Development of a Tetraplex qPCR for the Molecular Identification and Quantification of Human Enteric Viruses, NoV and HAV, in Fish Samples
Abstract
1. Introduction
2. Materials and Methods
2.1. Process Control Virus
2.2. Sample Processing and Total RNA Extraction
2.3. Taqman Probes and Primers
2.4. qPCR Standard Curve Construction
2.5. Single and Multiplex qPCR Assay
2.6. Analytical Specificity and Detection Limit Evaluation for the Single and Multiplex qPCR Assays
Evaluation of the Analytical Specificity and Detection Limit of the qPCR Assays with Previously Positive Samples
2.7. Nested PCR Assays for NoV GI, NoV GII, and HAV Detection
2.8. Dataset Compilation and Phylogenetic Analysis
3. Results
3.1. Multiplex qPCR Implementation
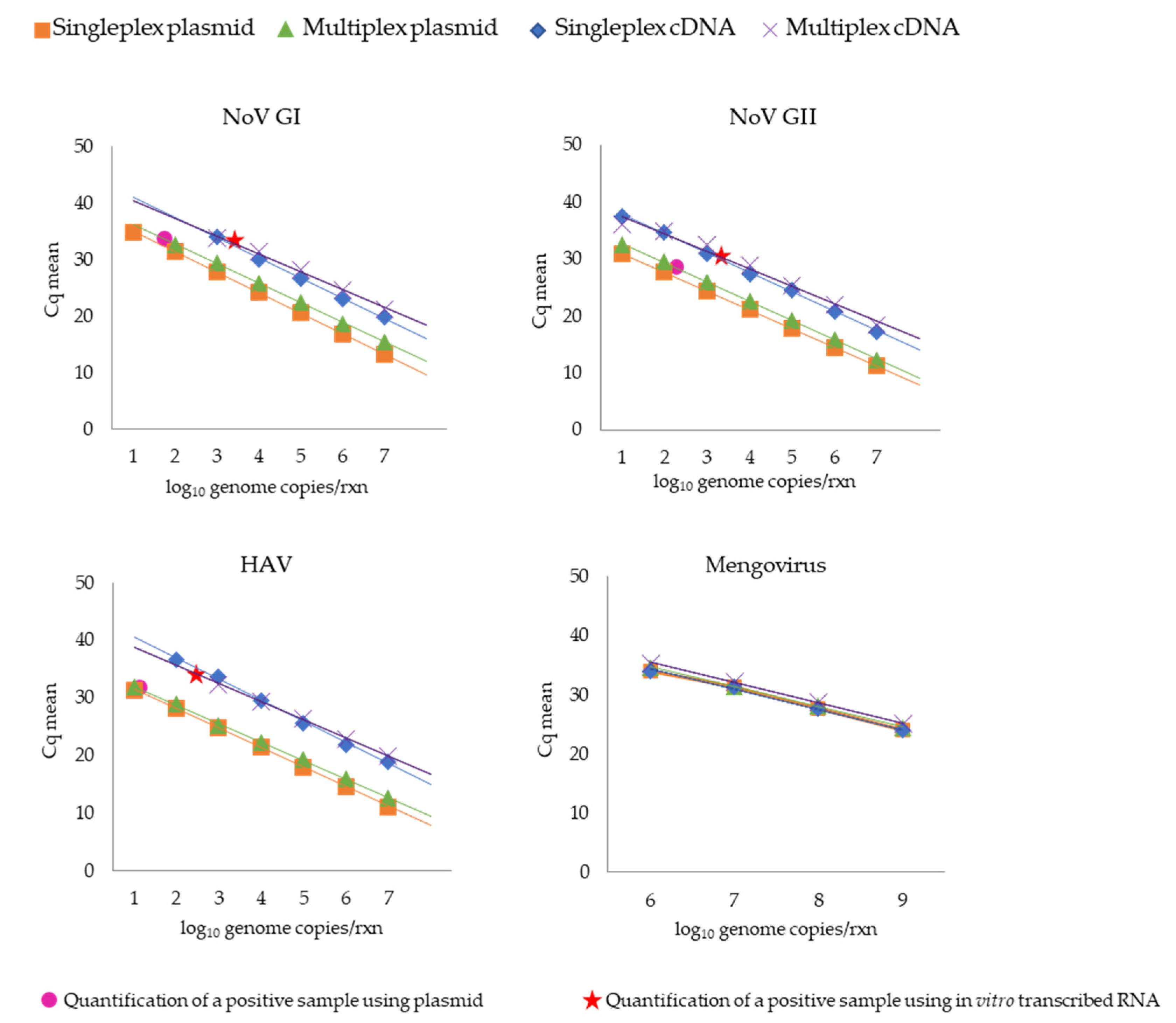
3.1.1. qPCR Efficiency, Analytical Specificity, and Sensitivity
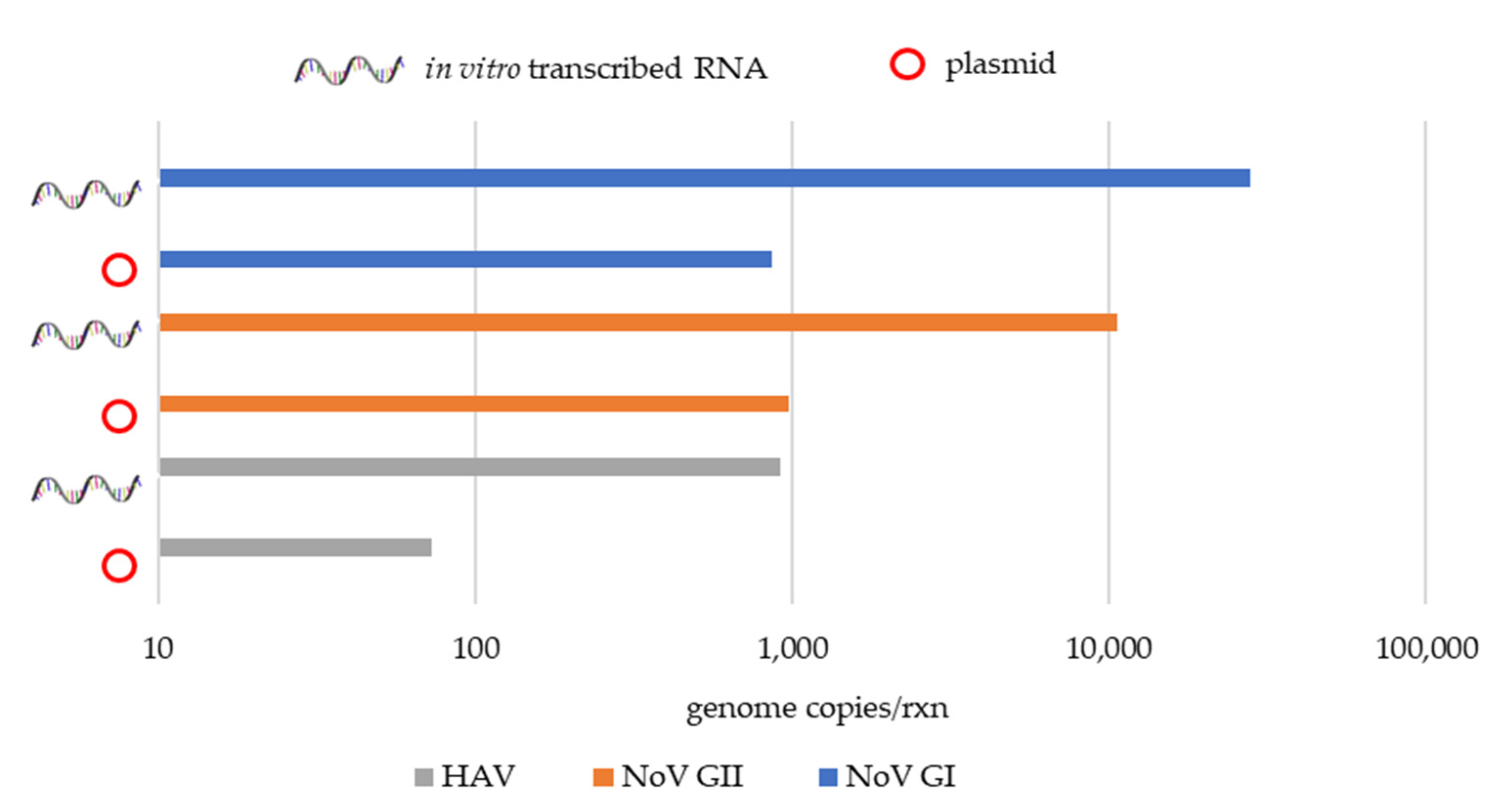
3.1.2. Quantification of the Wastewater Samples Positive for NoV GI, NoV GII, and HAV Genomes Using a Plasmid and an In Vitro Transcribed RNA Standard-Based Curve
3.2. Quantification of Mengovirus in Artificially Spiked Samples
3.3. Quantification and Characterisation of Human Pathogenic Viruses in Fish
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- ECDC and EFSA (European Food Safety Authority and European Centre for Disease Prevention and Control). The European Union One Health 2019 Zoonoses Report. EFSA J. 2021, 19, 6406. [Google Scholar] [CrossRef]
