Long-Term Effects of Dietary Supplementation with Olive Oil and Hydrogenated Vegetable Oil on the Rumen Microbiome of Dairy Cows
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animal Conditions and Experimental Design
2.2. Rumen Samples Analysis
2.3. Rumen Metataxonomic Analysis
2.4. Statistical Analysis
2.5. Ethics Statement
3. Results
3.1. Ruminal Fermentation Parameters
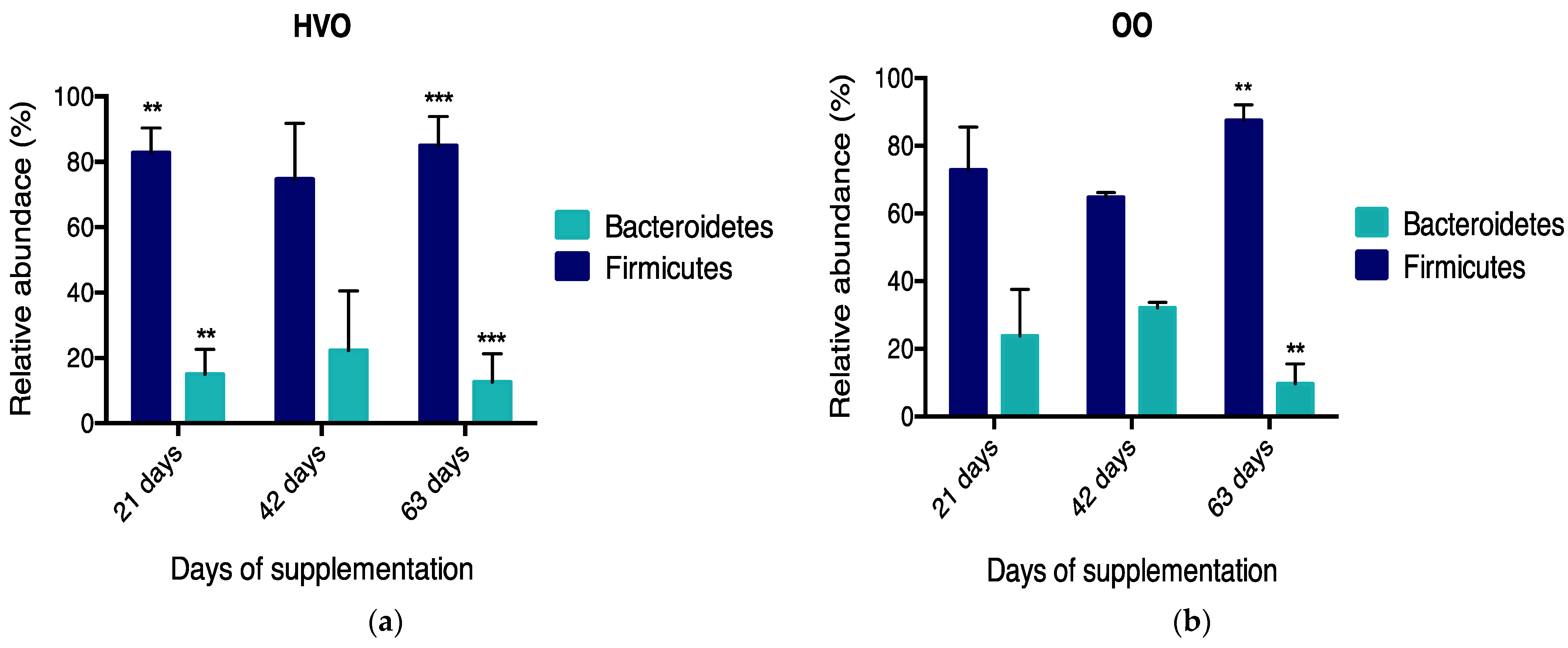
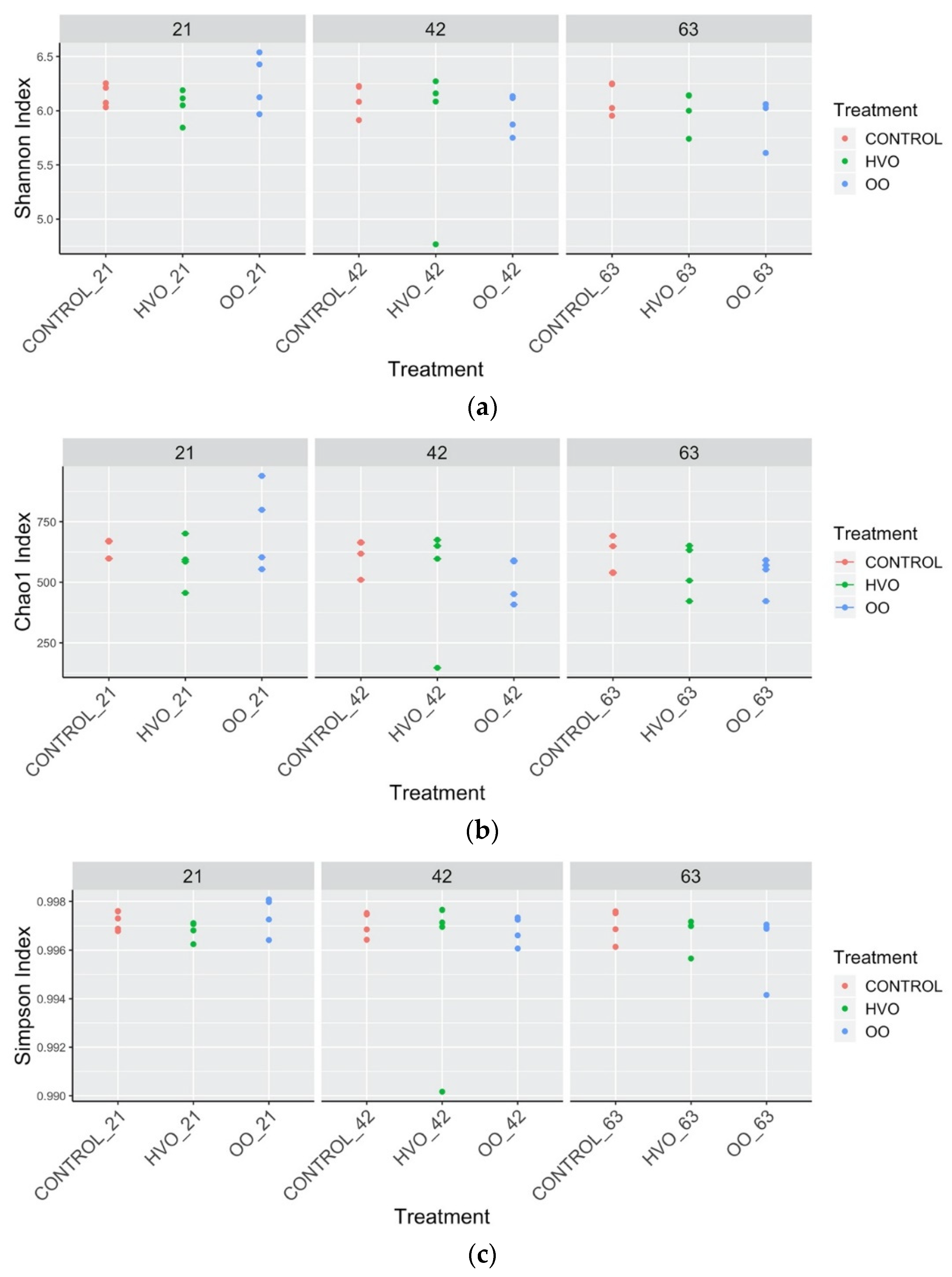
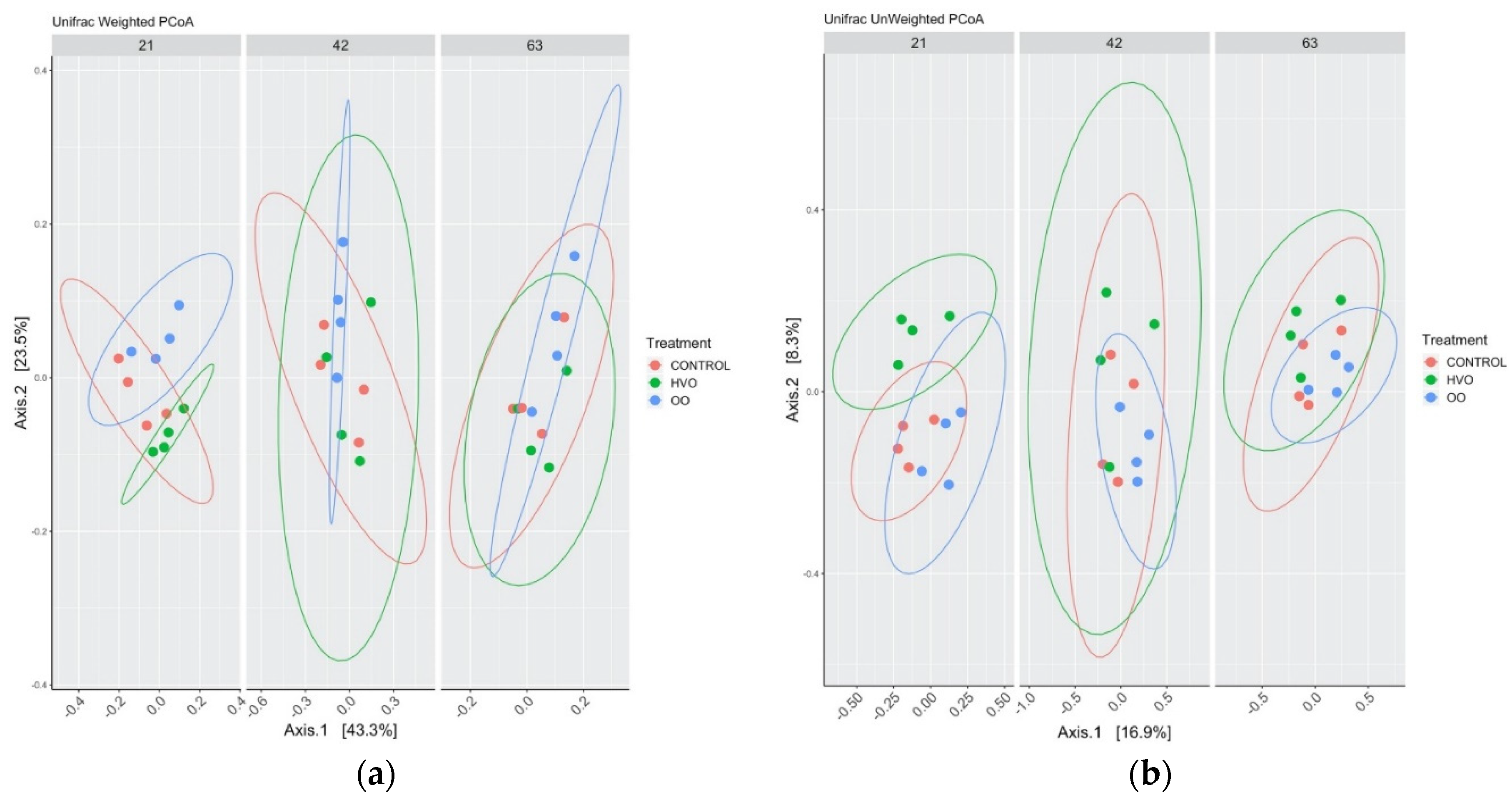
3.2. Rumen Metataxonomy
3.3. Prediction of Function
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Matthews, C.; Crispie, F.; Lewis, E.; Reid, M.; O’Toole, P.W.; Cotter, P.D. The rumen microbiome: A crucial consideration when optimising milk and meat production and nitrogen utilisation efficiency. Gut Microbes 2019, 10, 115–132. [Google Scholar] [CrossRef] [PubMed]
- Sirohi, S.; Singh, N.; Dagar, S.; Puniya, A. Molecular tools for deciphering the microbial community structure and diversity in rumen ecosystem. Appl. Microbiol. Biotechnol. 2012, 95, 1135–1154. [Google Scholar] [CrossRef] [PubMed]
- Huws, S.A.; Creevey, C.J.; Oyama, L.B.; Mizrahi, I.; Denman, S.E.; Popova, M.; Muñoz-Tamayo, R.; Forano, E.; Waters, S.M.; Hess, M.; et al. Addressing global ruminant agricultural challenges through understanding the rumen microbiome: Past, present, and future. Front. Microbiol. 2018, 9, 1–33. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Wang, R.; Xie, T.Y.; Janssen, P.H.; Zhao Sun, X.; Beauchemin, K.A.; Tan, Z.L.; Gao, M. Shifts in Rumen Fermentation and Microbiota Are Associated with Dissolved Ruminal Hydrogen Concentrations in Lactating Dairy Cows Fed Different Types of Carbohydrates. J. Nutr. 2016, 146, 1714–1721. [Google Scholar] [CrossRef]
- Maia, M.R.; Chaudhary, L.C.; Figueres, L.; Wallace, R.J. Metabolism of polyunsaturated fatty acids and their toxicity to the microflora of the rumen. Antonie Van Leeuwenhoek 2007, 91, 303–314. [Google Scholar] [CrossRef]
- Maia, M.R.; Chaudhary, L.C.; Bestwick, C.S.; Richardson, A.J.; McKain, N.; Larson, T.R. Toxicity of unsaturated fatty acids to the biohydrogenating ruminal bacterium, Butyrivibrio fibrisolvens. BMC Microbiol. 2010, 10, 52. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Toral, P.G.; Monahan, F.J.; Hervas, G.; Frutos, P.; Moloney, A.P. Review: Modulating ruminal lipid metabolism to improve the fatty acid composition of meat and milk. challenges and opportunities. Animal 2018, 12, S272–S281. [Google Scholar] [CrossRef] [Green Version]
