Phytoplankton Morpho-Functional Trait Variability along Coastal Environmental Gradients
Abstract
:1. Introduction
2. Materials and Methods
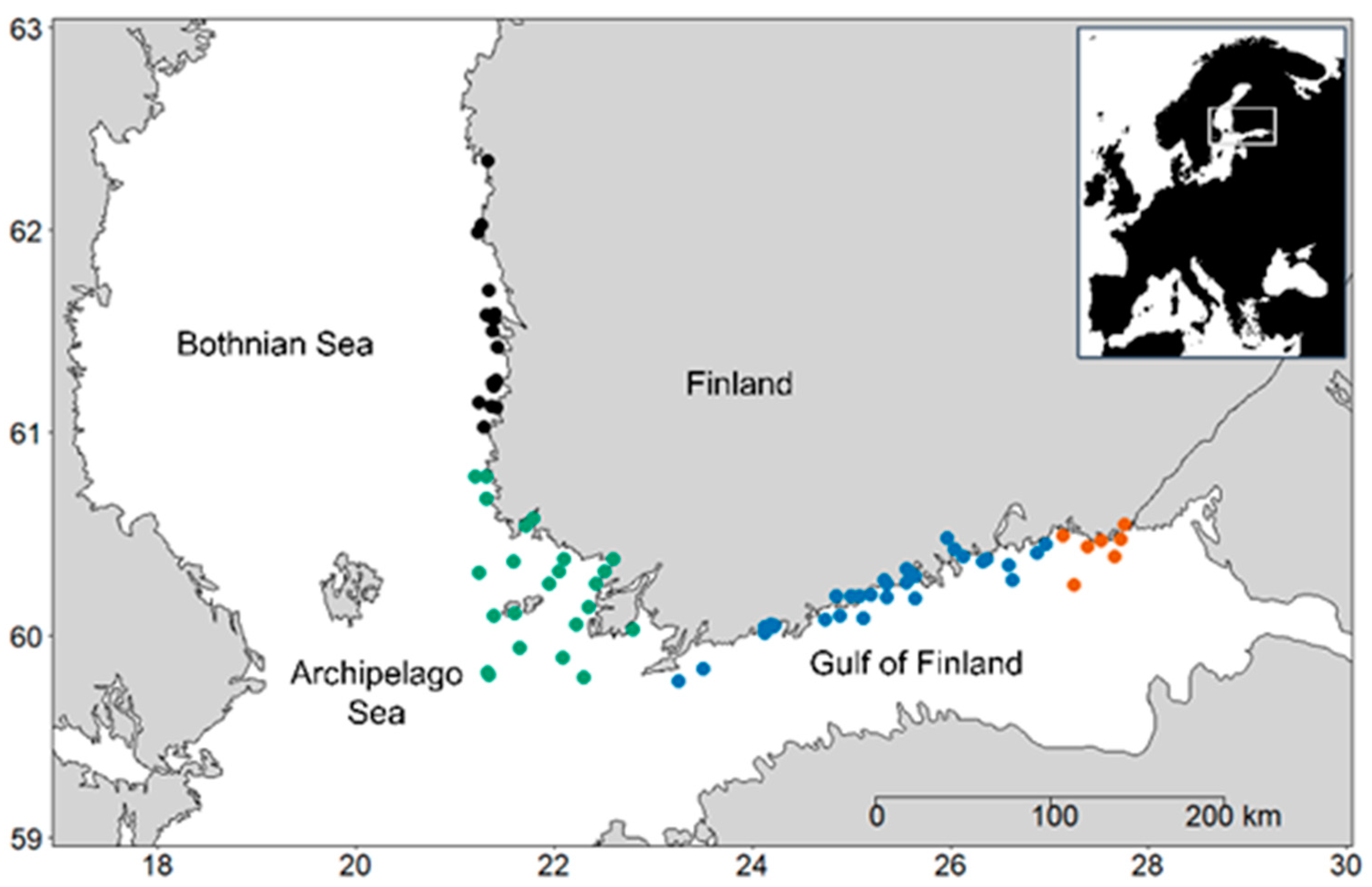
2.1. Study Area
2.2. Phytoplankton and Environmental Data
2.3. Morpho-Functional Traits
2.4. Statistical Analyses
3. Results
3.1. Comparison of the Coastal Areas
3.2. Effects of Environmental Variables on the Traits
3.3. Regional Variability in Trait Occurrences
4. Discussion
4.1. Physical Features Connected to Global Change vs. Traits
4.2. Water Quality Features Connected to Catchment Change vs. Traits
4.3. Nutrient Availability Connected to Nutrient Loading vs. Traits
4.4. Regionality
4.5. Future Research Directions
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pajares, S.; Ramos, R. Processes and microorganisms involved in the marine nitrogen cycle: Knowledge and gaps. Front. Mar. Sci. 2019, 6, 739. [Google Scholar] [CrossRef]
- Martini, S.; Larras, F.; Boyé, A.; Faure, E.; Aberle, N.; Archambault, P.; Bacouillard, L.; Beisner, B.E.; Bittner, L.; Castella, E.; et al. Functional trait-based approaches as a common framework for aquatic ecologists. Limnol. Oceanogr. 2021, 66, 965–994. [Google Scholar] [CrossRef]
- Litchman, E.; Klausmeier, C.A. Trait-based community ecology of phytoplankton. Annu. Rev. Ecol. Evol. Syst. 2008, 39, 615–639. [Google Scholar] [CrossRef] [Green Version]
- Leruste, A.; Villéger, S.; Malet, N.; De Wit, R.; Bec, B. Complementarity of the multidimensional functional and the taxonomic approaches to study phytoplankton communities in three Mediterranean coastal lagoons of different trophic status. Hydrobiologia 2018, 815, 207–227. [Google Scholar] [CrossRef] [Green Version]
- Graco-Roza, C.; Soininen, J.; Corrêa, G.; Pacheco, F.S.; Miranda, M.; Domingos, P.; Marinho, M.M. Functional rather than taxonomic diversity reveals changes in the phytoplankton community of a large dammed river. Ecol. Indic. 2021, 121, 107048. [Google Scholar] [CrossRef]
- Violle, C.; Navas, M.-L.; Vile, D.; Kazakou, E.; Fortunel, C.; Hummel, I.; Garnier, E. Let the concept of trait be functional! Oikos 2007, 116, 882–892. [Google Scholar] [CrossRef]
- Edwards, K.F.; Thomas, M.K.; Klausmeier, C.A.; Litchman, E. Light and growth in marine phytoplankton: Allometric, taxonomic, and environmental variation. Limnol. Oceanogr. 2015, 60, 540–552. [Google Scholar] [CrossRef] [Green Version]
- Klais, R.; Norros, V.; Lehtinen, S.; Tamminen, T.; Olli, K. Community assembly and drivers of phytoplankton functional structure. Funct. Ecol. 2017, 31, 760–767. [Google Scholar] [CrossRef]
