Seasonality and Vertical Structure of Microbial Communities in Alpine Wetlands
Abstract
1. Introduction
2. Materials and Methods
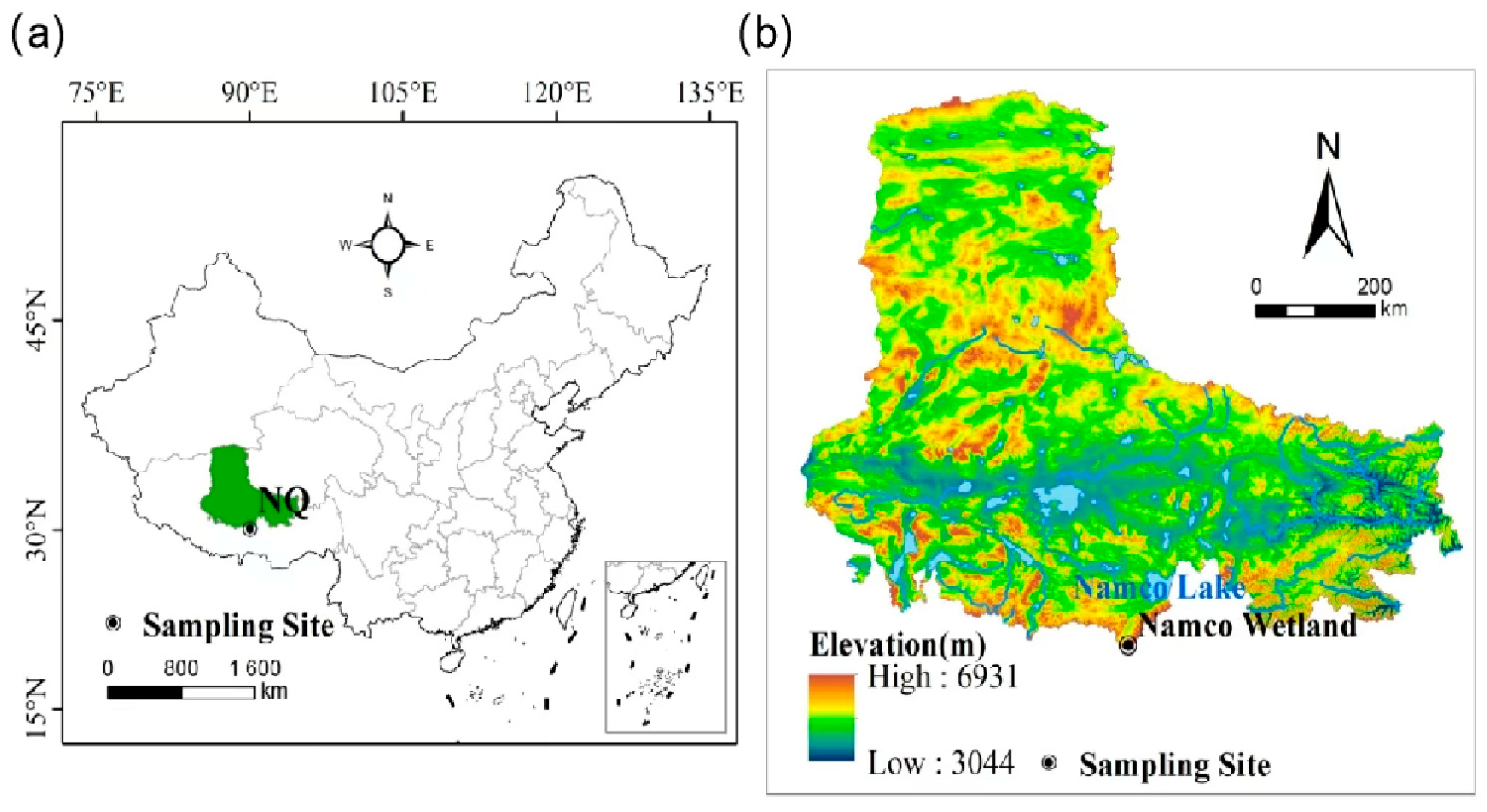
2.1. General Situation of Research Area
2.2. Sample Collection
2.3. Determination Methods
2.3.1. Determination of Soil Physical and Chemical Properties
2.3.2. High-Throughput Sequencing of 16S rRNA Gene in Soil Microbial Community
2.4. Data Analysis
3. Results
3.1. Soil Physicochemical Properties in Alpine Wetlands
3.2. Soil Microbial Community Composition in Alpine Wetlands Under Different Soil Depths and Seasons
3.3. Soil Microbial Alpha Diversity in Alpine Wetlands Under Different Soil Depths and Seasons
3.4. Soil Microbial Beta Diversity in Alpine Wetlands Under Different Soil Depths and Seasons
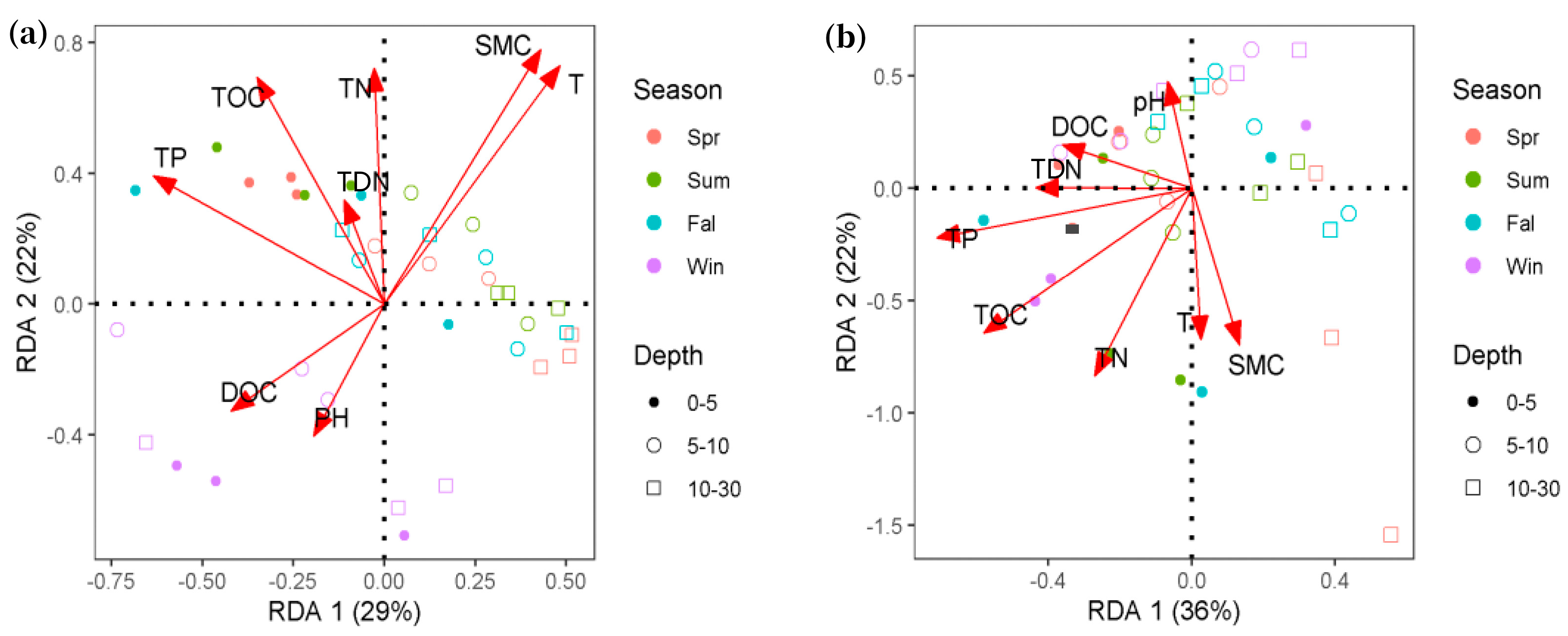
3.5. Main Environmental Factors Influencing Soil Microbial Communities in Alpine Wetlands
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Liang, S.; Li, H.; Wu, H.; Yan, B.; Song, A. Microorganisms in coastal wetland sediments: A review on microbial community structure, functional gene, and environmental potential. Front. Microbiol. 2023, 14, 1163896. [Google Scholar] [CrossRef] [PubMed]
- Philippot, L.; Chenu, C.; Kappler, A.; Rillig, M.C.; Fierer, N. The interplay between microbial communities and soil properties. Nat. Rev. Microbiol. 2024, 22, 226–239. [Google Scholar] [CrossRef]
- Zheng, Y.; Su, F.; Li, H.; Song, F.; Wei, C.; Cui, P. Structure and function of soil bacterial communities in the different wetland types of the Liaohe estuary wetland. Microorganisms 2024, 12, 2075. [Google Scholar] [CrossRef]
- Jia, T.; Guo, T.; Yao, Y.; Wang, R.; Chai, B. Seasonal microbial community characteristic and Its driving factors in a copper tailings dam in the Chinese Loess Plateau. Front. Microbiol. 2020, 11, 1574. [Google Scholar] [CrossRef] [PubMed]
- Kato, N.; Osaka, K.i.; Ohtsuka, T.; Iimura, Y. Root exudates in mangrove forests accelerate bicarbonate production in the soil environment. Sci. Rep. 2024, 14, 31765. [Google Scholar] [CrossRef] [PubMed]
