5-Methyl Furfural Reduces the Production of Malodors by Inhibiting Sodium l-Lactate Fermentation of Staphylococcus epidermidis: Implication for Deodorants Targeting the Fermenting Skin Microbiome
Abstract
1. Introduction
2. Materials and Methods
2.1. Fermentation of Bacterial Culture and Fermentation
2.2. Detection of Malodor Production
2.3. Inhibition of ALS Activity by 5MF
2.4. Inhibitory Effect of 5MF on Fermentation and Malodor Production
2.5. Cell Viability Assay
2.6. Statistical Analysis
3. Results
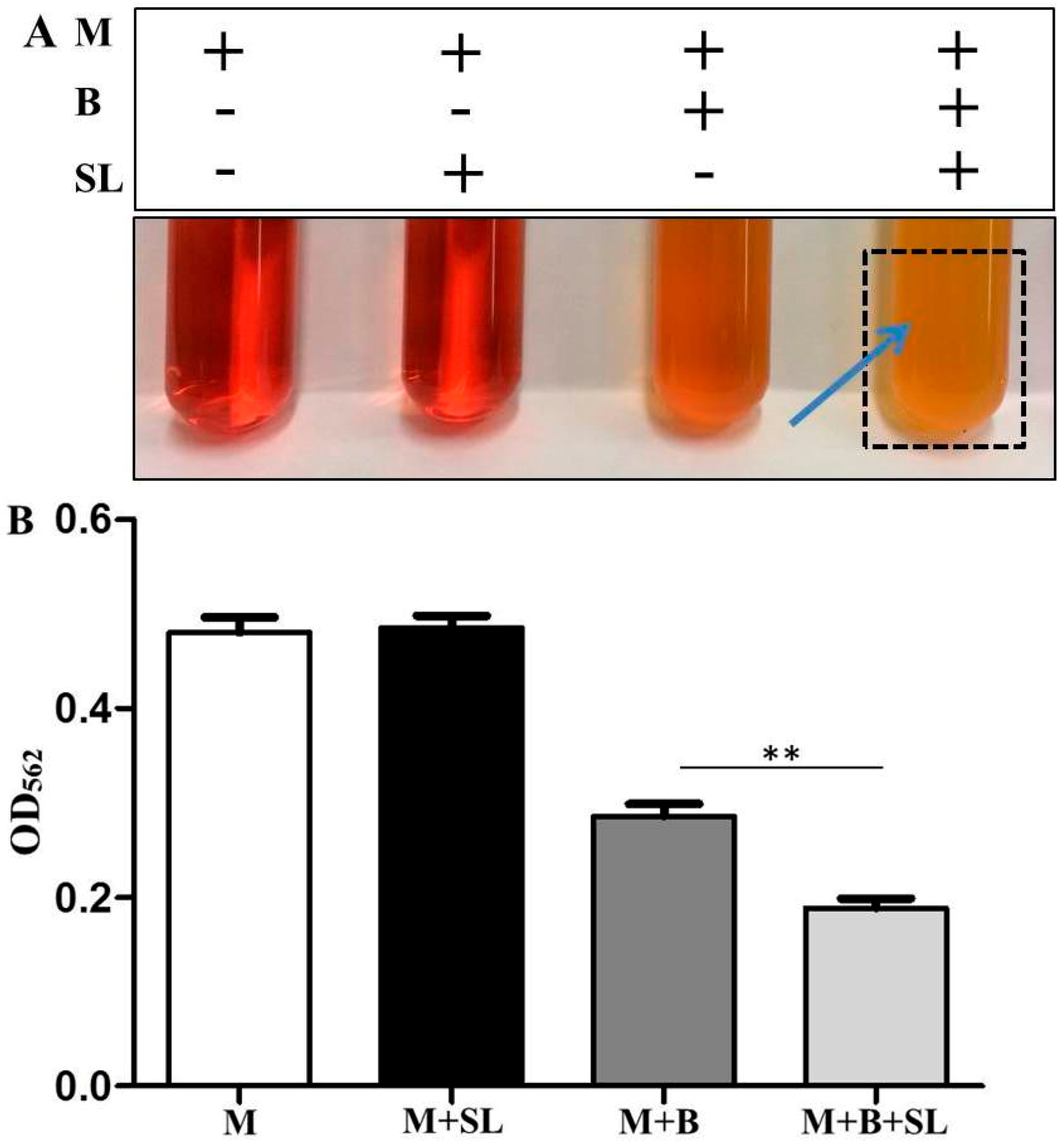
3.1. S. epidermidis Ferments Sodium l-Lactate
3.2. Detection of Malodors by A Gas Colorimetric Tube
3.3. Inhibition of ALS Activity by 5MF
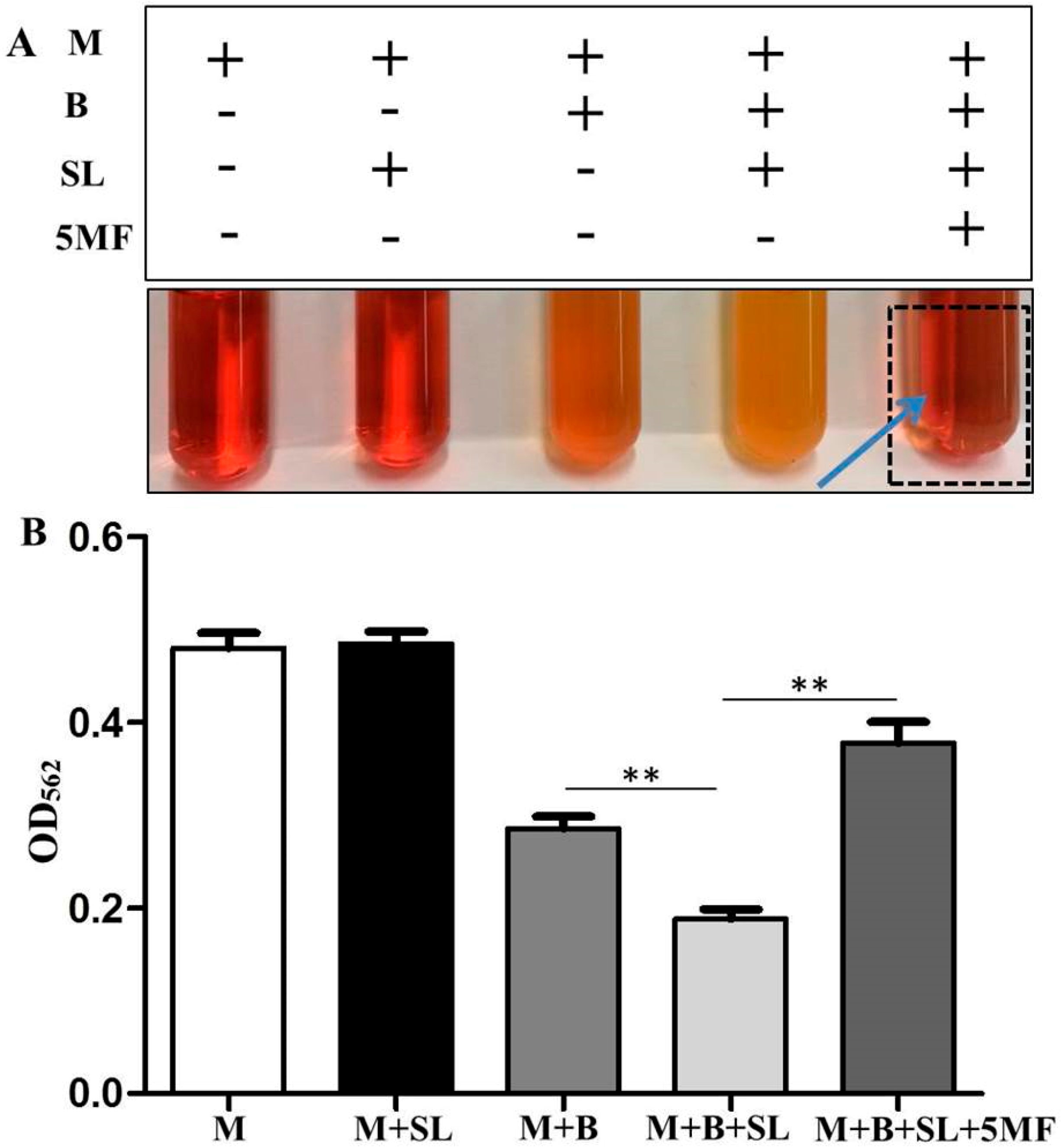
3.4. The Influence of 5MF on Sodium l-Lactate Fermentation of S. epidermidis
3.5. Reduction of Malodor Production by 5MF
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| ADH | Alcohol dehydrogenase |
| AlDH | Aldehyde dehydrogenase |
| ALS | Acetolactate synthease |
| ATCC | American Type Culture Collection |
| bp | Boiling point |
| CFU | Colony-forming unit |
| CO | Carbon monoxide |
| C. avidum | Cutibacterium avidum |
| C. striatum | Cutibacterium striatum |
| DMSO | Dimethyl sulfoxide |
| EDCs | Endocrine disruptors |
| GT-92L | Gastec detector Tubes-92L |
| GC-MS | Gas chromatography-mass spectrometry |
| HCO | Hydrogenated castor oil |
| 5MF | 5-methyl furfural |
| HMF | 5-hydorxymethyl furfural |
| HPLC | High-performance liquid chromatography |
| h | hours |
| KDa | Kilodalton |
| MBC assay | Minimum bactericidal concentration assay |
| min | minutes |
| M. elsdenii | Megasphaera elsdenii |
| 3M3SH | 3-methyl-3-sulfanylhexanol |
| MTT assay | 3,(4,5-Dimethyl thiazol-2-yl)-2-5 diphenyl tetrazolium bromide assay |
| MXC | Methoxychlor |
| NADH | Nicotinamide adenine dinucleotide |
| NCU | National Central University |
| NIH | National Institutes of Health |
| O.D. | Optical density |
| PBS | Phosphate buffer saline |
| PDH | Pyruvate dehydrogenase |
| ppm | Parts per millions |
| S. aureus | Staphylococcus aureus |
| SCFAs | Short chain fatty acids |
| SD | Standard deviation |
| S. epidermidis | Staphylococcus epidermidis |
| S. hominis | Staphylococcus hominis |
| TCS | Triclosan |
| TSB | Tryptic soy broth |
| VFAs | Volatile fatty acids |
