Virus-Associated Biomarkers in Oropharyngeal and Nasopharyngeal Cancers and Recurrent Respiratory Papillomatosis
Abstract
1. The First Human Virus-Associated Cancer: Epstein–Barr Virus and Nasopharyngeal Cancer
1.1. Plasma Cell-Free Epstein–Barr Virus DNA in Nasopharyngeal Cancer Patients
1.2. Other EBV-Associated Malignant Tumor
2. Human Papillomavirus as a Causative Agent Not Only for Cervical Cancer, but also for Oropharyngeal Cancer
3. Plasma Cell-Free HPV DNA in Oropharyngeal Cancer Patients
3.1. Oropharyngeal Cancer
3.2. Cervical Cancer
4. Pre-Treatment HPV DNA in Oral Rinses of Oropharyngeal Cancer Patients
5. Post-Treatment HPV DNA in Oral Rinse of Oropharyngeal Cancer Patients
6. Serum HPV Antibodies in HPV-Associated Oropharyngeal Cancer Patients
7. HPV DNA in Oral Rinses of Recurrent Respiratory Papillomatosis Patients
8. Future Perspective
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Murono, S.; Yoshizaki, T.; Tanaka, S.; Takeshita, H.; Park, C.S.; Furukawa, M. Detection of Epstein-Barr virus in nasopharyngeal carcinoma by in situ hybridization and polymerase chain reaction. Laryngoscope 1997, 197, 523–526. [Google Scholar] [CrossRef]
- Kondo, S.; Horikawa, T.; Takeshita, H.; Kanegane, C.; Kasahara, Y.; Sheen, T.; Sato, H.; Furukawa, M.; Yoshizaki, T. Diagnostic value of serum EBV-DNA quantification and antibody to viral capsid antigen in nasopharyngeal carcinoma patients. Cancer Sci. 2004, 85, 508–513. [Google Scholar] [CrossRef]
- Lo, Y.M.; Chan, L.Y.; Lo, K.W.; Leung, S.F.; Zhang, J.; Chan, A.T.; Lee, J.C.; Hjelm, N.M.; Johnson, P.J.; Huang, D.P. Quantitative analysis of cell-free Epstein-Barr virus DNA in plasma of patients with nasopharyngeal carcinoma. Cancer Res. 1999, 59, 1188–1191. [Google Scholar]
- Liu, Y.; Fang, Z.; Liu, L.; Yang, S.; Zhang, L. Detection of Epstein-Barr virus DNA in serum or plasma for nasopharyngeal cancer: A meta-analysis. Genet. Test Mol. Biomark. 2011, 15, 495–502. [Google Scholar] [CrossRef]
- Zhang, W.; Chen, T.; Chen, L.; Guo, R.; Zhou, G.; Tang, L.; Mao, Y.; Li, W.; Liu, X.; Du, X.; et al. The clinical utility of plasma Epstein-Barr virus DNA assays in nasopharyngeal carcinoma: The dawn of a new era? A systematic review and meta-analysis of 7836 cases. Medicine 2015, 94, e845. [Google Scholar] [CrossRef]
- Dürst, M.; Gissmann, L.; Ikenberg, H.; zur Hausen, H. A papillomavirus DNA from a cervical carcinoma and its prevalence in cancer biopsy samples from different geographic regions. Proc. Natl. Acad. Sci. USA 1983, 80, 3812–3815. [Google Scholar] [CrossRef]
- Wang, W.Y.; Twu, C.W.; Chen, H.H.; Jiang, R.S.; Wu, C.T.; Liang, K.L.; Shih, Y.T.; Chen, C.C.; Lin, P.J.; Liu, Y.C.; et al. Long-term survival analysis of nasopharyngeal carcinoma by plasma Epstein-Barr virus DNA levels. Cancer 2013, 119, 963–970. [Google Scholar] [CrossRef]
- Lei, K.I.; Chan, L.Y.; Chan, W.Y.; Johnson, P.J.; Lo, Y.M. Diagnostic and prognostic implications of circulating cell-free Epstein-Barr virus DNA in natural killer/T-cell lymphoma. Clin. Cancer Res. 2002, 8, 29–34. [Google Scholar]
