Elevated Expression of Toxin TisB Protects Persister Cells against Ciprofloxacin but Enhances Susceptibility to Mitomycin C
Abstract
1. Introduction
2. Materials and Methods
2.1. Growth Conditions
2.2. Plasmid and Strain Construction
2.3. Microplate Reader Experiments
2.4. Microscopy
2.5. Flow Cytometry
2.6. Persister Assays
2.7. ATP Measurements
2.8. DNA Damage Assay
2.9. RNA Methods
2.10. Western Blot Analysis
2.11. Statistical Analysis
3. Results
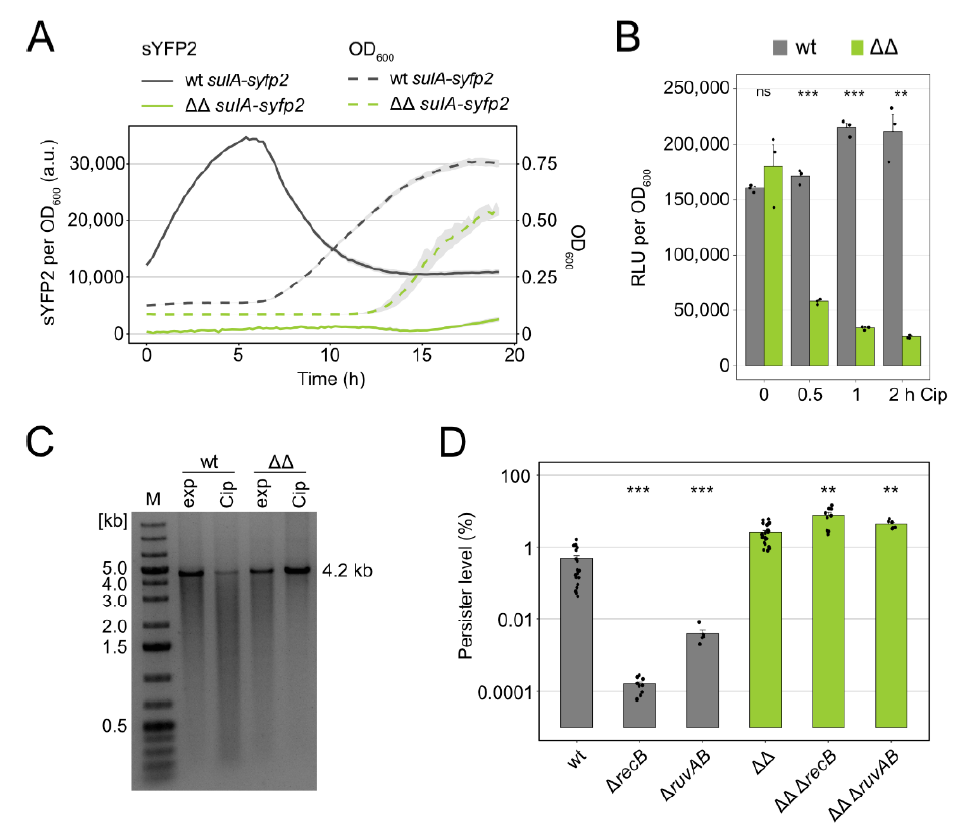
3.1. Persisters in Mutant Δ1-41 ΔistR Neither Experience Strong DNA Damage nor Rely on Double-Strand Break Repair upon Ciprofloxacin Treatment
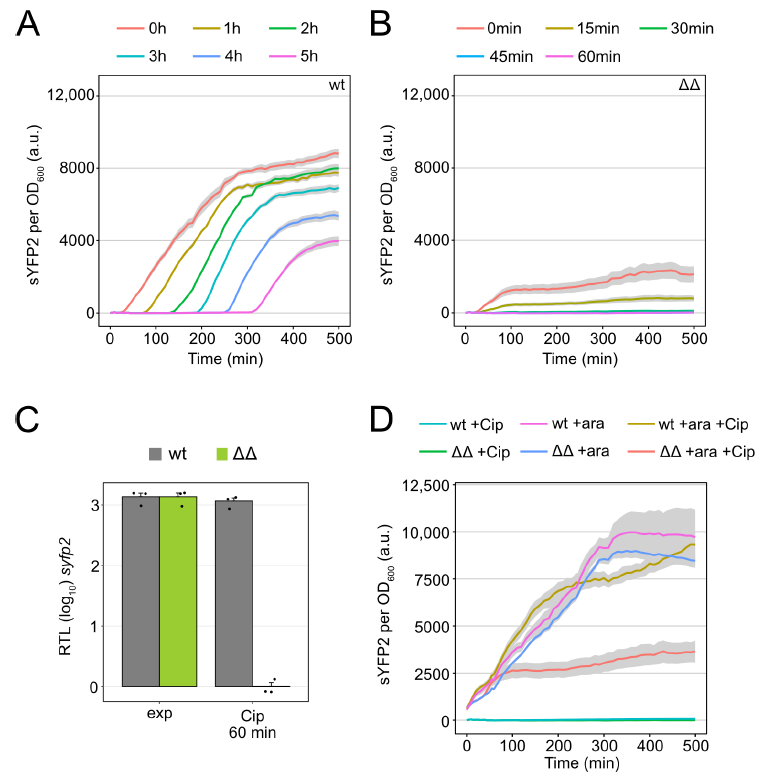
3.2. TisB Overexpression in Mutant Δ1-41 ΔistR upon Ciprofloxacin Treatment
3.3. Major Cellular Processes Are Inhibited in Mutant Δ1-41 ΔistR upon Ciprofloxacin Treatment
3.4. High TisB Levels Counteract Expression of SOS Genes
3.5. The Hip Phenotype of Mutant Δ1-41 ΔistR Is Lost upon Treatment with the DNA Cross-Linker Mitomycin C
4. Discussion
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Veening, J.-W.; Smits, W.K.; Kuipers, O.P. Bistability, Epigenetics, and Bet-Hedging in Bacteria. Annu. Rev. Microbiol. 2008, 62, 193–210. [Google Scholar] [CrossRef] [PubMed]
- Ackermann, M. A functional perspective on phenotypic heterogeneity in microorganisms. Nat. Rev. Genet. 2015, 13, 497–508. [Google Scholar] [CrossRef] [PubMed]
- Kussell, E.; Leibler, S. Phenotypic Diversity, Population Growth, and Information in Fluctuating Environments. Science 2005, 309, 2075–2078. [Google Scholar] [CrossRef] [PubMed]
- Balaban, N.Q.; Helaine, S.; Lewis, K.; Ackermann, M.; Aldridge, B.; Andersson, D.I.; Brynildsen, M.P.; Bumann, D.; Camilli, A.; Collins, J.J.; et al. Definitions and guidelines for research on antibiotic persistence. Nat. Rev. Genet. 2019, 17, 441–448. [Google Scholar] [CrossRef] [PubMed]
- Kaldalu, N.; Hauryliuk, V.; Turnbull, K.J.; La Mensa, A.; Putrinš, M.; Tenson, T. In Vitro Studies of Persister Cells. Microbiol. Mol. Biol. Rev. 2020, 84, e00070-20. [Google Scholar] [CrossRef] [PubMed]
- Lewis, K. Persister Cells. Annu. Rev. Microbiol. 2010, 64, 357–372. [Google Scholar] [CrossRef] [PubMed]
- Bigger, J. Treatment of staphylococcal infections with penicillin by intermittent sterilisation. Lancet 1944, 244, 497–500. [Google Scholar] [CrossRef]
- Hobby, G.L.; Meyer, K.; Chaffee, E. Observations on the Mechanism of Action of Penicillin. Exp. Biol. Med. 1942, 50, 281–285. [Google Scholar] [CrossRef]