- Vargas-Bello-Pérez, E.; Garnsworthy, P.C. Trans fatty acids and their role in the milk of dairy cows. Int. J. Agric. Nat. Resour. 2013, 40, 449–473. [Google Scholar] [CrossRef] [Green Version]
- Fuke, G.; Laerte Nornberg, J. Systematic evaluation on the effectiveness of conjugated linoleic acid in human health. Crit. Rev. Food Sci. Nutr. 2017, 57, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Vargas-Bello-Pérez, E.; Íñiguez-González, G.; Fehrmann-Cartes, K.; Toro-Mujica, P.; Garnsworthy, P.C. Influence of fish oil alone or in combination with hydrogenated palm oil on sensory characteristics and fatty acid composition of bovine cheese. Anim. Feed Sci. Technol. 2015, 205, 60–68. [Google Scholar] [CrossRef]
- Welter, K.C.; Marlon de Magalhães Rodrigues Martins, C.; Soligo Vizeu de Palma, A.; Martinson Martins, M.; Roqueto dos Reis, B.; Unglaube Schmidt, B.L.; Saran Netto, A. Canola Oil in Lactating Dairy Cow Diets Reduces Milk Saturated Fatty Acids and Improves Its Omega-3 and Oleic Fatty Acid Content. PLoS ONE 2016, 11, e0151876. [Google Scholar] [CrossRef] [Green Version]
- Vargas-Bello-Pérez, E.; Geldsetzer-Mendoza, C.; Morales, S.M.; Toro-Mujica, P.; Fellenberg, M.A.; Ibáñez, R.A. Effect of olive oil in dairy cow diets on the fatty acid profile and sensory characteristics of cheese. Int. Dairy J. 2018, 85, 8–15. [Google Scholar] [CrossRef]
- Vargas-Bello-Pérez, E.; Cancino-Padilla, N.; Geldsetzer-Mendoza, C.; Vyhmeister, S.; Morales, M.S.; Leskinen, H. Effect of Feeding Cows with Unsaturated Fatty Acid Sources on Milk Production, Milk Composition, Milk Fatty Acid Profile, and Physicochemical and Sensory Characteristics of Ice Cream. Animals 2019, 9, 568. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mannelli, F.; Cappucci, A.; Pini, F.; Pastorelli, R.; Decorosi, F.; Giovannetti, L. Effect of different types of olive oil pomace dietary supplementation on the rumen microbial community profile in Comisana ewes. Sci. Rep. 2018, 8, 8455. [Google Scholar] [CrossRef] [PubMed]