- Suzuki, R.; Yamaguchi, M.; Izutsu, K.; Yamamoto, G.; Takada, K.; Harabuchi, Y.; Isobe, Y.; Gomyo, H.; Koike, T.; NK-cell Tumor Study Group; et al. Prospective measurement of Epstein-Barr virus-DNA in plasma and peripheral blood mononuclear cells of extranodal NK/T-cell lymphoma, nasal type. Blood 2011, 118, 6018–6022. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.Y.; Liu, Q.F.; Wang, H.; Jin, J.; Wang, W.H.; Wang, S.L.; Song, Y.W.; Liu, Y.P.; Fang, H.; Ren, H.; et al. Clinical implications of plasma Epstein-Barr virus DNA in early-stage extranodal nasal-type NK/T-cell lymphoma patients receiving primary radiotherapy. Blood 2012, 120, 2003–2010. [Google Scholar] [CrossRef]
- Chow, L.Q.M. Head and neck cancer. N. Engl. J. Med. 2020, 382, 60–72. [Google Scholar] [CrossRef]
- Ang, K.K.; Harris, J.; Wheeler, R.; Weber, R.; Rosenthal, D.I.; Nguyen-Tân, P.F.; Westra, W.H.; Chung, C.H.; Jordan, R.C.; Lu, C.; et al. Human papillomavirus and survival of patients with oropharyngeal cancer. N. Engl. J. Med. 2010, 363, 24–35. [Google Scholar] [CrossRef]
- Global Burden of Disease Cancer Collaboration. Global, regional, and national cancer incidence, mortality, years of life lost, years lived with disability, and disability-adjusted life-years for 32 cancer groups, 1990 to 2015: A systematic analysis for the global burden of disease study. JAMA Oncol. 2017, 3, 524–548. [Google Scholar] [CrossRef] [PubMed]
- Chaturvedi, A.K.; Engels, E.A.; Pferffer, R.M.; Hernandez, B.Y.; Xiao, W.; Kim, E.; Jiang, B.; Goodman, M.T.; Sibug-Saber, M.; Cozen, W.; et al. Human Papillomavirus and Rising Oropharyngeal Cancer Incidence in the United States. J. Clin. Oncol. 2011, 29, 4294–4301. [Google Scholar] [CrossRef]
- Canfell, K. Towards the global elimination of cervical cancer. Papillomavirus Res. 2019, 8, 100170. [Google Scholar] [CrossRef]
- Lei, J.; Ploner, A.; Elfström, K.M.; Wang, J.; Roth, A.; Fang, F.; Sundström, K.; Dillner, J.; Sparén, P. HPV Vaccination and the Risk of Invasive Cervical Cancer. N. Engl. J. Med. 2020, 383, 1340–1348. [Google Scholar] [CrossRef]
- Bhatla, N.; Singhal, S. Primary HPV screening for cervical cancer. Best Pract. Res. Clin. Obstet. Gynaecol. 2020, 65, 98–108. [Google Scholar] [CrossRef]
- Capone, R.B.; Pai, S.I.; Koch, W.M.; Gillison, M.L.; Danish, H.N.; Westra, W.H.; Daniel, R.; Shah, K.V.; Sidransky, D. Detection and quantitation of human papillomavirus (HPV) DNA in the sera of patients with HPV-associated head and neck squamous cell carcinoma. Clin. Cancer Res. 2000, 6, 4171–4175. [Google Scholar]
- Jensen, K.K.; Grønhøj, C.; Jensen, D.H.; von Bucheald, C. Circulating human papillomavirus DNA as a surveillance tool in head and neck squamous cell carcinoma: A systematic review and meta-analysis. Clin. Otolaryngol. 2018, 43, 1242–1249. [Google Scholar] [CrossRef]
- Cao, H.; Bahn, A.; Kwok, S.; Shi, X.; Wu, S.; Krakow, T.; Khong, B.; Bavan, B.; Bala, R.; Pinsky, B.A.; et al. Quantitation of human papillomavirus DNA in plasma of oropharyngeal carcinoma patients. Int. J. Radiat. Oncol. Biol. Phys. 2012, 82, e351–e358. [Google Scholar] [CrossRef]