- Fuentes, C.; Guix, S.; Pérez-Rodriguez, F.J.; Fuster, N.; Carol, M.; Pintó, R.M.; Bosch, A. Standardized multiplex one-step qRT-PCR for hepatitis A virus, norovirus GI and GII quantification in bivalve mollusks and water. Food Microbiol. 2014, 40, 55–63. [Google Scholar] [CrossRef]
- Bosch, A.; Gkogka, E.; Le Guyader, F.S.; Loisy-Hamon, F.; Lee, A.; van Lieshout, L.; Marthi, B.; Myrmel, M.; Sansom, A.; Schultz, A.C.; et al. Foodborne viruses: Detection, risk assessment, and control options in food processing. Int. J. Food Microbiol. 2018, 285, 110–128. [Google Scholar] [CrossRef] [PubMed]
- Scharff, R.L. State Estimates for the Annual Cost of Foodborne Illness. J. Food Prot. 2015, 78, 1064–1071. [Google Scholar] [CrossRef] [PubMed]
- Mangen, M.J.; Friesema, I.H.M.; Pijnacker, R.; Mughini, L.; van Pelt, W. Disease Burden of Food-Related Pathogens in the Netherlands; RIVM Lett. Rep. 2018-0037 2018; National Institute for Public Health and the Environment: Bilthoven, The Netherlands, 2017. [CrossRef]
- Rimmer, A.E.; Becker, J.A.; Tweedie, A.; Lintermans, M.; Landos, M.; Stephens, F.; Whittington, R.J. Detection of dwarf gourami iridovirus (Infectious spleen and kidney necrosis virus) in populations of ornamental fish prior to and after importation into Australia, with the first evidence of infection in domestically farmed Platy (Xiphophorus maculatus). Prev. Vet. Med. 2015, 122, 181–194. [Google Scholar] [CrossRef] [PubMed]
- Gauthier, D.T. Bacterial zoonoses of fishes: A review and appraisal of evidence for linkages between fish and human infections. Vet. J. 2015, 203, 27–35. [Google Scholar] [CrossRef] [PubMed]