- Vargas-Bello-Pérez, E.; Cancino-Padilla, N.; Romero, J.; Garnsworthy, P.C. Quantitative analysis of ruminal bacterial populations involved in lipid metabolism in dairy cows fed different vegetable oils. Animal 2016, 10, 1821–1828. [Google Scholar] [CrossRef] [PubMed]
- Snelling, T.J.; Auffret, M.D.; Duthie, C.; Stewart, R.D.; Watson, M.; Dewhurst, R.J. Temporal stability of the rumen microbiota in beef cattle, and response to diet and supplements. Anim. Microbiome 2019, 1, 16. [Google Scholar] [CrossRef]
- Abecia, L.; Jiménez, E.; Martínez-Fernandez, G.; Martín-García, A.I.; Ramos-Morales, E.; Pinloche, E. Natural and artificial feeding management before weaning promote different rumen microbial colonization but not differences in gene expression levels at the rumen epithelium of newborn goats. PLoS ONE 2017, 12, e0182235. [Google Scholar] [CrossRef] [PubMed]
- Wildman, E.E.; Jones, G.M.; Wagner, P.E.; Boman, R.L.; Troutt, H.F., Jr.; Lesch, T.N. A dairy cow body condition scoring system and its relationship to selected production characteristics. J. Dairy Sci. 1982, 65, 495–501. [Google Scholar] [CrossRef]
- NRC. Nutrient Requirements of Dairy Cattle, 7th ed.; National Academy of Science: Washington, DC, USA, 2001. [Google Scholar]
- Geishauser, T.; Linhart, N.; Neidl, A.; Reimann, A. Factors associated with ruminal pH at herd level. J. Dairy Sci. 2012, 95, 4556–4567. [Google Scholar] [CrossRef] [PubMed]
- Caporaso, J.G.; Lauber, C.L.; Walters, W.A.; Berg-Lyons, D.; Lozupone, C.A.; Turnbaugh, P.J.; Fierer, N.; Knight, R. Global patterns of 16S rRNA diversity at a depth of millions of sequences per sample. Proc. Natl. Acad. Sci. USA 2011, 108, 4516–4522. [Google Scholar] [CrossRef] [Green Version]
- Lokesh, J.; Kiron, V. Transition from freshwater to seawater reshapes the skin-associated microbiota of Atlantic salmon. Sci. Rep. 2016, 6, 19707. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McMurdie, P.J.; Holmes, S. Phyloseq: An R Package for Reproducible Interactive Analysis and Graphics of Microbiome Census Data. PLoS ONE 2013, 8, e61217. [Google Scholar] [CrossRef] [Green Version]
- Wilkinson, T.J.; Huws, S.A.; Edwards, J.E.; Kingston-Smith, A.H.; Siu-Ting, K.; Hughes, M.; Rubino, F.; Friedersdorff, M.; Creevey, C.J. CowPI: A rumen microbiome focussed version of the PICRUSt functional inference software. Front. Microbiol. 2018, 9, 1095. [Google Scholar] [CrossRef] [PubMed]