- Lipson, D.A.; Schadt, C.W.; Schmidt, S.K. Changes in soil microbial community structure and function in an alpine dry meadow following spring snow melt. Microb. Ecol. 2002, 43, 307–314. [Google Scholar] [CrossRef]
- Lyu, Z.; Sommers, P.; Schmidt, S.K.; Magnani, M.; Cimpoiasu, M.; Kuras, O.; Zhuang, Q.; Oh, Y.; De La Fuente, M.; Cramm, M.; et al. Seasonal dynamics of Arctic soils: Capturing year-round processes in measurements and soil biogeochemical models. Earth-Sci. Rev. 2024, 254, 104820. [Google Scholar] [CrossRef]
- Wang, Z.; Li, J.; Xu, X.; Li, K.; Chen, Q. Denitrifying anaerobic methane oxidation and mechanisms influencing it in Yellow River Delta coastal wetland soil, China. Chemosphere 2022, 298, 134345. [Google Scholar] [CrossRef]
- Wang, Y.; Wu, W.; Ding, Y.; Liu, W.; Perera, A.; Chen, Y.; Devare, M. Methane oxidation activity and bacterial community composition in a simulated landfill cover soil is influenced by the growth of Chenopodium album L. Soil Biol. Biochem. 2008, 40, 2452–2459. [Google Scholar] [CrossRef]
- Iglesias-Carrasco, M.; Head, M.L.; Jennions, M.D.; Cabido, C. Condition-dependent trade-offs between sexual traits, body condition and immunity: The effect of novel habitats. BMC Evol. Biol. 2016, 16, 135. [Google Scholar] [CrossRef]
- Li, H.; Tan, L.; Li, X.; Cai, Q. Aquatic protected area system in the Qinghai-Tibet Plateau: Establishment, challenges and prospects. Front. Ecol. Evol. 2024, 12, 1204494. [Google Scholar] [CrossRef]
- Wu, P.; Zhang, H.; Cui, L.; Wickings, K.; Fu, S.; Wang, C. Impacts of alpine wetland degradation on the composition, diversity and trophic structure of soil nematodes on the Qinghai-Tibetan Plateau. Sci. Rep. 2017, 7, 5771. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Li, Y.; Wang, Y.; Chen, L. Typical alpine wetland system changes on the Qinghai-Tibet Plateau in recent 40 years. Acta Geogr. Sin. 2007, 62, 481–491. [Google Scholar]
- Murray, N.J.; Clemens, R.S.; Phinn, S.R.; Possingham, H.P.; Fuller, R.A. Tracking the rapid loss of tidal wetlands in the Yellow Sea. Front. Ecol. Environ. 2014, 12, 267–272. [Google Scholar] [CrossRef]
