Antileishmanial Activity of Ziziphus spina-christi Leaves Extract and Its Possible Cellular Mechanisms
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Preparing of Methanolic Extract
2.3. Phytochemical Analysis
2.4. Secondary Metabolites Contents
2.5. Parasite and Cell Culture
2.6. Anti-Intracellular Amastigote Effects
2.7. Evaluation of the Infection Rate in Macrophages
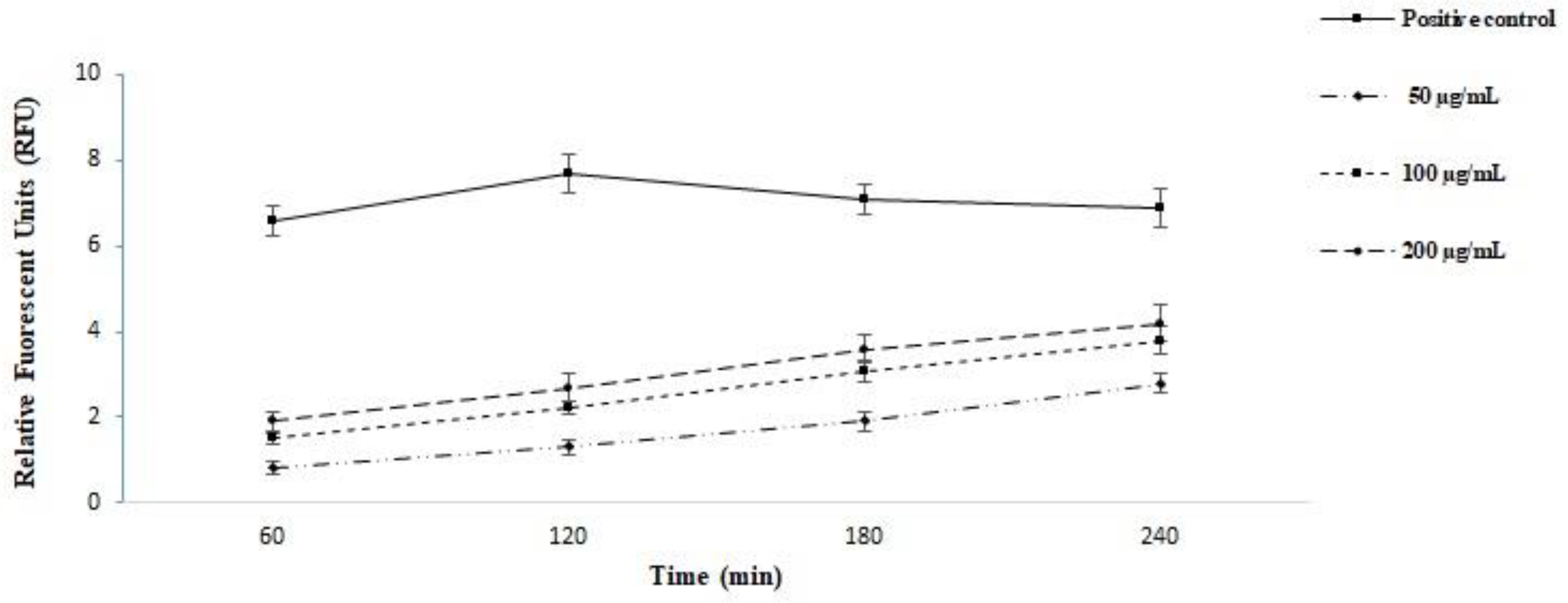
2.8. Plasma Membrane Permeability
2.9. Nitric Oxide (NO) Production
2.10. Evaluating the Caspase-3-like Activity of Extract-Treated Promastigotes
2.11. Cytotoxic Effects
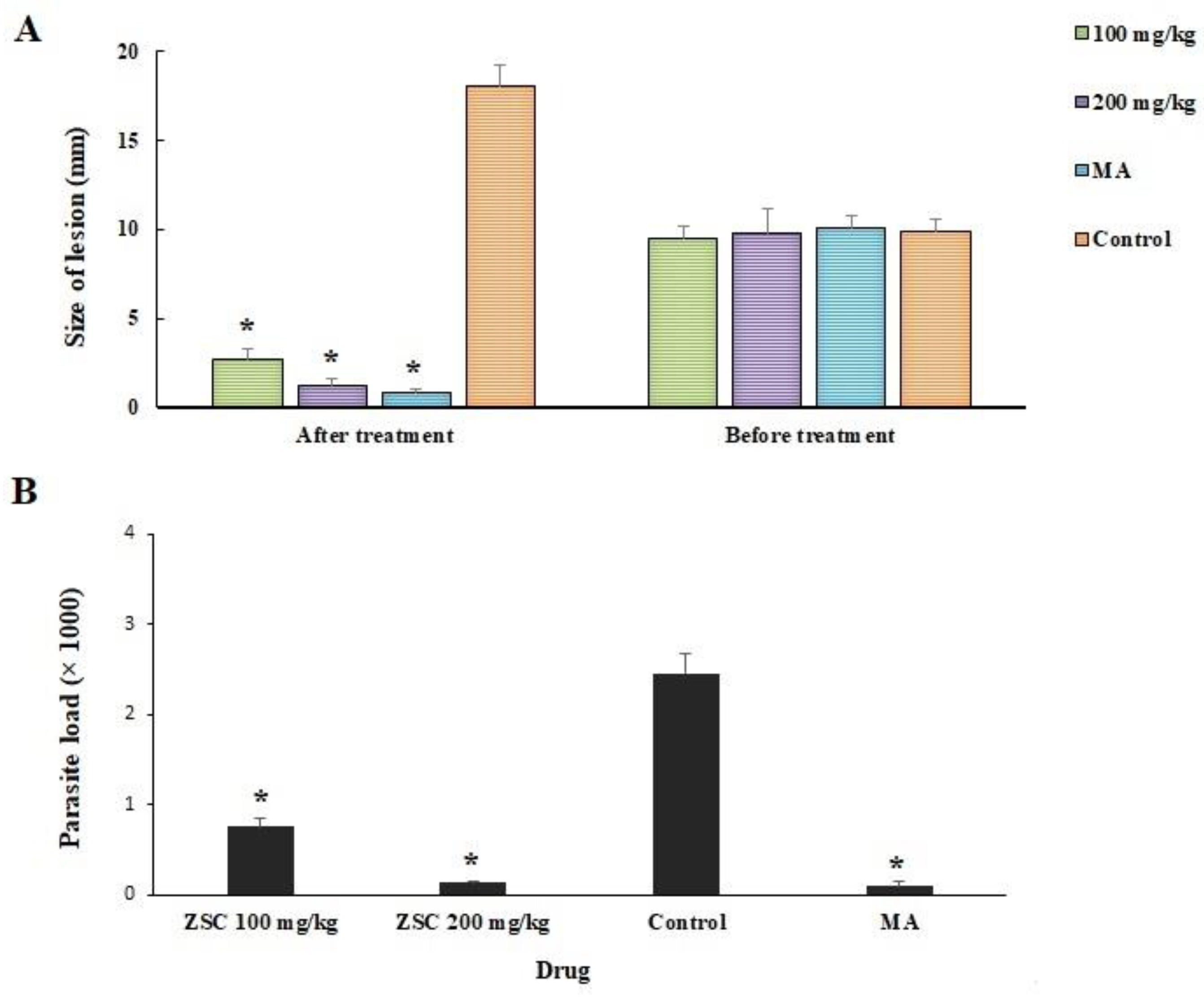
2.12. In Vivo Antileishmanial Effects against Cutaneous Leishmaniasis
2.12.1. Establishment of CL in BALB/c Mice
2.12.2. Treating Infected Mice
2.13. Statistical Analysis
3. Results
3.1. Phytochemical Analysis
3.2. Secondary Metabolites Contents
3.3. In Vitro Antileishmanial Effects
3.4. In Vivo Antileishmanial Effects
3.5. The Effect on the Plasma Membrane Permeability
3.6. The Effect of Wxtract on the Infectivity Rate of Promastigotes
3.7. Effect on the NO Production
3.8. Effect on the Caspase-3-like Activity
3.9. Cytotoxicity on the Macrophage Cells
4. Discussion
5. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kevric, I.; Cappel, M.A.; Keeling, J.H. New world and old world Leishmania infections: A practical review. Dermatologic clinics. 2015, 33, 579–593. [Google Scholar] [CrossRef]
