Changes in the Rumen Bacteriome Structure and Enzymatic Activities of Goats in Response to Dietary Supplementation with Schizochytrium spp.
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Design and Animals’ Diets
2.2. Rumen Samples Collection and DNA Extraction
2.3. PCR Amplification of the 16S rRNA Gene
2.4. Bioinformatic Analysis
2.5. Quantitative PCR Analysis for Validation
2.6. Rumen Enzymes and Ammonia Concentration
2.7. Statistical Evaluation of Data
3. Results
3.1. Dry Matter Intake
3.2. Sequencing, Quality Filtering, and Validation
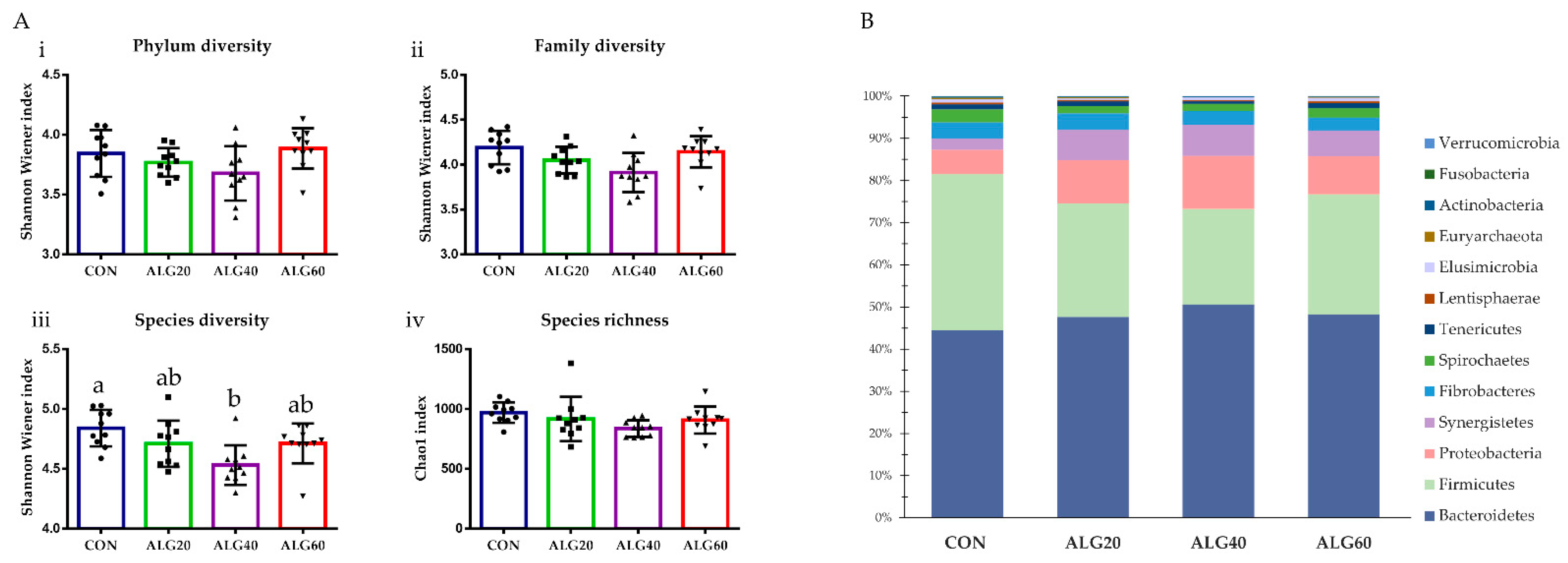
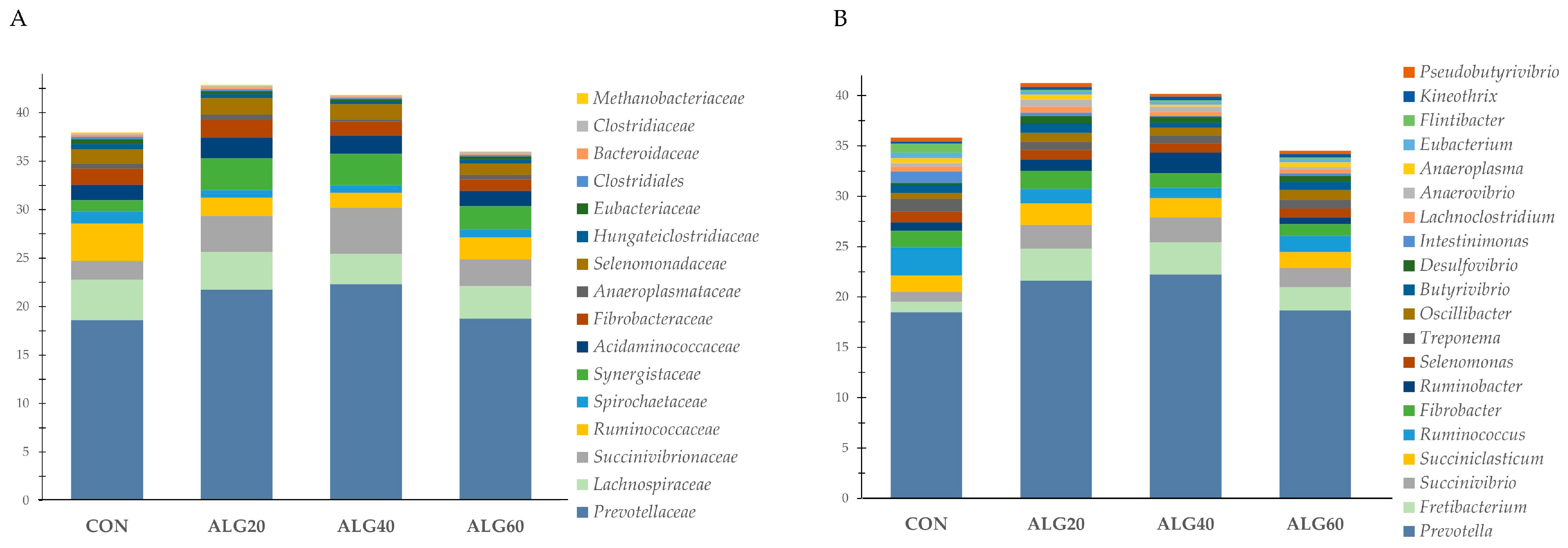
3.3. Diversity, Richness, and Composition of the Ruminal Bacteriome
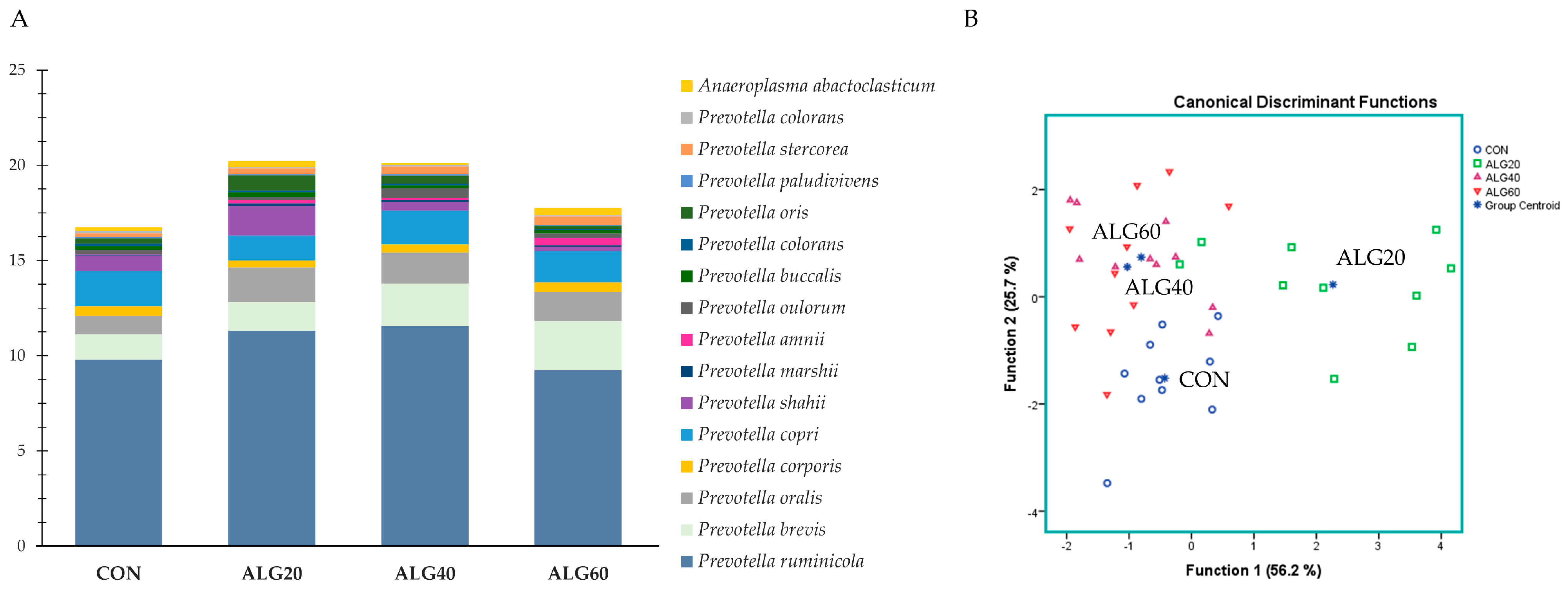
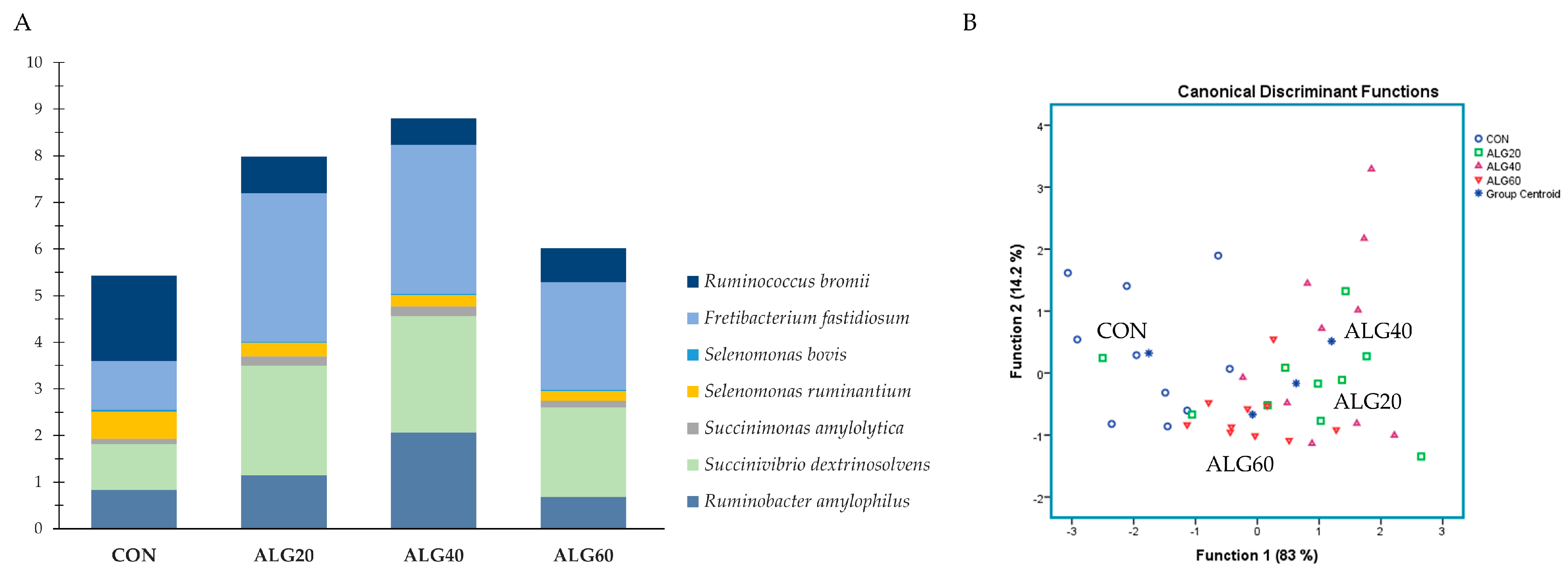
3.4. Relative Abundance of the Predominant Bacteria Species in the Goats’ Rumen
3.5. Enzyme Activities, Ammonia Concentration, and Ruminal pH
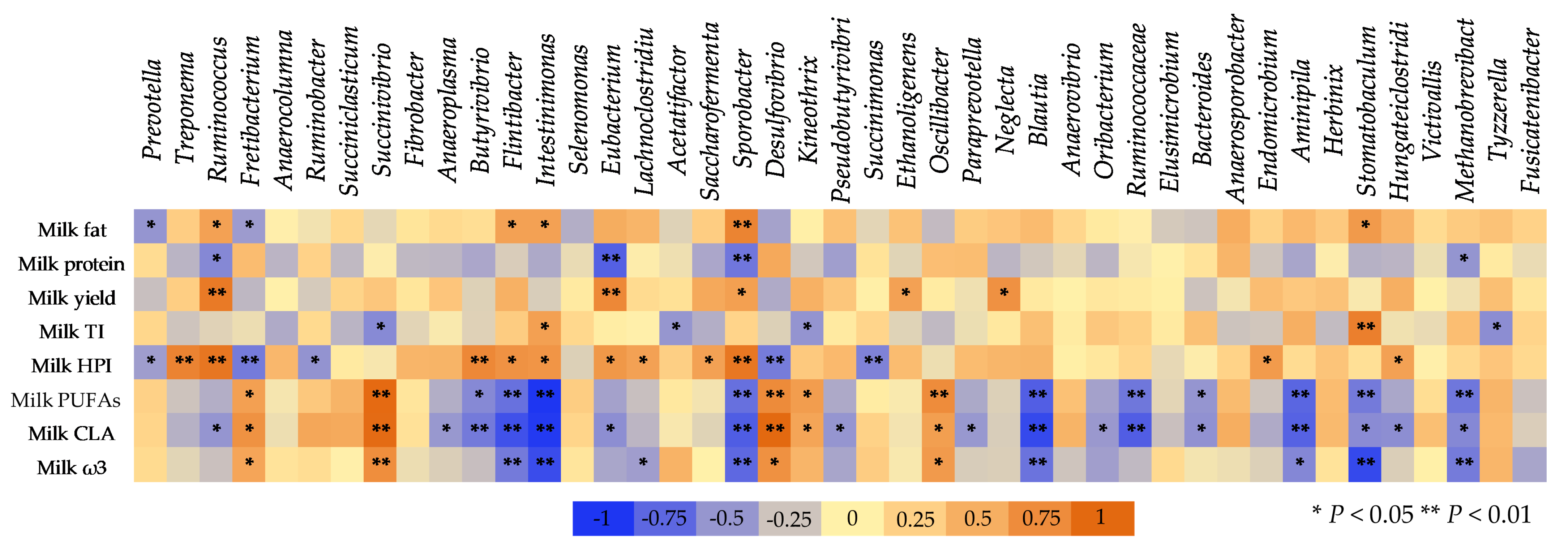
3.6. The Correlation between Ruminal Bacteria and Milk Composition
4. Discussion
4.1. Tight Linkage between the Rumen Microbiome and the Goats’ Performance
4.2. The Biohydrogenation Process and the Bacteria Species Involved Remain Unbridgeable
4.3. Microbiome Shifting Reflects Alterations in Rumen Biochemistry
4.4. Difficulties of Farm-Scale Implementation and Future Perspectives
5. Conclusions
Supplementary Materials
Author Contributions
Funding

Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Halmemies-Beauchet-Filleau, A.; Rinne, M.; Lamminen, M.; Mapato, C.; Ampapon, T.; Wanapat, M.; Vanhatalo, A. Review: Alternative and novel feeds for ruminants: Nutritive value, product quality and environmental aspects. Animal 2018, 12, s295–s309. [Google Scholar] [CrossRef] [Green Version]