- Yasin, A.; Niu, B.; Chen, Z.; Hu, Y.; Yang, X.; Li, Y.; Zhang, G.; Li, F.; Hou, W. Effect of warming on the carbon flux of the alpine wetland on the Qinghai-Tibet Plateau. Front. Earth Sci. 2022, 10, 935641. [Google Scholar] [CrossRef]
- Zhang, G.; Yao, T.; Xie, H.; Kang, S.; Lei, Y. Inland lake dynamics on the Tibetan Plateau between 2000 and 2010: Morphologic and climatic factors. J. Hydrol. 2013, 482, 336–344. [Google Scholar]
- Dong, S.; Peng, F.; You, Q.; Guo, J.; Xue, X. Lake dynamics and its relationship to climate change on the Tibetan Plateau over the last four decades. Reg. Environ. Change 2018, 18, 477–487. [Google Scholar] [CrossRef]
- Jones, D.L.; Willett, V.B. Experimental evaluation of methods to quantify dissolved organic nitrogen (DON) and dissolved organic carbon (DOC) in soil. Soil Biol. Biochem. 2006, 38, 991–999. [Google Scholar] [CrossRef]
- Lebret, K.; Schroeder, J.; Balestreri, C.; Highfield, A.; Cummings, D.; Smyth, T.; Schroeder, D. Choice of molecular barcode will affect species prevalence but not bacterial community composition. Mar. Genom. 2016, 29, 39–43. [Google Scholar] [CrossRef]
- Zhou, H.; Zhang, D.; Jiang, Z.; Sun, P.; Xiao, H.; Wu, Y.; Chen, J. Changes in the soil microbial communities of alpine steppe at Qinghai-Tibetan Plateau under different degradation levels. Sci. Total Environ. 2019, 651, 2281–2291. [Google Scholar] [CrossRef]
- Chen, S. Ultrafast one-pass FASTQ data preprocessing, quality control, and deduplication using fastp. Imeta 2023, 2, e107. [Google Scholar] [CrossRef]
- Magoč, T.; Salzberg, S.L. FLASH: Fast length adjustment of short reads to improve genome assemblies. Bioinformatics 2011, 27, 2957–2963. [Google Scholar] [CrossRef] [PubMed]
- Edgar, R.C. UPARSE: Highly accurate OTU sequences from microbial amplicon reads. Nat. Methods 2013, 10, 996–998. [Google Scholar] [CrossRef] [PubMed]
- Edgar, R.C.; Haas, B.J.; Clemente, J.C.; Quince, C.; Knight, R. UCHIME improves sensitivity and speed of chimera detection. Bioinformatics 2011, 27, 2194–2200. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Cole, J.R. Updated RDP taxonomy and RDP Classifier for more accurate taxonomic classification. Microbiol. Resour. Announc. 2024, 13, e01063-23. [Google Scholar] [CrossRef]
