The Role of Lysobacter antibioticus HS124 on the Control of Fall Webworm (Hyphantria cunea Drury) and Growth Promotion of Canadian Poplar (Populus canadensis Moench) at Saemangeum Reclaimed Land in Korea
Abstract
:1. Introduction
2. Materials and Methods
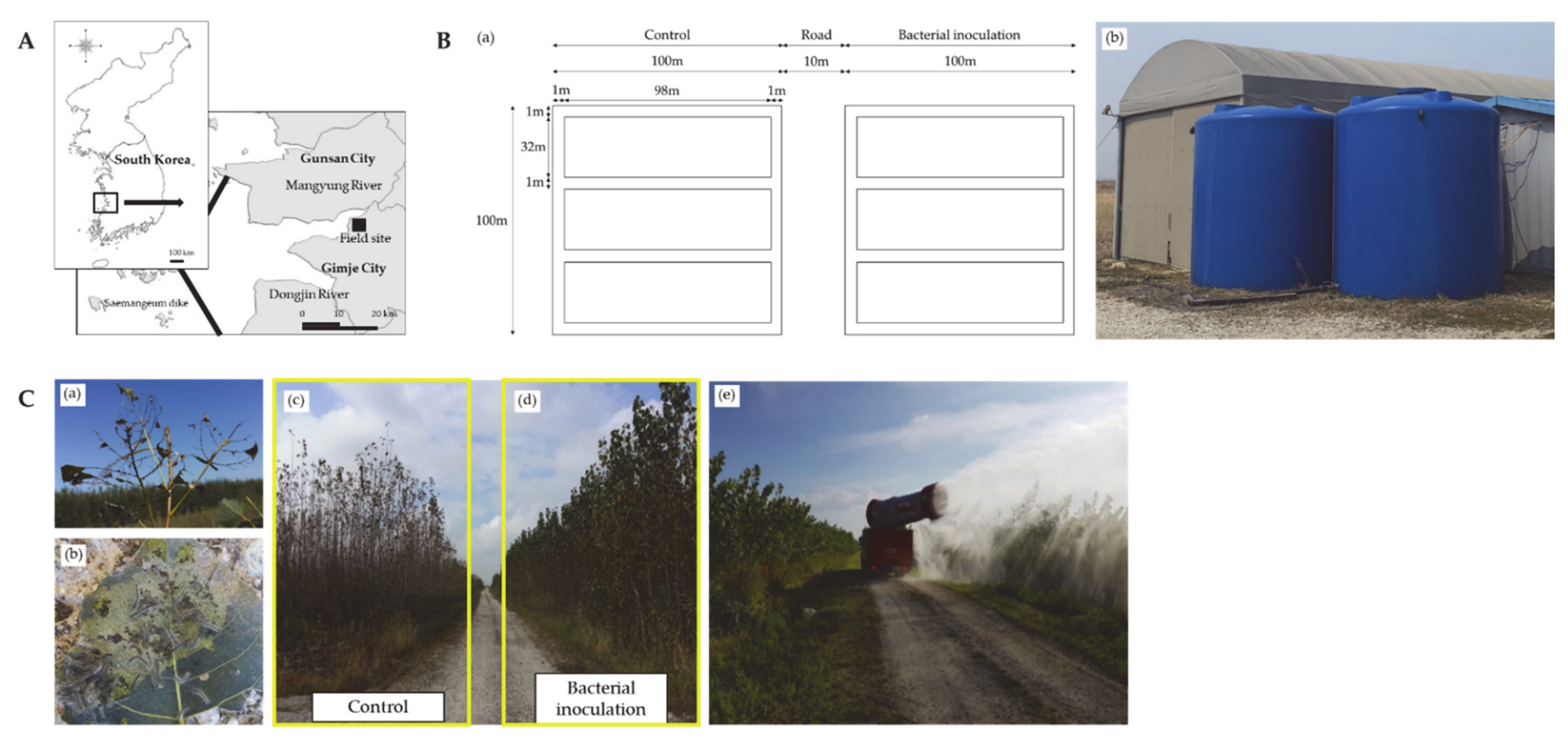
2.1. Study Area Description
2.2. Bacterial Culture and Growth Conditions
2.3. Production of Cuticle Degrading Enzymes by Lysobacter antibioticus HS124
2.4. Identification of Chitinase and Protease Genes from the Lysobacter antibioticus HS124 Genome
2.5. Preparation of Bacterial Culture and Crude Enzymes from Lysobacter antibioticus HS124
2.6. Larvicidal Activity of Lysobacter antibioticus HS124 against the Hyphantria cunea Larvae
2.7. Morphological Degradation of the Hyphantria cunea Larvae by Lysobacter antibioticus HS124
2.8. Indole-3-Acetic Acid Production by Lysobacter antibioticus HS124
