Changes in Energy Status of Saccharomyces cerevisiae Cells during Dehydration and Rehydration
Abstract
:1. Introduction
2. Materials and Methods
2.1. Strains and the Cultivation Conditions
2.2. Preparation of Cells for Experiments and Desiccation
2.3. Cell Rehydration and Reactivation
2.4. Electrochemical Measurements
2.5. ATP Measurements
3. Results
3.1. Studies of Dehydration
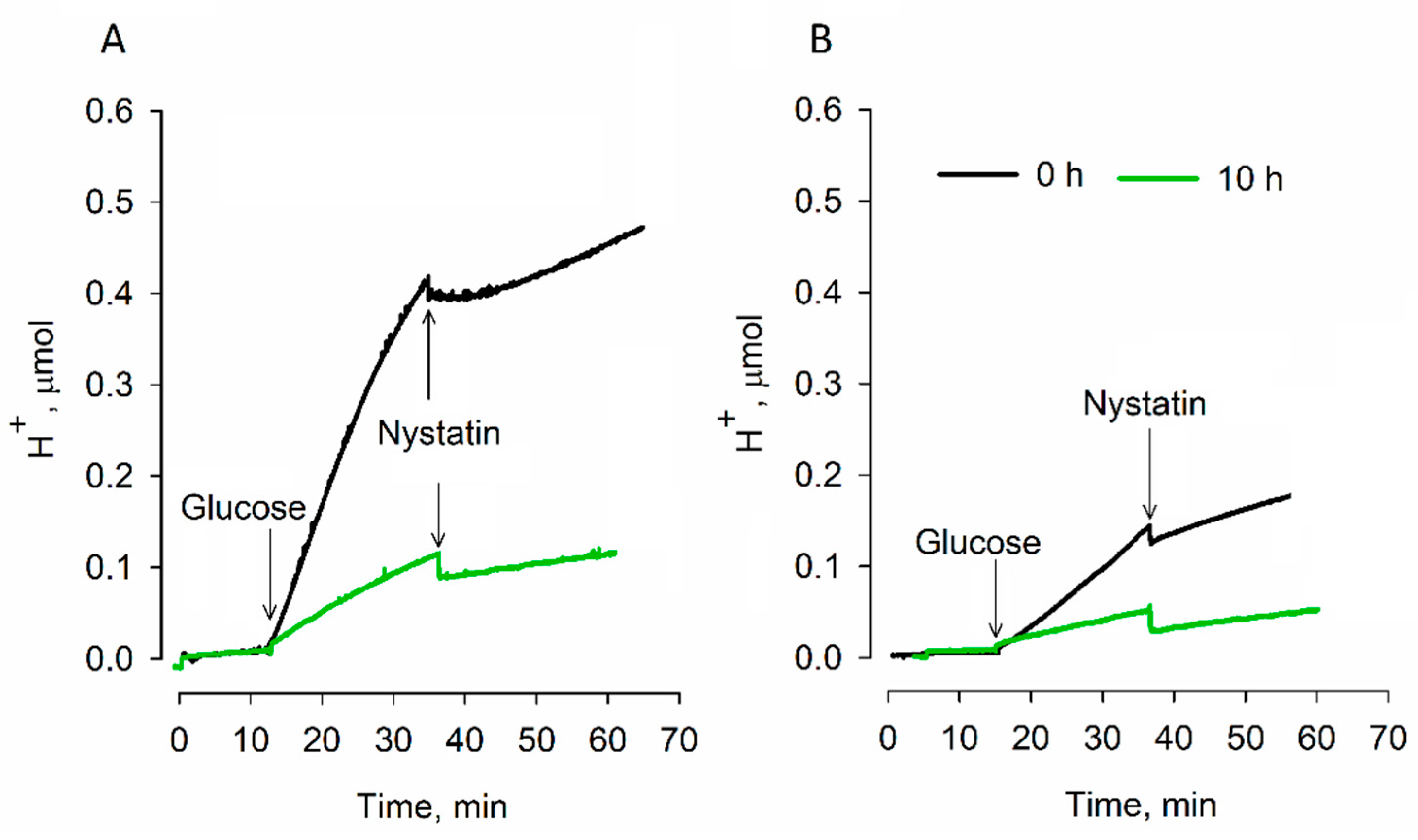
3.2. Effects of Dehydration on Acidification of the Medium
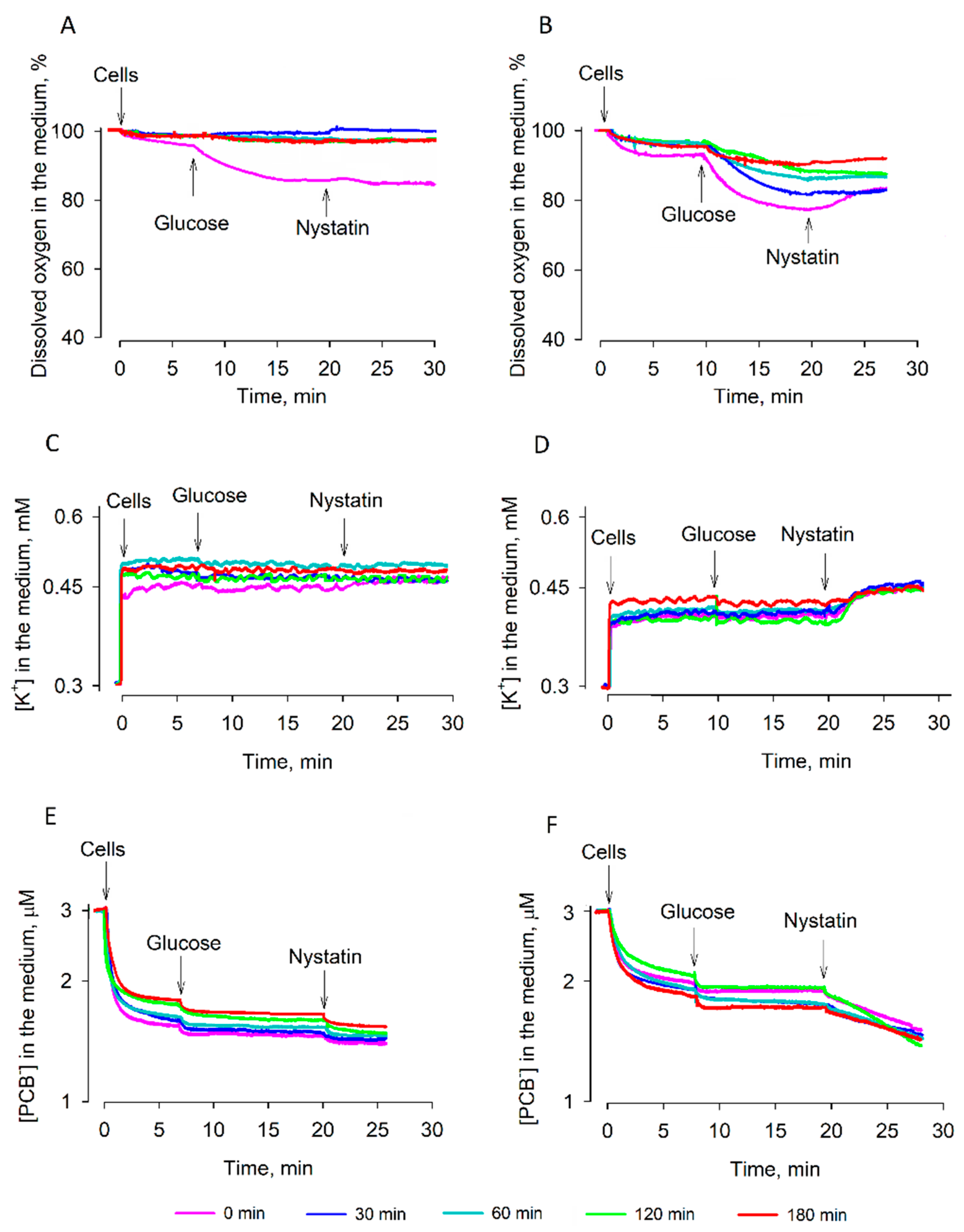
3.3. Efficiency of Rehydration
3.4. ATP Content of the Cells during Dehydration and Rehydration
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Beker, M.J.; Rapoport, A. Conservation of yeasts by dehydration. In Biotechnology Methods; Springer: Berlin/Heidelberg, Germany, 1987; pp. 127–171. [Google Scholar]
- Dupont, S.; Rapoport, A.; Gervais, P.; Beney, L. Survival kit of Saccharomyces cerevisiae for anhydrobiosis. Appl. Microbiol. Biotechnol. 2014, 98, 8821–8834. [Google Scholar] [CrossRef] [PubMed]
- Rapoport, A.; Golovina, E.A.; Gervais, P.; Dupont, S.; Beney, L. Anhydrobiosis: Inside yeast cells. Biotechnol. Adv. 2019, 37, 51–67. [Google Scholar] [CrossRef] [PubMed]
- Rapoport, A.; Turchetti, B.; Buzzini, P. Application of anhydrobiosis and dehydration of yeasts for non-conventional biotechnological goals. World J. Microbiol. Biotechnol. 2016, 32, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Rapoport, A. Anhydrobiosis and dehydration of yeasts. In Biotechnology of Yeasts and Filamentous Fungi; Sibirny, A., Ed.; Springer: Berlin/Heidelberg, Germany, 2017; pp. 87–116. [Google Scholar]
