Extreme Environments and High-Level Bacterial Tellurite Resistance
Abstract
:1. Introduction
1.1. Habitats of Highly Resistant Microbes
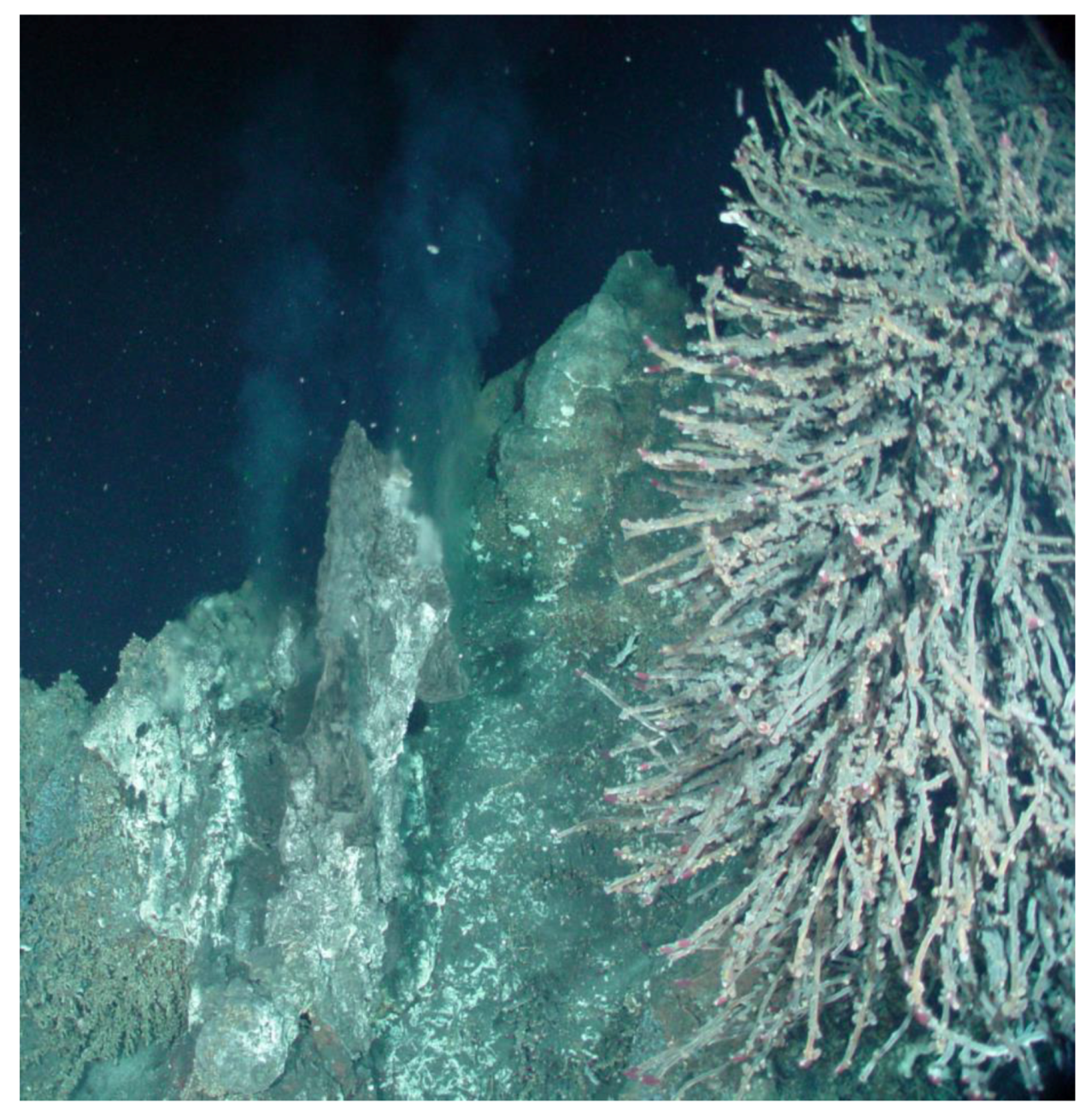
1.2. Deep-Sea Hydrothermal Vents
1.3. Thermal Springs
1.4. Hypersaline Environments
1.5. Mine Tailings
1.6. Diversity of Tellurite-Resistant Microbes Inhabiting Extreme Environments
2. Tellurium and Tellurite
2.1. Chemistry and Abundance
2.2. Interactions with Microbes
3. Mechanisms of Tellurite Resistance and Reduction
3.1. Aerobic Resistance
3.2. Anaerobic Resistance
3.3. Aerobic Anoxygenic Phototrophs and Tellurite Resistance
4. High-Level Tellurite Resistance
4.1. Strategies for High-Level Resistance
4.2. Ter Operon
4.3. Tellurite Reductases
5. Anaerobic Respiration Using Tellurite
6. Potential Role in Bioremediation and Biometallurgy
6.1. Bioremediation
6.2. Biometallurgy
7. Summary and Perspectives
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Bhatnagar, I.; Kim, S.K. Immense essence of Excellence: Marine Microbial Bioactive Compounds. Mar. Drugs 2010, 8, 2673–2701. [Google Scholar] [CrossRef] [PubMed]
- Yurkov, V.; Jappe, J.; Vermeglio, A. Tellurite Resistance and Reduction by Obligately Aerobic Photosynthetic Bacteria. Appl. Environ. Microbiol. 1996, 62, 4195–4198. [Google Scholar] [PubMed]
- Li, H.; Feng, Y.; Zou, X.; Luo, X. Study on Microbial Reduction of Vanadium Metallurgical Waste Water. Hydrometallurgy 2009, 99, 13–17. [Google Scholar] [CrossRef]
- Van Agteren, M.; Keuning, S.; Oosterhaven, J. Handbook on Biodegradation and Biological Treatment of Hazardous Organic Compounds, 1st ed.; Springer: Amsterdam, The Nedtherlands, 2013. [Google Scholar] [CrossRef]
- Arenas, F.; Pugin, B.; Henriquiez, N.; Arenas-Salinas, M.; Diaz-Vasquez, W.; Pozo, M.; Munoz, C.; Chasteen, T.; Perez-Donoso, J.; Vasquez, C. Isolation, Identification and Characterization of Highly Tellurite-Resistant, Tellurite-Reducing Bacteria from Antartica. Polar. Sci. 2014, 8, 40–52. [Google Scholar] [CrossRef]
- Schonheit, P.; Moll, J.; Thauer, R. Nickel, Cobalt, and Molybdenum Requirement for Growth of Methanobacterium Thermoautotrophicum. Arch. Microbiol. 1979, 123, 105–107. [Google Scholar] [CrossRef]
- Waldron, K.; Rutherford, J.; Ford, D.; Robinson, N. Metalloproteins and Metal Sensing. Nature 2009, 460, 823–830. [Google Scholar] [CrossRef]
- Glass, J.; Orphan, V. Trace Metal Requirements for Microbial Enzymes Involved in the Production and Consumption of Methane and Nitrous Oxide. Front. Microbiol. 2012, 3, 61. [Google Scholar] [CrossRef]
- Wadgaonkar, S.; Mal, J.; Nancharaiah, Y.; Maheshwari, N.; Esposito, G.; Lens, P. Formation of Se(0), Te(0), and Se(0)-Te(0) Nanostructures during simultaneous bioreduction of selenite and tellurite in a UASB Reactor. Appl. Microbiol. Biotechnol. 2018, 102, 2899–2911. [Google Scholar] [CrossRef]
- Zonaro, E.; Lampis, S.; Turner, R.; Qazi, S.; Vallini, G. Biogenic Selenium and Tellurium Nanoparticles Synthesized by Environmental Microbial Isolates Efficaciously Inhibit Bacterial Planktonic Cultures and Biofilms. Front. Microbiol. 2015, 6, 1–11. [Google Scholar] [CrossRef]
- Ueki, T. Bioaccumulation of Vanadium by Vanadium-Resistant Bacteria Isolated from the Intestine of Ascidia Sydneiensis Samea. Mar. Biotechnol. 2016, 18, 359–371. [Google Scholar] [CrossRef]
- Piacenza, E.; Presentato, A.; Zonaro, E.; Lampis, S.; Vallini, G.; Turner, R. Microbial-Based Bioremediation of Selenium and Tellurium Compounds. In Biosorption, 1st ed.; Derco, J., Vrana, B., Eds.; IntechOpen: London, UK, 2018. [Google Scholar] [CrossRef]
- Prakash, V.; Rao, N.; Bhatnagar, A.K. Linear Optical Properties of Niobium-Based Tellurite Glasses. Solid State Commun. 2001, 119, 39–44. [Google Scholar] [CrossRef]
- Maltman, C.; Piercey-Normore, M.; Yurkov, V. Tellurite-, Tellurate-, and Selenite-Based Anaerobic Respiration by Strain CM-3 Isolated from Gold Mine Tailings. Extremophiles 2015, 19, 1013–1019. [Google Scholar] [CrossRef] [PubMed]
- Yurkov, V.; Beatty, T. Aerobic Anoxygenic Phototrophic Bacteria. Microbiol. Mol. Biol. Rev. 1998, 62, 695–724. [Google Scholar] [PubMed]
- Javed, S.; Sarwar, A.; Tassawar, M.F.M. Conversion of Selenite to Elemental Selenium by Indigenous Bacteria Isolated from Polluted Areas. Chem. Spec. Bioavailab. 2016, 27, 162–168. [Google Scholar] [CrossRef]
- Maltman, C.; Yurkov, V. The Effect of Tellurite on Highly Resistant Freshwater Aerobic Anoxygenic Phototrophs and Their Strategies for Reduction. Microorganisms 2015, 3, 826–838. [Google Scholar] [CrossRef]
- Maltman, C.; Walter, G.; Yurkov, V. A Diverse Community of Metal(loid) Oxide Respiring Bacteria is Associated with Tube Worms in the Vicinity of the Juan De Fuca Ridge Black Smoker Field. PLoS ONE 2016, 11, e0149812. [Google Scholar] [CrossRef]
- Xie, H.; Xia, W.; Chen, M.; Wu, L.; Tong, J. Isolation and Characterization of the Tellurite-Reducing Photosynthetic Bacterium, Rhodopseudomonas palustris Strain TX618. Water Air Soil Pollut. 2018, 229, 158. [Google Scholar] [CrossRef]
- Rathgeber, C.; Yurkova, N.; Stackebrandt, E.; Beatty, T.; Yurkov, V. Isolation of Tellurite- and Selenite-Resistant Bacteria from Hydrothermal Vents of the Juan De Fuca Ridge in the Pacific Ocean. Appl. Environ. Microbiol. 2002, 68, 4613–4622. [Google Scholar] [CrossRef]
- Rathgeber, C.; Yurkova, N.; Stackebrandt, E.; Schumann, P.; Humphrey, E.; Beatty, T.; Yourkov, V. Metalloid Reducing Bacteria Isolated from Deep Ocean Hydrothermal Vents of the Juan De Fuca Ridge, Pseudoalteromonas Telluritireducens Sp. Nov. and Pseudoalteromonas Spiralis sp. Nov. Curr. Microbiol. 2006, 53, 449–456. [Google Scholar] [CrossRef]