- Laplace-Treyture, C.; Derot, J.; Prévost, E.; Le Mat, A.; Jamoneau, A. Phytoplankton morpho-functional trait dataset from French waterbodies. Sci. Data 2021, 8, 40. [Google Scholar] [CrossRef] [PubMed]
- Deutsch, C.; Sarmiento, J.L.; Sigman, D.M.; Gruber, N.; Dunne, J.P. Spatial coupling of nitrogen inputs and losses in the ocean. Nature 2007, 445, 163–167. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.L.; Moore, J.K.; Martiny, A.C.; Primeau, F.W. Convergent estimates of marine nitrogen fixation. Nature 2019, 566, 205–211. [Google Scholar] [CrossRef] [PubMed]
- Schindler, D.W. Evolution of phosphorus limitation in lakes. Science 1977, 195, 260–262. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tyrrell, T. The relative influences of nitrogen and phosphorus on oceanic primary production. Nature 1999, 400, 525–531. [Google Scholar] [CrossRef]
- Howarth, R.W.; Marino, R. Nitrogen as the limiting nutrient for eutrophication in coastal marine ecosystems: Evolving views over three decades. Limnol. Oceanogr. 2006, 51, 364–376. [Google Scholar] [CrossRef] [Green Version]
- Scott, J.T.; McCarthy, M.J. Nitrogen fixation may not balance the nitrogen pool in lakes over timescales relevant to eutrophication management. Limnol. Oceanogr. 2010, 55, 1265–1270. [Google Scholar] [CrossRef]
- Karlson, A.M.L.; Duberg, J.; Motwani, N.H.; Hogfors, H.; Klawonn, I.; Ploug, H.; Barthel, J.; Garbaras, S.A.; Sundelin, B.; Hajdu, S.; et al. Nitrogen fixation by cyanobacteria stimulates production in Baltic food webs. Ambio 2015, 44 (Suppl. 3), 413–426. [Google Scholar] [CrossRef] [Green Version]
- Adam, B.; Klaworn, I.; Svedén, J.B.; Bergkvist, J.; Nahar, N.; Walve, J.; Littmann, S.; Whitehouse, M.J.; Lavik, G.; Kuypers, M.M.; et al. N2-fixation, ammonium release and N-transfer to the microbial and classical food web within a plankton community. ISME J. 2016, 10, 450–459. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zehr, J.P.; Capone, D.G. Changing perspectives in marine nitrogen fixation. Science 2020, 368, 6492. [Google Scholar] [CrossRef] [PubMed]
- Walsby, A.E.; Hayes, P.K.; Boje, R. The gas vesicles, buoyancy and vertical distribution of cyanobacteria in the Baltic Sea. Eur. J. Phycol. 1995, 30, 87–94. [Google Scholar] [CrossRef] [Green Version]
- Walsby, A.E.; Hayes, P.K.; Boje, R.; Stal, L.J. The selective advantage of buoyancy provided by gas vesicles for planktonic cyanobacteria in the Baltic Sea. New Phytol. 1997, 136, 407–417. [Google Scholar] [CrossRef] [PubMed]
- Smayda, T.J. Harmful algal blooms: Their ecophysiology and general relevance to phytoplankton blooms in the sea. Limnol. Oceanogr. 1997, 42, 1137–1153. [Google Scholar] [CrossRef]