- Brauner, A.; Fridman, O.; Gefen, O.; Balaban, N.Q. Distinguishing between resistance, tolerance and persistence to antibiotic treatment. Nat. Rev. Genet. 2016, 14, 320–330. [Google Scholar] [CrossRef] [PubMed]
- Amato, S.M.; Brynildsen, M.P. Mechanisms of Stress-Activated Persister Formation in Escherichia coli. In Stress and Environmental Regulation of Gene Expression and Adaptation in Bacteria; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2016; pp. 446–453. [Google Scholar]
- Allison, K.R.; Brynildsen, M.P.; Collins, J.J. Heterogeneous bacterial persisters and engineering approaches to eliminate them. Curr. Opin. Microbiol. 2011, 14, 593–598. [Google Scholar] [CrossRef]
- Wilmaerts, D.; Bayoumi, M.; Dewachter, L.; Knapen, W.; Mika, J.T.; Hofkens, J.; Dedecker, P.; Maglia, G.; Verstraeten, N.; Michiels, J. The Persistence-Inducing Toxin HokB Forms Dynamic Pores That Cause ATP Leakage. mBio 2018, 9, e00744-18. [Google Scholar] [CrossRef]
- Radzikowski, J.L.; Vedelaar, S.; Siegel, D.; Ortega, Á.D.; Schmidt, A.; Heinemann, M. Bacterial Persistence Is an Active σS Stress Response to Metabolic Flux Limitation. Mol. Syst. Biol. 2016, 12, 882. [Google Scholar] [CrossRef]
- Orman, M.A.; Brynildsen, M.P. Dormancy Is Not Necessary or Sufficient for Bacterial Persistence. Antimicrob. Agents Chemother. 2013, 57, 3230–3239. [Google Scholar] [CrossRef]
- Pu, Y.; Zhao, Z.; Li, Y.; Zou, J.; Ma, Q.; Zhao, Y.; Ke, Y.; Zhu, Y.; Chen, H.; Baker, M.A.; et al. Enhanced Efflux Activity Facilitates Drug Tolerance in Dormant Bacterial Cells. Mol. Cell 2016, 62, 284–294. [Google Scholar] [CrossRef]
- Dillingham, M.S.; Kowalczykowski, S.C. RecBCD Enzyme and the Repair of Double-Stranded DNA Breaks. Microbiol. Mol. Biol. Rev. 2008, 72, 642–671. [Google Scholar] [CrossRef]
- Little, J. Mechanism of specific LexA cleavage: Autodigestion and the role of RecA coprotease. Biochimie 1991, 73, 411–421. [Google Scholar] [CrossRef]
- Lewis, L.K.; Harlow, G.R.; Gregg-Jolly, L.A.; Mount, D.W. Identification of High Affinity Binding Sites for LexA which Define New DNA Damage-inducible Genes in Escherichia coli. J. Mol. Biol. 1994, 241, 507–523. [Google Scholar] [CrossRef]
- De Henestrosa, A.R.F.; Ogi, T.; Aoyagi, S.; Chafin, D.; Hayes, J.J.; Ohmori, H.; Woodgate, R. Identification of additional genes belonging to the LexA regulon in Escherichia coli. Mol. Microbiol. 2002, 35, 1560–1572. [Google Scholar] [CrossRef]