- Csotonyi, J.; Stackebrandt, E.; Yurkov, V. Anaerobic Respiration on Tellurate and Other Metalloids in Bacteria from Hydrothermal Vent Fields in the Eastern Pacific Ocean. Appl. Environ. Microbiol. 2006, 72, 4950–4956. [Google Scholar] [CrossRef]
- Bajaj, M.; Winter, J. Se (IV) Triggers Faster Te (IV) Reduction by Soil Isolates of Heterotrophic Aerobic Bacteria: Formation of Extracellular SeTe Nanospheres. Microb. Cell. Fact. 2014, 13, 168–178. [Google Scholar] [CrossRef] [PubMed]
- Epelde, L.; Lanzen, A.; Blanco, F.; Urich, T.; Garbisu, C. Adaptation of Soil Microbial Community Structure and Function to Chronic Metal Contamination at an Abandonded Pb-Zn Mine. FEMS Microbiol. Ecol. 2015, 91, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Hughes, E.; Head, B.; Maltman, C.; Piercey-Normore, M.; Yurkov, V. Aerobic Anoxygenic Phototrophs in Gold Mine Tailings in Nopiming Provincial Park, Manitoba, Canada. Can. J. Microbiol. 2016, 63, 212–218. [Google Scholar] [CrossRef] [PubMed]
- Brock, T. Ecology of Saline Lakes. In Strategies of Microbial Life in Extreme Environments, 2nd ed.; Shilo, M., Ed.; Verlag Chemie: Weinheim, Germany, 1979; pp. 29–47. [Google Scholar]
- Li, S.J.; Hua, Z.S.; Huang, L.N.; Li, J.; Shi, S.H.; Chen, L.X.; Kuang, J.L.; Liu, J.; Hu, M.; Shu, W.S. Microbial Communities Evolve Faster in Extreme Environments. Sci. Rep. 2014, 4, 6205. [Google Scholar] [CrossRef]
- Nies, D. Heavy-Metal Resistant Bacteria as Extremophiles: Molecular Physiology and Biotechnological Use of Ralstonia Sp. CH34. Extremophiles 2000, 4, 77–82. [Google Scholar] [CrossRef]
- Malik, A.; Grohmann, E.; Alves, M. Management of Microbial Resources in the Environment, 1st ed.; Springer: Amsterdam, The Nedtherlands, 2013. [Google Scholar] [CrossRef]
- Patel, K.; Amaresan, N. Antimicrobial Compounds from Extreme Environment Rhizosphere Organisms for Plant Growth. Int. J. Curr. Microbiol. Appl. Sci. 2014, 3, 651–664. [Google Scholar]
- Yong, Y.C.; Zhong, J.J. Recent Advances in Biodegradation in China: New Microorganisms and Pathways, Biodegradation Engineering, and Bioenergy from Pollutant Biodegradation. Process Biochem. 2010, 45, 1937–1943. [Google Scholar] [CrossRef]
- Rogers, A.; Tyler, P.; Connelly, D.; Copley, J.; James, R.; Larter, R.; Linse, K.; Mills, R.; Garabato, A.; Pancost, R.; et al. The Discovery of New Deep-Sea Hydrothermal Vent Communities in the Southern Ocean and Implications for Biogeography. PLoS Biol. 2012, 10, 1–17. [Google Scholar] [CrossRef]
- Delaney, J.; Robigou, V.; McDuff, R. Geology of a Vigerous Hydrothermal System on the Endeavour Segment, Juan De Fuca Ridge. J. Geophys. Res. 1992, 97, 19663–19682. [Google Scholar] [CrossRef]
- Kelley, D.; Baross, J.; Delaney, J. Volcanoes, Fluids, and Life at Mid-Ocean Ridge Spreading Centers. Ann. Rev. Earth Planet. Sci. 2002, 30, 385–491. [Google Scholar] [CrossRef] [Green Version]
- Butler, I.; Nesbitt, R. Trace Element Distributions in the Chalcopyrite Wall of a Black Smoker Chimney: Insights from Laser Ablation Inductively Coupled Plasma Mass Spectrometry (LA-ICP-MS). Earth Planet. Sci. Lett. 1999, 167, 335–345. [Google Scholar] [CrossRef]
- Van Dover, C. The Ecology of Deep-Sea Hydrothermal Vents, 1st ed.; Princeton Universtiy Press: West Sussex, UK, 2000. [Google Scholar]
- Jeanthon, C.; Prieur, D. Susceptability to Heavy Metals and Characterization of Heterotrophic Bacteria Isolated from Two Hydrothermal Vent Polychaete Annelids, Alvinella Pompejana and Alvinella Caudate. Appl. Environ. Microbiol. 1990, 56, 3308–3314. [Google Scholar] [PubMed]
- Rajasabapathy, R.; Mohandass, C.; Colaco, A.; Dastager, S.; Santos, R.; Meena, R. Culturable Bacterial Phylogeny from a Shallow Water Hydrothermal Vent of Espalamaca (Faial, Azores) Reveals a Variety of Novel Taxa. Curr. Sci. 2014, 106, 58–69. [Google Scholar] [CrossRef]
- Yurkov, V.; Krieger, S.; Stackebrandt, E.; Beatty, T. Citromicrobium Bathyomarinum, a novel aerobic bacterium isolated from deep-sea hydrothermal vent plume waters that contains photosynthetic pigment-protein complexes. J. Bacteriol. 1999, 181, 4517–4525. [Google Scholar]
- Bonificio, W.; Clarke, D. Bacterial Recovery and Recycling of Tellurium from Tellurium-Containing Compounds by Pseudoalteromonas Sp. EPR3. J. Appl. Microbiol. 2014, 117, 1293–1304. [Google Scholar] [CrossRef]
- Yurkova, I.; Lyalikova, N. New Vanadate-Reducing Facultative Chemolithotrophic Bacteria. Mikrobiologiya 1990, 59, 968–975. [Google Scholar]
- Curewitz, D.; Karson, J. Structural Settings of Hydrothermal Outflow: Fracture Permeability Maintained by Fault Propagation and Interaction. J. Volcanol. Geotherm. Res. 1997, 79, 149–168. [Google Scholar] [CrossRef]
- Pentecost, A.; Jones, B.; Renaut, R. What is a Hot Spring? Can. J. Earth Sci. 2003, 40, 1443–1446. [Google Scholar] [CrossRef]
- Nelson, C.; Giles, D. Hydrothermal Eruption Mechanisms and Hot Spring Gold Deposits. Econ. Geol. 1985, 80, 1633–1639. [Google Scholar] [CrossRef]
- Ballantyne, J.; Moore, J. Arsenic Geochemistry in Geothermal Systems. Geochem. Cosmochim. Acta 1988, 52, 475–483. [Google Scholar] [CrossRef]
- Ebert, S.; Rye, R. Secondary Precious Metal Enrichment by Steam-Heated Fluids in the Crofoot-Lewis Hot Spring Gold-Silver Deposit and Relation to Paleoclimate. Econ. Geol. 1997, 92, 578–600. [Google Scholar] [CrossRef]
- Bundschuh, J.; Maity, J. Geothermal Arsenic: Occurence, Mobility and Environmental Implications. Renew. Sustain. Energy Rev. 2015, 42, 1214–1222. [Google Scholar] [CrossRef]