- Gemmell, B.J.; Oh, G.; Buskey, E.J.; Villareal, T.A. Dynamic sinking behaviour in marine phytoplankton: Rapid changes in buoyancy may aid in nutrient uptake. Proc. Biol. Sci. 2016, 283, 20161126. [Google Scholar] [CrossRef] [PubMed]
- Lee-Chang, K.J.; Albinsson, E.; Clementson, L.; Revill, A.T.; Jameson, I.; Blackburn, S.I. Australian strains of Botryococcus braunii examined for potential hydrocarbon and carotenoid pigment production and the effect of brackish water. Energies 2020, 13, 6644. [Google Scholar] [CrossRef]
- Stoecker, D.K. Conceptual models of mixotrophy in planktonic protists and some ecological and evolutionary implications. Eur. J. Protistol. 1998, 34, 81–290. [Google Scholar] [CrossRef]
- Ward, B.A. Mixotroph ecology: More than the sum of its parts. PNAS 2019, 116, 5846–5848. [Google Scholar] [CrossRef] [Green Version]
- Mitra, A.; Flynn, K.J.; Burkholder, J.M.; Berge, T.; Calbet, A.; Raven, J.A.; Granéli, E.; Glibert, P.M.; Hansen, P.J.; Stoecker, D.K.; et al. The role of mixotrophic protists in the biological carbon pump. Biogeosciences 2014, 11, 995–1005. [Google Scholar] [CrossRef] [Green Version]
- Kriest, I.; Oschlies, A. Modelling the effect of cell-size-dependent nutrient uptake and exudation on phytoplankton size spectra. Deep Sea Res. Part I 2007, 54, 1593–1618. [Google Scholar] [CrossRef]
- Finkel, Z.V.; Beardall, J.; Flynn, K.J.; Quihh, A.; Alwyn, T.; Rees, W.V.; Raven, J.A. Phytoplankton in a changing world: Cell size and elemental stoichiometry. J. Plankton Res. 2010, 32, 119–137. [Google Scholar] [CrossRef] [Green Version]
- Acevedo-Trejos, E.; Brandt, G.; Bruggeman, J.; Merico, A. Mechanisms shaping size structure and functional diversity of phytoplankton communities in the ocean. Sci. Rep. 2015, 5, 8918. [Google Scholar] [CrossRef] [PubMed]
- Stoecker, D.K.; Capuzzo, D.A. Predation on protozoa: Its importance to zooplankton. J. Plankton Res. 1990, 12, 891–908. [Google Scholar] [CrossRef] [Green Version]
- Sommer, U.; Hansen, T.; Blum, O.; Holzner, N.; Vadstein, O.; Stibor, H. Copepod and microzooplankton grazing in mesocosms fertilised with different Si:N ratios: No overlap between food spectra and Si:N influence on zooplankton trophic level. Oecologia 2005, 142, 274–283. [Google Scholar] [CrossRef]
- Sommer, U.; Sommer, F. Cladocerans versus copepods: The cause of contrasting top-down controls on fresh water and marine phytoplankton. Oecologia 2006, 147, 183–194. [Google Scholar] [CrossRef] [PubMed]
- Moestrup, Ø.; Akselmann-Cardella, R.; Fraga, S.; Hoppenrath, M.; Iwataki, M.; Komárek, J.; Larsen, J.; Lundholm, N.; Zingone, A. (Eds.) IOC-UNESCO Taxonomic Reference List of Harmful Micro Algae. 2009. Available online: http://www.marinespecies.org/hab (accessed on 11 November 2019).