- Quast, C.; Pruesse, E.; Yilmaz, P.; Gerken, J.; Schweer, T.; Yarza, P.; Peplies, J.; Glöckner, F.O. The SILVA ribosomal RNA gene database project: Improved data processing and web-based tools. Nucleic Acids Res. 2012, 41, D590–D596. [Google Scholar] [CrossRef]
- Gu, Y.; Bai, Y.; Xiang, Q.; Yu, X.; Zhao, K.; Zhang, X.; Li, C.; Liu, S.; Chen, Q. Degradation shaped bacterial and archaeal communities with predictable taxa and their association patterns in Zoige wetland at Tibet plateau. Sci. Rep. 2018, 8, 3884. [Google Scholar] [CrossRef]
- Yin, Y.; Yan, Z. Variations of soil bacterial diversity and metabolic function with tidal flat elevation gradient in an artificial mangrove wetland. Sci. Total Environ. 2020, 718, 137385. [Google Scholar] [CrossRef]
- Yue, P.; Zuo, X.; Li, K.; Li, X.; Wang, S.; Ma, X.; Qu, H.; Chen, M.; Liu, L.; Misselbrook, T.; et al. Responses of ecosystem respiration, methane uptake and nitrous oxide emission to drought in a temperate desert steppe. Plant Soil 2021, 469, 409–421. [Google Scholar] [CrossRef]
- Sun, Y.; Chen, X.; Zhong, A.; Guo, S.; Zhang, H. Variations in microbial residue and Its contribution to SOC between organic and mineral soil layers along an altitude gradient in the Wuyi mountains. Forests 2023, 14, 1678. [Google Scholar] [CrossRef]
- Rasche, F.; Knapp, D.; Kaiser, C.; Koranda, M.; Kitzler, B.; Zechmeister-Boltenstern, S.; Richter, A.; Sessitsch, A. Seasonality and resource availability control bacterial and archaeal communities in soils of a temperate beech forest. ISME J. 2011, 5, 389–402. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Pu, W.; Wang, S.; Zeng, X.; Sui, X.; Wang, X. pH-related changes in soil bacterial communities in the Sanjiang Plain, northeast China. Microorganisms 2023, 11, 2950. [Google Scholar] [CrossRef] [PubMed]
- Fang, Y.; Cheng, W.; Zhang, Y.; Wang, N.; Zhao, S.; Zhou, C.; Chen, X.; Bao, A. Changes in inland lakes on the Tibetan Plateau over the past 40 years. J. Geogr. Sci. 2016, 26, 415–438. [Google Scholar] [CrossRef]
- Liu, X.; Chen, H.; Zhu, Q.; Wu, J.; Frolking, S.; Zhu, D.; Wang, M.; Wu, N.; Peng, C.; He, Y. Holocene peatland development and carbon stock of Zoige peatlands, Tibetan Plateau: A modeling approach. J. Soils Sediments 2018, 18, 2032–2043. [Google Scholar] [CrossRef]