- Wade, J.T.; Reppas, N.B.; Church, G.M.; Struhl, K. Genomic Analysis of LexA Binding Reveals the Permissive Nature of the Escherichia coli Genome and Identifies Unconventional Target Sites. Genes Dev. 2005, 19, 2619–2630. [Google Scholar] [CrossRef]
- Courcelle, J.; Khodursky, A.; Peter, B.; Brown, P.O.; Hanawalt, P.C. Comparative Gene Expression Profiles Following UV Exposure in Wild-Type and SOS-Deficient Escherichia coli. Genetics 2001, 158, 41–64. [Google Scholar]
- Kreuzer, K.N. DNA Damage Responses in Prokaryotes: Regulating Gene Expression, Modulating Growth Patterns, and Manipulating Replication Forks. Cold Spring Harb. Perspect. Biol. 2013, 5, a012674. [Google Scholar] [CrossRef]
- Dörr, T.; Lewis, K.; Vulić, M. SOS Response Induces Persistence to Fluoroquinolones in Escherichia coli. PLoS Genet. 2009, 5, e1000760. [Google Scholar] [CrossRef]
- Theodore, A.; Lewis, K.; Vulić, M. Tolerance of Escherichia coli to Fluoroquinolone Antibiotics Depends on Specific Components of the SOS Response Pathway. Genetics 2013, 195, 1265–1276. [Google Scholar] [CrossRef]
- Berghoff, B.A.; Hoekzema, M.; Aulbach, L.; Wagner, E.G.H. Two Regulatory RNA Elements Affect TisB-Dependent Depolarization and Persister Formation. Mol. Microbiol. 2017, 103, 1020–1033. [Google Scholar] [CrossRef]
- Völzing, K.G.; Brynildsen, M.P. Stationary-Phase Persisters to Ofloxacin Sustain DNA Damage and Require Repair Systems Only during Recovery. mBio 2015, 6, e00731-15. [Google Scholar] [CrossRef]
- Mok, W.W.K.; Brynildsen, M.P. Timing of DNA damage responses impacts persistence to fluoroquinolones. Proc. Natl. Acad. Sci. USA 2018, 115, E6301–E6309. [Google Scholar] [CrossRef]
- Goormaghtigh, F.; Van Melderen, L. Single-cell imaging and characterization of Escherichia coli persister cells to ofloxacin in exponential cultures. Sci. Adv. 2019, 5, eaav9462. [Google Scholar] [CrossRef]
- Van Melderen, L. Toxin–Antitoxin Systems: Why so Many, What For? Curr. Opin. Microbiol. 2010, 13, 781–785. [Google Scholar] [CrossRef]
- Goeders, N.; Chai, R.; Chen, B.; Day, A.; Salmond, G.P. Structure, Evolution, and Functions of Bacterial Type III Toxin-Antitoxin Systems. Toxins 2016, 8, 282. [Google Scholar] [CrossRef]
- Harms, A.; Brodersen, D.E.; Mitarai, N.; Gerdes, K. Toxins, Targets, and Triggers: An Overview of Toxin-Antitoxin Biology. Mol. Cell 2018, 70, 768–784. [Google Scholar] [CrossRef]
- Page, R.; Peti, W. Toxin-antitoxin systems in bacterial growth arrest and persistence. Nat. Chem. Biol. 2016, 12, 208–214. [Google Scholar] [CrossRef] [PubMed]