- Ahn, S.M.; Chan, J.Y.; Zhang, Z.; Wang, H.; Khan, Z.; Bishop, J.A.; Westra, W.; Kock, W.M.; Califano, J.A. Saliva and plasma quantitative polymerase chain reaction-based detection and surveillance of human papillomavirus-related head and neck cancer. JAMA Otolaryngol. Head Neck Surg. 2014, 140, 846–854. [Google Scholar] [CrossRef]
- Dahlstrom, K.R.; Li, G.; Hussey, C.S.; Vo, J.T.; Wei, Q.; Zhao, C.; Sturgis, E.M. Circulating human papillomavirus DNA as a marker for disease extent and recurrence among patients with oropharyngeal cancer. Cancer 2015, 121, 3455–3464. [Google Scholar] [CrossRef]
- Lee, J.Y.; Garcia-Murillas, I.; Cutts, R.J.; De Castro, D.G.; Grove, L.; Hurley, T.; Wang, F.; Nutting, C.; Newbold, K.; Harrington, K.; et al. Predicting response to radical (chemo)radiotherapy with circulating HPV DNA in locally advanced head and neck squamous carcinoma. Br. J. Cancer 2017, 117, 876–883. [Google Scholar] [CrossRef]
- Chera, B.S.; Kumar, S.; Beaty, B.T.; Marron, D.; Jefferys, S.; Green, R.; Goldman, E.C.; Amdur, R.; Sheets, N.; Dagan, R.; et al. Rapid clearance profile of plasma circulating tumor HPV type 16 DNA during chemoradiotherapy correlates with disease control in HPV-associated oropharyngeal cancer. Clin. Cancer Res. 2019, 25, 4682–4690. [Google Scholar] [CrossRef]
- Pornthanakasem, W.; Shotelersuk, K.; Termrungruanglert, W.; Voravud, N.; Niruthisard, S.; Mutirangura, A. Human papillomavirus DAA in plasma of patients with cervical cancer. BMC Cancer 2001, 1, 2. [Google Scholar] [CrossRef] [PubMed]
- Dong, S.M.; Pai, S.I.; Rha, S.H.; Hildesheim, A.; Kurman, R.J.; Schwartz, P.E.; Mortel, R.; McGowan, L.; Greenberg, M.D.; Barnes, W.A.; et al. Detection and quantification of human papillomavirus DNA in the plasma of patients with cervical carcinoma. Cancer Epidemiol. Biomark. Prev. 2002, 11, 3–6. [Google Scholar]
- Jeannot, E.; Becette, V.; Campitelli, M.; Calméjane, M.A.; Lappartient, E.; Ruff, E.; Saada, S.; Holmes, A.; Bellet, D.; Sastre-Garau, X. Circulating human papillomavirus DNA detected using droplet digital PCR in the serum of patients diagnosed with early stage human papillomavirus-associated invasive carcinoma. J. Pathol. Clin. Res. 2016, 2, 201–209. [Google Scholar] [CrossRef]
- Cheung, T.H.; Yim, S.F.; Yu, M.Y.; Worley, M.J., Jr.; Fiascone, S.J.; Chiu, R.W.K.; Lo, K.W.K.; Siu, N.S.S.; Wong, M.C.S.; Yeung, A.C.M.; et al. Liquid biopsy of HPV DNA in cervical cancer. J. Clin. Virol. 2019, 114, 32–36. [Google Scholar] [CrossRef]
- Smith, E.M.; Ritchie, J.M.; Summersgill, K.F.; Klussmann, J.P.; Lee, J.H.; Wang, D.; Haugen, T.H.; Turek, L.P. Age, sexual behavior and human papillomavirus infection in oral cavity and oropharyngeal cancers. Int. J. Cancer 2004, 108, 766–772. [Google Scholar] [CrossRef] [PubMed]
- D’Souza, G.; Kreimer, A.R.; Viscidi, R.; Pawlita, M.; Fakhry, C.; Koch, W.M.; Westra, W.H.; Gillison, M.L. Case-control study of human papillomavirus and oropharyngeal cancer. N. Engl. J. Med. 2007, 356, 1944–1956. [Google Scholar] [CrossRef]