| V. filiformis | Vitreoscilla filiformis |
References
- Bawdon, D.; Thomas, G.H.; James, A.G.; Cox, D.S.; Ashford, D. Identification of axillary Staphylococcus sp. involved in the production of the malodorous thioalcohol 3-methyl-3-sufanylhexan-1-ol. FEMS Microbiol. Lett. 2015, 362, fnv111. [Google Scholar] [CrossRef] [PubMed]
- Austin, C.J.; Cox, D.S.; James, A.G.; Taylor, D.; Calvert, R. Microbiological and biochemical origins of human axillary odour. FEMS Microbiol. Ecol. 2013, 83, 527–540. [Google Scholar] [CrossRef]
- Fredrich, E.; Barzantny, H.; Brune, I.; Tauch, A. Daily battle against body odor: Towards the activity of the axillary microbiota. Trends Microbiol. 2013, 21, 305–312. [Google Scholar] [CrossRef] [PubMed]
- Beck, H.C.; Hansen, A.M.; Lauritsen, F.R. Catabolism of leucine to branched-chain fatty acids in Staphylococcus xylosus. J. Appl. Microbiol. 2004, 96, 1185–1193. [Google Scholar] [CrossRef] [PubMed]
- Lam, T.H.; Verzotto, D.; Brahma, P.; Ng, A.H.Q.; Hu, P.; Schnell, D.; Tiesman, J.; Kong, R.; Ton, T.M.U.; Li, J.; et al. Understanding the microbial basis of body odor in pre-pubescent children and teenagers. Microbiome 2018, 6, 213. [Google Scholar] [CrossRef] [PubMed]
- Hara, T.; Matsui, H.; Shimizu, H. Suppression of Microbial Metabolic Pathways Inhibits the Generation of the Human Body Odor Component Diacetyl by Staphylococcus spp. PLoS ONE 2014, 9, e111833. [Google Scholar] [CrossRef] [PubMed]
- Shu, M.; Wang, Y.; Yu, J.; Kuo, S.; Coda, A.; Jiang, Y.; Gallo, R.L.; Huang, C.-M. Fermentation of Propionibacterium acnes, a Commensal Bacterium in the Human Skin Microbiome, as Skin Probiotics against Methicillin-Resistant Staphylococcus aureus. PLoS ONE 2013, 8, e55380. [Google Scholar] [CrossRef]
- Christensen, G.J.M.; Scholz, C.F.P.; Enghild, J.; Rohde, H.; Kilian, M.; Thürmer, A.; Brzuszkiewicz, E.; Lomholt, H.B.; Brüggemann, H. Antagonism between Staphylococcus epidermidis and Propionibacterium acnes and its genomic basis. BMC Genom. 2016, 17, 152. [Google Scholar] [CrossRef]
- James, A.G.; Hyliands, D.; Johnston, H. Generation of volatile fatty acids by axillary bacteria1. Int. J. Cosmet. Sci. 2004, 26, 149–156. [Google Scholar] [CrossRef]
- Natsch, A.; Gfeller, H.; Gygax, P.; Schmid, J.; Acuna, G. A specific bacterial aminoacylase cleaves odorant precursors secreted in the human axilla. J. Biol. Chem. 2003, 278, 5718–5727. [Google Scholar] [CrossRef]