- Wanapat, M.; Cherdthong, A.; Phesatcha, K.; Kang, S. Dietary sources and their effects on animal production and environmental sustainability. Anim. Nutr. 2015, 1, 96–103. [Google Scholar] [CrossRef] [PubMed]
- Vargas, J.E.; Andrés, A.; López-Ferreras, L.; Snelling, T.J.; Yáñez-Ruíz, D.R.; García-Estrada, C. Dietary supplemental plant oils reduce methanogenesis from anaerobic microbial fermentation in the rumen. Sci. Rep. 2020, 10, 1613. [Google Scholar] [CrossRef] [Green Version]
- Szumacher-Strabel, M.; Potkański, A.; Kowalczyk, J.; Cieślak, A.; Czauderna, M.; Gubała, A. The influence of supplemental fat on rumen volatile fatty acid profile, ammonia and pH levels in sheep fed a standard diet. J. Anim. Feed Sci. 2002, 11, 577–587. [Google Scholar] [CrossRef] [Green Version]
- Altenhofer, C.; Spornraft, M.; Kienberger, H.; Rychlik, M.; Herrmann, J.; Meyer, H.H.D. Effects of rapeseed and soybean oil dietary supplementation on bovine fat metabolism, fatty acid composition and cholesterol levels in milk. J. Dairy Res. 2014, 81, 120–128. [Google Scholar] [CrossRef]
- Chamberlain, M.B.; DePeters, E.J. Impacts of feeding lipid supplements high in palmitic acid or stearic acid on performance of lactating dairy cows. J. Appl. Anim. Res. 2017, 45, 126–135. [Google Scholar] [CrossRef]
- Bougouin, A.; Martin, C.; Doreau, M.; Ferlay, A. Effects of starch-rich or lipid-supplemented diets that induce milk fat depression on rumen biohydrogenation of fatty acids and methanogenesis in lactating dairy cows. Animal 2019, 13, 1421–1431. [Google Scholar] [CrossRef] [PubMed]
- Grünberg, W.; Constable, P.D. Function and Dysfunction of the Ruminant Forestomach. In Current Veterinary Therapy; Elsevier Inc.: Philadelphia, PA, USA, 2009; pp. 12–19. [Google Scholar]
- Nur Atikah, I.; Alimon, A.R.; Yaakub, H.; Abdullah, N.; Jahromi, M.F.; Ivan, M.; Samsudin, A.A. Profiling of rumen fermentation, microbial population and digestibility in goats fed with dietary oils containing different fatty acids. BMC Vet. Res. 2018, 14, 1–9. [Google Scholar] [CrossRef]
- Bach, A.; Calsamiglia, S.; Stern, M. Nitrogen metabolism in the rumen. J. Dairy Sci. 2005, 88, E9–E21. [Google Scholar] [CrossRef] [Green Version]
