Viral Life Cycle That Is Blocked by Antiviral Substance(S). Bio-Protocol 2018, 8, e2830. [Google Scholar] [CrossRef]
- Fenard, D.; Lambeau, G.; Valentin, E.; Lefebvre, J.C.; Lazdunski, M.; Doglio, A. Secreted Phospholipases A(2), a New Class of HIV Inhibitors That Block Virus Entry into Host Cells. J. Clin. Investig. 1999, 104, 611–618. [Google Scholar] [CrossRef] [PubMed]
- Yao, Y.; Kang, T.; Jin, L.; Liu, Z.; Zhang, Z.; Xing, H.; Sun, P.; Li, M. Temperature-Dependent Growth and Hypericin Biosynthesis in Hypericum Perforatum. Plant Physiol. Biochem. 2019, 139, 613–619. [Google Scholar] [CrossRef]
- Romeo, A.; Cappelli, G.; Iacovelli, F.; Colizzi, V.; Falconi, M. Computational and Experimental Validation of Phthalocyanine and Hypericin as Effective SARS-CoV-2 Fusion Inhibitors. J. Biomol. Struct. Dyn. 2024, 42, 3920–3934. [Google Scholar] [CrossRef]
- Jin, Z.; Du, X.; Xu, Y.; Deng, Y.; Liu, M.; Zhao, Y.; Zhang, B.; Li, X.; Zhang, L.; Peng, C.; et al. Structure of Mpro from SARS-CoV-2 and Discovery of Its Inhibitors. Nature 2020, 582, 289–293. [Google Scholar] [CrossRef]
- Choi, M.H.; Wan, E.Y.F.; Wong, I.C.K.; Chan, E.W.Y.; Chu, W.M.; Tam, A.R.; Yuen, K.Y.; Hung, I.F.N. Comparative Effectiveness of Combination Therapy with Nirmatrelvir–Ritonavir and Remdesivir versus Monotherapy with Remdesivir or Nirmatrelvir–Ritonavir in Patients Hospitalised with COVID-19: A Target Trial Emulation Study. Lancet Infect. Dis. 2024, 24, 1213–1224. [Google Scholar] [CrossRef]
- Pitsillou, E.; Liang, J.; Karagiannis, C.; Ververis, K.; Darmawan, K.K.; Ng, K.; Hung, A.; Karagiannis, T.C. Interaction of Small Molecules with the SARS-CoV-2 Main Protease in Silico and in Vitro Validation of Potential Lead Compounds Using an Enzyme-Linked Immunosorbent Assay. Comput. Biol. Chem. 2020, 89, 107408. [Google Scholar] [CrossRef]
- Murali, M.; Gowtham, H.G.; Ansari, M.A.; Alomary, M.N.; Alghamdi, S.; Almehmadi, M.; Singh, S.B.; Shilpa, N.; Aiyaz, M.; Kalegowda, N.; et al. Repositioning Therapeutics for SARS-CoV-2: Virtual Screening of Plant-Based Anti-HIV Compounds as Possible Inhibitors against COVID-19 Viral RdRp. Curr. Pharm. Des. 2022, 28, 969–980. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Zhu, X.; Lu, Y.; Zhang, X.; Jia, X.; Yang, T. Potential Treatment with Chinese and Western Medicine Targeting NSP14 of SARS-CoV-2. J. Pharm. Anal. 2021, 11, 272. [Google Scholar] [CrossRef]
- Shivanika, C.; Kumar, D.; Ragunathan, V.; Tiwari, P.; Sumitha, A. Molecular Docking, Validation, Dynamics Simulations, and Pharmacokinetic Prediction of Natural Compounds against the SARS-CoV-2 Main-Protease. J. Biomol. Struct. Dyn. 2020, 40, 585–611. [Google Scholar] [CrossRef]
- Yalçın, S.; Yalçınkaya, S.; Ercan, F. Determination of Potential Drug Candidate Molecules of the Hypericum Perforatum for COVID-19 Treatment. Curr. Pharmacol. Rep. 2021, 7, 42–48. [Google Scholar] [CrossRef]
- Loschwitz, J.; Jäckering, A.; Keutmann, M.; Olagunju, M.; Eberle, R.J.; Coronado, M.A.; Olubiyi, O.O.; Strodel, B. Novel Inhibitors of the Main Protease Enzyme of SARS-CoV-2 Identified via Molecular Dynamics Simulation-Guided in Vitro Assay. Bioorg. Chem. 2021, 111, 104862. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, H.; Zou, M.; Oerlemans, R.; Shao, C.; Ren, Y.; Zhang, R.; Huang, X.; Li, G.; Cong, Y. Hypericin Inhibit Alpha-Coronavirus Replication by Targeting 3CL Protease. Viruses 2021, 13, 1825. [Google Scholar] [CrossRef]