- Sharifi, I.; Aflatoonian, M.R.; Parizi, M.H.D.; Hosseininasab, A.; Mostafavi, M.; Bamorovat, M.; Afshar, A.A.; Mohebali, M.; Keshavarz, H.; Daneshvar, H.; et al. Visceral Leishmaniasis in Southeastern Iran: A Narrative Review. Iran. J. Parasitol. 2017, 12, 1. [Google Scholar] [PubMed]
- Nafari, A.H.; Cheraghipour, K.; Sepahvand, M.; Shahrokhi, G.; Gabal, E.; Mahmoudvand, H. Nanoparticles: New agents toward treatment of leishmaniasis. Parasite Epidemiol. Control. 2020, 10, e00156. [Google Scholar] [CrossRef] [PubMed]
- Al Mohammed, H.I.; Khudair, K.A.; Albalawi, A.E.; Alanazi, A.D.; Baharvand, P.; Moghaddam, A.; Mahmoudvand, H. Chi-tosan-Based Nanomaterials as Valuable Sources of Anti-Leishmanial Agents: A Systematic Review. Nanomaterials 2021, 11, 689. [Google Scholar] [CrossRef] [PubMed]
- Amin, T.T.; Al-Mohammed, H.I.; Kaliyadan, F.; Mohammed, B.S. Cutaneous leishmaniasis in Al Hassa, Saudi Arabia: Epidemio-logical trends from 2000 to 2010. Asian Pac. J. Trop. Biomed. 2013, 6, 667–672. [Google Scholar] [CrossRef]
- Alanazi, A.D.; Alouffi, A.S.; Alyousif, M.S.; Rahi, A.A.; Ali, M.A.; Abdullah, H.H.A.M.; Brayner, F.A.; Mendoza-Roldan, J.A.; Bezerra-Santos, M.A.; Otranto, D. Molecular characterization of Leishmania species from stray dogs and human patients in Saudi Arabia. Parasitol. Res. 2021, 1–6. [Google Scholar] [CrossRef]