- Costa, G.; MacKenzie, K.; Oliva, M.E. A Review of the Parasites Infecting Fishes of the Genus Trachurus (Pisces: Carangidae). Rev. Fish. Sci. Aquac. 2017, 25, 297–315. [Google Scholar] [CrossRef]
- Chintagari, S.; Hazard, N.; Edwards, G.; Jadeja, R.; Janes, M. Risks Associated with Fish and Seafood. Preharvest Food Saf. 2017, 123–142. [Google Scholar] [CrossRef]
- ISO 15216-1:2017. Microbiology of the Food Chain—Horizontal Method for Determination of Hepatitis A Virus and Norovirus Using Real-Time RT-PCR—Part 1: Method for Quantification; European Committee for Standardization: Geneva, Switzerland, 2017. [Google Scholar]
- Bowers, R.M.; Dhar, A.K. Effect of template on generating a standard curve for absolute quantification of an RNA virus by real-time reverse transcriptase-polymerase chain reaction. Mol. Cell. Probes 2011, 25, 60–64. [Google Scholar] [CrossRef]
- Terlizzi, M.E.; Bergallo, M.; Astegiano, S.; Sidoti, F.; Gambarino, S.; Solidoro, P.; Costa, C.; Cavallo, R. Improvement of HRV quantification using cRNA-based standards for real time RT-PCR. Mol. Biotechnol. 2011, 48, 15–18. [Google Scholar] [CrossRef]
- Costafreda, M.I.; Bosch, A.; Pintó, R.M. Development, evaluation, and standardization of a real-time TaqMan reverse transcription-PCR assay for quantification of hepatitis A virus in clinical and shellfish samples. Appl. Environ. Microbiol. 2006, 72, 3846–3855. [Google Scholar] [CrossRef]
- Katoh, K.; Standley, D.M. MAFFT Multiple Sequence Alignment Software Version 7: Improvements in Performance and Usability Article Fast Track. Mol. Biol. Evol. 2013, 30, 772–780. [Google Scholar] [CrossRef]