- Sacco, M.D.; Hu, Y.; Gongora, M.V.; Meilleur, F.; Kemp, M.T.; Zhang, X.; Wang, J.; Chen, Y. The P132H Mutation in the Main Protease of Omicron SARS-CoV-2 Decreases Thermal Stability without Compromising Catalysis or Small-Molecule Drug Inhibition. Cell Res. 2022, 32, 498–500. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.T.; Yang, Q.; Gribenko, A.; Perrin, B.S., Jr.; Zhu, Y.; Cardin, R.; Liberator, P.A.; Anderson, A.S.; Hao, L. Genetic Surveillance of SARS-CoV-2 Mpro Reveals High Sequence and Structural Conservation Prior to the Introduction of Protease Inhibitor Paxlovid. mBio 2022, 13, e0086922. [Google Scholar] [CrossRef]
- Blair, H.A. Correction to: Remdesivir: A Review in COVID-19. Drugs 2023, 83, 1349. [Google Scholar] [CrossRef] [PubMed]
- Gandhi, S.; Klein, J.; Robertson, A.J.; Peña-Hernández, M.A.; Lin, M.J.; Roychoudhury, P.; Lu, P.; Fournier, J.; Ferguson, D.; Mohamed Bakhash, S.A.K.; et al. De Novo Emergence of a Remdesivir Resistance Mutation during Treatment of Persistent SARS-CoV-2 Infection in an Immunocompromised Patient: A Case Report. Nat. Commun. 2022, 13, 1547. [Google Scholar] [CrossRef] [PubMed]
- Sanderson, T.; Hisner, R.; Donovan-Banfield, I.; Hartman, H.; Løchen, A.; Peacock, T.P.; Ruis, C. A Molnupiravir-Associated Mutational Signature in Global SARS-CoV-2 Genomes. Nature 2023, 623, 594–600. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Souza, H.d.S.; Martins, J.S.C.C.; Sousa, T.d.C.; Sardar, S.; Fintelman-Rodrigues, N.; Silva-Trujillo, L.; Souza, T.M.L.e.; Siqueira, M.M.; Fernandes, J.H.; Matos, A.d.R. Hypericin Suppresses SARS-CoV-2 Replication and Synergizes with Antivirals via Dual Targeting of RdRp and 3CLpro. Microorganisms 2025, 13, 1004. https://doi.org/10.3390/microorganisms13051004
Souza HdS, Martins JSCC, Sousa TdC, Sardar S, Fintelman-Rodrigues N, Silva-Trujillo L, Souza TMLe, Siqueira MM, Fernandes JH, Matos AdR. Hypericin Suppresses SARS-CoV-2 Replication and Synergizes with Antivirals via Dual Targeting of RdRp and 3CLpro. Microorganisms. 2025; 13(5):1004. https://doi.org/10.3390/microorganisms13051004
Chicago/Turabian StyleSouza, Helena da Silva, Jéssica Santa Cruz Carvalho Martins, Thiagos das Chagas Sousa, Saiqa Sardar, Natalia Fintelman-Rodrigues, Lina Silva-Trujillo, Thiago Moreno Lopes e Souza, Marilda Mendonça Siqueira, Jorge Hernandes Fernandes, and Aline da Rocha Matos. 2025. "Hypericin Suppresses SARS-CoV-2 Replication and Synergizes with Antivirals via Dual Targeting of RdRp and 3CLpro" Microorganisms 13, no. 5: 1004. https://doi.org/10.3390/microorganisms13051004
APA StyleSouza, H. d. S., Martins, J. S. C. C., Sousa, T. d. C., Sardar, S., Fintelman-Rodrigues, N., Silva-Trujillo, L., Souza, T. M. L. e., Siqueira, M. M., Fernandes, J. H., & Matos, A. d. R. (2025). Hypericin Suppresses SARS-CoV-2 Replication and Synergizes with Antivirals via Dual Targeting of RdRp and 3CLpro. Microorganisms, 13(5), 1004. https://doi.org/10.3390/microorganisms13051004