- Le Bars, D.; Yvon, M. Formation of diacetyl and acetoin by Lactococcus lactis via aspartate catabolism. J. Appl. Microbiol. 2008, 104, 171–177. [Google Scholar] [CrossRef]
- Hara, T.M.H.; Kyuka, A.; Shimizu, H. Diacetyl, a novel body odor compound, is effectively suppressed by inhibition of microbial metabolic pathways leading to the genration of malodor. In Proceedings of the 22nd Conference of International Federation of Societies of Cosmetic Chemists, Rio de Janeiro, Brazil, 30 October–1 November 2013; pp. 213–214. [Google Scholar]
- Petersen, L.J. Interstitial lactate levels in human skin at rest and during an oral glucose load: A microdialysis study. Clin. Physiol. 1999, 19, 246–250. [Google Scholar] [CrossRef]
- Sakharov, D.A.; Shkurnikov, M.U.; Vagin, M.Y.; Yashina, E.I.; Karyakin, A.A.; Tonevitsky, A.G. Relationship between lactate concentrations in active muscle sweat and whole blood. Bull. Exp. Biol. Med. 2010, 150, 83–85. [Google Scholar] [CrossRef]
- Natsch, A. What Makes Us Smell: The Biochemistry of Body Odour and the Design of New Deodorant Ingredients. Chim. Int. J. Chem. 2015, 69, 414–420. [Google Scholar] [CrossRef]
- Walton, J.R. Aluminum disruption of calcium homeostasis and signal transduction resembles change that occurs in aging and Alzheimer’s disease. J. Alzheimers Dis. 2012, 29, 255–273. [Google Scholar] [CrossRef]
- McGrath, K.G. Apocrine sweat gland obstruction by antiperspirants allowing transdermal absorption of cutaneous generated hormones and pheromones as a link to the observed incidence rates of breast and prostate cancer in the 20th century. Med. Hypotheses 2009, 72, 665–674. [Google Scholar] [CrossRef]
- Willhite, C.C.; Ball, G.L.; McLellan, C.J. Total allowable concentrations of monomeric inorganic aluminum and hydrated aluminum silicates in drinking water. Crit. Rev. Toxicol. 2012, 42, 358–442. [Google Scholar] [CrossRef]
- Taghipour, K.; Tatnall, F.; Orton, D. Allergic axillary dermatitis due to hydrogenated castor oil in a deodorant. Contact Dermat. 2008, 58, 168–169. [Google Scholar] [CrossRef]
- Kim, J.-Y.; Yi, B.-R.; Go, R.-E.; Hwang, K.-A.; Nam, K.-H.; Choi, K.-C. Methoxychlor and triclosan stimulates ovarian cancer growth by regulating cell cycle- and apoptosis-related genes via an estrogen receptor-dependent pathway. Environ. Toxicol. Pharmacol. 2014, 37, 1264–1274. [Google Scholar] [CrossRef]