- Abuzaid, A.A.; Abdoon, A.M.; Aldahan, M.A.; Alzahrani, A.G.; AlHakeem, R.F.; Asiri, A.M.; Alzahrani, M.H.; Memish, Z.A. Cutaneous Leishmaniasis in Saudi Arabia: A Comprehensive Overview. Vector Borne Zoonotic Dis. 2017, 17, 673–684. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Monzote, L. Current treatment of leishmaniasis: A review. Open Antimicrob. Agents J. 2009, 1, 9–19. [Google Scholar]
- Palumbo, E. Current treatment for cutaneous leishmaniasis: A review. Am. J. Ther. 2009, 16, 178–182. [Google Scholar] [CrossRef]
- Copeland, N.K.; Aronson, N.E. Leishmaniasis: Treatment updates and clinical practice guidelines review. Current Opin. Infect. Dis. 2015, 28, 426–437. [Google Scholar] [CrossRef]
- Oliveira, L.F.; Schubach, A.O.; Martins, M.M.; Passos, S.L.; Oliveira, R.V.; Marzochi, M.C.; Andrade, C.A. Systematic review of the adverse effects of cutaneous leishmaniasis treatment in the New World. Actatropica 2011, 118, 87–96. [Google Scholar] [CrossRef]
- Passero, F.; Cruz, L.A.; Santos-Gomes, G.; Rodrigues, E.; Laurenti, M.D.; Lago, J.H.G. Conventional Versus Natural Alternative Treatments for Leishmaniasis: A Review. Curr. Top. Med. Chem. 2018, 18, 1275–1286. [Google Scholar] [CrossRef] [PubMed]
- World Health Organisation. Leishmaniasis Fact Sheet. 2020. Available online: https://www.who.int/news-room/fact-sheets/detail/leishmaniasis (accessed on 31 May 2020).