- Granéli, E.; Turner, J.T. (Eds.) Ecology of Harmful Algae; Ecological Studies 189; Springer: Berlin/Heidelberg, Germany, 2008; p. 416. [Google Scholar]
- Suikkanen, S.; Fistarol, G.O.; Granéli, E. Effects of cyanobacterial allelochemicals on a natural plankton community. Mar. Ecol. Prog. Ser. 2005, 287, 1–9. [Google Scholar] [CrossRef]
- Suikkanen, S.; Hakanen, P.; Spilling, K.; Kremp, A. Allelopathic effects of Baltic Sea spring bloom dinoflagellates on co-occurring phytoplankton. Mar. Ecol. Prog. Ser. 2011, 439, 45–55. Available online: https://www.jstor.org/stable/24875557 (accessed on 27 October 2019). [CrossRef] [Green Version]
- Hakanen, P.; Suikkanen, S.; Kremp, A. Allelopathic activity of the toxic dinoflagellate Alexandrium ostenfeldii: Intra-population variability and response of co-occurring dinoflagellates. Harmful Algae 2014, 39, 287–294. [Google Scholar] [CrossRef]
- Kozlowsky-Suzuki, B.; Karjalainen, M.; Lehtiniemi, M.; Engström-Öst, J.; Koski, M.; Carlsson, P. Feeding, reproduction and toxin accumulation by the copepods Acartia bifilosa and Eurytemora affinis in the presence of the toxic cyanobacterium Nodularia spumigena. Mar. Ecol. Prog. Ser. 2003, 249, 237–249. [Google Scholar] [CrossRef] [Green Version]
- Sopanen, S.; Koski, M.; Uronen, P.; Kuuppo, P.; Lehtinen, S.; Legrand, C.; Tamminen, T. Prymnesium parvum exotoxins affect the grazing and viability of the calanoid copepod Eurytemora affinis. Mar. Ecol. Prog. Ser. 2008, 361, 191–202. [Google Scholar] [CrossRef]
- Henrikson, J.C.; Gharfeh, M.S.; Easton, A.C.; Easton, J.D.; Glenn, K.L.; Shadfan, M.; Mooberry, S.L.; Hambright, K.D.; Cichewicz, R.H. Reassessing the ichthyotoxin profile of cultured Prymnesium parvum (golden algae) and comparing it to samples collected from recent freshwater bloom and fish kill events in North America. Toxicon 2010, 55, 1396–1404. [Google Scholar] [CrossRef] [PubMed]
- Andersen, N.G.; Hansen, P.J.; Engell-Sørensen, K.; Nørremark, L.H.; Andersen, P.; Lorenzen, E.; Lorenzen, N. Ichthyotoxicity of the microalga Pseudochattonella farcimen under laboratory and field conditions in Danish waters. Dis. Aquat. Org. 2015, 16, 165–172. [Google Scholar] [CrossRef] [PubMed]
- Shumway, S.E.; Allen, S.M.; Boersma, P.D. Marine birds and harmful algal blooms: Sporadic victims or under-reported events? Harmful Algae 2003, 2, 1–17. [Google Scholar] [CrossRef]
- Broadwater, M.H.; Van Dolah, F.M.; Fire, S.E. Vulnerabilities of marine mammals to harmful algal blooms. In Harmful Algal Blooms: A Compendium Desk Reference; Shumway, S.E., Burkholder, J.M., Morton, S.L., Eds.; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2018; ISBN 9781118994672. [Google Scholar] [CrossRef]
- Baselga-Cervera, B.; Balboa, C.G.; Costas, E.; Lopez-Rodas, V. Why Cyanobacteria produce toxins? evolutionary game theory suggests the key. Int. J. Biol. Arch. 2015, 7, 1. [Google Scholar] [CrossRef] [Green Version]
- Glibert, P.M.; Burford, M.A. Globally changing nutrient loads and harmful algal blooms: Recent advances, new paradigms, and continuing challenges. Oceanography 2017, 30, 58–69. [Google Scholar] [CrossRef] [Green Version]
- Hallegraeff, G.M.; Anderson, D.M.; Belin, C.; Dechraoui Bottein, M.-Y.; Bresnan, E.; Chinain, M.; Enevoldsen, H.; Iwataki, M.; Karlson, B.; McKenzie, C.H.; et al. Perceived global increase in algal blooms is attributable to intensified monitoring and emerging bloom impacts. Commun. Earth Environ. 2021, 2, 117. [Google Scholar] [CrossRef]
- Andersson, A.; Tamminen, T.; Lehtinen, S.; Jürgens, K.; Labrenz, M.; Viitasalo, M. Chapter: The pelagic food web. In Biological Oceanography of the Baltic Sea; Snoeijs-Leijonmalm, P., Schubert, H., Radziejewska, T., Eds.; Springer Nature: Dordrecht, The Netherlands, 2017; pp. 281–332. [Google Scholar]
- Ross, O.N.; Sharples, J. Swimming for survival: A role of phytoplankton motility in a stratified turbulent environment. J. Mar. Syst. 2008, 70, 248–262. [Google Scholar] [CrossRef]