- Dörr, T.; Vulić, M.; Lewis, K. Ciprofloxacin Causes Persister Formation by Inducing the TisB toxin in Escherichia coli. PLoS Biol. 2010, 8, e1000317. [Google Scholar] [CrossRef] [PubMed]
- Wagner, E.G.H.; Unoson, C. The Toxin-Antitoxin System TisB-IstR1: Expression, Regulation, and Biological Role in Persister Phenotypes. RNA Biol. 2012, 9, 1513–1519. [Google Scholar] [CrossRef] [PubMed]
- Berghoff, B.A.; Karlsson, T.; Källman, T.; Wagner, E.G.H.; Grabherr, M.G. RNA-sequence data normalization through in silico prediction of reference genes: The bacterial response to DNA damage as case study. BioData Min. 2017, 10, 30. [Google Scholar] [CrossRef]
- Darfeuille, F.; Unoson, C.; Vogel, J.; Wagner, E.G.H. An Antisense RNA Inhibits Translation by Competing with Standby Ribosomes. Mol. Cell 2007, 26, 381–392. [Google Scholar] [CrossRef]
- Romilly, C.; Deindl, S.; Wagner, E.G.H. The ribosomal protein S1-dependent standby site in tisB mRNA consists of a single-stranded region and a 5’ structure element. Proc. Natl. Acad. Sci. USA 2019, 116, 15901–15906. [Google Scholar] [CrossRef]
- Romilly, C.; Lippegaus, A.; Wagner, E.G.H. An RNA pseudoknot is essential for standby-mediated translation of the tisB toxin mRNA in Escherichia coli. Nucleic Acids Res. 2020, 48, 12336–12347. [Google Scholar] [CrossRef]
- Vogel, J.; Argaman, L.; Wagner, E.H.; Altuvia, S. The Small RNA IstR Inhibits Synthesis of an SOS-Induced Toxic Peptide. Curr. Biol. 2004, 14, 2271–2276. [Google Scholar] [CrossRef]
- Berghoff, B.A.; Wagner, E.G.H. Persister Formation Driven by TisB-Dependent Membrane Depolarization. In Persister Cells and Infectious Disease; Lewis, K., Ed.; Springer: Cham, Switzerland, 2019; pp. 77–97. [Google Scholar]
- Berghoff, B.A.; Wagner, E.G.H. RNA-based regulation in type I toxin–antitoxin systems and its implication for bacterial persistence. Curr. Genet. 2017, 63, 1011–1016. [Google Scholar] [CrossRef]
- Edelmann, D.; Oberpaul, M.; Schäberle, T.F.; Berghoff, B.A. Post-transcriptional Deregulation of the tisB/istR-1 Toxin–Antitoxin System Promotes SOS-independent Persister Formation in Escherichia coli. Environ. Microbiol. Rep. 2021, 13, 159–168. [Google Scholar] [CrossRef]
- Unoson, C.; Wagner, E.G.H. A small SOS-induced toxin is targeted against the inner membrane in Escherichia coli. Mol. Microbiol. 2008, 70, 258–270. [Google Scholar] [CrossRef]
- Gurnev, P.A.; Ortenberg, R.; Dörr, T.; Lewis, K.; Bezrukov, S.M. Persister-promoting bacterial toxin TisB produces anion-selective pores in planar lipid bilayers. FEBS Lett. 2012, 586, 2529–2534. [Google Scholar] [CrossRef]