- Gillison, M.L.; D’Souza, G.; Westra, W.; Sugar, E.; Xiao, W.; Begum, S.; Viscidi, R. Distinct risk factor profiles for human papillomavirus type 16-positive and human papillomavirus type 16-negative head and neck cancers. J. Natl. Cancer Inst. 2008, 100, 407–420. [Google Scholar] [CrossRef]
- Agrawal, Y.; Koch, W.M.; Xiao, W.; Westra, W.H.; Trivett, A.L.; Symer, D.E.; Gillison, M.L. Oral human papillomavirus infection before and after treatment for human papillomavirus 16-positive and human papillomavirus 16-negative head and neck squamous cell carcinoma. Clin. Cancer Res. 2008, 14, 7143–7150. [Google Scholar] [CrossRef]
- Koslabova, E.; Hamsikova, E.; Salakova, M.; Klozar, J.; Foltynova, E.; Salkova, E.; Rotnaglova, E.; Ludvikova, V.; Tachezy, R. Markers of HPV infection and survival in patients with head and neck tumors. Int. J. Cancer. 2013, 133, 1832–1839. [Google Scholar] [CrossRef]
- D’Souza, G.; Gross, N.D.; Pai, S.I.; Haddad, R.; Anderson, K.S.; Rajan, S.; Gerber, J.; Gillison, M.L.; Posner, M.R. Oral human papillomavirus (HPV) infection in HPV-positive patients with oropharyngeal cancer and their partners. J. Clin. Oncol. 2014, 32, 2408–2415. [Google Scholar] [CrossRef]
- Dang, J.; Feng, Q.; Eaton, K.D.; Jang, H.; Kiviat, N.B. Detection of HPV in oral rinse samples from OPSCC and non-OPSCC patients. BMC Oral Health. 2015, 15, 126. [Google Scholar] [CrossRef]
- Rettig, E.M.; Wentz, A.; Posner, M.R.; Gross, N.D.; Haddad, R.I.; Gillison, M.L.; Fakhry, C.; Quon, H.; Sikora, A.G.; Stott, W.J.; et al. Prognostic implication of persistent human papillomavirus type 16 DNA detection in oral rinses for human papillomavirus-related oropharyngeal carcinoma. JAMA Oncol. 2015, 1, 907–915. [Google Scholar] [CrossRef] [PubMed]
- Tsao, A.S.; Papadimitrakopoulou, V.; Lin, H.; Guo, M.; Lee, J.J.; Holsinger, F.C.; Hong, W.K.; Sturgis, E.M. Concordance of oral HPV prevalence between patients with oropharyngeal cancer and their partners. Infect. Agent Cancer. 2016, 11, 21. [Google Scholar] [CrossRef] [PubMed]
- Chai, R.C.; Lim, Y.; Frazer, I.H.; Wan, Y.; Perry, C.; Jones, L.; Lambie, D.; Punyadeera, C. A pilot study to compare the detection of HPV-16 biomarkers in salivary oral rinses with tumour p16(INK4a) expression in head and neck squamous cell carcinoma patients. BMC Cancer. 2016, 16, 178. [Google Scholar] [CrossRef]
- Imai, T.; Sato, I.; Matsumoto, K.; Asada, Y.; Kato, K.; Sogai, S.; Watanabe, K.; Sadayasu, R.; Saijo, S.; Matsuura, K. Human papilloma virus detection in oropharyngeal cancer with gargle samples. B-ENT 2016, 12, 263–269. [Google Scholar]
- Yoshida, H.; Murono, S.; Ueno, T.; Nakanishi, Y.; Tsuji, A.; Hatano, M.; Endo, K.; Kondo, S.; Sugimoto, H.; Wakisaka, N.; et al. Usefulness of human papillomavirus detection in oral rinse as a biomarker of oropharyngeal cancer. Acta Otolaryngol. 2017, 137, 773–777. [Google Scholar] [CrossRef]