- Saied, A.S.; Gebauer, J.; Hammer, K.; Buerkert, A. Ziziphus spinachristi (L.) Willd.: A multipurpose fruit tree. Genet. Resour. Crop. Evol. 2007, 55, 929–937. [Google Scholar] [CrossRef]
- Farooqi, A. Plants of Quraan; Sidrah Publishers: Lucknow, India, 1997; pp. 65–74. [Google Scholar]
- Abdel-Zaher, A.O.; Salim, S.Y.; Assaf, M.H.; Abdel-Hady, R.H. Antidiabetic activity and toxicity of Zizyphus spina-christi leaves. J Ethnopharmacol. 2005, 101, 129–138. [Google Scholar] [CrossRef]
- Asgarpanah, J.; Haghighat, E. Phytochemistry and pharmacologic properties of Ziziphus spina-christi (L.) Willd. Afr. J. Pharm. Pharmacol. 2012, 6, 2332–2339. [Google Scholar] [CrossRef] [Green Version]
- Ezatpour, B.; Saedi, D.E.; Mahmoudvand, H.; Azadpour, M.; Ezzatkhah, F. In vitro and in vivo antileishmanial effects of Pistacia khinjuk against Leishmania tropica and Leishmania major. Evid. Based Complementary Altern. Med. 2015, 2015, 149707. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nazer, M.R.; Jahanbakhsh, S.; Ebrahimi, K.; Niazi, M.; Sepahvand, M.; Khatami, M.; Kharazi, S. Cytotoxic and antileishmanial effects of various extracts of Capparisspinosa L. Turk. J. Pharm. Sci. 2021, 18, 146. [Google Scholar] [CrossRef]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventós, M. Analysis of total phenols and other oxidation substrates and antioxidants by means of Folin-Ciocalteu reagent. Method Enzymol. 1999, 299, 152–178. [Google Scholar]
- Phuyal, N.; Jha, P.K.; Raturi, P.P.; Rajbhandary, S. Total Phenolic, Flavonoid Contents, and Antioxidant Activities of Fruit, Seed, and Bark Extracts of Zanthoxylum armatum DC. Sci. World J. 2020, 2020, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Broadhurst, R.B.; Jones, W.T. Analysis of condensed tannins using acidified vanillin. J. Sci. Food Agric. 1978, 29, 788–794. [Google Scholar] [CrossRef]
- Mahmoudvand, H.; Dezaki, E.S.; Ezatpour, B.; Sharifi, I.; Kheirandish, F.; Rashidipour, M. In vitro and in vivo antileishmanial ac-tivities of Pistacia vera essential oil. Planta Med. 2016, 82, 279–284. [Google Scholar] [PubMed] [Green Version]
- Albalawi, A.E.; Abdel-Shafy, S.; KhudairKhalaf, A.; Alanazi, A.D.; Baharvand, P.; Ebrahimi, K.; Mahmoudvand, H. Therapeutic po-tential of green synthesized copper nanoparticles alone or combined with meglumineantimoniate (glucantime®) in cutaneous leishmaniasis. Nanomaterials 2021, 11, 891. [Google Scholar] [CrossRef] [PubMed]
- Albalawi, A.E.; Khalaf, A.K.; Alyousif, M.S.; Alanazi, A.D.; Baharvand, P.; Shakibaie, M.; Mahmoudvand, H. Fe3O4@ piroctoneolamine magnetic nanoparticles: Synthesize and therapeutic potential in cutaneous leishmaniasis. Biomed. Pharmacother. 2021, 139, 111566. [Google Scholar] [CrossRef] [PubMed]
- Ezzatkhah, F.; Khalaf, A.K.; Mahmoudvand, H. Copper nanoparticles: Biosynthesis, characterization, and protoscolicidal effects alone and combined with albendazole against hydatid cyst protoscoleces. Biomed. Pharmacother. 2021, 136, 111257. [Google Scholar] [CrossRef]