- Madeira, M.S.; Cardoso, C.; Lopes, P.A.; Coelho, D.; Afonso, C.; Bandarra, N.M.; Prates, J.A. Microalgae as feed ingredients for livestock production and meat quality: A review. Livest. Sci. 2017, 205, 111–121. [Google Scholar] [CrossRef]
- Dineshbabu, G.; Goswamia, G.; Kumara, R.; Sinhala, A.; Dasa, D. Microalgae-nutritious, sustainable aqua and animal feed source. J. Funct. Foods 2019, 62, 103545. [Google Scholar] [CrossRef]
- Catalá, A. (Ed.) Chapter 3: Algal-Derived Polyunsaturated Fatty Acids: Implications for Human Health. In Polyunsaturated Fatty Acids: Sources, Antioxidant Properties and Health Benefits; Nova Sciences Publishers, Inc.: Hauppauge, NY, USA, 2013. [Google Scholar]
- Franklin, S.; Martin, K.; Baer, R.; Schingoethe, D.; Hippen, A. Dietary Marine Algae (Schizochytrium sp.) Increases Concentrations of Conjugated Linoleic, Docosahexaenoic and Transvaccenic Acids in Milk of Dairy Cows. J. Nutr. 2019, 12, 2048–2054. [Google Scholar] [CrossRef] [PubMed]
- Bichi, E.; Hervas, G.; Toral, P.G.; Loor, J.J.; Frutos, P. Milk fat depression induced by dietary marine algae in dairy ewes: Persistency of milk fatty acid composition and animal performance responses. J. Dairy Sci. 2013, 96, 524–532. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mavrommatis, A.; Tsiplakou, E. The impact of the dietary supplementation level with Schizochytrium sp., on milk chemical composition and fatty acid profile of both blood plasma and milk of goats. Small Rum. Res. 2020, 193, 106252. [Google Scholar] [CrossRef]