- Tong, J.; Zhang, H.; Yang, D.; Zhang, Y.; Xiong, Y.; Jiang, L. Illumina sequencing analysis of the ruminal microbiota in high-yield and low-yield lactating dairy cows. PLoS ONE 2018, 13, 1–15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Benchaar, C.; Romero-Pérez, G.A.; Chouinard, P.Y.; Hassanat, F.; Eugene, M.; Petit, H.V.; Côrtes, C. Supplementation of increasing amounts of linseed oil to dairy cows fed total mixed rations: Effects on digestion, ruminal fermentation characteristics, protozoal populations, and milk fatty acid composition. J. Dairy Sci. 2012, 95, 4578–4590. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vargas-Bello-Pérez, E.; Geldsetzer-Mendoza, C.; Cancino-Padilla, N.; Morales, M.S.; Leskinen, H.; Garnsworthy, P.C. Effects of Dietary Vegetable Oils on Mammary Lipid-Related Genes in Holstein Dairy Cows. Animals 2020, 10, 57. [Google Scholar] [CrossRef] [Green Version]
- Lyons, T.; Boland, T.; Storey, S.; Doyle, E. Linseed oil supplementation of lambs’ diet in early life leads to persistent changes in rumen microbiome structure. Front. Microbiol. 2017, 8, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Kim, M.; Morrison, M.; Yu, Z. Status of the phylogenetic diversity census of ruminal microbiomes. FEMS Microbiol. Ecol. 2011, 76, 49–63. [Google Scholar] [CrossRef] [Green Version]
- Jewell, K.A.; McCormick, C.A.; Odt, C.L.; Weimer, P.J.; Suen, G. Ruminal Bacterial Community Composition in Dairy Cows Is Dynamic over the Course of Two Lactations and Correlates with Feed Efficiency. Appl. Environ. Microbiol. 2015, 81, 4697–4710. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhu, Z.; Kristensen, L.; Difford, G.F.; Poulsen, M.; Noel, S.J.; Al-Soud, W.A.; Sørensen, S.J.; Lassen, J.; Løvendahl, P.; Højberg, O. Changes in rumen bacterial and archaeal communities over the transition period in primiparous Holstein dairy cows. J. Dairy Sci. 2018, 101, 9847–9862. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.; Wang, Y.; Li, Y.; Zhang, Y.; Liu, T.; Wang, Y.; Sharpton, T.J.; Zhu, W. Progressive colonization of bacteria and degradation of rice straw in the rumen by Illumina sequencing. Front. Microbiol. 2017, 8, 1–10. [Google Scholar] [CrossRef]
- Weimer, P.J. Redundancy, resilience, and host specificity of the ruminal microbiota: Implications for engineering improved ruminal fermentations. Front. Microbiol. 2015, 6, 1–16. [Google Scholar] [CrossRef] [Green Version]