- Taghipour, M.T.; Nameni, R.; Taghipour, M.; Ghorat, F. Phytochemical Analysis and Antimicrobial Activity of Ziziphus spina-christi and Tamarix aphylla Leaves’ Extracts as Effective Treatment for Coronavirus Disease 2019 (COVID-19). Thrita 2020, 9, e107776. [Google Scholar] [CrossRef]
- Xie, Y.; Yang, W.; Tang, F.; Chen, X.; Ren, L. Antibacterial Activities of Flavonoids: Structure-Activity Relationship and Mechanism. Curr. Med. Chem. 2014, 22, 132–149. [Google Scholar] [CrossRef]
- Kaczmarek, B. Tannic Acid with Antiviral and Antibacterial Activity as A Promising Component of Biomaterials—A Minireview. Materials 2020, 13, 3224. [Google Scholar] [CrossRef]
- Cushnie, T.T.; Cushnie, B.; Lamb, A.J. Alkaloids: An overview of their antibacterial, antibiotic-enhancing and antivirulence activ-ities. Int. J. Antimicrob. Agents 2014, 44, 377–386. [Google Scholar] [CrossRef]
- Astuti, S.M.; Sakinah, A.M.; Andayani, B.M.R.; Risch, A. Determination of Saponin Compound from Anredera cordifolia (Ten) Steenis Plant (Binahong) to Potential Treatment for Several Diseases. J. Agric. Sci. 2011, 3, 224. [Google Scholar] [CrossRef]
- Haddad, M.H.F.; Khodkar, I.; Samie, M. In Vitro Anti-leishmanial Effects of Hydroalcoholic Extracts from Six Iranian Medicinal Herbs on Leishmania major (MRHO/IR/75/ER) Promastigotes. Jentashapir J. Health Res. 2016, 7, e33465. [Google Scholar] [CrossRef]
- Alzahrani, F.; Al-Shaebi, E.M.; Dkhil, M.A.; Al-Quraishy, S. In vivo anti-eimeria and in vitro anthelmintic activity of Ziziphusspina-christi leaf extracts. Pak J Zool 2016, 48, 409–413. [Google Scholar]
- Hafiz, T.A.; Mubaraki, M.A.; Diab, M.S.; Dkhil, M.A.; Al-Quraishy, S. Ameliorative role of Ziziphus spina-christi leaf extracts against hepatic injury induced by Plasmodium chabaudi infected erythrocytes. Saudi J. Biol. Sci. 2019, 26, 490–494. [Google Scholar] [CrossRef] [PubMed]
- Almeer, R.S.; El-Khadragy, M.F.; Abdelhabib, S.; Moneim, A.E.A. Ziziphus spina-christi leaf extract ameliorates schistosomiasis liver granuloma, fibrosis, and oxidative stress through downregulation of fibrinogenic signaling in mice. PLoS ONE 2018, 13, e0204923. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fadladdin, Y.A. Antischistosomal Activity of Origanum majorana, Ziziphus spina-christi, and Salvia fruticosa Plant Extracts on Hamster Infected with Schistosoma haematobium. BioMed Res. Int. 2021, 2021, 5545331. [Google Scholar] [CrossRef]
- Karar, M.E.; Quiet, L.; Rezk, A.; Jaiswal, R.; Rehders, M.; Ullrich, M.S.; Brix, K.; Kuhnert, N. Phenolic profile and in vitro assessment of cytotoxicity and antibacterial activity of Ziziphus spina-christi leaf extracts. Med. Chem. 2016, 6, 143–156. [Google Scholar]
- Mahmoudvand, H.; Kheirandish, F.; Mirbadie, S.R.; Kayedi, M.H.; Riabi, T.R.; Ghasemi, A.A.; Bamorovat, M.; Sharifi, I. The potential use of methotrexate in the treatment of cutaneous leishmaniasis: In vitro assays against sensitive and meglumineantimoni-ate-resistant strains of Leishmania tropica. Iran. J. Parasitol. 2017, 12, 339. [Google Scholar]