- Chatziefthimiou, A.; Crespo-Medina, M.; Wang, Y.; Vetriani, C.; Barkay, T. The Isolation and Initial Characterization of Mercury Resistant Chemolithotrophic Thermophilic Bacteria from Mercury Rich Geothermal Springs. Extremophiles 2007, 11, 469–479. [Google Scholar] [CrossRef] [PubMed]
- Zakeri, F.; Noghabi, K.; Sadeghizadeh, M.; Kardan, M.; Masoomi, F.; Farshidpour, M.; Atarilar, A. Serratia sp. ZF03: An Efficient Radium Biosorbent Isolated from Hot-Spring Waters in High Background Radiation Areas. Bioresour. Technol. 2010, 101, 9163–9170. [Google Scholar] [CrossRef] [PubMed]
- Masoudzadeh, N.; Zakeri, F.; Lotfabad, T.; Sharafi, H.; Masoomi, F.; Zahiri, H.; Ahmadian, G.; Noghabi, K. Biosorption of Cadmium by Brevundimonas sp. ZF12 Strain, a Novel Biosorbent Isolated from Hot-Spring Waters in High Background Radiation Areas. J. Hazerd. Mater. 2011, 197, 190–198. [Google Scholar] [CrossRef] [PubMed]
- Yurkov, V.; Gorlenko, V. Erythrobacter Sibiricus sp. Nov., a New Freshwater Aerobic Bacterial Species Containing Bacteriochlorophyll a. Mikrobiologiya 1990, 59, 85–89. [Google Scholar]
- Yurkov, V.; Lysenko, A.; Gorlenko, V. Hybridization Analysis of the Classification of Bacteriochlorophyll a - Containing Freshwater Aerobic bacteria. Mikrobiologiya 1991, 60, 362–366. [Google Scholar]
- Yurkov, V.; Gorlenko, V.; Kompantseva, E. A New Genus of Orange-Colored Bacteria Containing Bacteriochlorophyll a: Erythromicrobium Gen. Nov. Mikrobiologiya 1992, 61, 256–260. [Google Scholar]
- Yurkov, V.; Gorlenko, V. New Species of Aerobic Bacteria from the Genus Erythromicrobium Containing Bacteriochlorophyll a. Mikrobiologiya 1992, 61, 163–168. [Google Scholar]
- Yurkov, V.; Stackebrandt, E.; Holmes, A.; Fuerst, J.; Hugenholtz, P.; Golecki, J.; Gad’on, N.; Gorlenko, V.; Kompantseva, E.; Drews, G. Phylogenetic Positions of Novel Aerobic Bacteriochlorophyll a - Containing Bacteria and Descriptions of Roseococcus Thiosulfatophilus Gen. Nov., sp. Nov., Erythromicrobium Ramosum Gen. Nov., sp. Nov., and Erythrobacter Litoralis sp. nov. Int. J. Syst. Bacteriol. 1994, 44, 427–434. [Google Scholar] [CrossRef]
- Rodriguez-Valera, F. Introduction to Saline Environments. In The Biology of Halophilic Bacteria, 1st ed.; Vreeland, R.H., Hochstein, L.I., Eds.; CRC Press: Boca Raton, FL, USA, 1992; pp. 1–23. [Google Scholar]
- Shiba, T.; Simidu, U.; Taga, N. Distribution of Aerobic Bacteria which Contain Bacteriochlorophyll a. Appl. Environ. Microbiol. 1979, 38, 43–45. [Google Scholar] [PubMed]
- Yurkov, V.; Van Gemerden, H. Abundance and Salt Tolerance of Obligately Aerobic, Phototrophic Bacteria in a Marine Microbial Mat. Netherl. J. Sea Res. 1993, 31, 57–62. [Google Scholar] [CrossRef]
- Labrenz, M.; Hirsch, P. Physiological Diversity and Adaptations of Aerobic Heterotrophic Bacteria from Different Depths of Hypersaline, Heliothermal and Meromictic Ekho Lake (East Antarctica). Polar Biol. 2001, 24, 320–327. [Google Scholar] [CrossRef]
- Yurkova, N.; Rathgeber, C.; Swiderski, J.; Stackebrandt, E.; Beatty, T.; Hall, K.; Yurkov, V. Diversity, Distribution and Physiology of the Aerobic Phototrophic Bacteria in the Mixolimnion of a Meromictic Lake. FEMS Microbiol. Ecol. 2002, 40, 191–204. [Google Scholar] [CrossRef] [PubMed]
- Csotonyi, F.; Swiderski, J.; Stackebrandt, E.; Yurkov, V. Novel Halophilic Aerobic Anoxygenic Phototrophs from a Canadian Hypersaline Spring System. Extremophiles 2008, 12, 529–539. [Google Scholar] [CrossRef] [PubMed]
- Javor, B. Hypersaline Environments: Microbiology and Biogeochemistry, 1st ed.; Springer-Verlag: New York, NY, USA, 1989. [Google Scholar]
- Ong, C.; Herbel, M.; Dahlgren, R.; Tanji, K. Trace Element (Se, As, Mo, B) Contamination of Vaporates in Hypersaline Agricultural Evaporation Ponds. Environ. Sci. Technol. 1997, 31, 831–836. [Google Scholar] [CrossRef]
- Csotonyi, J.; Maltman, C.; Swiderski, C.; Stackebrandt, E. Extremely Vanadiphilic Multiply Metal-Resistant and Halophilic Aerobic Anoxygenic Photrophs, Strains EG13 and EG8, from Hypersaline Springs in Canada. Extremophiles 2015, 19, 127–134. [Google Scholar] [CrossRef]
- Pearion, C.; Jablonski, P. High Level, Intrinsic Resistance of Natronococcus Occultus to Potassium Tellurite. FEMS Microbiol. Lett. 1999, 174, 19–23. [Google Scholar] [CrossRef]
- Baesman, S.S.J.; Kulp, T. Enrichment and Isolation of Bacillus Beveridgei Sp. Nov., a Facultative Anaerobic Haloalkaliphile from Mono Lake, California, that Respires Oxyanions of Tellurium, Selenium, and Arsenic. Extremophiles 2009, 13, 695–705. [Google Scholar] [CrossRef]
- Cooper, W. Tellurium, 1st ed.; Van Nostrand Reinhold Company: New York, NY, USA, 1971. [Google Scholar]
- Andseron, B. Materials Availability for Large-Scale Thin-Film Photovoltaics. Prog. Photovol. Res. Appl. 2000, 8, 61–76. [Google Scholar] [CrossRef]
- Siddique, T.; Arocena, J.; Thring, R.; Zhang, Y. Bacterial Reduction of Selenium in Coal Mine Tailings Pond Sediment. J. Environ. Qual. 2007, 36, 621–627. [Google Scholar] [CrossRef] [PubMed]
- Green, M. Estimates of Te and In Prices from Direct Mining of Known Ores. Prog. Photovol. Res. Appl. 2009, 17, 347–359. [Google Scholar] [CrossRef]
- Sracek, O.; Mihaljevic, M.; Kribek, B.; Majer, V.; Filip, J.; Vanek, A.; Penizek, V.; Ettler, V.; Mapani, B. Geochemistry and Mineralogy of Vanadium in Mine Tailings at Berg Aukas, Northern Namibia. J. Afri. Earth Sci. 2014, 96, 180–189. [Google Scholar] [CrossRef]
- Yang, J.; Tang, Y.; Yang, K.; Rouff, A.; Elzinga, E.; Huang, J. Leaching Characteristics of Vanadium in Mine Tailings and Soils Near a Vanadium Titanomangetite Mining Site. J. Hazard. Mater. 2014, 264, 498–504. [Google Scholar] [CrossRef]