- Aly, H.A.; Azhar, A.S. Methoxychlor induced biochemical alterations and disruption of spermatogenesis in adult rats. Reprod. Toxicol. 2013, 40, 8–15. [Google Scholar] [CrossRef]
- Aoyama, H.; Chapin, R.E. Reproductive toxicities of methoxychlor based on estrogenic properties of the compound and its estrogenic metabolite, hydroxyphenyltrichloroethane. Vitam. Horm. 2014, 94, 193–210. [Google Scholar] [CrossRef]
- Callewaert, C.; Lambert, J.; Van de Wiele, T. Towards a bacterial treatment for armpit malodour. Exp. Dermatol. 2017, 26, 388–391. [Google Scholar] [CrossRef]
- Almeida, J.R.; Karhumaa, K.; Bengtsson, O.; Gorwa-Grauslund, M.F. Screening of Saccharomyces cerevisiae strains with respect to anaerobic growth in non-detoxified lignocellulose hydrolysate. Bioresour. Technol. 2009, 100, 3674–3677. [Google Scholar] [CrossRef]
- Palmqvist, E.; Hahn-Hägerdal, B. Fermentation of lignocellulosic hydrolysates. II: Inhibitors and mechanisms of inhibition. Bioresour. Technol. 2000, 74, 25–33. [Google Scholar] [CrossRef]
- Thomsen, M.H.; Thygesen, A.; Thomsen, A.B. Identification and characterization of fermentation inhibitors formed during hydrothermal treatment and following SSF of wheat straw. Appl. Microbiol. Biotechnol. 2009, 83, 447–455. [Google Scholar] [CrossRef]
- Ohnishi, A.; Hasegawa, Y.; Abe, S.; Bando, Y.; Fujimoto, N.; Suzuki, M. Hydrogen fermentation using lactate as the sole carbon source: Solution for ‘blind spots’ in biofuel production. RSC Adv. 2012, 2, 8332–8340. [Google Scholar] [CrossRef]
- Bruce, N.; McCracken, J.; Albalak, R.; Schei, M.A.; Smith, K.R.; Lopez, V.; West, C. Impact of improved stoves, house construction and child location on levels of indoor air pollution exposure in young Guatemalan children. J. Expo Anal. Environ. Epidemiol. 2004, 14 (Suppl. 1), S26–S33. [Google Scholar] [CrossRef]
- Cogan, T.M.; Fitzgerald, R.J.; Doonan, S. Acetolactate synthase of Leuconostoc lactis and its regulation of acetoin production. J. Dairy Res. 1984, 51, 597–604. [Google Scholar] [CrossRef]
- Stormer, F.C. Evidence for induction of the 2,3-butanediol-forming enzymes in Aerobacter aerogenes. FEBS Lett. 1968, 2, 36–38. [Google Scholar] [CrossRef]