- Rapoport, A.; Khrustaleva, G.; Chamanis, G.; Beker, M.E. Yeast anhydrobiosis: Permeability of the cytoplasmic membrane. Mikrobiologiia 1995, 64, 275–278. [Google Scholar] [PubMed]
- Rapoport, A. Anhydrobiosis in Non-conventional Yeasts. In Non-Conventional Yeasts: From Basic Research to Application; Sibirny, A., Ed.; Springer: Berlin/Heidelberg, Germany, 2019; pp. 341–359. [Google Scholar]
- Rapoport, A.; Rusakova, A.; Khroustalyova, G.; Walker, G. Thermotolerance in Saccharomyces cerevisiae is linked to resistance to anhydrobiosis. Process. Biochem. 2014, 49, 1889–1892. [Google Scholar] [CrossRef]
- Khroustalyova, G.; Giovannitti, G.; Severini, D.; Scherbaka, R.; Turchetti, B.; Buzzini, P.; Rapoport, A. Anhydrobiosis in yeasts: Psychrotolerant yeasts are highly resistant to dehydration. Yeast 2019, 36, 375–379. [Google Scholar] [CrossRef] [PubMed]
- Daugelavicius, R.; Gaidelyte, A.; Cvirkaite-Krupovic, V.; Bamford, D.H. On-line monitoring of changes in host cell physiology during the one-step growth cycle of Bacillus phage Bam35. J. Microbiol. Methods 2007, 69, 174–179. [Google Scholar] [CrossRef] [PubMed]
- Flewelling, R.F.; Hubbell, W.L. The membrane dipole potential in a total applications to hydrophobic ion interactions with membranes. Biophys. J. 1986, 49, 541–552. [Google Scholar] [CrossRef] [Green Version]
- Daugelavicius, R.; Bakiene, E.; Berzinskiene, J.; Bamford, D.H. Binding of lipophilic anions to microbial cells. Bioelectrochem. Bioenerg. 1997, 42, 263–274. [Google Scholar] [CrossRef]
- Daugelavicius, R.; Bakiene, E.; Berzinskiene, J.; Bamford, D.H. Use of lipophilic anions for estimation of biomass and cell viability. Biotechnol. Bioeng. 2000, 71, 208–216. [Google Scholar] [CrossRef]