- Bautista-Hernandez, D.; Ramirez-Burgos, L.; Duran-Paramo, E.; Fernandez-Linares, L. Zinc and Lead Biosorption by Delftia Tsuruhatensis: A Bacteria Strain Resistant to Metals Isolated from Mine Tailings. J. Water Resour. Protec. 2012, 4, 207–216. [Google Scholar] [CrossRef] [Green Version]
- Rothschild, L.; Mancinelli, R. Life in Extreme Environments. Nature 2001, 409, 1092–1101. [Google Scholar] [CrossRef]
- Moyer, C.; Dobbs, F.; Karl, D. Phylogenetic Diversity of the Bacterial Community from a Microbial Mat at an Active, Hydrothermal Vent System, Loihi Seamount, Hawaii. Appl. Environ. Microbiol. 1995, 61, 1555–1562. [Google Scholar]
- Teske, A.; Hinrich, K.; Edgcomb, V.; De Vera Gomez, A.; Kysela, D.; Sylva, S.; Sogin, M.; Jannasch, H. Microbial Diversity of Hydrothermal Sediments in the Guaymas Basin: Evidence for Anaerobic Methanotrophic Communities. Appl. Environ. Microbiol. 2002, 68, 1994–2007. [Google Scholar] [CrossRef] [Green Version]
- Huber, J.; Cantin, H.; Huse, S.; Welch, D.; Sogin, M.; Butterfield, D. Isolated Communities of Epsilonproteobacteria in Hydrothermal Vent Fluids of the Mariana Arc Seamounts. FEMS Microbiol. Ecol. 2010, 73, 538–540. [Google Scholar] [CrossRef]
- Campbell, B.; Polson, S.; Zeigler Allen, L.; Williamson, S.; Lee, C.; Wommack, K.; Cary, S. Diffuse Flow Environments Within Basalt- and Sediment-Based Hydrothermal Vent Ecosystems Harbor Specialized Microbial Communities. Front. Microbiol. 2013, 4, 182. [Google Scholar] [CrossRef] [Green Version]
- Makhalanyane, T.; Valverde, A.; Birkeland, N.; Cary, S.; Tuffin, I.; Cowan, D. Evidence for Successional Development in Antarctic Hypolithic Bacterial Communities. ISME J. 2013, 7, 2080–2090. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Piubeli, F.; De Lourdes Moreno, M.; Kishi, L.; Henrique-Silva, F.; Garcia, M.; Mellado, E. Phylogenetic Profiling and Diversity of Bacterial Communities in the Death Valley, an Extreme Habitat in the Atacama Desert. Indian. J. Microbiol. 2015, 55, 392–399. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jones, D.; Grettenberger, C.; Larson, L.; Burgos, W.; Macaladya, J. Geochemical Niches of Iron-Oxidizing Acidophiles in Acidic Coal Mine Drainage. Appl. Environ. Microbiol. 2015, 81, 1242–1250. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, W.; Xiao, T.; Sun, M.; Dong, Y.; Nign, Z.; Xiao, E.; Tang, S.; Li, J. Diversity of the Sediment Microbial Community in the Aha Watershed (Southwest China) in Response to Acid Mine Drainage Pollution Gradients. Appl. Environ. Microbiol. 2015, 81, 4874–4884. [Google Scholar] [CrossRef] [Green Version]
- Walker, J.; Spear, J.; Pace, N. Geobiology of a Microbial Endolithic Community in the Yellowstone Geothermal Environment. Nature 2005, 434, 1011–1114. [Google Scholar] [CrossRef]
- Roane, T.; Kellogg, S. Characterization of bacterial communities in heavy metal contaminated soils. Can. J. Microbiol. 1996, 42, 593–603. [Google Scholar] [CrossRef]
- Sauvain, L.; Bueche, M.; Junier, T.; Masson, M.; Wunderlin, T.; Kohler-Milleret, R.; Diez, E.; Loizeau, J.L.; Tercier-Waeber, M.L.; Juiner, P. Bacterial Communities in Trace Metal Contaminated Lake Sediments are Dominated by Endospore-Forming Bacteria. Aquat. Sci. 2014, 76, 33–46. [Google Scholar] [CrossRef] [Green Version]
- Brito, E.; De La Cruz Barron, M.; Caretta, C.; Goni-Urriza, M.; Andrade, L.; Cuevas-Rodriguez, G.; Malm, O.; Torres, J.; Simon, M.; Guyoneaud, R. Impact of Hydrocarbons, PCBs and Heavy Metals on Bacterial Communities in Lerma River, Salamanca, Mexico: Investigation of Hydrocarbon Degradation Potential. Sci. Total. Environ. 2015, 521, 1–10. [Google Scholar] [CrossRef]
- Huber, J.; Mark Welch, D.; Morrison, H.; Huse, S.; Neal, P.; Butterfield, D.; Sogin, M. Microbial Population Structures in the Deep Marine Biosphere. Science 2007, 318, 97–100. [Google Scholar] [CrossRef] [Green Version]
- Brazelton, W.; Baross, J. Abundant Transposases Encoded by the Metagenome of a Hydrothermal Chimney Biofilm. ISME J. 2009, 3, 1420–1424. [Google Scholar] [CrossRef] [Green Version]
- Xie, W.; Wang, F.; Guo, L.; Chen, Z.; Sievert, S.; Meng, J.; Huang, G.; Li, Y.; Yan, Q.; Wu, S.; et al. Comparative Metagenomics of Microbial Communities Inhabiting Deep-Sea Hydrothermal Vent Chimneys with Contrasting Chemistry. ISME J. 2011, 5, 414–426. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Zhou, H.; Meng, J.; Peng, X.; Jiang, L.; Sun, P.; Zhang, C.; Van Nostrand, J.; Deng, Y.; He, Z.; et al. GeoChip-Based Analysis of Metabolic Diversity of Microbial Communities at the Juan De Fuca Ridge Hydrothermal Vent. Proc. Natl. Acad. Sci. USA 2009, 106, 4840–4845. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jeanthon, C. Molecular Ecology of Hydrothermal Vent Microbial Communities. Antoni. Van Leeuwenhoek 2000, 71, 117–133. [Google Scholar] [CrossRef] [PubMed]
- Cox, P. The Elements, 1st ed.; Oxford University Press: New York, NY, USA, 1989. [Google Scholar]
- Ba, L.; Doring, M.; Jamier, V.; Jacob, C. Tellurium: An Element with Great Biological Potency and Potential. Org. Biomol. Chem. 2010, 8, 4203–4216. [Google Scholar] [CrossRef]
- Belzile, N.; Chen, Y.W. Tellurium in the Environment: A Critical Review Focused on Natural Waters, Soils, Sediments and Airborne Particles. Appl. Geochem. 2015, 63, 83–92. [Google Scholar] [CrossRef]
- Wray, D. The Impact of Unconfined Mine Tailings and Anthropogenic Pollution on a Semi-Arid Environment - An Initial Study of the Rodalquilar Mine District, Southeast Spain. Environ. Geochem. Health 1998, 20, 29–38. [Google Scholar] [CrossRef]