- Gao, H.; Chen, H.; Jin, Y.; Gao, R.; Wei, C.; Zhang, C.; Zhang, W. Occurrence and speciation of pollutants in Guilin Huixian wetland: Nutrients, microplastics, heavy metals, and emerging contaminants. Water 2024, 16, 2816. [Google Scholar] [CrossRef]
- Liu, J.; Sui, Y.; Yu, Z.; Shi, Y.; Chu, H.; Jin, J.; Liu, X.; Wang, G. High throughput sequencing analysis of biogeographical distribution of bacterial communities in the black soils of northeast China. Soil Biol. Biochem. 2014, 70, 113–122. [Google Scholar] [CrossRef]
- Rogers, B.F.; Tate, R.L. Temporal analysis of the soil microbial community along a toposequence in Pineland soils. Soil Biol. Biochem. 2001, 33, 1389–1401. [Google Scholar] [CrossRef]
- Bai, Z.; Zhang, M.; Liu, N.; Zhang, X. Effect of long-term fertilization on microbial community in a Chinese arable Mollisol. J. Grad. Sch. Chin. Acad. Sci. 2008, 25, 479–486. [Google Scholar]
- Si, G.; Wang, J.; Xia, Y.; Yuan, Y.; Zhang, G.; Lei, T. Change characteristics of microbial communities and enzyme activities in soils of marshes in Nyaiqentanglha mountains with heights above sea level. Wetl. Sci. 2014, 12, 340–348. [Google Scholar]
- Anthony, K.W.; Daanen, R.; Anthony, P.; von Deimling, T.S.; Ping, C.-L.; Chanton, J.P.; Grosse, G. Methane emissions proportional to permafrost carbon thawed in Arctic lakes since the 1950s. Nat. Geosci. 2016, 9, 679–682. [Google Scholar] [CrossRef]
- Chan, O.C.; Yang, X.; Fu, Y.; Feng, Z.; Sha, L.; Casper, P.; Zou, X. 16S rRNA gene analyses of bacterial community structures in the soils of evergreen broad-leaved forests in south-west China. FEMS Microbiol. Ecol. 2006, 58, 247–259. [Google Scholar] [CrossRef] [PubMed]
- Cordova-Kreylos, A.L.; Cao, Y.; Green, P.G.; Hwang, H.-M.; Kuivila, K.M.; LaMontagne, M.G.; Van De Werfhorst, L.C.; Holden, P.A.; Scow, K.M. Diversity, composition, and geographical distribution of microbial communities in california salt marsh Sediments. Appl. Environ. Microbiol. 2006, 72, 3357–3366. [Google Scholar] [CrossRef]