- Fievez, V.; Boeckaert, C.; Vlaeminck, B.; Mestdagh, J.; Demeyer, D. In vitro examination of DHA-edible micro-algae. Anim. Feed Sci. Technol. 2007, 136, 80–95. [Google Scholar] [CrossRef]
- Mavrommatis, A.; Skliros, D.; Simoni, M.; Righi, F.; Flemetakis, E.; Tsiplakou, E. Alterations in the Rumen Particle-Associated Microbiota of Goats in Response to Dietary Supplementation Levels of Schizochytrium spp. Sustainability 2021, 13, 607. [Google Scholar] [CrossRef]
- Mavrommatis, A.; Sotirakoglou, K.; Skliros, D.; Flemetakis, E.; Tsiplakou, E. Dose and time response of dietary supplementation with Schizochytrium sp. on the abundances of several microorganisms in the rumen liquid of dairy goats. Livest. Sci. 2021, 247, 104489. [Google Scholar] [CrossRef]
- Martin, C.; Morgavi, D.P.; Doreau, M. Methane mitigation in ruminants: From microbe to the farm scale. Animal 2009, 4, 351–365. [Google Scholar] [CrossRef] [Green Version]
- Maia, M.R.; Chaudhary, L.C.; Figueres, L.; Wallace, R.J. Metabolism of polyunsaturated fatty acids and their toxicity to the microflora of the rumen. Antonie Leeuwenhoek 2007, 91, 303–314. [Google Scholar] [CrossRef]
- Varijakshapanicker, P.; Mckune, S.; Miller, L.; Hendrickx, S.; Balehegn, M.; Dahl, G.E.; Adesogan, A.T. Sustainable livestock systems to improve human health, nutrition, and economic status. Anim. Front. 2019, 9, 39–50. [Google Scholar] [CrossRef] [Green Version]
- Wirth, R.; Kádár, G.; Kakuk, B.; Maróti, G.; Bagi, Z.; Szilágyi, A.; Rákhely, G.; Horváth, J.; Kovács, K.L. The planktonic core microbiome and core functions in the cattle rumen by next generation sequencing. Front. Microbiol. 2018, 9, 1–19. [Google Scholar] [CrossRef] [Green Version]