- Jansson, M.; Blomqvist, P.; Jonsson, A.; Bergström, A.-K. Nutrient limitation of bacterioplankton, autotrophic and mixotrophic phytoplankton, and heterotrophic nanoflagellates inLake Örträsket. Limnol. Oceanogr. 1996, 41, 1552–1559. [Google Scholar] [CrossRef]
- Peltomaa, E.; Ojala, A. Size-related photosynthesis of algae in a strongly stratified humic lake. J. Plankton Res. 2010, 32, 341–355. [Google Scholar] [CrossRef] [Green Version]
- Wulff, F.V.; Rahm, L.; Larsson, P. (Eds.) A Systems Analysis of the Baltic Sea; Springer: Berlin/Heidelberg, Germany, 2001; p. 457. [Google Scholar]
- Snoeijs-Leijonmalm, P.; Andrén, E. Chapter: Why is the Baltic Sea so special to live in. In Biological Oceanography of the Baltic Sea; Snoeijs-Leijonmalm, P., Schubert, H., Radziejewska, T., Eds.; Springer Nature: Dordrecht, The Netherlands, 2017; pp. 23–84. [Google Scholar]
- Hällfors, G. Checklist of Baltic Sea phytoplankton species (including some heterotrophic protists). Balt. Sea Environ. Proc. 2004, 95, 208. [Google Scholar]
- Räike, A.; Taskinen, A.; Knuuttila, S. Nutrient export from Finnish rivers into the Baltic Sea has not decreased despite water protection measures. Ambio 2020, 49, 460–474. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lehtinen, S.; Tamminen, T.; Ptacnik, R.; Andersen, T. Phytoplankton species richness, evenness, and production in relation to nutrient availability and imbalance. Limnol. Oceanogr. 2017, 62, 1393–1408. [Google Scholar] [CrossRef] [Green Version]
- European Union. Directive 2000/60/EC of the European Parliament and of the Council of 23 October 2000 establishing a framework for Community action in the field of water policy. Off. J. 2000, 327, 1–73. [Google Scholar]
- Fleming, V.; Kuosa, H.; Hoikkala, L.; Räike, A.; Huttunen, M.; Miettunen, E.; Virtanen, E.; Tuomi, L.; Nygård, H.; Kauppila, P. Rannikkovesiemme Vedenlaadun ja Rehevöitymistilan Tulevaisuus ja sen Arvioiminen [The Future of the Water Quality and Eutrophication Status of Finnish Coastal Waters, and Assessing It]. Publications of the Government’s Analysis, Assessment and Research Activities. 2021, Volume 14, p. 123. (In Finnish). Available online: https://julkaisut.valtioneuvosto.fi/handle/10024/162906 (accessed on 11 November 2021).
- HELCOM. Guidelines for Monitoring of Phytoplankton Species Composition, Abundance and Biomass. Version 13.9.2021. 2021; p. 22p. Available online: https://helcom.fi/wp-content/uploads/2020/01/HELCOM-Guidelines-for-monitoring-of-phytoplankton-species-composition-abundance-and-biomass.pdf (accessed on 11 November 2021).
- Utermöhl, H. Zur Vervollkommung der quantitativen Phytoplankton-Methodik. Mitt. Int. Ver. Limnol. 1958, 9, 1–38. [Google Scholar]
- Olenina, I.; Hajdu, S.; Edler, L.; Andersson, A.; Wasmund, N.; Busch, S.; Göbel, J.; Gromisz, S.; Huseby, S.; Huttunen, M.; et al. Biovolumes and size-classes of phytoplankton in the Baltic Sea. Balt. Sea Environ. Proc. 2006, 106, 142. [Google Scholar]
- Wacklin, P.; Hoffmann, L.; Komárek, J. Nomenclatural validation of the genetically revised cyanobacterial genus Dolichospermum (Ralfs ex Bornet et Flahault) comb. nova. Fottea 2009, 9, 59–64. [Google Scholar] [CrossRef] [Green Version]
- Koroleff, F. Meriveden yleisimmät kemialliset analyysimenetelmät [The most common analysis methods for sea water]. Institute of Marine Research, Finland. Meri 1979, 7, 60. (In Finnish) [Google Scholar]
- Grasshoff, K.; Kremling, K.; Ehrhardt, M. Methods of Seawater Analysis; Wiley-VCH Verlag: Weinheim, Germany, 1999. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2021; Available online: https://www.R-project.org/ (accessed on 11 November 2021).
- Winslow, L.; Read, J.; Woolway, R.; Brentrup, J.; Leach, T.; Zwart, J.; Albers, S.; Collinge, D. rLakeAnalyzer: Lake Physics Tools. R Package Version 1.11.4.1. 2019. Available online: https://CRAN.R-project.org/package=rLakeAnalyzer (accessed on 11 November 2021).