2.9. Field Experimental Conditions and Plant Sampling
2.10. Statistical Analysis
3. Results
3.1. Larvicidal Activity of Lysobacter antibioticus HS124 on the Hyphantria cunea Larvae
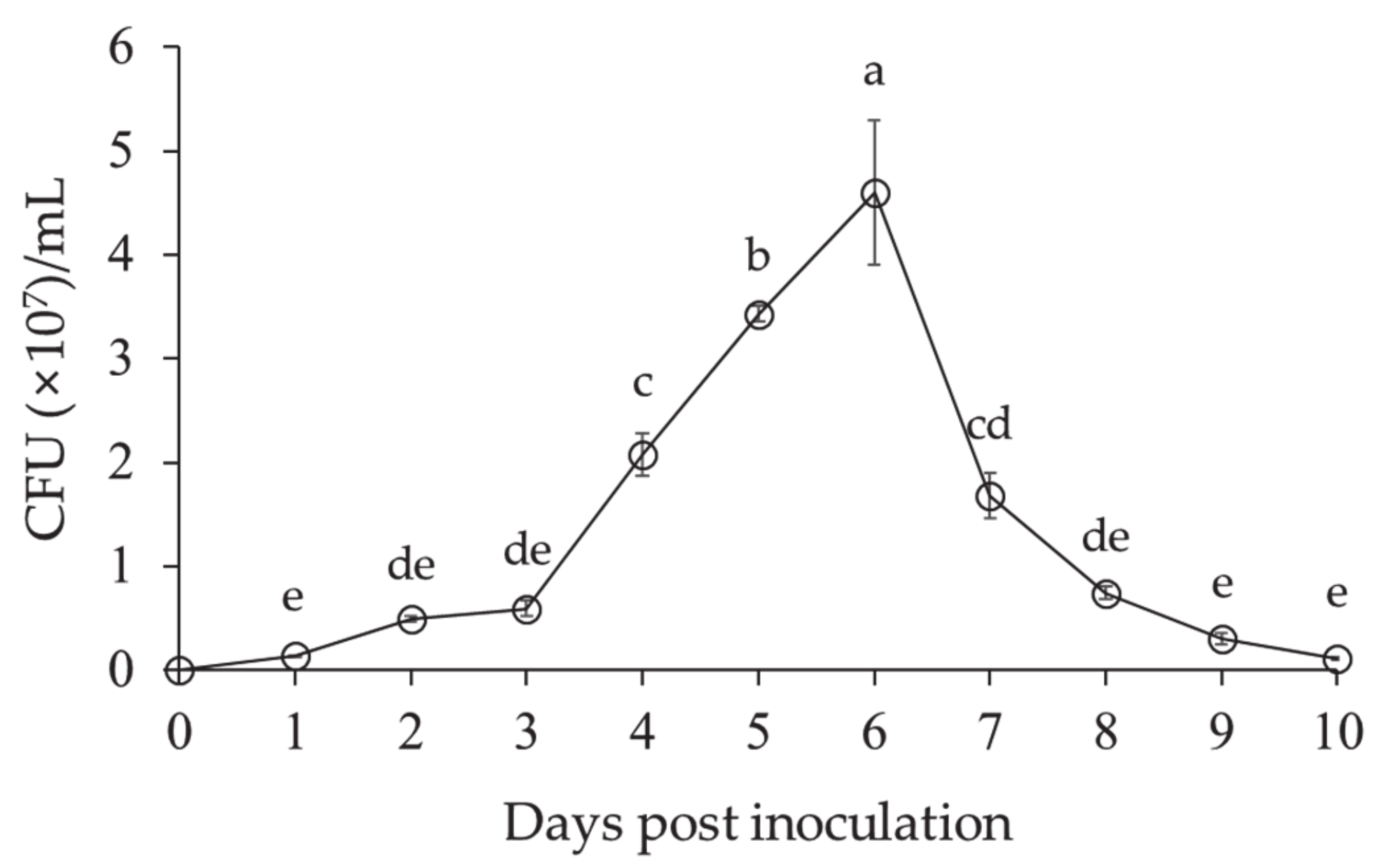
3.1.1. Growth Pattern of Lysobacter antibioticus HS124
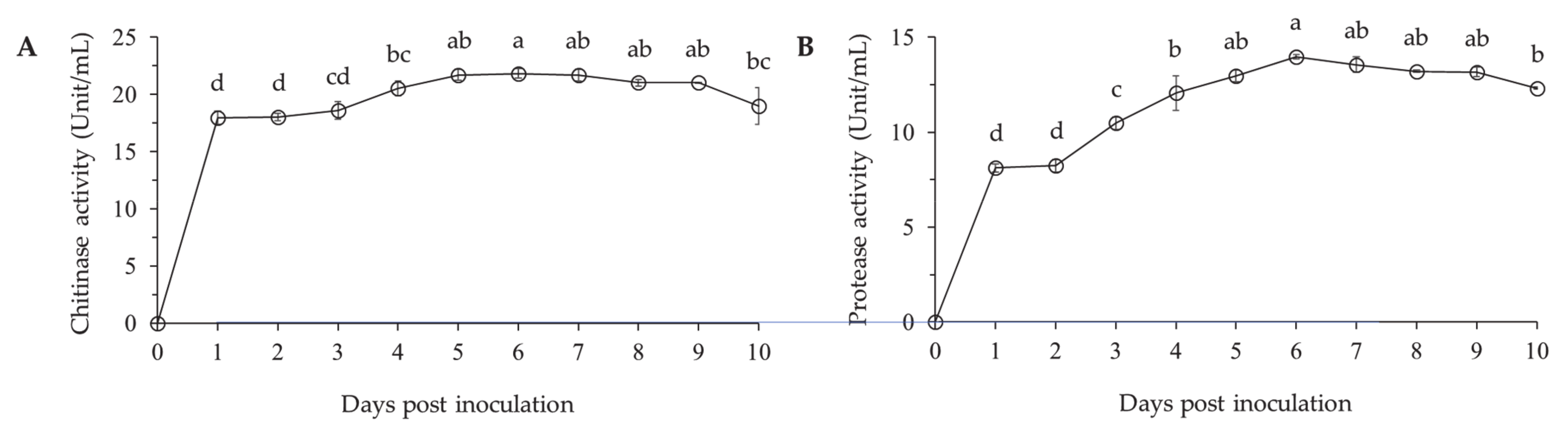
3.1.2. Cuticle Degrading Enzymes Production by Lysobacter antibioticus HS124
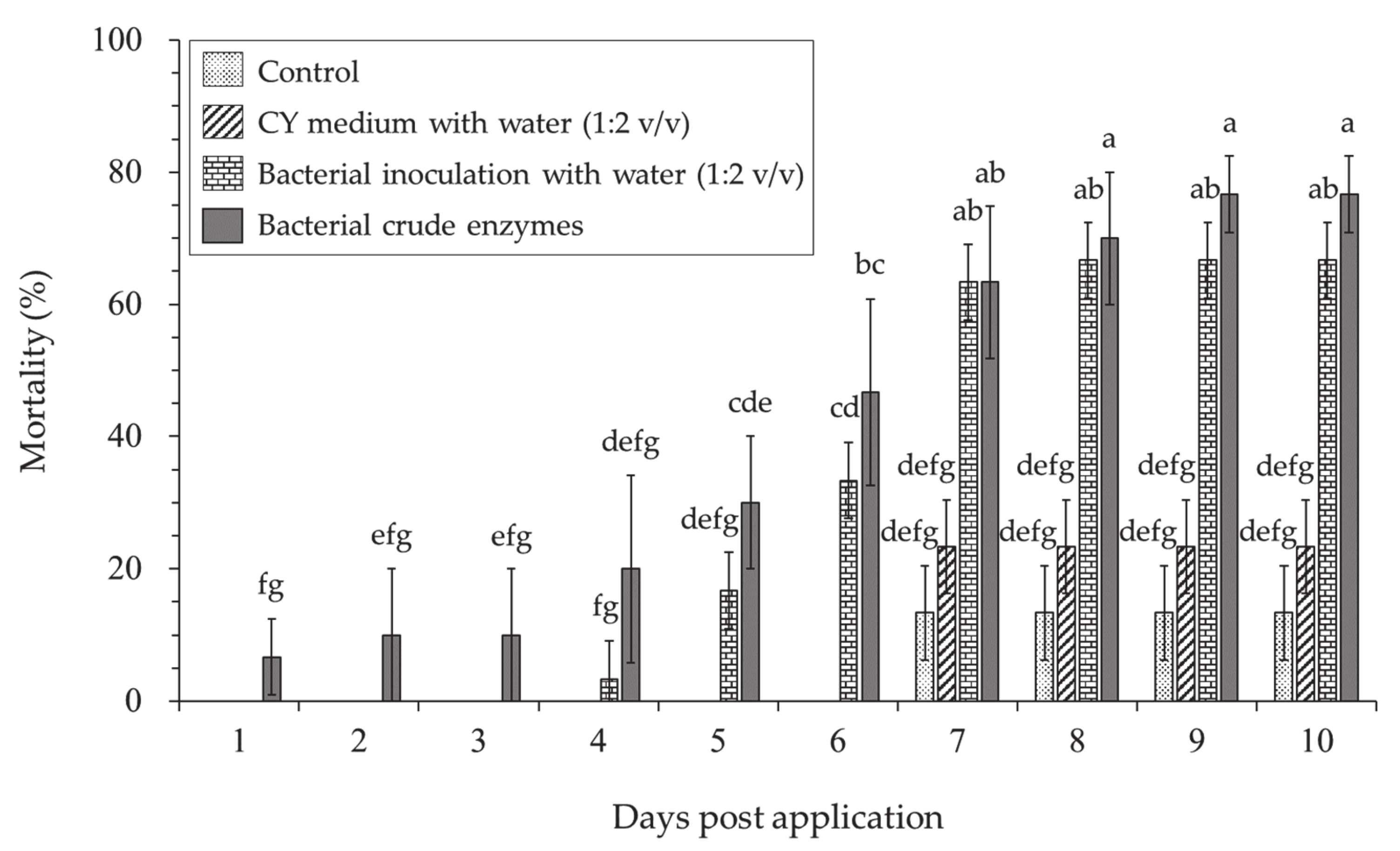
3.1.3. Hyphantria cunea Larvae Mortality of Lysobacter antibioticus HS124
3.1.4. Morphological Deformation of the Hyphantria cunea Larvae
3.2. Promotion Effects of Lysobacter antibioticus HS124 on Growth of Populus canadensis
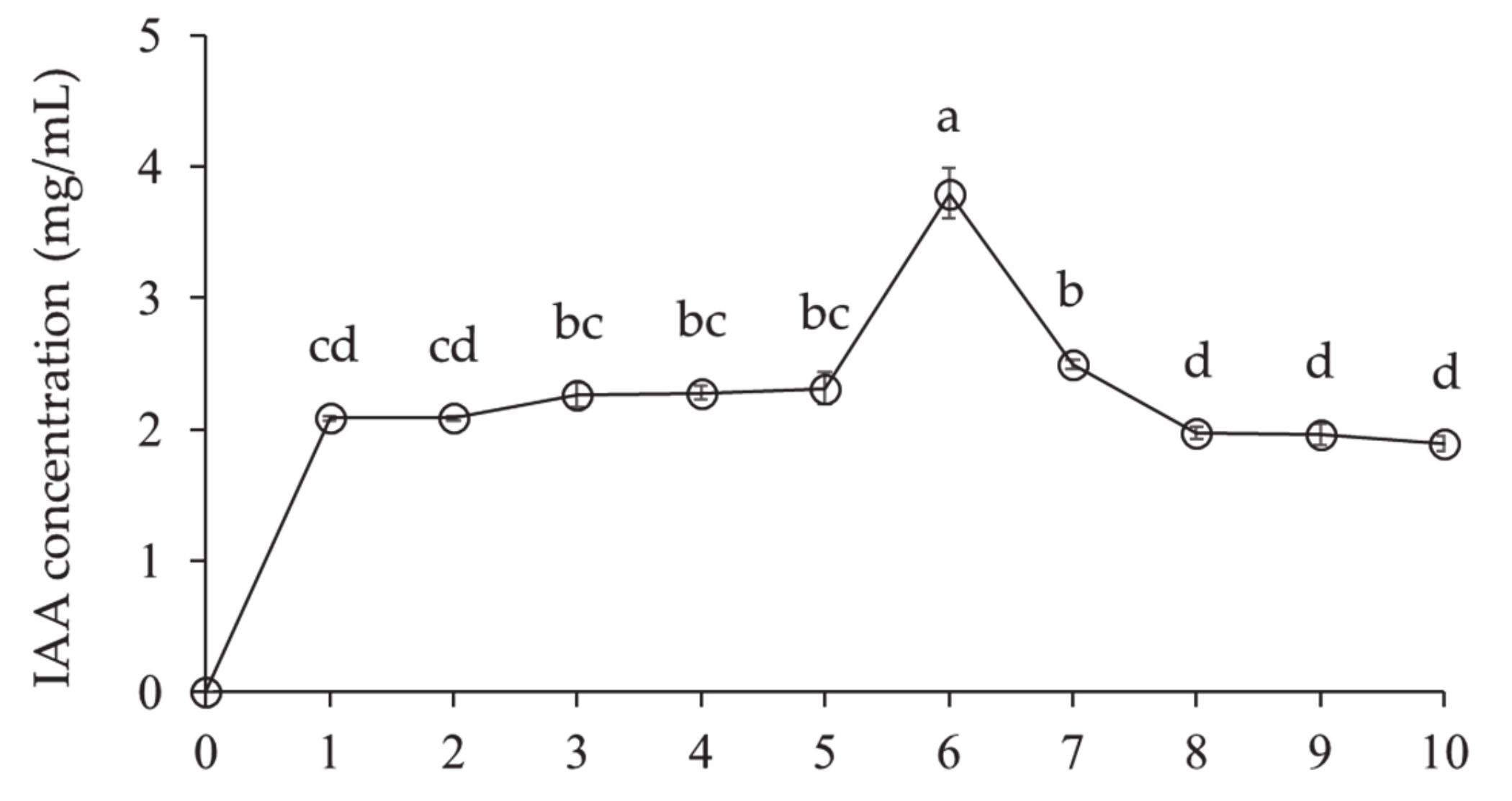
3.2.1. Indole-3-Acetic Acid Production of Lysobacter antibioticus HS124
3.2.2. Growth and Biomass Yield of Populus canadensis Trees
4. Discussion
4.1. Larvicidal Activity of Lysobacter antibioticus HS124 against the Hyphantria cunea Larvae