- Carreño, D.; Toral, P.G.; Pinloche, E.; Belenguer, A.; Yáñez-Ruiz, D.R.; Hervás, G.; McEwan, N.R.; Newbold, C.J.; Frutos, P. Rumen bacterial community responses to DPA, EPA and DHA in cattle and sheep: A comparative in vitro study. Sci. Rep. 2019, 9, 1–9. [Google Scholar]
- Dewanckele, L.; Vlaeminck, B.; Hernandez-Sanabria, E.; Ruiz-González, A.; Debruyne, S.; Jeyanathan, J.; Fievez, V. Rumen Biohydrogenation and Microbial Community Changes Upon Early Life Supplementation of 22:6n−3 Enriched Microalgae to Goats. Front. Microbiol. 2018, 27, 573. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, L.; Jiang, X.; Xu, H.; Zhang, Y. Metagenomics Analyses of Cellulose and Volatile Fatty Acids Metabolism by Microorganisms in the Cow Rumen. bioRxiv 2018. [Google Scholar] [CrossRef]
- Abbott, D.W.; Aasen, I.M.; Beauchemin, K.A.; Grondahl, F.; Gruninger, R.; Hayes, M.; Huws, S.; Kenny, D.A.; Krizsan, S.J.; Kirwan, S.F.; et al. Seaweed and Seaweed Bioactives for Mitigation of Enteric Methane: Challenges and Opportunities. Animals 2020, 10, 2432. [Google Scholar] [CrossRef] [PubMed]
- Newbold, C.; Ramos-Morales, E. Review: Ruminal microbiome and microbial metabolome: Effects of diet and ruminant host. Animal 2020, 14, S78–S86. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mavrommatis, A.; Chronopoulou, G.; Sotirakoglou, K.; Labrou, N.; Zervas, G.; Tsiplakou, E. The impact of the dietary supplementation level with Schizochytrium sp, on the oxidative capacity of both goats’ organism and milk. Livest. Sci. 2018, 218, 37–43. [Google Scholar] [CrossRef]