- Shan, Y.; Gandt, A.B.; Rowe, S.E.; Deisinger, J.P.; Conlon, B.P.; Lewis, K. ATP-Dependent Persister Formation in Escherichia coli. mBio 2017, 8, e02267-16. [Google Scholar] [CrossRef]
- Conlon, B.P.; Rowe, S.E.; Gandt, A.B.; Nuxoll, A.S.; Donegan, N.P.; Zalis, E.A.; Clair, G.; Adkins, J.N.; Cheung, A.L.; Lewis, K. Persister formation in Staphylococcus aureus is associated with ATP depletion. Nat. Microbiol. 2016, 1, 1–7. [Google Scholar] [CrossRef]
- Edelmann, D.; Berghoff, B.A. Type I toxin-dependent generation of superoxide affects the persister life cycle of Escherichia coli. Sci. Rep. 2019, 9, 1–10. [Google Scholar] [CrossRef]
- Spanka, D.-T.; Konzer, A.; Edelmann, D.; Berghoff, B.A. High-Throughput Proteomics Identifies Proteins with Importance to Postantibiotic Recovery in Depolarized Persister Cells. Front. Microbiol. 2019, 10, 378. [Google Scholar] [CrossRef]
- Datta, S.; Costantino, N.; Court, D.L. A set of recombineering plasmids for gram-negative bacteria. Gene 2006, 379, 109–115. [Google Scholar] [CrossRef]
- Datsenko, K.A.; Wanner, B.L. One-step inactivation of chromosomal genes in Escherichia coli K-12 using PCR products. Proc. Natl. Acad. Sci. USA 2000, 97, 6640–6645. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of Relative Gene Expression Data Using Real-Time Quantitative PCR and the 2(-Delta Delta C(T)). Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Zhou, K.; Zhou, L.; Lim, Q.E.; Zou, R.; Stephanopoulos, G.; Too, H.-P. Novel reference genes for quantifying transcriptional responses of Escherichia coli to protein overexpression by quantitative PCR. BMC Mol. Biol. 2011, 12, 18. [Google Scholar] [CrossRef]
- Schägger, H. Tricine–SDS-PAGE. Nat. Protoc. 2006, 1, 16–22. [Google Scholar] [CrossRef] [PubMed]
- Bi, E.; Lutkenhaus, J. Cell division inhibitors SulA and MinCD prevent formation of the FtsZ ring. J. Bacteriol. 1993, 175, 1118–1125. [Google Scholar] [CrossRef] [PubMed]
- Bos, J.; Zhang, Q.; Vyawahare, S.; Rogers, E.; Rosenberg, S.M.; Austin, R.H. Emergence of antibiotic resistance from multinucleated bacterial filaments. Proc. Natl. Acad. Sci. USA 2015, 112, 178–183. [Google Scholar] [CrossRef] [PubMed]
- Kwan, B.W.; Chowdhury, N.; Wood, T.K. Combatting bacterial infections by killing persister cells with mitomycin C. Environ. Microbiol. 2015, 17, 4406–4414. [Google Scholar] [CrossRef]
- McKay, S.L.; Portnoy, D.A. Ribosome Hibernation Facilitates Tolerance of Stationary-Phase Bacteria to Aminoglycosides. Antimicrob. Agents Chemother. 2015, 59, 6992–6999. [Google Scholar] [CrossRef]