4.2. Promotion Effects of Lysobacter antibioticus HS124 on Growth of Populus canadensis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Karp, A.; Shield, I. Bioenergy from plants and the sustainable yield challenge. New Phytol. 2008, 179, 15–32. [Google Scholar] [CrossRef] [PubMed]
- Tian, X.; Sohngen, B.; Kim, J.B.; Ohrel, S.; Cole, J. Global climate change impacts on forests and markets. Environ. Res. Lett. 2016, 11, 035011. [Google Scholar] [CrossRef]
- Wang, D.; LeBauer, D.; Dietze, M. Predicting yields of short-rotation hybrid poplar (Populus spp.) for the United States through model–data synthesis. Ecol. Appl. 2013, 23, 944–958. [Google Scholar] [CrossRef] [Green Version]
- Mosquera-Losada, M.R.; Morán-Zuloaga, D.; Rigueiro-Rodriguez, A. Effects of lime and sewage sludge on soil, pasture production and tree growth in a six-year-old Populus canadensis Moench silvopastoral system. J. Plant. Nutr. Soil Sci. 2011, 174, 145–153. [Google Scholar] [CrossRef]
- Rubio, A.; Loetti, V.; Bellocq, I. Effect of defoliation intensity and timing on the growth of Populus alba and Salix babylonica x Salix alba. Bosque 2013, 34, 353–358. [Google Scholar] [CrossRef] [Green Version]
- Chen, S.; Hawighorst, P.; Sun, J.; Polle, A. Salt tolerance in Populus: Significance of stress signaling networks, mycorrhization and soil amendments for cellular and whole-plant nutrition. Environ. Exp. Bot. 2014, 107, 113–124. [Google Scholar] [CrossRef]
- Park, H.-G.; Lee, Y.-S.; Kim, K.-Y.; Park, Y.-S.; Park, K.-H.; Han, T.-H.; Park, C.-M.; Ahn, Y.S. Inoculation with Bacillus licheniformis MH48 promotes nutrient uptake in seedlings of the ornamental plant Camellia japonica grown in Korean reclaimed coastal lands. Korean J. Hortic. Sci. Technol. 2017, 35, 11–20. [Google Scholar] [CrossRef]