- Ramos-Morales, E.; Arco-Pérez, A.; Martín-García, A.I.; Yáñez-Ruiz, D.R.; Frutos, P.; Hervás, G. Use of stomach tubing as an alternative to rumen cannulation to study ruminal fermentation and microbiota in sheep and goats. Anim. Feed Sci. Technol. 2014, 198, 57–66. [Google Scholar] [CrossRef] [Green Version]
- Klindworth, A.; Pruesse, E.; Schweer, T.; Peplies, J.; Quast, C.; Horn, M.; Glöckner, F.O. Evaluation of general 16S ribosomal RNA gene PCR primers for classical and next-generation sequencing-based diversity studies. Nucleic Acids Res. 2012, 41, e1. [Google Scholar] [CrossRef] [PubMed]
- 16S Metagenomic Sequencing Library Preparation Protocol. (ILLUMINA). Available online: https://support.illumina.com/documents/documentation/chemistry_documentation/16s/16s-metagenomic-library-prep-guide-15044223-b.pdf (accessed on 15 January 2021).
- Zhang, J.; Kobert, K.; Flouri, T.; Stamatakis, A. PEAR: A fast and accurate Illumina Paired-End reAd mergeR. Bioinformatics 2014, 30, 614–620. [Google Scholar] [CrossRef] [Green Version]
- Martin, M. Cutadapt removes adapter sequences from high-throughput sequencing reads. EMBnet 2011, 17, 10. [Google Scholar] [CrossRef]
- Edgar, R.C.; Haas, B.J.; Clemente, J.C.; Quince, C.; Knight, R. UCHIME improves sensitivity and speed of chimera detection. Bioinformatics 2011, 27, 2194–2200. [Google Scholar] [CrossRef] [Green Version]
- Li, W.; Godzik, A. Cd-hit: A fast program for clustering and comparing large sets of protein or nucleotide sequences. Bioinformatics 2006, 12, 1658–1659. [Google Scholar] [CrossRef] [Green Version]
- R Development Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2008; ISBN 3-900051-07-0. Available online: http://www.Rproject.Org (accessed on 15 January 2021).