- Holzmuller, P.; Sereno, D.; Cavaleyra, M.; Mangot, I.; Daulouede, S.; Vincendeau, P.; Lemesre, J.L. Nitric oxide-mediated proteasome-dependent oligonucleosomal DNA fragmentation in Leishmania amazonensis amastigotes. Infect. Immun. 2002, 70, 3727–3735. [Google Scholar] [CrossRef] [Green Version]
- Elmore, S. Apoptosis: A review of programmed cell death. Toxicol. Pathol. 2007, 35, 495–516. [Google Scholar] [CrossRef]
- Budihardjo, I.; Oliver, G.; Lutter, M.; Luo, X.; Wang, X. Biochemical Pathways of Caspase Activation During Apoptosis. Annu. Rev. Cell Dev. Biol. 1999, 15, 269–290. [Google Scholar] [CrossRef] [Green Version]
- Porter, A.G.; Jänicke, R.U. Emerging roles of caspase-3 in apoptosis. Cell Death Differ. 1999, 6, 99–104. [Google Scholar] [CrossRef]
- Weniger, B.; Robledo, S.; Arango, G.J.; Deharo, E.; Aragón, R.; Muñoz, V.; Callapa, J.; Lobstein, A.; Anton, R. Antiprotozoal activities of Colombian plants. J. Ethnopharmacol. 2001, 78, 193–200. [Google Scholar] [CrossRef]


| Phytochemical | Test | Presence |
|---|---|---|
| Alkaloids | Mayer and Dragendorff’s reagents test | + |
| Flavonoids | Ammonia test, alkaline reagent test | + |
| Glycosides | Nitroprusside test | + |
| Saponins | Frothing test | - |
| Tannins | FeCl3 solutions | + |
| Terpenoids | Salkowski test | + |
| Total Content | Test | Amount |
|---|---|---|
| Phenolic | Folin–Ciocalteau’s reagent colorimetric | 51.33 ± 0.41 mg GEA/g DW |
| Flavonoids | Aluminum chloride (AlCl3 2%) colorimetric | 14.78 ± 0.36 mg QE/g DW |
| Tannins | Vanillin-HCl colorimetric | 21.6 ± 1.51 mg CE/g DW |
| Tested Material | IC50 (µg/mL) for L. major Amastigote | CC50 (µg/mL) of the J774-A1 Cells | SI |
|---|---|---|---|
| Z. spina-christi extract | 54.6 ± 3.15 | 563.3 ± 8.63 | 10.31 |
| MA | 47.3 ± 2.15 | 914.6 ± 11.60 | 19.33 |
| Promastigotes | Percentage of Infected Macrophages | Infectiveness Reduction (%) |
|---|---|---|
| Non-treated | 78.6± 3.15 | - |
| Treated with Z. spina-christi extract (5 µg/mL) | 30.3± 2.51 | 61.4 * |
| Concentration (µg/mL) | Production of Nitric Oxide (nM) |
|---|---|
| 10 | 8.6 ± 0.55 |
| 25 | 9.4 ± 0.64 |
| 50 | 16 ± 1.15 * |
| Non-treated | 5.6 ± 1.15 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Albalawi, A.E. Antileishmanial Activity of Ziziphus spina-christi Leaves Extract and Its Possible Cellular Mechanisms. Microorganisms 2021, 9, 2113. https://doi.org/10.3390/microorganisms9102113
Albalawi AE. Antileishmanial Activity of Ziziphus spina-christi Leaves Extract and Its Possible Cellular Mechanisms. Microorganisms. 2021; 9(10):2113. https://doi.org/10.3390/microorganisms9102113
Chicago/Turabian StyleAlbalawi, Aishah E. 2021. "Antileishmanial Activity of Ziziphus spina-christi Leaves Extract and Its Possible Cellular Mechanisms" Microorganisms 9, no. 10: 2113. https://doi.org/10.3390/microorganisms9102113
APA StyleAlbalawi, A. E. (2021). Antileishmanial Activity of Ziziphus spina-christi Leaves Extract and Its Possible Cellular Mechanisms. Microorganisms, 9(10), 2113. https://doi.org/10.3390/microorganisms9102113