- Isaac, A.; Kostiuk, M.; Zhang, H.; Lindsay, C.; Makki, F.; O’Connell, D.A.; Harris, J.R.; Cote, D.W.; Seikaly, H.; Biron, V.L. Ultrasensitive detection of oncogenic human papillomavirus in oropharyngeal tissue swabs. J. Otolaryngol. Head Neck Surg. 2017, 46, 5. [Google Scholar] [CrossRef]
- Rosenthal, M.; Huang, B.; Katabi, N.; Migliacci, J.; Bryant, R.; Kaplan, S.; Blackwell, T.; Patel, S.; Yang, L.; Pei, Z.; et al. Detection of HPV related oropharyngeal cancer in oral rinse specimens. Oncotarget. 2017, 8, 109393–109401. [Google Scholar] [CrossRef]
- Wasserman, J.K.; Rourke, R.; Purgina, B.; Caulley, L.; Dimitroulakos, J.; Corsten, M.; Johnson-Obaseki, S. HPV DNA in saliva from patients with SCC of the head and neck is specific for p16-positive oropharyngeal tumours. J. Otolaryngol. Head Neck Surg. 2017, 46, 3. [Google Scholar] [CrossRef]
- Tang, K.D.; Baeten, K.; Kenny, L.; Frazer, I.H.; Scheper, G.; Punyadeera, C. Unlocking the potential of saliva-based test to detect HPV-16-deriven oropharyngeal cancer. Cancers 2019, 11, 473. [Google Scholar] [CrossRef] [PubMed]
- Fakhry, C.; Blackford, A.L.; Neuner, G.; Xiao, W.; Jiang, B.; Agrawal, A.; Gillison, M.L. Association of oral human papillomavirus DNA persistence with cancer progression after primary treatment for oral cavity and oropharyngeal squamous cell carcinoma. JAMA Oncol. 2019, 5, 985–992. [Google Scholar] [CrossRef]
- Gipson, B.J.; Robbins, H.A.; Fakhry, C.; D’Souza, G. Sensitivity and specificity of oral HPV detection for HPV-positive head and neck cancer. Oral Oncol. 2018, 77, 52–56. [Google Scholar] [CrossRef]
- Murono, S. Viral DNA as a biomarker of nasopharyngeal and oropharyngeal cancers. Oto-Rhino-Laryngol. Tokyo 2019, 62, 252–260. [Google Scholar]
- Chuang, A.Y.; Chuang, T.C.; Chang, S.; Zhou, S.; Begum, S.; Westra, W.H.; Ha, P.K.; Koch, W.M.; Califano, J.A. Presence of HPV DNA in convalescent salivary rinses is an adverse prognostic marker in head and neck squamous cell carcinoma. Oral Oncol. 2008, 44, 915–919. [Google Scholar] [CrossRef]
- Mirghani, H.; Lang Kuhs, K.A.; Waterboer, T. Biomarkers for early identification of recurrences in HPV-driven oropharyngeal cancer. Oral Oncol. 2018, 82, 108–114. [Google Scholar] [CrossRef]
- Dahlstrom, K.R.; Anderson, K.S.; Cheng, J.N.; Chowell, D.; Li, G.; Posner, M.; Sturgis, E.M. HPV serum antibodies as predictors of survival and disease progression in patients with HPV-positive squamous cell carcinoma of the oropharynx. Clin. Cancer Res. 2015, 21, 2861–2869. [Google Scholar] [CrossRef]
- Fakhry, C.; Qualliotine, J.R.; Zhang, Z.; Agrawal, N.; Gaykalova, D.A.; Bishop, J.A.; Subramaniam, R.M.; Koch, W.M.; Chung, C.H.; Eisele, D.W.; et al. Serum antibodies to HPV16 early proteins warrant investigation as potential biomarkers for risk stratification and recurrence of HPV-associated oropharyngeal cancer. Cancer Prev. Res. 2016, 9, 135–141. [Google Scholar] [CrossRef]