- Ikutakazuhiro, K.; Okadakeiji, N.; Yasuda, O. Simplified Method Using Rumen Ammonium Nitrogen as a Blood-test Reagent. JVMA 2004, 57, 435–439. [Google Scholar]
- Baintner, K. Determination of Proteolytic Activity of Rumen Liquor with Azocasein. Zent. Veterinärmed. Reihe A 2010, 28, 796–802. [Google Scholar] [CrossRef]
- Abe, C.A.L.; Faria, C.B.; De Castro, F.F.; De Souza, S.R.; Santos, F.C.d.; Da Silva, C.N.; Tessmann, D.J.; Barbosa-Tessmann, I.P. Fungi Isolated from Maize (Zea mays L.) Grains and Production of Associated Enzyme Activities. Int. J. Mol. Sci. 2015, 16, 15328–15346. [Google Scholar] [CrossRef] [PubMed]
- Kalim, B.; Ali, N.M. Optimization of fermentation media and growth conditions for microbial xylanase production. Biotechnology 2016, 6, 122. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 2012, 9, 671. [Google Scholar] [CrossRef] [PubMed]
- Zeng, H.; Guo, C.; Sun, D.; Seddik, H.-E.; Mao, S. The Ruminal Microbiome and Metabolome Alterations Associated with Diet-Induced Milk Fat Depression in Dairy Cows. Metabolites 2019, 9, 154. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Delgado, B.; Bach, A.; Guasch, I.; Gonzalez, C.; Elcoso, G.; Pryce, J.E.; Gonzalez-Recio, O. Whole rumen metagenome sequencing allows classifying and predicting feed efficiency and intake levels in cattle. Sci. Rep. 2019, 9, 11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jami, E.; White, B.A.; Mizrahi, I. Potential Role of the Bovine Rumen Microbiome in Modulating Milk Composition and Feed Efficiency. PLoS ONE 2014, 9, e85423. [Google Scholar] [CrossRef]
- Castro-Carrera, T.; Toral, P.G.; Frutos, P.; McEwan, N.R.; Hervás, G.; Abecia, L.; Belenguer, A. Rumen bacterial community evaluated by 454 pyrosequencing and terminal restriction fragment length polymorphism analyses in dairy sheep fed marine algae. J. Dairy Sci. 2014, 97, 1661–1669. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wallace, R.J.; Chaudhary, L.C.; McKain, N.; McEwan, N.R.; Richardson, A.J.; Vercoe, P.E.; Walker, N.D.; Paillard, D. Clostridium proteoclasticum: A ruminal bacterium that forms stearic acid from linoleic acid. FEMS Microbiol. Lett. 2006, 265, 195–201. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McKain, N.; Shingfield, K.J.; Wallace, R.J. Metabolism of conjugated linoleic acids and 18: 1 fatty acids by ruminal bacteria: Products and mechanisms. Microbiol. SGM 2010, 156, 579–588. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huws, S.A.; Lee, M.R.F.; Muetzel, S.M.; Scott, M.B.; Wallace, R.J.; Scollan, N.D. Forage type and fish oil cause shifts in rumen bacterial diversity. FEMS Microbiol. Ecol. 2010, 73, 396–702. [Google Scholar] [CrossRef] [Green Version]
- Toral, P.G.; Belenguer, A.; Shingfield, K.J.; Hervás, G.; Toivonen, V.; Frutos, P. Fatty acid composition and bacterial community changes in the rumen fluid of lactating sheep fed sunflower oil plus incremental levels of marine algae. J. Dairy Sci. 2012, 95, 794–806. [Google Scholar] [CrossRef] [Green Version]
- Zhu, H.; Fievez, V.; Mao, S.; He, W.; Zhu, W. Dose and time response of ruminally infused algae on rumen fermentation characteristics, biohydrogenation and Butyrivibrio group bacteria in goats. J. Anim. Sci. Biotechnol. 2016, 7, 22. [Google Scholar] [CrossRef] [Green Version]
- Huws, S.A.; Kim, E.J.; Lee, M.R.; Scott, M.B.; Tweed, J.K.; Pinloche, E.; Wallace, R.J.; Scollan, N.D. As yet uncultured bacteria phylogenetically classified as Prevotella, Lachnospiraceae incertae sedis and unclassified Bacteroidales, Clostridiales and Ruminococcaceae may play a predominant role in ruminal biohydrogenation. Environ. Microbiol. 2011, 13, 1500–1512. [Google Scholar] [CrossRef]
- Wang, X.; Martin, G.B.; Liu, S.; Shi, B.; Guo, X.; Zhao, Y.; Yan, S. The mechanism through which dietary supplementation with heated linseed grain increases n-3 long-chain polyunsaturated fatty acid concentration in subcutaneous adipose tissue of cashmere kids. J. Anim. Sci. 2019, 97, 385–397. [Google Scholar] [CrossRef]
- Toral, P.G.; Hervás, G.; Carreño, D.; Leskinen, H.; Belenguer, A.; Shingfield, K.J.; Frutos, P. In vitro response to EPA, DPA, and DHA: Comparison of effects on ruminal fermentation and biohydrogenation of 18-carbon fatty acids in cows and ewes. J. Dairy Sci. 2017, 100, 6187–6198. [Google Scholar] [CrossRef]