- Loor, J.J.; Elolimy, A.A.; McCann, J.C. Dietary impacts on rumen microbiota in beef and dairy production. Anim. Front. 2016, 6, 22–29. [Google Scholar] [CrossRef] [Green Version]
- Cremonesi, P.; Conte, G.; Severgnini, M.; Turri, F.; Monni, A.; Capra, E.; Rapetti, L.; Colombini, S.; Chessa, S.; Battelli, G.; et al. Evaluation of the effects of different diets on microbiome diversity and fatty acid composition of rumen liquor in dairy goat. Animal 2018, 12, 1856–1866. [Google Scholar] [CrossRef] [PubMed]
- McCann, J.C.; Wickersham, T.A.; Loor, J.J. High-throughput methods redefine the rumen microbiome and its relationship with nutrition and metabolism. Bioinform. Biol. Insights 2014, 8, 109–125. [Google Scholar] [CrossRef] [PubMed]
- Paz, H.A.; Hales, K.E.; Wells, J.E.; Kuehn, L.A.; Freetly, H.C.; Berry, E.D.; Flythe, M.D.; Spangler, M.L.; Fernando, S.C. Rumen bacterial community structure impacts feed efficiency in beef cattle. J. Anim. Sci. 2018, 96, 1045–1058. [Google Scholar] [CrossRef]
- Pitta, D.W.; Indugu, N.; Vecchiarelli, B.; Rico, D.E.; Harvatine, K.J. Alterations in ruminal bacterial populations at induction and recovery from diet-induced milk fat depression in dairy cows. J. Dairy Sci. 2018, 101, 295–309. [Google Scholar] [CrossRef] [Green Version]
- Bayat, A.R.; Tapio, I.; Vilkki, J.; Shingfield, K.J.; Leskinen, H. Plant oil supplements reduce methane emissions and improve milk fatty acid composition in dairy cows fed grass silage-based diets without affecting milk yield. J. Dairy Sci. 2017, 101, 1136–1151. [Google Scholar] [CrossRef] [Green Version]
- Huws, S.A.; Kim, E.J.; Cameron, S.J.S.; Girdwood, S.E.; Davies, L.; Tweed, J.; Vallin, H.; Scollan, N.D. Characterization of the rumen lipidome and microbiome of steers fed a diet supplemented with flax and echium oil. Microb. Biotechnol. 2015, 8, 331–341. [Google Scholar] [CrossRef] [Green Version]
- Myer, P.R.; Smith, T.P.L.; Wells, J.E.; Kuehn, L.A.; Freetly, H.C. Rumen Microbiome from Steers Differing in Feed Efficiency. PLoS ONE 2015, 10, e0129174. [Google Scholar] [CrossRef] [Green Version]
- Pitta, D.W.; Pinchak, E.; Dowd, S.E.; Osterstock, J.; Gontcharova, V.; Youn, E.; Dorton, K.; Yoon, I.; Min, B.R.; Fulford, J.D.; et al. Rumen bacterial diversity dynamics associated with changing from bermudagrass hay to grazed winter wheat diets. Microb. Ecol. 2010, 59, 511–522. [Google Scholar] [CrossRef]