- Mookerjee, S.A.; Brand, M.D. Measurements and analysis of extracellular acid production to determine glycolytic rate. J. Vis. Exp. 2015, 106, 53464. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Camara, A.A., Jr.; Sant’Ana, A.S. Advances in yeast preservation: Physiological aspects for cell perpetuation. Curr. Opin. Food Sci. 2021, 38, 62–70. [Google Scholar] [CrossRef]
- Marini, G.; Nuske, E.; Leng, W.; Alberti, S.; Pigino, G. Reorganization of budding yeast cytoplasm upon energy depletion. Mol. Biol. Cell 2020, 31, 1232–1245. [Google Scholar] [CrossRef] [PubMed]
- Munder, M.C.; Midtvedt, D.; Franzmann, T.; Nüske, E.; Otto, O.; Herbig, M.; Ulbricht, E.; Müller, P.; Taubenberger, A.; Maharana, S.; et al. A pH-driven transition of the cytoplasm from a fluid-to a solid-like state promotes entry into dormancy. eLife 2016, 5, e09347. [Google Scholar] [CrossRef] [PubMed]
- Roca-Domènech, G.; Poblet, M.; Rozès, N.; Cordero-Otero, R. Magnesium enhances dehydration tolerance in Schizosaccharomyces pombe by promoting intracellular 5′-methylthioadenosine accumulation. Yeast 2019, 36, 449–461. [Google Scholar] [CrossRef] [PubMed]
- Erkut, C.; Gade, V.R.; Laxman, S.; Kurzchalia, T.V. The glyoxylate shunt is essential for desiccation tolerance in C. elegans and budding yeast. eLife 2016, 5, e13614. [Google Scholar] [CrossRef] [PubMed]
- Ariño, J.; Ramos, J.; Sychrová, H. Alkali metal cation transport and homeostasis in yeasts. Microbiol. Mol. Biol. Rev. 2010, 74, 95–120. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ariño, J.; Ramos, J.; Sychrova, H. Monovalent cation transporters at the plasma membrane in yeasts. Yeast 2019, 36, 177–193. [Google Scholar] [CrossRef] [PubMed]