- Lang Kuhs, K.A.; Kreimer, A.R.; Trivedi, S.; Holzinger, D.; Pawlita, M.; Pfeiffer, R.M.; Gibson, S.P.; Schmitt, N.C.; Hildesheim, A.; Waterboer, T.; et al. Human papillomavirus 16 E6 antibodies are sensitive for human papillomavirus-driven oropharyngeal cancer and are associated with recurrence. Cancer 2017, 123, 4382–4390. [Google Scholar] [CrossRef]
- Huang, C.G.; Lee, L.A.; Liao, C.T.; Yen, T.C.; Yang, S.L.; Liu, Y.C.; Li, J.C.; Gong, Y.N.; Kang, C.J.; Huang, S.F.; et al. Molecular and serologic markers of HPV 16 infection are associated with local recurrence in patients with oral cavity squamous cell carcinoma. Oncotarget 2017, 8, 34820–34835. [Google Scholar] [CrossRef]
- Spector, M.E.; Sacco, A.G.; Bellile, E.; Taylor, J.M.G.; Jones, T.; Sun, K.; Brown, W.C.; Birkeland, A.C.; Bradford, C.R.; Wolf, G.T.; et al. E6 and E7 antibody levels are potential biomarkers of recurrence in patients with advanced-stage human papillomavirus-positive oropharyngeal squamous cell carcinoma. Clin. Cancer Res. 2017, 23, 2723–2729. [Google Scholar] [CrossRef]
- Zhang, Y.; Waterboer, T.; Haddad, R.I.; Miles, B.A.; Wentz, A.; Gross, N.D.; Fakhry, C.; Quon, H.; Lorch, J.H.; Gourin, C.G.; et al. Human papillomavirus (HPV) 16 antibodies at diagnosis of HPV-related oropharyngeal cancer and antibody trajectories after treatment. Oral Oncol. 2017, 67, 77–82. [Google Scholar] [CrossRef]
- Mounts, P.; Shah, K.V.; Kashima, H. Viral etiology of juvenile- and adult-onset squamous papilloma of the larynx. Proc. Natl. Acad. Sci. USA 1982, 79, 5425–5429. [Google Scholar] [CrossRef] [PubMed]
- San Giorgi, M.R.; van den Heuvel, E.R.; Tjon Pian Gi, R.E.; Brunings, J.W.; Chirila, M.; Friedrich, G.; Golusinski, W.; Graupp, M.; Horcasitas Pous, R.A.; Ilmarinen, T.; et al. Age of onset of recurrent respiratory papillomatosis: A distribution analysis. Clin. Otolaryngol. 2016, 41, 448–453. [Google Scholar] [CrossRef]
- Buchinsky, F.J.; Valentino, W.L.; Ruszkay, N.; Powell, E.; Derkay, C.S.; Seedat, R.Y.; Uloza, V.; Dikkers, F.G.; Tunkel, D.E.; Choi, S.S.; et al. Age at diagnosis, but not HPV type, is strongly associated with clinical course in recurrent respiratory papillomatosis. PLoS ONE 2019, 14, e0216697. [Google Scholar] [CrossRef]
- Born, H.; Ruiz, R.; Verma, A.; Taliercio, S.; Achlatis, S.; Pitman, M.; Gandonu, S.; Bing, R.; Amin, M.R.; Branski, R.C. Concurrent oral human papilloma virus infection in patients with recurrent respiratory papillomatosis: A preliminary study. Laryngoscope 2014, 124, 2785–2790. [Google Scholar] [CrossRef]
- Hao, Y.; Ruiz, R.; Yang, L.; Neto, A.G.; Amin, M.R.; Kelly, D.; Achlatis, S.; Roof, S.; Bing, R.; Kannan, K.; et al. Mitochondrial somatic mutations and the lack of viral genomic variation in recurrent respiratory papillomatosis. Sci. Rep. 2019, 9, 16625. [Google Scholar] [CrossRef]
- Gillison, M.L.; Broutian, T.; Pickard, R.K.; Tong, Z.Y.; Xiao, W.; Kahle, L.; Graubard, B.I.; Chaturvedi, A.K. Prevalence of oral HPV infection in the United States, 2009–2010. JAMA 2012, 307, 693–703. [Google Scholar] [CrossRef]