- Kuosa, H.; Fleming-Lehtinen, V.; Lehtinen, S.; Lehtiniemi, M.; Nygård, H.; Raateoja, M.; Raitaniemi, J.; Tuimala, J.; Uusitalo, L.; Suikkanen, S. A retrospective view of the development of the Gulf of Bothnia ecosystem. Mar. Syst. 2017, 167, 78–92. [Google Scholar] [CrossRef]
- Karlson, B.; Andersen, P.; Arneborg, L.; Cembella, A.; Eikrem, W.; John, U.; West, J.J.; Klemm, K.; Kobos, J.; Lehtinen, S.; et al. Harmful algal blooms and their effects in coastal seas of Northern Europe. Harmful Algae 2021, 102, 101989. [Google Scholar] [CrossRef]
- Moestrup, Ø.; Thomsen, H.A. Taxonomy of toxic haptophytes (prymnesiophytes). In Manual on Harmful Marine Microalgae; Hallegraeff, G.M., Anderson, D.M., Cembella, A.D., Eds.; Monographs on Oceanographic Methology; UNESCO Publishing: Paris, France, 2004; pp. 433–463. [Google Scholar]
- Box, G.E.P.; Cox, D.R. An analysis of transformations. J. R. Stat. Soc. Ser. B 1964, 26, 211–252. [Google Scholar] [CrossRef]
- Wood, S.N. Generalized Additive Models: An Introduction with R, 2nd ed.; Chapman and Hall/CRC: London, UK, 2017. [Google Scholar]
- Lenth, R.V. emmeans: Estimated Marginal Means, aka Least-Squares Means. R Package Version 1.6.3. 2021. Available online: https://CRAN.R-project.org/package=emmeans (accessed on 11 November 2021).
- Paerl, H.W.; Otten, T.G. Harmful cyanobacterial blooms: Causes, consequences, and controls. Microb. Ecol. 2013, 65, 995–1010. [Google Scholar] [CrossRef] [PubMed]
- Gasiūnaitė, Z.R.; Cardoso, A.C.; Heiskanen, A.-S.; Henriksen, P.; Kauppila, P.; Olenina, I.; Pilkaitytė, R.; Purina, I.; Razinkovas, A.; Sagert, S.; et al. Seasonality of coastal phytoplankton in the Baltic Sea: Influence of salinity and eutrophication. Est. Coast. Shelf Sci. 2005, 65, 239–252. [Google Scholar] [CrossRef]
- Sommer, U.; Charalampous, E.; Genitsaris, S.; Moustaka-Gouni, M. Benefits, costs and taxonomic distributionof marine phytoplankton body size. J. Plankton Res. 2017, 39, 494–508. [Google Scholar] [CrossRef]
- HELCOM. Water Clarity. HELCOM Core Indicator Report. Version July 2018. Available online: https://helcom.fi/media/core%20indicators/Water-clarity-HELCOM-core-indicator-2018.pdf (accessed on 11 November 2021).
- Berglund, J.; Müren, U.; Båmstedt, U.; Andersson, A. Efficiency of a phytoplankton-based and a bacteria-based food web in a pelagic marine system. Limnol. Oceanogr. 2007, 52, 121–131. [Google Scholar] [CrossRef]
- HELCOM. Total Phosphorus. HELCOM Core Indicator Report. Version July 2018. Available online: https://helcom.fi/media/core%20indicators/Total-phosphorous-HELCOM-core-indicator-2018.pdf (accessed on 11 November 2021).
- Xu, X.; Lemmen, C.; Wirtz, K.W. Less nutrients but more phytoplankton: Long-term ecosystem dynamics of the southern North Sea. Front. Mar. Sci. 2020, 7, 662. [Google Scholar] [CrossRef]
- Olli, K.; Klais, R.; Tamminen, T. Rehabilitating the cyanobacteria–niche partitioning, resource use efficiency and phytoplankton community structure during diazotrophic cyanobacterial blooms. J. Ecol. 2015, 103, 1153–1164. [Google Scholar] [CrossRef] [PubMed]
- Suikkanen, S.; Uusitalo, L.; Lehtinen, S.; Lehtiniemi, M.; Kauppila, P.; Makinen, K.; Kuosa, H. Diazotrophic cyanobacteria in planktonic food webs. Food Webs 2021, 28, e00202. [Google Scholar] [CrossRef]
- Kuosa, H. Picoplanktonic algae in the northern Baltic Sea: Seasonal dynamics and flagellate grazing. Mar. Ecol. Prog. Ser. 1991, 73, 269–276. [Google Scholar] [CrossRef]
- Paczkowska, J.; Brugel, S.; Rowe, O.; Lefébure, R.; Brutemark, A.; Andersson, A. Response of Coastal Phytoplankton to High Inflows of Terrestrial Matter. Front. Mar. Sci. 2020, 7, 80. [Google Scholar] [CrossRef] [Green Version]