- Zhang, L.; Yuan, L.; Xiang, J.; Liao, Q.; Zhang, D.; Liu, J. Response of the microbial community structure to the environmental factors during the extreme flood season in Poyang Lake, the largest freshwater lake in China. Front. Microbiol. 2024, 15, 1362968. [Google Scholar] [CrossRef]
- Wei, Z.; Lin, C.; Xu, C.; Xiong, D.; Liu, X.; Chen, S.; Lin, T.; Yang, Z.; Yang, Y. Soil respiration in planted and naturally regenerated castanopis carelesii forests during three years post-establishment. Forests 2022, 13, 931. [Google Scholar] [CrossRef]
- Chen, Y.-L.; Deng, Y.; Ding, J.-Z.; Hu, H.-W.; Xu, T.-L.; Li, F.; Yang, G.-B.; Yang, Y.-H. Distinct microbial communities in the active and permafrost layers on the Tibetan Plateau. Mol. Ecol. 2017, 26, 6608–6620. [Google Scholar] [CrossRef]
- Liu, B.; Wu, W.F.; Lin, S.Z.; Lin, K.M. Characteristics of soil microbial biomass carbon and nitrogen and its seasonal dynamics in four mid-subtropical forests. J. Appl. Ecol. 2019, 30, 1901–1910, (In Chinese with English Abstract). [Google Scholar] [CrossRef]
- Wintsche, B.; Glaser, K.; Straeuber, H.; Centler, F.; Liebetrau, J.; Harms, H.; Kleinsteuber, S. Trace elements Induce predominance among methanogenic activity in anaerobic digestion. Front. Microbiol. 2016, 7, 2034. [Google Scholar] [CrossRef]
- Feng, Y.; Grogan, P.; Caporaso, J.G.; Zhang, H.; Lin, X.; Knight, R.; Chu, H. pH is a good predictor of the distribution of anoxygenic purple phototrophic bacteria in Arctic soils. Soil Biol. Biochem. 2014, 74, 193–200. [Google Scholar] [CrossRef]



| Index Parameter | Sample Depth | Spring | Summer | Fall | Winter |
|---|---|---|---|---|---|
| TN (mg/kg) | 0–5 cm | 10.29 ± 1.91 Aab | 11.29 ± 1.10 Aa | 8.35 ± 0.43 Ac | 7.63 ± 1.30 Ad |
| TP (mg/kg) | 1.80 ± 0.22 Aa | 1.87 ± 0.20 Aa | 1.46 ± 0.19 Aa | 1.65 ± 0.21 Aa | |
| DOC (mg/kg) | 26.88 ± 12.08 Abc | 18.34 ± 1.77 Ac | 37.15 ± 1.98 Abc | 41.38 ± 12.79 Aa | |
| TDN (mg/kg) | 5.81 ± 3.05 Aa | 3.06 ± 0.31 Aab | 5.48 ± 1.16 Aa | 4.15 ± 0.46 Aa | |
| TOC (mg/kg) | 0.15 ± 0.025 Aa | 0.14 ± 0.007 Aab | 0.10 ± 0.006 Ab | 0.09 ± 0.026 Ab | |
| pH | 7.43 ± 0.01 Aa | 7.22 ± 0.16 Aa | 7.32 ± 0.07 Aa | 7.50 ± 0.25 Aa | |