- Dupont, S.; Lemetais, G.; Ferreira, T.; Cayot, P.; Gervais, P.; Beney, L. Ergosterol biosynthesis: A fungal pathway for life on land? Evolution 2012, 66, 2961–2968. [Google Scholar] [CrossRef] [PubMed]
- Ramos, J.; Ariño, J.; Sychrová, H. Alkali–metal–cation influx and efflux systems in nonconventional yeast species. FEMS Microbiol. Lett. 2011, 317, 1–8. [Google Scholar] [CrossRef] [PubMed] [Green Version]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kuliešienė, N.; Žūkienė, R.; Khroustalyova, G.; Chang, C.-R.; Rapoport, A.; Daugelavičius, R. Changes in Energy Status of Saccharomyces cerevisiae Cells during Dehydration and Rehydration. Microorganisms 2021, 9, 444. https://doi.org/10.3390/microorganisms9020444
Kuliešienė N, Žūkienė R, Khroustalyova G, Chang C-R, Rapoport A, Daugelavičius R. Changes in Energy Status of Saccharomyces cerevisiae Cells during Dehydration and Rehydration. Microorganisms. 2021; 9(2):444. https://doi.org/10.3390/microorganisms9020444
Chicago/Turabian StyleKuliešienė, Neringa, Rasa Žūkienė, Galina Khroustalyova, Chuang-Rung Chang, Alexander Rapoport, and Rimantas Daugelavičius. 2021. "Changes in Energy Status of Saccharomyces cerevisiae Cells during Dehydration and Rehydration" Microorganisms 9, no. 2: 444. https://doi.org/10.3390/microorganisms9020444
APA StyleKuliešienė, N., Žūkienė, R., Khroustalyova, G., Chang, C.-R., Rapoport, A., & Daugelavičius, R. (2021). Changes in Energy Status of Saccharomyces cerevisiae Cells during Dehydration and Rehydration. Microorganisms, 9(2), 444. https://doi.org/10.3390/microorganisms9020444