- Ye, J.; Coulouris, G.; Zaretskaya, I.; Cutcutache, I.; Rozen, S.; Madden, T.L. Primer-BLAST: A tool to design target-specific primers for polymerase chain reaction. BMC Bioinform. 2012. [Google Scholar] [CrossRef]
- Pintó, R.M.; Costafreda, M.I.; Bosch, A. Risk assessment in shellfish-borne outbreaks of hepatitis A. Appl. Environ. Microbiol. 2009, 75, 7350–7355. [Google Scholar] [CrossRef]
- Ramanan, P.; Espy, M.J.; Khare, R.; Binnicker, M.J. Detection and differentiation of norovirus genogroups I and II from clinical stool specimens using real-time PCR. Diagn. Microbiol. Infect. Dis. 2017, 87, 325–327. [Google Scholar] [CrossRef]
- Kageyama, T.; Kojima, S.; Shinohara, M.; Uchida, K.; Fukushi, S.; Hoshino, F.B.; Takeda, N.; Katayama, K. Broadly reactive and highly sensitive assay for Norwalk-like viruses based on real-time quantitative reverse transcription-PCR. J. Clin. Microbiol. 2003, 41, 1548–1557. [Google Scholar] [CrossRef] [PubMed]
- Kojima, S.; Kageyama, T.; Fukushi, S.; Hoshino, F.B.; Shinohara, M.; Uchida, K.; Natori, K.; Takeda, N.; Katayama, K. Genogroup-specific PCR primers for detection of Norwalk-like viruses. J. Virol. Methods 2002, 100, 107–114. [Google Scholar] [CrossRef]
- RVIM:Protocol Molecular Detection and Typing of Vp1region of Hepatitis A Virus (HAV)|Enhanced Reader. Available online: moz-extension://c1d2a850-d283-44bc-884c-6a5e8dbae832/enhanced-reader.html?openApp&pdf=https%3A%2F%2Fwww.rivm.nl%2Fsites%2Fdefault%2Ffiles%2F2018-11%2FTyping%2520protocol%2520HAVNET%2520VP1P2A%2520a1a.pdf (accessed on 29 January 2021).