- Fuge, R. Anthropogenic Sources. In Essentials of Medical Geology, 2nd ed.; Selinus, O., Ed.; Springer: Amsterdam, The Netherlands, 2013; pp. 59–74. [Google Scholar]
- Mokmeli, M.; Dreisinger, D.; Wassink, B.; Difley, B. Reduction mechanism of tellurium species from copper electrowinning solutions. Int. J. Chem. Kinet. 2016, 48, 204–211. [Google Scholar] [CrossRef]
- Taylor, D. Bacterial Tellurite Resistance. Trends Microbiol. 1999, 7, 111–115. [Google Scholar] [CrossRef]
- Lohmeir-Vogel, E.; Ung, S.; Turner, R. In Vivo 31P Nuclear Magnetic Resonance Investigation of Tellurite Toxicity in Escherichia Coli. Appl. Environ. Microbiol. 2004, 70, 7324–7347. [Google Scholar] [CrossRef] [Green Version]
- Sandoval, J.; Leveque, P.; Gallez, B.; Vasquez, C.; Buc Calderon, P. Tellurite-Induced Oxidative Stress Leads to Cell Death of Murine Hepatocarcinoma Cells. Biometals 2010, 23, 623–632. [Google Scholar] [CrossRef]
- Maltman, C.; Yurkov, V. The Impact of Tellurite on Highly Resistant Marine Bacteria and Strategies for Its Reduction. Int. J. Environ. Eng. Nat. Resour. 2014, 1, 109–119. [Google Scholar]
- Turner, R.; Weiner, J.; Taylor, D. Tellurite-Mediated Thiol Oxidation in Escherichia Coli. Microbiology 1999, 145, 2549–2557. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chasteen, T.; Fuentes, D.; Tantalean, J.; Vasquez, C. Tellurite: History, Oxidative Stress, and Molecular Mechanisms of Resistance. FEMS Microbiol. Rev. 2009, 33, 820–832. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Burkholz, T.; Jacob, C. Tellurium in Nature. In Encyclopedia of Metalloproteins, 1st ed.; Kretsinger, R.H., Uversky, V.N., Permyakov, E.A., Eds.; Springer: New York, NY, USA, 2013; pp. 2163–2174. [Google Scholar] [CrossRef]
- Moroder, L. Isoteric Replacement of Sulfur with Other Chalcogens in Peptides and Proteins. J. Pept. Sci. 2005, 11, 187–214. [Google Scholar] [CrossRef]
- Ramadan, S.; Razak, R.; Ragab, A.; El-Meleigy, M. Incorporation of Tellurium into Amino Acids and Proteins in a Tellurium-Tolerant Fungi. Biol. Trace Elem. Res. 1989, 20, 225–232. [Google Scholar] [CrossRef]
- Boles, J.O.; Lebioda, L.; Dunlap, R.B.; Odom, J.D. Telluromethionine in Structural Biochemistry. SAAS Bull. Biochem. Biotech. 1995, 8, 29–34. [Google Scholar]
- Budisa, N.; Stepie, B.; Demanger, P.; Eckerskorn, C.; Kellermann, J.; Huber, R. High-Level Biosynthetic Substitution of Methionine in Proteins by its Analogs 2-Aminohexanoic Acid, Selenotethionine, Telluromethionine, and Ethionine in Escherichia coli. Eur. J. Biochem. 1995, 230, 788–796. [Google Scholar] [CrossRef]
- Fleming, A. On the Specific Antibacterial Properties of Penicillin and Potassium Tellurite. Incorporating a Method of Demonstrating Some Bacterial Antagonisms. Path. Bacteriol. 1932, 35, 831–842. [Google Scholar] [CrossRef]
- Zannoni, D.; Borsetti, F.; Harrison, J.; Turner, R. The Bacterial Response to the Chalcogen Metalloids Se and Te. In Advances in Microbial Physiology, 1st ed.; Poole, R., Ed.; Elsevier: Amsterdam, The Netherlands, 2008; Volume 54, pp. 1–71. [Google Scholar] [CrossRef]
- Maltman, C.; Donald, L.; Yurkov, V. Two Distinct Periplasmic Enzymes are Responsible for Tellurite/Tellurate and Selenite Reduction by Strain ER-Te-48 Isolated from a Deep Sea Hydrothermal Vent Tube Worm. Arch. Microbiol. 2017, 199, 1113–1120. [Google Scholar] [CrossRef]
- Maltman, C.; Donald, L.; Yurkov, V. Tellurite and Tellurate Reduction by the Aerobic Anoxygenic Phototroph Erythromonas Ursincola, Strain KR99 is Carried Out by a Novel Membrane Associated Enzyme. Microorganisms 2017, 5, 20. [Google Scholar] [CrossRef] [Green Version]
- Moore, M.; Kaplan, S. Identification of Intrinsic High-Level Resistance to Rare-Earth Oxides and Oxyanions in Members of the Class Proteobacteria: Characterization of Tellurite, Selenite, and Rhodium Sesquioxide Reduction in Rhodobacter Sphaeroides. J. Bacteriol. 1992, 174, 1505–1514. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Horiike, T.; Otsuka, O.; Tanaka, Y.; Terahara, T.; Imada, C.; Yamashita, M. Diversity of Salt-Tolerant Tellurite-Reducing Bacteria in a Marine Environment. J. Gen. Appl. Microbiol. 2019. [Google Scholar] [CrossRef] [PubMed]
- Agranoc, D.; Krishna, S. Metal Ion Homeostasis and Intracellular Parasitism. Mol. Microbiol. 1998, 28, 403–412. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Daubin, V.; Gouy, M.; Perriere, G. A Phylogenomic Approach to Bacterial Phylogeny: Evidence of a Core of Genes Sharing a Common History. Genome Res. 2002, 12, 1080–1090. [Google Scholar] [CrossRef] [Green Version]
- Coombs, J.; Barkay, T. Molecular Evidence for the Evolution of Metal Homeostasis Genes by Lateral Gene Transfer in Bacteria from the Deep Terrestrial Subsurface. Appl. Environ. Microbiol. 2003, 70, 1698–1707. [Google Scholar] [CrossRef] [Green Version]
- Lebaron, P.; Batailler, N.; Baleux, B. Recombination of a Recombinant Nonconjugative Plasmid at the Interface Between Wastewater and the Marine Coastal Environment. FEMS Microbiol. Ecol. 1994, 15, 61–70. [Google Scholar] [CrossRef]
- Valdivia-Gonzalez, M.; Diaz-Vasquez, W.; Ruiz-Leon, D.; Becerra, A.; Aguayo, D.; Perez-Donoso, J.; Vasquez, C. A Comparative Analysis of Tellurite Detoxification by Members of the Genus Shewanella. Arch. Microbiol. 2018, 200, 267–273. [Google Scholar] [CrossRef]
- Perez, J.; Calderon, I.; Arenase, F.; Fuentes, D.; Pradenas, G.; Fuentes, E.; Sandoval, J.; Castro, M.; Elias, A.; Vasquez, C. Bacterial Toxicity of Potassium Tellurite: Unveiling an Ancient Enigma. PLoS ONE 2007, 2, e211. [Google Scholar] [CrossRef]