- Wasmund, N.; Voss, M.; Lochte, K. Evidence of nitrogen fixation by non-heterocystous cyanobacteria in the Baltic Sea and re-calculation of a budget of nitrogen fixation. Mar. Ecol. Prog. Ser. 2001, 214, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Martínez-Pérez, C.; Mohr, W.; Löscher, C.R.; Dekaezemacker, J.; Littmann, S.; Yilmaz, P.; Lehnen, N.; Fuchs, B.M.; Lavik, G.; Schmitz, R.A.; et al. The small unicellular diazotrophic symbiont, UCYN-A, is a key player in the marine nitrogen cycle. Nat. Microbiol. 2016, 1, 16163. [Google Scholar] [CrossRef] [Green Version]
- Harding, K.; Turk-Kubo, K.A.; Sipler, R.E.; Mills, M.M.; Bronk, D.A.; Zehr, J.P. Symbiotic unicellular cyanobacteria fix nitrogen in the Arctic Ocean. Proc. Natl. Acad. Sci. USA 2018, 115, 13371–13375. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bianchi, T.S.; Engelhaupt, E.; Westman, P.; Andrén, T.; Rolff, C.; Elmgren, R. Cyanobacterial blooms in the Baltic Sea: Natural or human-induced? Limnol. Oceanogr. 2000, 45, 716–726. [Google Scholar] [CrossRef]
- Paerl, H. The cyanobacterial nitrogen fixation paradox in natural waters. F1000Research 2017, 6, 244. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kahru, M.; Elmgren, R.; Kaiser, J.; Wasmund, N.; Savchuk, O. Cyanobacterial blooms in the Baltic Sea: Correlations with environmental factors. Harmful Algae 2020, 92, 101739. [Google Scholar] [CrossRef] [PubMed]
- Anttila, S.; Fleming-Lehtinen, V.; Attila, J.; Junttila, S.; Alasalmi, H.; Hällfors, H.; Kervinen, M.; Koponen, S. A novel earth observation based ecological indicator for cyanobacterial blooms. Int. J. Appl. Earth Obs. Geoinf. 2018, 64, 145–155. [Google Scholar] [CrossRef]
- Anttila, S.; Fleming-Lehtinen, V.; Attila, J.; Junttila, S.; Hällfors, H.; Wassmund, N.; Kaitaranta, J. Cyanobacterial Bloom Index (CyaBI). In HELCOM Pre-Core Indicator Report; HELCOM: Helsinki, Finland, 2018; p. 22. Available online: https://www.helcom.fi/wp-content/uploads/2019/08/Cyanobacterial-bloom-index-HELCOM-pre-core-indicator-2018.pdf (accessed on 22 October 2021).
- Griffiths, J.R.; Hajdu, S.; Downing, A.S.; Hjerne, O.; Larsson, U.; Winder, M. Phytoplankton community interactions and environmental sensitivity in coastal and offshore habitats. Oikos 2016, 125, 1134–1143. [Google Scholar] [CrossRef]


| Explanatory Variable | |||||||
|---|---|---|---|---|---|---|---|
| Response Variable 1 | Temp | Sal | Secchi | E | TP | L | Area |
| Nfix biom | <0.001 | 0.073 | <0.001 | 0.101 | 0.007 | 0.779 | 0.049 |
| Nfix share | <0.001 | 0.221 | <0.001 | 0.089 | <0.001 | 0.513 | 0.086 |
| nonNfix biom | 0.004 | 0.845 | <0.001 | 0.008 | <0.001 | 0.632 | 0.002 |
| nonNfix share | <0.001 | 0.563 | <0.001 | 0.167 | <0.001 | 0.518 | 0.199 |
| Buo biom | <0.001 | 0.193 | <0.001 | 0.043 | 0.022 | 0.769 | 0.109 |
| Buo share | <0.001 | 0.424 | <0.001 | 0.067 | <0.001 | 0.483 | 0.103 |
| nonBuo biom | 0.003 | 0.765 | <0.001 | 0.006 | <0.001 | 0.630 | 0.001 |
| nonBuo share | <0.001 | 0.775 | <0.001 | 0.120 | <0.001 | 0.489 | 0.188 |
| Mot biom | <0.001 | 0.606 | <0.001 | 0.104 | 0.050 | 0.987 | <0.001 |
| Mot share | <0.001 | 0.107 | <0.001 | 0.681 | 0.355 | 0.388 | 0.010 |
| nonMot biom | <0.001 | 0.301 | <0.001 | 0.096 | 0.001 | 0.820 | 0.228 |
| nonMot share | <0.001 | 0.241 | <0.001 | 0.997 | 0.841 | 0.456 | 0.011 |
| MX biom | <0.001 | 0.010 | 0.009 | 0.509 | 0.049 | 0.361 | <0.001 |
| MX share | <0.001 | 0.156 | <0.001 | 0.298 | <0.001 | 0.570 | <0.001 |