- Steinberg, B.M.; Topp, W.C.; Schneider, P.S.; Abramson, A.L. Laryngeal papillomavirus infection during clinical remission. N. Engl. J. Med. 1983, 308, 1261–1264. [Google Scholar] [CrossRef]
- D’Souza, G.; Clemens, G.; Troy, T.; Castillo, R.G.; Struijk, L.; Waterboer, T.; Bender, N.; Pierorazio, P.M.; Best, S.R.; Strickler, H.; et al. Evaluating the utility and prevalence of HPV biomarkers in oral rinses and serology for HPV-related oropharyngeal cancer. Cancer Prev. Res. 2019, 12, 689–700. [Google Scholar] [CrossRef] [PubMed]
- Murono, S.; Yoshida, H.; Kobayashi, T.; Kawase, T.; Kikuchi, D.; Suzuki, T.; Nakanishi, Y.; Endo, K.; Kondo, S.; Wakisaka, N.; et al. Multifocal human papillomavirus detection in palatine and pharyngeal tonsils. Acta Otolaryngol. 2018, 138, 483–486. [Google Scholar] [CrossRef] [PubMed]
- Rotondo, J.C.; Oton-Gonzalez, L.; Mazziotta, C.; Lanzillotti, C.; Iaquinta, M.L.; Tognon, M.; Martini, F. Simultaneous detection and viral DNA load quantification of different human papillomavirus type in clinical specimens by the high analytical droplet digital PCR method. Front. Microbiol. 2020, 11, 591452. [Google Scholar] [CrossRef] [PubMed]
- Larsson, G.L.; Helenius, G. Digital droplet PCR (ddPCR) for the detection and quantification of HPV 16, 18, 33 and 45—A short report. Cell Oncol. 2017, 40, 521–527. [Google Scholar] [CrossRef]

| Author (Reference) | Year | Number of Cases (HPV-Positive: HPV-Negative) | Tissue HPV Status | Detection Method | Sensitivity 1 | Specificity 1 |
|---|---|---|---|---|---|---|
| Cao [20] | 2012 | HPV-positive OPC 2 40 + HPV-negative HNC 3 24 | p16 | qPCR 4 | 65% | 100% |
| Ahn [21] | 2014 | OPC 87 (75:12) + unknown primary 6 (6:0) | ISH 5 or p16 | qPCR | 67% (pre-treatment) 55% (post-treatment) | 100% (pre-treatment) 96% (post-treatment) |
| Dahlstrom [22] | 2015 | OPC 141 (114:27) | PCR | qPCR | 61% | 67% |
| Lee [23] | 2017 | (Test cohort) OPC 47 + LC 6 4 + HPC 7 4 (27:28) (Validation cohort) OPC 28 + LC 4 + HPC 1 (20:13) | p16 | Amplicon-based next generation sequencing assay | 100% 90% | 93% 100% |
| Chera [24] | 2019 | OPC 103 (44:10:49 unknown) + HV 8 103 | p16 | Digital PCR | 89% | 97% |
| Author [Reference] | Year | Sample | HPV DNA Viral Load |
|---|---|---|---|
| Cao [20] | 2012 | plasma | <500 copies/mL in 13 patients and >500 copies/mL in 13 patients among 26 patients with detectable HPV DNA |
| Dahlstrom [22] | 2015 | plasma | 0 copies/mL in 114 patients, 0.1 to <10 copies/mL in 23 patients, 10 to <100 copies/mL in 59 patients; 100 to <1000 copies/mL in 47 patients and ≥1000 copies/mL in 19 patients among all 262 OPC 1 patients, including 114 HPV-positive, 27 HPV-negative and 121 with patients missing data |
| Chera [24] | 2019 | plasma | Median 419 copies/mL, ranging from 8 to 22,579, in 84 HPV-positive OPC patients with detectable HPV16 DNA in plasma |
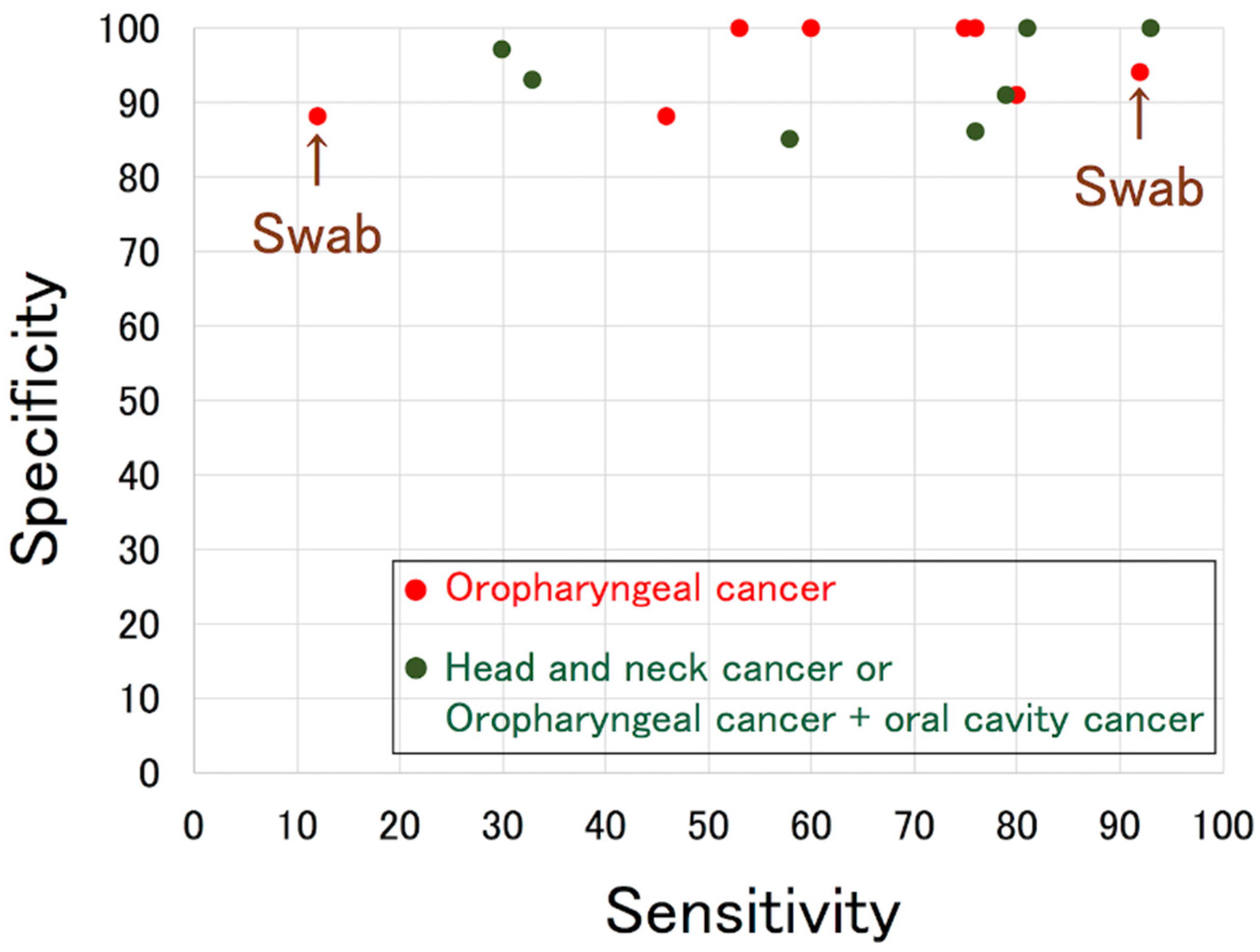
| Author [Reference] | Year | Number of Cases (HPV-Positive: HPV-Negative) | Tissue HPV Status | Detection Method | Sensitivity 2 | Specificity 2 |
|---|---|---|---|---|---|---|