- Allison, K.R.; Brynildsen, M.P.; Collins, J.J. Metabolite-enabled eradication of bacterial persisters by aminoglycosides. Nat. Cell Biol. 2011, 473, 216–220. [Google Scholar] [CrossRef]
- Kwan, B.W.; Valenta, J.A.; Benedik, M.J.; Wood, T.K. Arrested Protein Synthesis Increases Persister-Like Cell Formation. Antimicrob. Agents Chemother. 2013, 57, 1468–1473. [Google Scholar] [CrossRef]
- Harms, A.; Maisonneuve, E.; Gerdes, K. Mechanisms of bacterial persistence during stress and antibiotic exposure. Science 2016, 354, aaf4268. [Google Scholar] [CrossRef] [PubMed]
- Ronneau, S.; Helaine, S. Clarifying the Link between Toxin–Antitoxin Modules and Bacterial Persistence. J. Mol. Biol. 2019, 431, 3462–3471. [Google Scholar] [CrossRef] [PubMed]
- Verstraeten, N.; Knapen, W.J.; Kint, C.I.; Liebens, V.; Bergh, B.V.D.; Dewachter, L.; Michiels, J.E.; Fu, Q.; David, C.C.; Fierro, A.C.; et al. Obg and Membrane Depolarization Are Part of a Microbial Bet-Hedging Strategy that Leads to Antibiotic Tolerance. Mol. Cell 2015, 59, 9–21. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Lord, D.M.; Cheng, H.-Y.; Osbourne, D.O.; Hong, S.H.; Sanchez-Torres, V.; Quiroga, C.; Zheng, K.; Herrmann, T.; Peti, W.; et al. A new type V toxin-antitoxin system where mRNA for toxin GhoT is cleaved by antitoxin GhoS. Nat. Chem. Biol. 2012, 8, 855–861. [Google Scholar] [CrossRef]
- Cheng, H.-Y.; Soo, V.W.C.; Islam, S.; McAnulty, M.J.; Benedik, M.J.; Wood, T.K. Toxin GhoT of the GhoT/GhoS toxin/antitoxin system damages the cell membrane to reduce adenosine triphosphate and to reduce growth under stress. Environ. Microbiol. 2014, 16, 1741–1754. [Google Scholar] [CrossRef]
- Arnion, H.; Korkut, D.N.; Gelo, S.M.; Chabas, S.; Reignier, J.; Iost, I.; Darfeuille, F. Mechanistic insights into type I toxin antitoxin systems in Helicobacter pylori: The importance of mRNA folding in controlling toxin expression. Nucleic Acids Res. 2017, 45, 4782–4795. [Google Scholar] [CrossRef]
- Kristiansen, K.I.; Weel-Sneve, R.; Booth, J.A.; Bjørås, M. Mutually exclusive RNA secondary structures regulate translation initiation of DinQ in Escherichia coli. RNA 2016, 22, 1739–1749. [Google Scholar] [CrossRef]
- Wen, J.; Harp, J.R.; Fozo, E.M. The 5′ UTR of the type I toxin ZorO can both inhibit and enhance translation. Nucleic Acids Res. 2017, 45, 4006–4020. [Google Scholar] [CrossRef]