- Castresana, J. Selection of conserved blocks from multiple alignments for their use in phylogenetic analysis. Mol. Biol. Evol. 2000, 17, 540–552. [Google Scholar] [CrossRef] [PubMed]
- Trifinopoulos, J.; Nguyen, L.-T.; von Haeseler, A.; Quang Minh, B. W-IQ-TREE: A fast online phylogenetic tool for maximum likelihood analysis. Nucleic Acids Res. 2016, 44, W232–W235. [Google Scholar] [CrossRef]
- Chen, H. Nucleic Acid Detection of Major Foodborne Viral Pathogens: Human Noroviruses and Hepatitis A Virus. Nucleic Acids—From Basic Asp. to Lab. Tools. Marcelo L. Larramendy Sonia Soloneski; IntechOpen: London, UK, 2016. [Google Scholar] [CrossRef]
- Martin-Latil, S.; Hennechart-Collette, C.; Delannoy, S.; Guillier, L.; Fach, P.; Perelle, S. Quantification of hepatitis E virus in naturally-contaminated pig liver products. Front. Microbiol. 2016, 7, 1–10. [Google Scholar] [CrossRef]
- Li, D.; Butot, S.; Zuber, S.; Uyttendaele, M. Monitoring of foodborne viruses in berries and considerations on the use of RT-PCR methods in surveillance. Food Control 2018, 89, 235–240. [Google Scholar] [CrossRef]
- Spackman, E.; Kapczynski, D.; Sellers, H. Multiplex Real-Time Reverse Transcription–Polymerase Chain Reaction for the Detection of Three Viruses Associated with Poult Enteritis Complex: Turkey Astrovirus, Turkey Coronavirus, and Turkey Reovirus. Avian Dis. 2006, 49, 86–91. [Google Scholar] [CrossRef] [PubMed]
- Hou, Y.; Zhang, H.; Miranda, L.; Lin, S. Serious Overestimation in Quantitative PCR by Circular (Supercoiled) Plasmid Standard: Microalgal pcna as the Model Gene. PLoS ONE 2010, 5, e9545. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Kadlubar, F.F.; Chen, J.Z. DNA supercoiling suppresses real-time PCR: A new approach to the quantification of mitochondrial DNA damage and repair. Nucleic Acids Res. 2007, 35, 1377–1388. [Google Scholar] [CrossRef] [PubMed]
- Levesque-Sergerie, J.P.; Duquette, M.; Thibault, C.; Delbecchi, L.; Bissonnette, N. Detection limits of several commercial reverse transcriptase enzymes: Impact on the low- and high-abundance transcript levels assessed by quantitative RT-PCR. BMC Mol. Biol. 2007, 8, 93. [Google Scholar] [CrossRef]
- Campos, C.J.A.; Lees, D.N. Environmental transmission of human noroviruses in shellfish waters. Appl. Environ. Microbiol. 2014, 80, 3552–3561. [Google Scholar] [CrossRef]
- La Bella, G.; Martella, V.; Basanisi, M.G.; Nobili, G.; Terio, V.; La Salandra, G. Food-Borne Viruses in Shellfish: Investigation on Norovirus and HAV Presence in Apulia (SE Italy). Food Environ. Virol. 2017, 9, 179–186. [Google Scholar] [CrossRef]
- Li, D.; Stals, A.; Tang, Q.-J.; Uyttendaele, M. Detection of Noroviruses in Shellfish and Semiprocessed Fishery Products from a Belgian Seafood Company. J. Food Prot. 2014, 77, 1342–1347. [Google Scholar] [CrossRef] [PubMed]