| AU biom | <0.001 | 0.989 | <0.001 | 0.021 | <0.001 | 0.886 | 0.238 |
| AU share | <0.001 | 0.246 | <0.001 | 0.728 | 0.349 | 0.779 | <0.001 |
| Small biom | 0.454 | 0.457 | <0.001 | 0.016 | 0.842 | 0.842 | <0.001 |
| Small share | 0.082 | 0.643 | <0.001 | 0.955 | <0.001 | 0.227 | <0.001 |
| Large biom | 0.006 | 0.240 | <0.001 | 0.089 | <0.001 | 0.748 | <0.001 |
| Large share | 0.049 | 0.823 | <0.001 | 0.793 | <0.001 | 0.179 | <0.001 |
| aveESD | 0.001 | 0.128 | <0.001 | 0.405 | 0.135 | 0.027 | 0.186 |
| HABalg biom | <0.001 | 0.540 | <0.001 | 0.047 | 0.002 | 0.470 | <0.001 |
| HABalg share | <0.001 | 0.809 | 0.001 | 0.003 | <0.001 | 0.208 | 0.001 |
| HABcyano biom | <0.001 | 0.160 | <0.001 | 0.058 | 0.010 | 0.704 | 0.102 |
| HABcyano share | <0.001 | 0.404 | <0.001 | 0.040 | <0.001 | 0.419 | 0.137 |
| nonHAB biom | 0.007 | 0.731 | <0.001 | 0.004 | <0.001 | 0.547 | 0.004 |
| nonHAB share | <0.001 | 0.133 | <0.001 | 0.088 | <0.001 | 0.437 | 0.835 |
| Sea Area Pairs | ||||||
|---|---|---|---|---|---|---|
| Response Variable 1 | BS–eGF | BS–wGF | BS–AS | eGF–wGF | eGF–AS | wGF–AS |
| Nfix biom | 0.283 | 0.160 | 0.984 | 0.984 | 0.227 | 0.077 |
| Nfix share | 0.781 | 0.809 | 0.847 | 0.974 | 0.201 | 0.068 |
| nonNfix biom | 0.070 | 0.001 | 0.002 | 0.966 | 0.987 | 1.000 |
| nonNfix share | 0.946 | 0.950 | 0.798 | 0.996 | 0.411 | 0.159 |
| Buo biom | 0.496 | 0.320 | 0.999 | 0.997 | 0.352 | 0.122 |
| Buo share | 0.915 | 0.931 | 0.713 | 0.990 | 0.264 | 0.080 |
| nonBuo biom | 0.066 | 0.001 | 0.001 | 0.973 | 0.988 | 1.000 |
| nonBuo share | 0.973 | 0.971 | 0.732 | 0.999 | 0.438 | 0.148 |
| Mot biom | 0.014 | <0.001 | <0.001 | 0.068 | 0.805 | 0.285 |
| Mot share | 0.898 | 0.329 | 0.299 | 0.031 | 0.122 | 1.000 |
| nonMot biom | 0.264 | 0.283 | 0.783 | 0.870 | 0.490 | 0.612 |
| nonMot share | 0.858 | 0.433 | 0.241 | 0.037 | 0.060 | 0.965 |
| MX biom | <0.001 | <0.001 | <0.001 | 0.719 | 0.276 | <0.001 |
| MX share | 0.005 | <0.001 | 0.002 | 0.652 | 0.746 | 0.005 |
| AU biom | 0.657 | 0.219 | 0.251 | 0.980 | 0.993 | 1.000 |
| AU share | 0.017 | <0.001 | 0.033 | 0.713 | 0.565 | 0.001 |
| Small biom | 0.333 | 0.741 | 0.110 | 0.003 | <0.001 | 0.234 |
| Small share | 0.002 | 0.121 | 0.993 | 0.033 | <0.001 | 0.003 |
| Large biom | <0.001 | <0.001 | 0.027 | 0.838 | 0.017 | 0.003 |
| Large share | 0.005 | 0.107 | 0.997 | 0.075 | <0.001 | 0.003 |
| aveESD | 0.442 | 0.126 | 0.346 | 0.999 | 0.989 | 0.895 |
| HABalg biom | 0.083 | <0.001 | <0.001 | 0.642 | 0.776 | 1.000 |
| HABalg share | 0.553 | 0.019 | 0.001 | 0.624 | 0.289 | 0.585 |
| HABcyano biom | 0.423 | 0.268 | 0.994 | 0.993 | 0.337 | 0.131 |
| HABcyano share | 0.890 | 0.926 | 0.785 | 0.980 | 0.279 | 0.113 |
| nonHAB biom | 0.081 | 0.003 | 0.006 | 0.989 | 1.000 | 0.986 |
| nonHAB share | 0.984 | 0.888 | 0.998 | 0.994 | 0.993 | 0.872 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lehtinen, S.; Suikkanen, S.; Hällfors, H.; Tuimala, J.; Kuosa, H. Phytoplankton Morpho-Functional Trait Variability along Coastal Environmental Gradients. Microorganisms 2021, 9, 2477. https://doi.org/10.3390/microorganisms9122477
Lehtinen S, Suikkanen S, Hällfors H, Tuimala J, Kuosa H. Phytoplankton Morpho-Functional Trait Variability along Coastal Environmental Gradients. Microorganisms. 2021; 9(12):2477. https://doi.org/10.3390/microorganisms9122477
Chicago/Turabian StyleLehtinen, Sirpa, Sanna Suikkanen, Heidi Hällfors, Jarno Tuimala, and Harri Kuosa. 2021. "Phytoplankton Morpho-Functional Trait Variability along Coastal Environmental Gradients" Microorganisms 9, no. 12: 2477. https://doi.org/10.3390/microorganisms9122477