- Wirth, R.; Kádár, G.; Kakuk, B.; Maróti, G.; Bagi, Z.; Szilágyi, A.; Rákhely, G.; Horváth, J.; Kovács, K.L. The planktonic core microbiome and core functions in the cattle rumen by next generation sequencing. Front. Microbiol. 2018, 9, 1–19. [Google Scholar] [CrossRef] [Green Version]
- Koringa, P.G.; Thakkar, J.R.; Pandit, R.J.; Hinsu, A.T.; Parekh, M.J.; Shah, R.K.; Jakhesara, S.J.; Joshi, C.G. Metagenomic characterisation of ruminal bacterial diversity in buffaloes from birth to adulthood using 16S rRNA gene amplicon sequencing. Funct. Integr. Genom. 2019, 19, 237–247. [Google Scholar] [CrossRef] [PubMed]
- Bi, Y.; Zeng, S.; Zhang, R.; Diao, Q.; Tu, Y. Effects of dietary energy levels on rumen bacterial community composition in Holstein heifers under the same forage to concentrate ratio condition. BMC Microbiol. 2018, 18, 1–11. [Google Scholar] [CrossRef]
- Ren, H.; Su, X.; Bai, H.; Yang, Y.; Wang, H.; Dan, Z.; Lu, J.; Wu, S.; Cai, C.; Cao, Y.; et al. Specific enrichment of microbes and increased ruminal propionate production: The potential mechanism underlying the high energy efficiency of Holstein heifers fed steam-flaked corn. AMB Expr. 2019, 9, 209. [Google Scholar] [CrossRef] [Green Version]
- Huang, S.; Ji, S.; Yan, H.; Hao, Y.; Zhang, J.; Wang, Y.; Cao, Z.; Li, S. The day-to-day stability of the ruminal and fecal microbiota in lactating dairy cows. Microbiologyopen 2020, 9, e990. [Google Scholar] [CrossRef] [PubMed]
- Vargas-Bello-Pérez, E.; Bionaz, M.; Sciarresi-Arechabala, P.; Cancino-Padilla, N.; Morales, M.S.; Romero, J.; Leskinen, H.; Garnsworthy, P.C.; Loor, J.J. Long-Term Effects of Dietary Olive Oil and Hydrogenated Vegetable Oil on Expression of Lipogenic Genes in Subcutaneous Adipose Tissue of Dairy Cows. Vet. Sci. 2019, 6, 74. [Google Scholar] [CrossRef] [Green Version]
- Vargas-Bello-Pérez, E.; Cancino-Padilla, N.; Geldsetzer-Mendoza, C.; Morales, M.S.; Leskinen, H.; Garnsworthy, P.C.; Loor, J.J.; Romero, J. Effects of dietary polyunsaturated fatty acid sources on expression of lipid-related genes in bovine milk somatic cells. Sci Rep. 2020, 10, 14850. [Google Scholar] [CrossRef] [PubMed]