| SMC (%) | 91.47 ± 3.86 Aa | 121.77 ± 7.53 Aab | 139.71 ± 17.47 Aab | 88.39 ± 0.1.98 Ab | |
| TN (mg/kg) | 5–10 cm | 10.12 ± 0.85 Aab | 8.35 ± 2.80 Aab | 8.91 ± 0.31 Aab | 7.53 ± 2.66 bc |
| TP (mg/kg) | 1.66 ± 0.28 Aa | 1.44 ± 0.08 Aab | 1.45 ± 0.23 Aab | 1.69 ± 0.45 ABab | |
| DOC (mg/kg) | 27.11 ± 6.71 Aab | 25.96 ± 4.78 Ab | 26.78 ± 10.23 Bb | 27.83 ± 12.09 ABab | |
| TDN (mg/kg) | 4.51 ± 0.58 Aab | 5.40 ± 1.41 Aa | 3.76 ± 1.45 Ac | 4.54 ± 2.60 ab | |
| TOC (mg/kg) | 0.12 ± 0.009 Aa | 0.09 ± 0.046 Aa | 0.09 ± 0.023 Aa | 0.72 ± 0.024 Aa | |
| pH | 7.35 ± 0.09 Abc | 7.38 ± 0.13 Aabc | 7.28 ± 0.05 Abc | 7.60 ± 0.09 Aa | |
| SMC (%) | 121 ± 22.72 Aa | 133.07 ± 7.37 Aa | 124.68 ± 22.97 Aa | 56.28 ± 20.47 ABb | |
| TN (mg/kg) | 10–30 cm | 8.03 ± 2.22 Ab | 8.74 ± 0.76 Aab | 8.96 ± 1.31 Aab | 4.93 ± 0.92 Bc |
| TP (mg/kg) | 1.34 ± 0.11 Abc | 1.46 ± 0.26 Aabc | 1.57 ± 0.24 Aabc | 1.31 ± 0.09 Ac | |
| DOC (mg/kg) | 18.01 ± 1.51 Ac | 20.84 ± 4.98 Bc | 21.83 ± 2.51 Ab | 28.66 ± 6.64 Cab | |
| TDN (mg/kg) | 3.37 ± 0.43 Aab | 3.45 ± 1.09 Aab | 3.22 ± 0.98 Aab | 1.02 ± 0.20 Bc | |
| TOC (mg/kg) | 0.08 ± 0.041 Aab | 0.08 ± 0.009 Aab | 0.09 ± 0.037 Aa | 0.05 ± 0.019 d | |
| pH | 7.42 ± 0.21 Aabc | 7.29 ± 0.02 Abc | 7.19 ± 0.25 Ac | 7.35 ± 0.09 Abc | |
| SMC (%) | 111.12 ± 12.28 Ab | 136.41 ± 19.16 Aa | 111.45 ± 3.94 Ab | 34.15 ± 0.83 Cc | |
| T (°C) | 2.94 ± 0.91 b | 11.16 ± 0.66 a | −1 ± 2.68 b | −9.88 ± 1.63 c |
| Sample Name | Index Parameter | Mean Squares | F.Model | Variation | Pr (>F) | |
|---|---|---|---|---|---|---|
| Bacteria | Soil depth (cm) | 0–5/5–10 | 0.272 | 2.418 | 0.099 | 0.001 ** |
| 0–5/10–30 | 0.348 | 2.898 | 0.116 | 0.001 ** | ||
| 5–10/10–30 | 0.127 | 1.207 | 0.052 | 0.169 | ||
| Season | Spr/Sum | 0.106 | 1.019 | 0.060 | 0.359 | |
| Spr/Fal | 0.155 | 1.480 | 0.085 | 0.067 | ||
| Spr/Win | 0.295 | 2.438 | 0.132 | 0.002 ** | ||
| Sum/Fal | 0.106 | 1.035 | 0.061 | 0.383 | ||
| Sum/Win | 0.306 | 2.567 | 0.138 | 0.003 ** | ||
| Fal/Win | 0.301 | 2.516 | 0.136 | 0.003 ** | ||
| Archaea | Soil depth (cm) | 0–5/5–10 | 0.254 | 2.860 | 0.115 | 0.007 ** |
| 0–5/10–30 | 0.387 | 3.626 | 0.141 | 0.003 ** | ||