- Huang, G.; Zhang, Y.; Xu, Q.; Zheng, N.; Zhao, S.; Liu, K.; Qu, X.; Yu, J.; Wang, J. DHA content in milk and biohydrogenation pathway in rumen: A review. PeerJ 2020, 8, e10230. [Google Scholar] [CrossRef] [PubMed]
- Escobar, M.; Vlaeminck, B.; Jeyanathan, J.; Thanh, L.P.; Shingfield, K.J.; Wallace, R.J.; Fievez, V. Effect of adsorbants on in vitro biohydrogenation of 22:6n−3 by mixed cultures of rumen microorganisms. Animal 2016, 10, 1439–1447. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Mulder, T.; Goossens, K.; Peiren, N.; Vandaele, L.; Haegeman, A.; De Tender, C.; Ruttink, T.; Van De Wiele, T.; De Campeneere, S. Exploring the methanogen and bacterial communities of rumen environments: Solid adherent, fluid and epimural. FEMS Microbiol. Ecol. 2017, 93, 1–12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Auffret, M.D.; Dewhurst, R.J.; Duthie, C.-A.; Rooke, J.A.; Wallace, R.J.; Freeman, T.C.; Stewart, R.D.; Watson, M.; Roehe, R. The rumen microbiome as a reservoir of antimicrobial resistance and pathogenicity genes is directly affected by diet in beef cattle. Microbiome 2017, 5, 1–11. [Google Scholar] [CrossRef]
- Zhong, Y.; Xue, M.; Liu, J. Composition of Rumen Bacterial Community in Dairy Cows with Different Levels of Somatic Cell Counts. Front. Microbiol. 2018, 9, 3217. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kamke, J.; Soni, P.; Li, Y.; Ganesh, S.; Kelly, W.J.; Leahy, S.C.; Shi, W.; Froula, J.; Rubin, E.M.; Attwood, G.T. Gene and transcript abundances of bacterial type III secretion systems from the rumen microbiome are correlated with methane yield in sheep. BMC Res. Notes 2017, 8, 367. [Google Scholar] [CrossRef] [PubMed]
- Snelling, T.J.; Genç, B.; McKain, N.; Watson, M.; Waters, S.M.; Creevey, C.J.; Wallace, R.J. Diversity and community composition of methanogenic archaea in the rumen of Scottish upland sheep assessed by different methods. PLoS ONE 2014, 9, e106491. [Google Scholar] [CrossRef] [Green Version]
- Lu, Z.; Xu, Z.; Shen, Z.; Tian, Y.; Shen, H. Dietary Energy Level Promotes Rumen Microbial Protein Synthesis by Improving the Energy Productivity of the Ruminal Microbiome. Front. Microbiol. 2019, 10, 847. [Google Scholar] [CrossRef]
- Méheust, R.; Castelle, C.J.; Matheus Carnevali, P.B.; Farag, I.F.; He, C.; Chen, L.X.; Amano, Y.; Hug, L.A.; Banfield, J.F. Groundwater Elusimicrobia are metabolically diverse compared to gut microbiome Elusimicrobia and some have a novel nitrogenase paralog. ISME J. 2020, 14, 2907–2922. [Google Scholar] [CrossRef]
- Hristov, A.N.; Jouany, J.P. Factors affecting the efficiency of nitrogen utilization in the rumen. In Nitrogen and Phosphorus Nutrition of Cattle: Reducing the Environmental Impact of Cattle Operations; Pfeffer, E., Hristov, A., Eds.; CABI: Wallingford, UK, 2005; pp. 117–166. [Google Scholar]
- Hackmann, T.J.; Firkins, J.L. Maximizing efficiency of rumen microbial protein production. Front. Microbiol. 2015, 6, 465. [Google Scholar] [CrossRef] [Green Version]
- Popova, M.; McGovern, E.; McCabe, M.S.; Martin, C.; Doreau, M.; Arbre, M.; Meale, S.J.; Morgavi, D.P.; Waters, S.M. The Structural and Functional Capacity of Ruminal and Cecal Microbiota in Growing Cattle Was Unaffected by Dietary Supplementation of Linseed Oil and Nitrate. Front. Microbiol. 2017, 8, 937. [Google Scholar] [CrossRef] [Green Version]
- Difford, G.F.; Plichta, D.R.; Løvendahl, P.; Lassen, J.; Noel, S.J.; Højberg, O.; Wright, A.G.; Zhu, Z.; Kristensen, L.; Nielsen, H.B.; et al. Host genetics and the rumen microbiome jointly associate with methane emissions in dairy cows. PLoS Genet. 2018, 14, e1007580. [Google Scholar] [CrossRef] [Green Version]
- Min, B.R.; Gurung, N.; Shange, R.; Solaiman, S.; Shange, R. Potential role of rumen microbiota in altering average daily gain and feed efficiency in meat goats fed simple and mixed pastures using bacterial tag-encoded FLX amplicon pyrosequencing1. J. Anim. Sci. 2019, 97, 3523–3534. [Google Scholar] [CrossRef] [PubMed]
- Klieve, A.V.; O’Leary, M.N.; McMillen, L.; Ouwerkerk, D. Ruminococcus bromii, identification and isolation as a dominant community member in the rumen of cattle fed a barley diet. J. Appl. Microbiol. 2007, 103, 2065–2073. [Google Scholar] [CrossRef] [PubMed]
- Keweloh, H.; Heipieper, H.J. Trans unsaturated fatty acids in bacteria. Lipids 1996, 31, 129–137. [Google Scholar] [CrossRef] [PubMed]