- Tremaroli, V.; Fedi, S.; Zannoni, D. Evidence for a Tellurite-Dependent Generation of reactive Oxygen Species and Absence of a Tellurite-Mediated Adaptive Response to Oxidative Stress in Cells of Pseudomonas Pseudoalcaligenes KF707. Arch. Microbiol. 2007, 187, 127–135. [Google Scholar] [CrossRef]
- Calderon, I.; Arenas, F.; Perez, J.; Fuentes, D.; Araya, M.; Saavedra, C.; Tantalean, J.; Pichuantes, S.; Youderian, P.; Vasquez, C. Catalases are NAD(P)H-Dependant Tellurite Reductases. PLoS ONE 2006, 1, 1–8. [Google Scholar] [CrossRef]
- Avazeri, C.; Turner, R.; Pommier, J.; Weiner, J.; Giordana, G.; Vermeglio, A. Tellurite Reductase Activity of Nitrate Reductase is Responsible for the Basal Resistance of Escherichia Coli to Tellurite. Microbiology 1997, 143, 1181–1189. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sabaty, M.; Avazeri, C.; Pignol, D.; Vermeglio, A. Characterization of the Reduction of Selenate and Tellurite by Nitrate Reductases. Appl. Environ. Microbiol. 2001, 67, 5122–5126. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Borsetti, F.; Francia, F.; Turner, R.; Zannoni, D. The Thiol: Disulfide Oxidoreductase DsbB Mediates the Oxidizing Effects of the Toxic Metalloid Tellurite (TeO32-) on the Plasma Membrane Redox System of the Facultative Phototroph Rhodobacter Capsulatus. J. Bacteriol. 2007, 189, 851–859. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guzzo, J.; Dubow, M. A Novel Selenite- and Tellurite-Inducible Gene in Escherichia Coli. Appl. Environ. Microbiol. 2000, 66, 4972–4978. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Araya, M.; Tantalena, J.; Perez, J.; Fuentes, D.; Calderon, I.; Saavedra, C.; Burra, R.; Chasteen, T.; Vasquez, C. Cloning, Purification and Characterization of Geobacillus Stearothermophilus V Uroporphurinogen-III C-Methyltransferase: Evaluation of its Role in Resistance to Potassium Tellurite in Escherichia Coli. Res. Microbiol. 2009, 160, 125–133. [Google Scholar] [CrossRef] [PubMed]
- Castro, M.; Molina, R.; Diaz, W.; Pichuantes, S.; Vasquez, C. The Dihydrolipoamide Dehydrogenase of Aeromonas Caviae ST exhibits NADH-Dependant Tellurite Reductase Activity. Biochem. Biophys. Res. Commun. 2008, 375, 91–94. [Google Scholar] [CrossRef] [PubMed]
- Terai, T.; Kamahora, T.; Yamamura, Y. Tellurite Reductase from Mycobacterium Avium. J. Bacteriol. 1958, 75, 535–539. [Google Scholar]
- Chiong, M.; Gonzalez, E.; Barra, R.; Vasquez, C. Purification and Biochemical Characterization of Tellurite-Reducing Activites from Thermus Thermophilus HB8. J. Bacteriol. 1988, 170, 3269–3273. [Google Scholar] [CrossRef] [Green Version]
- Kabiri, M.; Amoozegar, M.; Tabebordbar, M.; Gilany, K.; Salekdeh, G. Effects of Selenite and Tellurite on Growth, Physiology, and Proteome of a Moderately Halophilic Bacterium. J. Proteome Res. 2009, 8, 3095–3108. [Google Scholar] [CrossRef]
- Walter, G.; Thomas, C.; Ibbotson, J.; Taylor, D. Transcriptional Analysis, Translational Analysis, and Sequence of the KilA-Tellurite Resistance Region of Plasmid RK2Ter. J. Bacteriol. 1991, 173, 1111–1119. [Google Scholar] [CrossRef] [Green Version]
- Turner, R.; Hou, Y.; Weiner, J.; Taylor, D. The Arsenical ATPase Efflux Pump Mediates Tellurite Resistance. J. Bacteriol. 1992, 174, 3092–3094. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- O’Gara, J.; Gomelsky, M.; Kaplan, S. Identification and Molecular Genetic Analysis of Multiple Loci Contributing to High-Level Tellurite Resistance in Rhodobacter Sphaeroides. Appl. Environ. Microbiol. 1997, 63, 4713–4720. [Google Scholar] [PubMed]
- Coumoyer, B.; Watanabe, S.; Vivian, A. A Tellurite-Resistance Genetic Determinant from Phytopathogenic Pseudomonads Encodes a Thiopurine Methyltransferase: Evidence of a Widely-Conserved Family of Methyltransferases. Biochim. Biophys. Acta 1998, 1397, 161–168. [Google Scholar] [CrossRef]
- Valkovicova, L.; Vavrova, S.; Mravec, J.; Grones, J.; Turna, J. Protein-Protein Association and Cellular Localization of Four Essential Gene Products Encoded by Tellurite Resistance-Conferring Cluster Ter from Pathogenic Escherichia Coli. Anton. Van Leeuwenhoek 2013, 104, 899–911. [Google Scholar] [CrossRef]
- Moore, M.; Kaplan, S. Members of the Family Rhodospirillaceae Reduce Heavy-Metal Oxyanions to Maintain Redox Poise During Photosynthetic Growth. Am. Soc. Microbiol. News 1994, 60, 17–23. [Google Scholar]
- Borghese, R.; Zannoni, D. Acetate Permease (ActP) is Responsible for Tellurite (TeO32-) Uptake and Resistance in Cells of the Facultative Phototroph Rhodobacter Capsulatus. Appl. Environ. Microbiol. 2010, 76, 942–944. [Google Scholar] [CrossRef] [Green Version]
- Borghese, R.; Canducci, L.; Musiani, F.; Cappelletti, M.; Ciurli, S.; Rurner, R.; Zannoni, D. On the Role of a Specific Insert in Acetate Permease (ActP) for Tellurite Uptake in Bacteria: Functional and Structural Studies. J. Inorg. Biochem. 2016, 163, 103–109. [Google Scholar] [CrossRef]
- Borsetti, F.; Toninello, A.; Zannoni, D. Tellurite Uptake by Cells of the Facultative Phototroph Rhodobacter Capsulatus is a pH-Dependant Process. FEBS Lett. 2003, 554, 315–318. [Google Scholar] [CrossRef] [Green Version]
- Elias, A.; Abarca, M.; Montes, R.; Chasteen, T.; Perez-Donoso, J.; Vasquez, C. Tellurite enters Escherichia Coli Mainly Through the PitA Phosphate Transporter. Microbiologyopen 2012, 1, 259–267. [Google Scholar] [CrossRef]
- Thomas, J.; Kay, W. Tellurite Susceptibility and Non-Plasmid Mediated Resistance in Escherichia Coli. Antimicrob. Agents Chemother. 1986, 30, 127–131. [Google Scholar] [CrossRef] [Green Version]