| 5–10/10–30 | 0.172 | 2.054 | 0.085 | 0.061 | ||
| Season | Spr/Sum | 0.064 | 0.598 | 0.036 | 0.755 | |
| Spr/Fal | 0.078 | 0.693 | 0.042 | 0.653 | ||
| Spr/Win | 0.168 | 1.481 | 0.085 | 0.212 | ||
| Sum/Fal | 0.051 | 0.541 | 0.033 | 0.882 | ||
| Sum/Win | 0.154 | 1.635 | 0.093 | 0.114 | ||
| Fal/Win | 0.102 | 1.030 | 0.060 | 0.361 | ||
| Environmental Variables | Bacteria | Archaea | ||||
|---|---|---|---|---|---|---|
| Variance | F | Pr (>F) | Variance | F | Pr (>F) | |
| DOC | 0.01 | 1.24 | 0.13 | 0.01 | 1.05 | 0.34 |
| pH | 0.01 | 1.20 | 0.15 | 0.01 | 0.82 | 0.61 |
| SMC | 0.02 | 2.25 | 0.001 *** | 0.02 | 1.00 | 0.53 |
| TDN | 0.01 | 1.41 | 0.06 | 0.01 | 1.45 | 0.12 |
| TOC | 0.01 | 1.42 | 0.05 * | 0.02 | 2.30 | 0.01 * |
| TN | 0.01 | 1.84 | 0.01 ** | 0.01 | 27 | 0.02 * |
| TP | 0.02 | 2.22 | 0.01 ** | 0.01 | 3.00 | 0.01 * |
| T | 0.02 | 2.21 | 0.001 *** | 0.02 | 1.04 | 0.35 |
| T | 0.02 | 2.21 | 0.001 *** | 0.02 | 1.04 | 0.35 |
| Inertia | Proportion | Inertia | Proportion | |||
| Constrained | 0.10 | 0.30 | 0.07 | 3.00 | ||
| Unconstrained | 0.21 | 0.70 | 0.18 | 0.70 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, H.; Li, Y.; Yang, X.; Niu, B.; Jiao, H.; Yang, Y.; Huang, G.; Hou, W.; Zhang, G. Seasonality and Vertical Structure of Microbial Communities in Alpine Wetlands. Microorganisms 2025, 13, 962. https://doi.org/10.3390/microorganisms13050962
Wang H, Li Y, Yang X, Niu B, Jiao H, Yang Y, Huang G, Hou W, Zhang G. Seasonality and Vertical Structure of Microbial Communities in Alpine Wetlands. Microorganisms. 2025; 13(5):962. https://doi.org/10.3390/microorganisms13050962
Chicago/Turabian StyleWang, Huiyuan, Yue Li, Xiaoqin Yang, Bin Niu, Hongzhe Jiao, Ya Yang, Guoqiang Huang, Weiguo Hou, and Gengxin Zhang. 2025. "Seasonality and Vertical Structure of Microbial Communities in Alpine Wetlands" Microorganisms 13, no. 5: 962. https://doi.org/10.3390/microorganisms13050962
APA StyleWang, H., Li, Y., Yang, X., Niu, B., Jiao, H., Yang, Y., Huang, G., Hou, W., & Zhang, G. (2025). Seasonality and Vertical Structure of Microbial Communities in Alpine Wetlands. Microorganisms, 13(5), 962. https://doi.org/10.3390/microorganisms13050962