| Smith [29] | 2004 | OPC 3 67 (25:42) + OCC 4 126 (13:113) | PCR and direct sequencing | PCR and direct sequencing | 58% | 85% |
| D’Souza [30] | 2007 | OPC 100 (72:28) | ISH 5 | PCR | 32% (HPV16-positive rate) | NA 6 |
| Gillison [31] * | 2008 | HNC 7 240 (92:148) including OPC 114 (82:32) | ISH | qPCR 8 | 33% | 93% |
| Agrawal [32] | 2008 | HNC 135 (44:91) including OPC 52 (41:11) | ISH | PCR + linear probe assay | 30% (HPV16) | 97% (HPV16) |
| Koslabova [33] * | 2013 | OPC 118 + OCC 24 (84:58) | PCR | PCR + line blot hybridization | 76% | 86% |
| Ahn [21] * | 2014 | OPC 87 (75:12) + UP 9 6 (6:0) | qPCR | qPCR | 53% | 100% |
| D’Souza [34] | 2014 | HPV-positive OPC 164 | ISH or p16 | PCR and qPCR | 61% (oncogenic HPV-positive rate) | NA |
| Dang [35] | 2015 | Mostly OPC 56 (48:8) | p16 | qPCR | 46% | 88% |
| Rettig [36] | 2015 | HPV-positive OPC 124 | ISH and p16 | PCR + line blot hybridization | 54% (HPV16-positive rate) | NA |
| Tsao [37] *,1 | 2016 | OPC 144 (128:16) | ISH or PCR | PCR + Easy-Chip HPV blot | 12% | 88% |
| Chai [38] * | 2016 | HNC 82 (42:40) including OPC 50 (38:12) | p16 and ISH | qPCR | 93% | 100% |
| Imai [39] | 2016 | OPC 15 (5:10) | p16 and ISH | Cobas | 60% | 100% |
| Yoshida [40] * | 2017 | OPC 19 (12:7) | p16 | Auto-nested PCR | 75% | 100% |
| Isaac [41] *,1 | 2017 | OPC 52 (36:16) | p16 | Droplet digital PCR | 92% | 94% |
| Rosenthal [42] | 2017 | OPC 45 + OCC 61 (43:63) | p16 | Cobas | 79% | 91% |
| Wasserman [43] | 2017 | OPC 24 (17:7) | p16 | Nested PCR | 76% | 100% |
| Tang [44] | 2019 | OPC 121 (89:32) | p16 | qPCR | 80% | 91% |
| Fakhry [45] | 2019 | OPC 217 (187:30) + OCC 170 (7:163) + UP 9 (8:1) | mRNA | PCR + line blot hybridization | 84% | 88% |
| Author [Reference] | Year | Sample | HPV DNA Viral Load |
|---|---|---|---|
| Agrawal [32] | 2008 | Oral rinse | Median 4.6 copies/1000 cells, ranging from 0.2 to 19, in positive samples |
| Rettig [36] | 2015 | Oral rinse | Median 161 copies/2 µL, ranging from 21 to 846 |
| Tang [44] | 2019 | Oral rinse | Mean 774.1 copies/50 ng in advanced stage of HPV-positive OPC 1 and 232.0 copies/50 ng in early stage of HPV-positive OPC |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Murono, S. Virus-Associated Biomarkers in Oropharyngeal and Nasopharyngeal Cancers and Recurrent Respiratory Papillomatosis. Microorganisms 2021, 9, 1150. https://doi.org/10.3390/microorganisms9061150
Murono S. Virus-Associated Biomarkers in Oropharyngeal and Nasopharyngeal Cancers and Recurrent Respiratory Papillomatosis. Microorganisms. 2021; 9(6):1150. https://doi.org/10.3390/microorganisms9061150
Chicago/Turabian StyleMurono, Shigeyuki. 2021. "Virus-Associated Biomarkers in Oropharyngeal and Nasopharyngeal Cancers and Recurrent Respiratory Papillomatosis" Microorganisms 9, no. 6: 1150. https://doi.org/10.3390/microorganisms9061150
APA StyleMurono, S. (2021). Virus-Associated Biomarkers in Oropharyngeal and Nasopharyngeal Cancers and Recurrent Respiratory Papillomatosis. Microorganisms, 9(6), 1150. https://doi.org/10.3390/microorganisms9061150