- Ollivier, P.; Bahrou, A.; Marcus, S.; Cox, T.; Church, T.; Hanson, T. Volitization and Precipitation of Tellurium by Aerobic Tellurite-Resistant Marine Microbes. Appl. Environ. Microbiol. 2008, 74, 7163–7173. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Borghese, R.; Borsetti, F.; Foladori, P.; Ziglio, G.; Zannoni, D. Effects of the Metalloid Oxyanion Tellurite (TeO32-) on Growth Characteristics of the Phototrophic Bacterium Rhodobacter Capsulatus. Appl. Environ. Microbiol. 2004, 70, 6595–6602. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yurkov, V.; Csotonyi, J. Aerobic Anoxygenic Phototrophs and Heavy Metalloid Reducers from Extreme Environments. Recent Res. Dev. Bacteriol. 2003, 1, 247–300. [Google Scholar]
- Biebl, H.; Tindall, B.; Pukall, R.; Lunsdorf, H.; Allgaier, M.; Wagner-Dobler, I. Hoeflea Phototrophifa sp. Nov., a Novel Marine Aerobic Alphaproteobacterium that Forms Bacteriochlorophyll a. Int. J. Syst. Evol. Microbiol. 2006, 56, 821–826. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yurkov, V.; Csotonyi, J. New Light on Aerobic Anoxygenic Phototrophs. In The Purple Phototrophic Bacteria, 1st ed.; Hunter, N., Daldal, F., Thurnauer, B., Beatty, T., Eds.; Springer: New York, NY, USA, 2009; pp. 31–55. [Google Scholar] [CrossRef]
- Sabaty, M.; Bebien, M.; Avazeri, C.; Yurkov, V.; Richaud, P.; Vermeglio, A. Reduction of Tellurite and Selenite by Photosynthetic Bacteria. In Photosynthesis: Mechanisms and Effects, 1st ed.; Garab, G., Ed.; Kluwer Academic Publishers: Dordrecht, The Netherlands, 1998; pp. 4123–4128. [Google Scholar]
- Beatty, T. On the Natural Selection and Evolution of the Aerobic Phototrophic Bacteria. Photosynth. Res. 2002, 73, 109–114. [Google Scholar] [CrossRef]
- El-Agamey, A.; McGarvey, D. Carotenoid Radicals and Radical Ions. In Carotenoids: Natural Functions, 1st ed.; Britton, G., Liaaen-Jensen, S., Pfander, H., Eds.; Springer: Basel, Switzerland, 2008; Volume 4, pp. 119–154. [Google Scholar] [CrossRef]
- Yurkov, V.; Gad’on, N.; Drews, G. The Major Part of Polar Carotenoids of the Aerobic Bacteria Roseococcus Thiosulfatophilus RB3 and Erythromicrobium Ramosum E5 is Not Bound to the Bacteriochlorophyll A-Complexes of the Photosynthetic Apparatus. Arch. Microbiol. 1993, 160, 372–376. [Google Scholar] [CrossRef]
- Csotonyi, J.; Maltman, C.; Yurkov, V. Influence of Tellurite on Synthesis of Bacteriochlorophyll and Carotenoids in Aerobic Anoxygenic Phototrophic Bacteria. Trends Photochem. Photobiol. 2014, 16, 1–17. [Google Scholar]
- Whelan, K.; Sherburne, R.; Taylor, D. Characterization of a Region of the IncHI2 Plasmid R478 which Protects Escherichia Coli from Toxic Effects Specified by Components of the Tellurite, Phage, and Colicin Resistance Cluster. J. Bacteriol. 1997, 179, 63–71. [Google Scholar] [CrossRef] [Green Version]
- Ponnusamy, D.; Hartson, S.; Clinkenbeard, K. Intracellular Yersinia Pestis Expresses General Stress Response and Tellurite Resistance Proteins in Mouse Macrophages. Vet. Microbiol. 2011, 150, 146–151. [Google Scholar] [CrossRef]
- Soudi, M.; Ghazvini, P.; Khajeh, K.; Gharavi, S. Bioprocessing of Seleno-Oxyanions and Tellurite in a Novel Bacillus sp. Strain STG-83: A Solution to Removal of Toxic Oxyanions in the Presence of Nitrate. J. Hazard. Mater. 2009, 165, 71–77. [Google Scholar] [CrossRef]
- Etezad, S.; Khajeh, K.; Soudi, M.; Ghazvini, P.; Dabirmanesh, B. Evidence on the Presence of Two Distinct Enzymes Responsible for the Reduction of Selenate and Tellurite in Bacillus sp. STG-83. Enzyme Microbial. Technol. 2009, 45, 1–6. [Google Scholar] [CrossRef]
- Lovely, D. Dissimilatory Metal Reduction. Annu. Rev. Microbiol. 1993, 47, 263–290. [Google Scholar] [CrossRef] [PubMed]
- Stoltz, J.; Oremland, R. Bacterial Respiration of Arsenic and Selenium. FEMS Microbiol. Rev. 1999, 23, 615–627. [Google Scholar] [CrossRef] [PubMed]
- Bouroushian, M. Electrochemistry of Metal Chalcogenides; Springer: Berlin, Germany, 2010. [Google Scholar] [CrossRef]
- Nancharaiah, Y.; Lens, P. Ecology and Biotechnology of Selenium-Respiring Bacteria. Microbiol. Mol. Biol. Rev. 2015, 79, 61–80. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Terry, L.; Kulp, T.; Wiatrowski, H.; Miller, L.; Oremland, R. Microbiological Oxidation of Antimony(III) with Oxygen or Nitrate by Bacteria Isolated from Contaminated Mine Sediments. Appl. Environ. Microbiol. 2015, 81, 8478–8488. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moroz, O.; Hnatush, S.; Bohoslavets, C.; Yavorska, G.; Truchym, N. Usage of Ferrum (III) and Manganese (IV) Ions as Electron Acceptors by Desulfuromonas sp. Bacteria. Biosyst. Divers. 2016, 24, 87–95. [Google Scholar] [CrossRef] [Green Version]
- Klonowska, A.; Heulin, T.; Vermeglio, A. Selenite and Tellurite Reduction by Shewanella Oneidensis. Appl. Environ. Microbiol. 2005, 71, 5607–5609. [Google Scholar] [CrossRef] [Green Version]
- Baesman, S.; Bullen, T.; Dewald, J.; Zhang, D.; Curran, S.; Islam, F.; Beveridge, T.; Oremland, R. Formation of Tellurium Nanocrystals During Anaerobic Growth of Bacteria That Use Te Oxyanions As Respiratory Electron Acceptors. Appl. Environ. Microbiol. 2007, 73, 2135–2143. [Google Scholar] [CrossRef] [Green Version]
- Edwards, K.; Bach, W.; Rogers, D. Geomicrobiology of the Ocean Crust: A Role for Chemoautotrophic Fe-Bacteria. Biol. Bull. 2003, 204, 180–185. [Google Scholar] [CrossRef]
- Grimalt, J.; Ferrer, M.; Macpherson, E. The Mine Tailing Accident in Aznalcollar. Sci. Total. Environ. 1999, 242, 3–11. [Google Scholar] [CrossRef]