- Mesquita, J.R.; Vaz, L.; Cerqueira, S.; Castilho, F.; Santos, R.; Monteiro, S.; Manso, C.F.; Romalde, J.L.; Nascimento, M.S.J. Norovirus, hepatitis A virus and enterovirus presence in shellfish from high quality harvesting areas in Portugal. Food Microbiol. 2011, 28, 936–941. [Google Scholar] [CrossRef]
- Polo, D.; Varela, M.F.; Romalde, J.L. Detection and quantification of hepatitis A virus and norovirus in Spanish authorized shellfish harvesting areas. Int. J. Food Microbiol. 2015, 193, 43–50. [Google Scholar] [CrossRef]
- Tao, J.; Chunhui, H.; Fanning, S.; Nan, L.; Jiahui, W.; Hongyuan, Z.; Jing, Z.; Fengqin, L. Norovirus contamination in retail oysters from Beijing and Qingdao, China. Food Control 2018, 86, 415–419. [Google Scholar] [CrossRef]
- Kimura, E.; Goto, H.; Migita, A.; Harada, S.; Yamashita, S.; Hirano, T.; Uchino, M. An adult norovirus-related encephalitis/encephalopathy with mild clinical manifestation. BMJ Case Rep. 2010, 10–12. [Google Scholar] [CrossRef] [PubMed]
- Shima, T.; Okumura, A.; Kurahashi, H.; Numoto, S.; Abe, S.; Ikeno, M.; Shimizu, T. A nationwide survey of norovirus-associated encephalitis/encephalopathy in Japan. Brain Dev. 2019, 41, 263–270. [Google Scholar] [CrossRef] [PubMed]
- Karst, S.M.; Wobus, C.E.; Goodfellow, I.G.; Green, K.Y.; Virgin, H.W. Advances in norovirus biology. Cell Host Microbe 2014, 15, 668–680. [Google Scholar] [CrossRef]
- Van Dycke, J.; Ny, A.; Conceição-Neto, N.; Maes, J.; Hosmillo, M.; Cuvry, A.; Goodfellow, I.; Nogueira, T.C.; Verbeken, E.; Matthijnssens, J.; et al. A robust human norovirus replication model in zebrafish larvae. PLoS Pathog. 2019, 15, e1008009. [Google Scholar] [CrossRef] [PubMed]
- Chhabra, P.; de Graaf, M.; Parra, G.I.; Chan, M.C.W.; Green, K.; Martella, V.; Wang, Q.; White, P.A.; Katayama, K.; Vennema, H.; et al. Updated classification of norovirus genogroups and genotypes. J. Gen. Virol. 2019, 100, 1393–1406. [Google Scholar] [CrossRef]
- Kwan, H.S.; Chan, P.K.S.; Chan, M.C.W. Overview of Norovirus as a Foodborne Pathogen; Elsevier Inc.: Amsterdam, The Netherlands, 2016; ISBN 9780128041949. [Google Scholar]


| Species | Source | Fishery Type | N° of Specimens | N° of Pools |
|---|---|---|---|---|
| Trachurus trachurus (Atlantic horse mackerel) | Figueira da Foz fish market | Wild fisheries | 30 | 3 |
| Trachurus trachurus (Atlantic horse mackerel) | Peniche fish market | Wild fisheries | 20 | 2 |
| Trachurus trachurus (Atlantic horse mackerel) | Algarve fish market | Wild fisheries | 30 | 3 |
| Trachurus trachurus (Atlantic horse mackerel) | Supermarket | Wild fisheries | 10 | 1 |
| Trachurus trachurus (Atlantic horse mackerel) | Discarded from fish markets | Wild fisheries | 6 | 1 |
| Sardina pilchardus (sardine) | Algarve fish market | Wild fisheries | 30 | 3 |
| Sardina pilchardus (sardine) | Sagres fish market | Wild fisheries | 30 | 3 |
| Sparus aurata (gilthead seabream) | Algarve fish market | Wild fisheries | 15 | 3 |