- Bionaz, M.; Vargas-Bello-Pérez, E.; Busato, S. Advances in fatty acids nutrition in dairy cows: From gut to cells and effects on performance. J. Anim. Sci. Biotechnol. 2020, 11, 110. [Google Scholar] [CrossRef] [PubMed]



| Rumen Parameter | Treatment | p-Value | |||||
|---|---|---|---|---|---|---|---|
| Control | HVO | OO | SEM | Diet (D) | Time (T) | D × T | |
| pH | 6.98 | 7.10 | 6.97 | 0.07 | 0.157 | 0.901 | 0.432 |
| NH3-N, mg/dL | 5.42 | 4.39 | 4.76 | 1.21 | 0.217 | 0.064 | 0.291 |
| Total VFA (mmol/L) | 96.1 | 73.0 | 82.5 | 19.5 | 0.069 | 0.001 | 0.605 |
| Molar proportion (mol/100 mol) | |||||||
| Acetate | 66.3 | 65.4 | 64.4 | 3.69 | 0.559 | 0.021 | 0.300 |
| Propionate | 18.6 | 19.3 | 20.0 | 4.03 | 0.746 | 0.001 | 0.184 |
| Butyrate | 9.62 | 9.04 | 8.85 | 1.26 | 0.412 | 0.024 | 0.234 |
| Valerate | 1.66 | 1.84 | 2.00 | 0.52 | 0.376 | 0.104 | 0.692 |
| Isovalerate | 1.59 | 2.16 | 1.71 | 0.63 | 0.164 | 0.279 | 0.480 |
| Isobutyric | 1.97 | 2.05 | 2.54 | 0.83 | 0.310 | 0.013 | 0.301 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cancino-Padilla, N.; Catalán, N.; Siu-Ting, K.; Creevey, C.J.; Huws, S.A.; Romero, J.; Vargas-Bello-Pérez, E. Long-Term Effects of Dietary Supplementation with Olive Oil and Hydrogenated Vegetable Oil on the Rumen Microbiome of Dairy Cows. Microorganisms 2021, 9, 1121. https://doi.org/10.3390/microorganisms9061121
Cancino-Padilla N, Catalán N, Siu-Ting K, Creevey CJ, Huws SA, Romero J, Vargas-Bello-Pérez E. Long-Term Effects of Dietary Supplementation with Olive Oil and Hydrogenated Vegetable Oil on the Rumen Microbiome of Dairy Cows. Microorganisms. 2021; 9(6):1121. https://doi.org/10.3390/microorganisms9061121
Chicago/Turabian StyleCancino-Padilla, Nathaly, Natalia Catalán, Karen Siu-Ting, Christopher J. Creevey, Sharon A. Huws, Jaime Romero, and Einar Vargas-Bello-Pérez. 2021. "Long-Term Effects of Dietary Supplementation with Olive Oil and Hydrogenated Vegetable Oil on the Rumen Microbiome of Dairy Cows" Microorganisms 9, no. 6: 1121. https://doi.org/10.3390/microorganisms9061121
APA StyleCancino-Padilla, N., Catalán, N., Siu-Ting, K., Creevey, C. J., Huws, S. A., Romero, J., & Vargas-Bello-Pérez, E. (2021). Long-Term Effects of Dietary Supplementation with Olive Oil and Hydrogenated Vegetable Oil on the Rumen Microbiome of Dairy Cows. Microorganisms, 9(6), 1121. https://doi.org/10.3390/microorganisms9061121