- Kossoff, D.; Dubbin, W.; Alfredsson, M.; Edwards, S.; Macklin, M.; Hudson-Edwards, K. Mine Tailings Dams: Characteristics, Failure, Environmental Impacts, and Remediation. Appl. Geochem. 2014, 51, 229–245. [Google Scholar] [CrossRef] [Green Version]
- De Santiago-Martin, A.; Guesdon, G.; Diaz-Sanz, J.; Galvez-Cloutier, R. Oil Spill in Lac-Megantic, Canada: Environmental Monitoring and Remediation. Int. J. Water Wastewater Treat. 2015, 2, 1–6. [Google Scholar] [CrossRef]
- Kim, Y.; Kim, C.; Choi, I.; Rengaraj, S.; Yi, J. Arsenic Removal Using Mesoporous Alumina Prepared Via a Templating Method. Environ. Sci. Technol. 2004, 38, 924–931. [Google Scholar] [CrossRef] [PubMed]
- Elwakeel, K.; Atia, A.; Donia, A. Removal of Mo(VI) as Oxoanions from Aqueous Solutions Using Chemically Modified Magnetic Chitosan Resins. Hydrometallurgy 2009, 97, 21–28. [Google Scholar] [CrossRef]
- Gadd, G. Metals, Minerals and Microbes: Geomicrobiology and Bioremediation. Microbiology 2010, 156, 609–643. [Google Scholar] [CrossRef] [PubMed]
- Pieper, D.; Reineke, W. Engineering Bacteria for Bioremediation. Curr. Opin. Biotechnol. 2000, 11, 262–270. [Google Scholar] [CrossRef]
- Ruggiero, C.; Boukhalfa, H.; Forsythe, J.; Lack, J.; Hersman, L.; New, M. Actinide and Metal Toxicity to Prospective Bioremediation Bacteria. Environ. Microbiol. 2005, 7, 88–97. [Google Scholar] [CrossRef]
- Shah, K.; Nongkynrih, J. Metal Hyperaccumulation and Bioremediation. Biol. Plant. 2007, 51, 618–634. [Google Scholar] [CrossRef]
- Jadhav, J.; Kalyani, D.; Telke, A.; Phugare, S.; Govindwar, S. Evaluation of the Efficacy of a Bacterial Consortium for the Removal of Color, Reduction of Heavy Metals, and Toxicity from Textile Dye Effluent. Bioresour. Technol. 2010, 101, 165–173. [Google Scholar] [CrossRef]
- Dogan, N.; Kantar, C.; Gulcan, S.; Dodge, C.; Yilmaz, B.; Mazmanci, M. Chromium(VI) Bioremoval by Pseudomonas Bacteria: Role of Microbial Exudates for Natural Attenuation and Biotreatment of Cr(VI) Contamination. Environ. Sci. Technol. 2011, 45, 2278–2285. [Google Scholar] [CrossRef]
- Wasi, S.; Tabrez, S.; Ahmad, M. Use of Pseudomonas Spp. for the Bioremediation of Environmental Pollutants: A Review. Environ. Monit. Assess. 2013, 185, 8147–8155. [Google Scholar] [CrossRef] [PubMed]
- Luek, A.; Brock, C.; Rowan, D.; Rasmussen, J. A Simplified Anaerobic Bioreactor for the Treatment of Selenium-Laden Discharges from Non-Acidic, End-Pit Lakes. Mine. Water Environ. 2014, 33, 295–306. [Google Scholar] [CrossRef]
- Ramon-Ruiz, A.; Field, J.; Wilkening, J.S.A.R. Recovery of Elemental Tellurium Nanoparticles by the Reduction of Tellurium Oxyanions in a Methanogenic Microbial Consortium. Environ. Sci. Technol. 2016, 50, 1492–1500. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rajwade, J.; Paknikar, K. Bioreduction of Tellurite to Elemental Tellurium by Pseudomonas Mendocina MCM B-180 and its Practical Application. Hydrometallurgy 2003, 71, 243–248. [Google Scholar] [CrossRef]
- Maltman, C.; Yurkov, V. Bioremediation Potential of Bacteria Able to Reduce High Levels of Selenium and Tellurium Oxyanions. Arch. Microbiol. 2018, 200, 1411–1417. [Google Scholar] [CrossRef]
- Ilyas, S.; Lee, J.C. Biometallurical Recovery of Metals from Waste Electrical and Electronic Equipment: A Review. ChemBioEng Rev. 2014, 1, 148–169. [Google Scholar] [CrossRef]
- Rawlings, D.; Silver, S. Mining with Microbes. Nat. Biotechnol. 1995, 13, 773–778. [Google Scholar] [CrossRef]
- Zhuang, W.Q.; Fitts, J.; Ajo-Franklin, C.; Maes, S.; Alvarez0Cohen, L.; Hennebel, T. Recovery of Critical Metals Using Biometallurgy. Curr. Opin. Biotechnol. 2015, 33, 327–335. [Google Scholar] [CrossRef] [Green Version]
- Brierley, C.; Brierley, J. Microbiology for the Metal Mining Industry. In Manual of Environmental Microbiology, 2nd ed.; Hurst, C., Ed.; ASM Press: Washington, WA, USA, 2002; pp. 1057–1071. [Google Scholar] [CrossRef]
- Krebs, W.; Brombacher, C.; Bosshard, P.; Bachofen, R.; Brandl, H. Microbial Recovery of Metals from Solids. FEMS Microbiol. Rev. 1997, 20, 605–617. [Google Scholar] [CrossRef]
- Turner, R.; Borghese, R.; Zannoni, D. Microbial Processing of Tellurium as a Tool in Biotechnology. Biotechnol. Adv. 2012, 30, 954–963. [Google Scholar] [CrossRef]
- Claessens, P.; White, C. Method of Tellurium Separation from Copper Electrorefining Slime. U.S. Patent 5,271,909, 21 December 1993. [Google Scholar]
- Marwede, M.; Reller, A. Future Recycling Flows of Tellurium from Cadmium Telluride Photovoltaic Waste. Resour. Conserv. Recycl. 2012, 69, 35–49. [Google Scholar] [CrossRef] [Green Version]



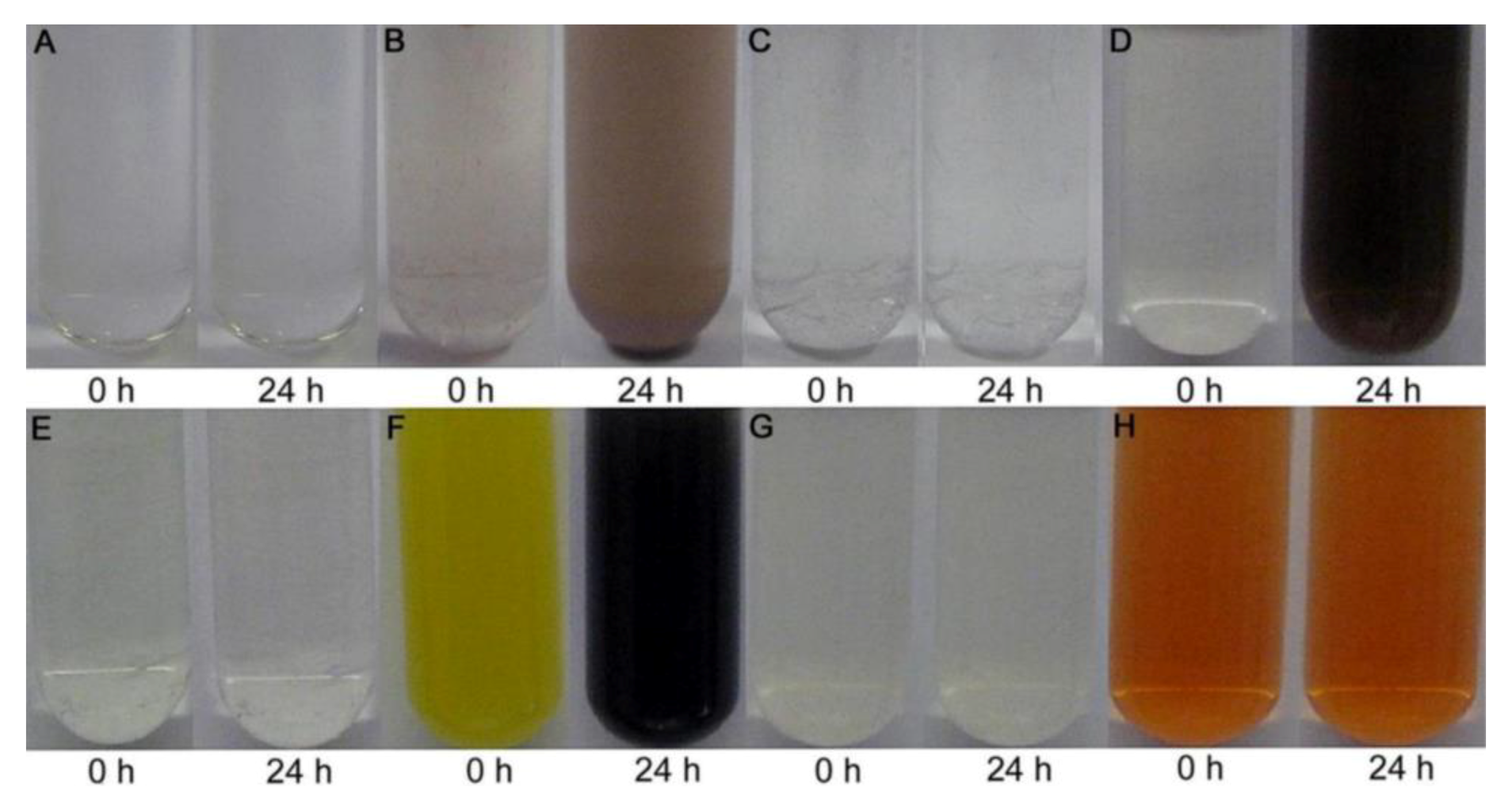


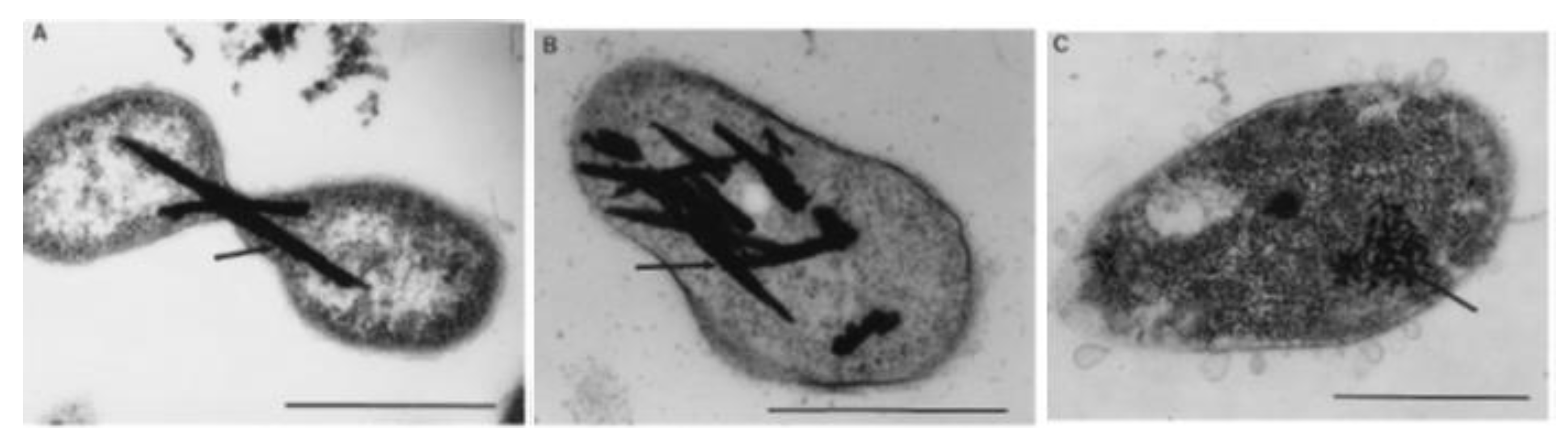
| Strain | Aerobic | Anaerobic |
|---|---|---|
| P. mendocina MCM B-180 | 1.4 | NA |
| Erythomonas ursincola KR99 | 4.2 | NA |
| Erythromicrobium ramosum E5 | 5.1 | NA |
| AV-Te-18 | 2.1 | 0.4 |
| ER-V-8 | 2.0 | 0.2 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maltman, C.; Yurkov, V. Extreme Environments and High-Level Bacterial Tellurite Resistance. Microorganisms 2019, 7, 601. https://doi.org/10.3390/microorganisms7120601
Maltman C, Yurkov V. Extreme Environments and High-Level Bacterial Tellurite Resistance. Microorganisms. 2019; 7(12):601. https://doi.org/10.3390/microorganisms7120601
Chicago/Turabian StyleMaltman, Chris, and Vladimir Yurkov. 2019. "Extreme Environments and High-Level Bacterial Tellurite Resistance" Microorganisms 7, no. 12: 601. https://doi.org/10.3390/microorganisms7120601
APA StyleMaltman, C., & Yurkov, V. (2019). Extreme Environments and High-Level Bacterial Tellurite Resistance. Microorganisms, 7(12), 601. https://doi.org/10.3390/microorganisms7120601