| Sparus aurata (gilthead seabream) | Algarve fish market | Aquaculture | 15 | 3 |
| Sparus aurata (gilthead seabream) | Setúbal fish market | Aquaculture | 15 | 3 |
| Sparus aurata (gilthead seabream) | Peniche fish market | Wild fisheries | 15 | 3 |
| Sparus aurata (gilthead seabream) | Supermarket | Aquaculture | 7 | 1 |
| Sparus aurata (gilthead seabream) | Discarded from fish markets | Wild fisheries | 5 | 1 |
| Sparus aurata (gilthead seabream) | Discarded from fish markets | Aquaculture | 5 | 1 |
| Dicentrarchus labrax (seabass) | Algarve fish market | Aquaculture | 15 | 3 |
| Dicentrarchus labrax (seabass) | Setúbal fish market | Aquaculture | 15 | 3 |
| Dicentrarchus labrax (seabass) | Peniche fish market | Wild fisheries | 15 | 3 |
| Dicentrarchus labrax (seabass) | Figueira da Foz fish market | Wild fisheries | 15 | 3 |
| Dicentrarchus labrax (seabass) | Supermarket | Aquaculture | 7 | 1 |
| Dicentrarchus labrax (seabass) | Discarded from fish markets | Aquaculture | 4 | 1 |
| Merluccius merluccius (European hake) | Discarded from fish markets | Wild fisheries | 6 | 1 |
| Mullus surmuletus (mullet) | Discarded from fish markets | Wild fisheries | 5 | 1 |
| Mugil cephalus (rooster) | Discarded from fish markets | Wild fisheries | 2 | 1 |
| Chelidonichthys lucerna (redfish) | Discarded from fish markets | Wild fisheries | 3 | 1 |
| Mugil cephalus (flathead grey mullet) | Discarded from fish markets | Wild fisheries | 3 | 1 |
| Target | Primers/Probes (5′-3′) | Reference | Reference Sequence |
|---|---|---|---|
| Fw_Mengo (vMC0) | GCGGGTCCTGCCGAAAGT | [16] | L22089 |
| Rv_Mengo (vMC0) | GAAGTAACATATAGACAGACGCACAC | ||
| P_Mengo (vMC0) | ATCACATTACTGGCCGAAGC | ||
| Fw_NoV GI | CCATGTTCCGBTGGATGC a | [17] | M87661 |
| Rv_NoV GI | CCTTAGACGCCATCATCATTTAC | [18] | |
| P_NoV GI | AGATRGCGATCTCCTGTCCACA a | [18] | |
| Fw_NoV GII | ATGTTYAGRTGGATGAGATTCTC a | [17] | AF145896 |
| Rv_NoV GII | TCGACGCCATCTTCATTCACA | [18] | |
| P_NoV GII | TGGGAGGGCGATCGCAATCT | [18] | |
| Fw_HAV | TCACCGCCGTTTGCCTAG | [13] | M14707 |
| Rv_HAV | GGAGAGCCCTGGAAGAAAG | [13] | |
| P_HAV | GATTCCTGCAGGTTCAGGGTTCT | This study | |
| NoV GI_nFw1 | CGYTGGATGCGNTTYCATGA a | [18] | M87661 |
| NoV GI_nRv1/2 | CCAACCCARCCATTRTACA a | [19] | |
| NoV GI_nFw2 | CTGCCCGAATTYGTAAATGA a | [19] | |
| NoV GII_nFw1 | CARGARBCNATGTTYAGRTGGATGAG a | [18] | AF145896 |
| NoV GII_nRv1/2 | CCRCCNGCATRHCCRTTRTACAT a | [19] | X86557 |
| NoV GII_nFw2 | CNTGGGAGGGCGATCGCAA a | [19] | X86557 |
| HAV_nFw1 | TATGCYGTITCWGGIGCIYTRGAYGG a | [20] | NC_001489 |
| HAV_nRv1 | TCYTTCATYTCWGTCCAYTTYTCATCATT a | ||
| HAV1_nFw2 | GGATTGGTTTCCATTCARATTGCNAAYTA a | ||
| HAV2_nrv2 | CTGCCAGTCAGAACTCCRGCWTCCATYTC a |
| Reagents | NoV GII, HAV, Mengovirus Single Reaction | NoV GI Single Reaction | HAV, NoV GII Multiplex Reactions | NoV GI Multiplex Reaction | Mengovirus Multiplex Reaction |