- Maia, M.R.; Chaudhary, L.C.; Bestwick, C.S.; Richardson, A.J.; McKain, N.; Larson, T.R.; Wallace, R.J. Toxicity of unsaturated fatty acids to the biohydrogenating ruminal bacterium, Butyrivibrio fibrisolvens. BMC Microbiol. 2010, 10, 52. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yuyama, K.T.; Rohde, M.; Molinari, G.; Stadler, M.; Abraham, W.-R. Unsaturated Fatty Acids Control Biofilm Formation of Staphylococcus aureus and Other Gram-Positive Bacteria. Antibiotics 2020, 9, 788. [Google Scholar] [CrossRef]
- Koeck, D.E.; Hahnke, S.; Zverlov, V.V. Herbinix luporum sp. Nov., a thermophilic cellulose-degrading bacterium isolated from a thermophilic biogas reactor. Int. J. Syst. Evol. Microbiol. 2016, 66, 4132–4137. [Google Scholar] [CrossRef]
- Krause, D.O.; Denman, S.E.; Mackie, R.I.; Morrison, M.; Rae, A.L.; Attwood, G.T.; McSweeney, C.S. Opportunities to improve fiber degradation in the rumen: Microbiology, ecology, and genomics. FEMS Microbiol. Rev. 2003, 27, 663–693. [Google Scholar] [CrossRef] [Green Version]
- Shah, A.M.; Ma, J.; Wang, Z.; Hu, R.; Wang, X.; Peng, Q.; Amevor, F.K.; Goswami, N. Production of Hydrogen Sulfide by Fermentation in Rumen and Its Impact on Health and Production of Animals. Processes 2020, 8, 1169. [Google Scholar] [CrossRef]
- Morine, S.; Drewnoski, M.; Johnson, A.; Hansen, S. Determining the influence of dietary roughage concentration and source on ruminal parameters related to sulfur toxicity. J. Anim. Sci. 2014, 92, 4068–4076. [Google Scholar] [CrossRef]
- Mavrommatis, A.; Sotirakoglou, K.; Kamilaris, C.; Tsiplakou, E. Effects of Inclusion of Schizochytrium spp. and Forage-to Concentrate Ratios on Goats’ Milk Quality and Oxidative Status. Foods 2021, 10, 1322. [Google Scholar] [CrossRef] [PubMed]
- Xin, Y.; Shen, C.; She, Y.; Chen, H.; Wang, C.; Wei, L.; Yoon, K.; Han, D.; Hu, Q.; Xu, J. Biosynthesis of Triacylglycerol Molecules with a Tailored PUFA Profile in Industrial Microalgae. Mol. Plant 2019, 1, 474–488. [Google Scholar] [CrossRef] [PubMed]
- Patelou, M.; Infante, C.; Dardelle, F.; Randewig, D.; Kouri, E.D.; Udvardi, M.K.; Tsiplakou, E.; Mantecón, L.; Flemetakis, E. Transcriptomic and metabolomic adaptation of Nannochloropsis gaditana grown under different light regimes. Algal Res. 2020, 45, 101735. [Google Scholar] [CrossRef]




| Chemical composition of feeds (g/kg) | ||||||
| Alfalfa hay | DHAgold™ | CON | ALG20 | ALG40 | ALG60 | |
| Dry matter | 927 | 980 | 922 | 927 | 918 | 926 |
| Crude protein | 134 | 167 | 126 | 127 | 127 | 127 |
| Crude fat | 13 | 556 | 43 | 57 | 63 | 79 |
| Crude fiber | 319 | 45 | 50 | 52 | 52 | 56 |
| NDF | 503 | - | 157 | 152 | 149 | 169 |
| ADF | 381 | - | 61 | 56 | 57 | 64 |
| Ash | 72 | 88 | 92 | 95 | 93 | 89 |
| Fatty acid composition (g/100g total FA) | ||||||
| C14:0 | 2.3 | 5.9 | 0.1 | 1.4 | 2.8 | 3.7 |
| C16:0 | 31.6 | 13.1 | 14.3 | 15.2 | 17.1 | 18.7 |
| C18:0 | 5.8 | 0.3 | 3.9 | 3.2 | 2.7 | 2.5 |
| C18:1 cis−9 | 11.6 | n.d. | 36.1 | 29.8 | 24.2 | 21.8 |
| C18:2 n−6 | 26.4 | n.d. | 42.2 | 36.5 | 31.1 | 28.6 |
| C18:3 n−3 | 19.7 | 0.1 | 1.7 | 1.6 | 1.3 | 1.2 |
| C22:5 n−6 | n.d. | 6.8 | n.d. | 2.9 | 5.3 | 6.2 |
| C22:6 n−3 | n.d. | 21.8 | 0.1 | 7.3 | 13.4 | 15.5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mavrommatis, A.; Skliros, D.; Flemetakis, E.; Tsiplakou, E. Changes in the Rumen Bacteriome Structure and Enzymatic Activities of Goats in Response to Dietary Supplementation with Schizochytrium spp. Microorganisms 2021, 9, 1528. https://doi.org/10.3390/microorganisms9071528
Mavrommatis A, Skliros D, Flemetakis E, Tsiplakou E. Changes in the Rumen Bacteriome Structure and Enzymatic Activities of Goats in Response to Dietary Supplementation with Schizochytrium spp. Microorganisms. 2021; 9(7):1528. https://doi.org/10.3390/microorganisms9071528
Chicago/Turabian StyleMavrommatis, Alexandros, Dimitrios Skliros, Emmanouil Flemetakis, and Eleni Tsiplakou. 2021. "Changes in the Rumen Bacteriome Structure and Enzymatic Activities of Goats in Response to Dietary Supplementation with Schizochytrium spp." Microorganisms 9, no. 7: 1528. https://doi.org/10.3390/microorganisms9071528
APA StyleMavrommatis, A., Skliros, D., Flemetakis, E., & Tsiplakou, E. (2021). Changes in the Rumen Bacteriome Structure and Enzymatic Activities of Goats in Response to Dietary Supplementation with Schizochytrium spp. Microorganisms, 9(7), 1528. https://doi.org/10.3390/microorganisms9071528