- Burtenshaw, J.M.L. The Mechanism of Self-Disinfection of the Human Skin and its Appendages. J. Hyg. 1942, 42, 184–210. [Google Scholar] [CrossRef]
- Mickelsen, O.; Keys, A. The composition of sweat with special reference to the vitamins. J. Biol. Chem. 1943, 149, 479–490. [Google Scholar]
- Chotigarpa, R.; Na Lampang, K.; Pikulkaew, S.; Okonogi, S.; Ajariyakhajorn, K.; Mektrirat, R. Inhibitory Effects and Killing Kinetics of Lactic Acid Rice Gel Against Pathogenic Bacteria Causing Bovine Mastitis. Sci. Pharm. 2018, 86, 29. [Google Scholar] [CrossRef]
- Seeliger, S.; Janssen, P.H.; Schink, B. Energetics and kinetics of lactate fermentation to acetate and propionate via methylmalonyl-CoA or acrylyl-CoA. FEMS Microbiol. Lett. 2002, 211, 65–70. [Google Scholar] [CrossRef]
- Duncan, S.H.; Louis, P.; Flint, H.J. Lactate-utilizing bacteria, isolated from human feces, that produce butyrate as a major fermentation product. Appl. Environ. Microbiol. 2004, 70, 5810–5817. [Google Scholar] [CrossRef]
- Antal, M.J.; Mok, W.S.L.; Richards, G.N. Mechanism of formation of 5-(hydroxymethyl)-2-furaldehyde from d-fructose and sucrose. Carbohydr. Res. 1990, 199, 91–109. [Google Scholar] [CrossRef]
- Antal, M.J.; Leesomboon, T.; Mok, W.S.; Richards, G.N. Mechanism of formation of 2-furaldehyde from d-xylose. Carbohydr. Res. 1991, 217, 71–85. [Google Scholar] [CrossRef]
- Almeida, J.R.; Modig, T.; Petersson, A.; Hähn-Hägerdal, B.; Lidén, G.; Gorwa-Grauslund, M.F. Increased tolerance and conversion of inhibitors in lignocellulosic hydrolysates by Saccharomyces cerevisiae. J. Chem. Technol. Biotechnol. Int. Res. Process Environ. Clean Technol. 2007, 82, 340–349. [Google Scholar] [CrossRef]
- Giordano, L.; Calabrese, R.; Davoli, E.; Rotilio, D. Quantitative analysis of 2-furfural and 5-methylfurfural in different Italian vinegars by headspace solid-phase microextraction coupled to gas chromatography–mass spectrometry using isotope dilution. J. Chromatogr. A 2003, 1017, 141–149. [Google Scholar] [CrossRef]
- Modig, T.; Lidén, G.; Taherzadeh, M.J. Inhibition effects of furfural on alcohol dehydrogenase, aldehyde dehydrogenase and pyruvate dehydrogenase. Biochem. J. 2002, 363, 769–776. [Google Scholar] [CrossRef]