- Thisted, T.; Nielsen, A.; Gerdes, K. Mechanism of post-segregational killing: Translation of Hok, SrnB and Pnd mRNAs of plasmids R1, F and R483 is activated by 3′-end processing. EMBO J. 1994, 13, 1950–1959. [Google Scholar] [CrossRef]
- Gerdes, K.; Wagner, E.G.H. RNA antitoxins. Curr. Opin. Microbiol. 2007, 10, 117–124. [Google Scholar] [CrossRef]
- Wen, J.; Fozo, E.M. sRNA Antitoxins: More than One Way to Repress a Toxin. Toxins 2014, 6, 2310–2335. [Google Scholar] [CrossRef]
- Masachis, S.; Darfeuille, F. Type I Toxin-Antitoxin Systems: Regulating Toxin Expression via Shine-Dalgarno Sequence Sequestration and Small RNA Binding. In Regulating with RNA in Bacteria and Archaea; American Society for Microbiology: Washington, DC, USA, 2019; Volume 6, pp. 173–190. [Google Scholar]
- Brantl, S.; Jahn, N. sRNAs in bacterial type I and type III toxin-antitoxin systems. FEMS Microbiol. Rev. 2015, 39, 413–427. [Google Scholar] [CrossRef]
- Szaflarski, W.; Nierhaus, K.H. Question 7: Optimized Energy Consumption for Protein Synthesis. Orig. Life Evol. Biosphere 2007, 37, 423–428. [Google Scholar] [CrossRef]
- Nikaido, H. Molecular Basis of Bacterial Outer Membrane Permeability Revisited. Microbiol. Mol. Biol. Rev. 2003, 67, 593–656. [Google Scholar] [CrossRef] [PubMed]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Edelmann, D.; Leinberger, F.H.; Schmid, N.E.; Oberpaul, M.; Schäberle, T.F.; Berghoff, B.A. Elevated Expression of Toxin TisB Protects Persister Cells against Ciprofloxacin but Enhances Susceptibility to Mitomycin C. Microorganisms 2021, 9, 943. https://doi.org/10.3390/microorganisms9050943
Edelmann D, Leinberger FH, Schmid NE, Oberpaul M, Schäberle TF, Berghoff BA. Elevated Expression of Toxin TisB Protects Persister Cells against Ciprofloxacin but Enhances Susceptibility to Mitomycin C. Microorganisms. 2021; 9(5):943. https://doi.org/10.3390/microorganisms9050943
Chicago/Turabian StyleEdelmann, Daniel, Florian H. Leinberger, Nicole E. Schmid, Markus Oberpaul, Till F. Schäberle, and Bork A. Berghoff. 2021. "Elevated Expression of Toxin TisB Protects Persister Cells against Ciprofloxacin but Enhances Susceptibility to Mitomycin C" Microorganisms 9, no. 5: 943. https://doi.org/10.3390/microorganisms9050943
APA StyleEdelmann, D., Leinberger, F. H., Schmid, N. E., Oberpaul, M., Schäberle, T. F., & Berghoff, B. A. (2021). Elevated Expression of Toxin TisB Protects Persister Cells against Ciprofloxacin but Enhances Susceptibility to Mitomycin C. Microorganisms, 9(5), 943. https://doi.org/10.3390/microorganisms9050943