|---|---|---|---|---|---|
| Reverse primer | 900 nM | 500 nM | 400 nM * | 400 nM | 900 nM |
| Forward primer | 500 nM | 100 nM | 100 nM | 100 nM | 500 nM |
| Probe | 250 nM | 250 nM | 100 nM | 250 nM | 250 nM |
| Plasmid | In Vitro Transcribed RNA | |||||||
|---|---|---|---|---|---|---|---|---|
| Single | Multiplex | Single | Multiplex | |||||
| qPCR Efficiency (%) | R2 | qPCR Efficiency (%) | R2 | qPCR Efficiency (%) | R2 | qPCR Efficiency (%) | R2 | |
| Nov GI | 89.3 | 0.999 | 94.4 | 0.999 | 90.8 | 0.999 | 106.9 | 0.995 |
| NoV GII | 100.8 | 1 | 98.0 | 0.999 | 97.0 | 0.999 | 111.6 | 0.985 |
| HAV | 97.6 | 0.999 | 105.5 | 0.999 | 88.0 | 0.997 | 108.8 | 0.999 |
| Mengovirus | 97.1 | 0.996 | 97.6 | 0.999 | 97.1 | 0.996 | 96.3 | 0.999 |
| Plasmid | In Vitro Transcribed RNA | |||
|---|---|---|---|---|
| Viruses | Cq Mean | Genome Copies/rxn | Cq Mean | Genome Copies/rxn |
| Nov GI | 34.94 | 8.60 × 102 | 34.43 | 2.80 × 104 |
| NoV GII | 31.52 | 9.82 × 102 | 31.63 | 1.06 × 104 |
| HAV | 33.76 | 7.30 × 101 | 33.07 | 9.18 × 102 |
| Plasmid | In Vitro Transcribed RNA | |||||
|---|---|---|---|---|---|---|
| Tissue | Amount (in Genome Copies/µL) | Cq Mean | Recovery Rates (%) | Amount (in Genome Copies/µL) | Cq Mean | Recovery Rates (%) |
| liver | 1.12 × 106 | 29.23 | 11.18 | 1.29 × 106 | 28.92 | 12.90 |
| 1.58 × 105 | 32.86 | 15.78 | 9.66 × 105 | 32.91 | 9.66 | |
| 2.95 × 104 | 35.53 | 29.46 | 1.56 × 104 | 35.91 | 15.63 | |
| gills | 2.10 × 106 | 27.58 | 21.00 | 2.74 × 106 | 27.12 | 27.37 |
| 1.34 × 105 | 29.51 | 13.37 | 1.42 × 105 | 29.67 | 14.21 | |
| 3.22 × 104 | 32.22 | 32.20 | 2.24 × 104 | 32.23 | 22.40 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Filipa-Silva, A.; Nunes, M.; Parreira, R.; Barreto Crespo, M.T. Development of a Tetraplex qPCR for the Molecular Identification and Quantification of Human Enteric Viruses, NoV and HAV, in Fish Samples. Microorganisms 2021, 9, 1149. https://doi.org/10.3390/microorganisms9061149
Filipa-Silva A, Nunes M, Parreira R, Barreto Crespo MT. Development of a Tetraplex qPCR for the Molecular Identification and Quantification of Human Enteric Viruses, NoV and HAV, in Fish Samples. Microorganisms. 2021; 9(6):1149. https://doi.org/10.3390/microorganisms9061149
Chicago/Turabian StyleFilipa-Silva, Andreia, Mónica Nunes, Ricardo Parreira, and Maria Teresa Barreto Crespo. 2021. "Development of a Tetraplex qPCR for the Molecular Identification and Quantification of Human Enteric Viruses, NoV and HAV, in Fish Samples" Microorganisms 9, no. 6: 1149. https://doi.org/10.3390/microorganisms9061149
APA StyleFilipa-Silva, A., Nunes, M., Parreira, R., & Barreto Crespo, M. T. (2021). Development of a Tetraplex qPCR for the Molecular Identification and Quantification of Human Enteric Viruses, NoV and HAV, in Fish Samples. Microorganisms, 9(6), 1149. https://doi.org/10.3390/microorganisms9061149