- McCourt, J.A.; Duggleby, R.G. Acetohydroxyacid synthase and its role in the biosynthetic pathway for branched-chain amino acids. Amino Acids 2006, 31, 173–210. [Google Scholar] [CrossRef]
- Singh, B.K.; Shaner, D.L. Biosynthesis of Branched Chain Amino Acids: From Test Tube to Field. Plant Cell 1995, 7, 935–944. [Google Scholar] [CrossRef]
- Richie, D.L.; Thompson, K.V.; Studer, C.; Prindle, V.C.; Aust, T.; Riedl, R.; Estoppey, D.; Tao, J.; Sexton, J.A.; Zabawa, T.; et al. Identification and evaluation of novel acetolactate synthase inhibitors as antifungal agents. Antimicrob. Agents Chemother. 2013, 57, 2272–2280. [Google Scholar] [CrossRef]
- Pang, S.S.; Duggleby, R.G.; Schowen, R.L.; Guddat, L.W. The crystal structures of Klebsiella pneumoniae acetolactate synthase with enzyme-bound cofactor and with an unusual intermediate. J. Biol. Chem. 2004, 279, 2242–2253. [Google Scholar] [CrossRef]



© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kumar, M.; Myagmardoloonjin, B.; Keshari, S.; Negari, I.P.; Huang, C.-M. 5-Methyl Furfural Reduces the Production of Malodors by Inhibiting Sodium l-Lactate Fermentation of Staphylococcus epidermidis: Implication for Deodorants Targeting the Fermenting Skin Microbiome. Microorganisms 2019, 7, 239. https://doi.org/10.3390/microorganisms7080239
Kumar M, Myagmardoloonjin B, Keshari S, Negari IP, Huang C-M. 5-Methyl Furfural Reduces the Production of Malodors by Inhibiting Sodium l-Lactate Fermentation of Staphylococcus epidermidis: Implication for Deodorants Targeting the Fermenting Skin Microbiome. Microorganisms. 2019; 7(8):239. https://doi.org/10.3390/microorganisms7080239
Chicago/Turabian StyleKumar, Manish, Binderiya Myagmardoloonjin, Sunita Keshari, Indira Putri Negari, and Chun-Ming Huang. 2019. "5-Methyl Furfural Reduces the Production of Malodors by Inhibiting Sodium l-Lactate Fermentation of Staphylococcus epidermidis: Implication for Deodorants Targeting the Fermenting Skin Microbiome" Microorganisms 7, no. 8: 239. https://doi.org/10.3390/microorganisms7080239
APA StyleKumar, M., Myagmardoloonjin, B., Keshari, S., Negari, I. P., & Huang, C.-M. (2019). 5-Methyl Furfural Reduces the Production of Malodors by Inhibiting Sodium l-Lactate Fermentation of Staphylococcus epidermidis: Implication for Deodorants Targeting the Fermenting Skin Microbiome. Microorganisms, 7(8), 239. https://doi.org/10.3390/microorganisms7080239