- Won, S.-J.; Kwon, J.-H.; Kim, D.-H.; Ahn, Y.-S. The effect of Bacillus licheniformis MH48 on control of foliar fungal diseases and growth promotion of Camellia oleifera seedlings in the coastal reclaimed land of Korea. Pathogens 2019, 8, 6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jang, J.; Woo, S.Y.; Kwak, M.J.; Je, S.M.; Lee, J.K.; Kim, I.R. Evaluation of bioenergy potential and relative impact of microclimate conditions for sustainable fuel pellets production and carbon sequestration of short-rotation forestry (Populus × Canadensis Moench.) in reclaimed land, South Korea: Three-year monitoring. Sustainability 2020, 12, 6244. [Google Scholar] [CrossRef]
- Cho, K.H.; Beon, M.-S.; Jeong, J.-C. Dynamics of soil salinity and vegetation in a reclaimed area in Saemangeum, Republic of Korea. Geoderma 2018, 321, 42–51. [Google Scholar] [CrossRef]
- Edosa, T.T.; Jo, Y.H.; Keshavarz, M.; Anh, Y.S.; Noh, M.Y.; Han, Y.S. Current status of the management of fall webworm, Hyphantria cunea: Towards the integrated pest management development. J. Appl. Entomol. 2019, 143, 1–10. [Google Scholar] [CrossRef] [Green Version]
- LaštůVka, Z. Climate change and its possible influence on the occurrence and importance of insect pests. Plant Prot. Sci. 2009, 45, S53–S62. [Google Scholar] [CrossRef] [Green Version]
- Forestry Statistical Yearbook. Available online: https://www.forest.go.kr/kfsweb/cop/bbs/selectBoardArticle.do;jsessionid=CdQnCFhg3Ef0jHdTFpRAK9ZQsjaKK0nSirDxUlWiBCyILXm1JV6kPsv3PTPYXG2D.frswas02_servlet_engine5?nttId=3122752&bbsId=BBSMSTR_1064&pageIndex=1&pageUnit=10&searchtitle=title&searchcont=&searchkey=&searchwriter=&searchdept=&searchWrd=&ctgryLrcls=&ctgryMdcls=&ctgrySmcls=&ntcStartDt=&ntcEndDt=&orgId=&mn=NKFS_04_05_09&component= (accessed on 5 October 2018).
- Climate change scenario. Available online: http://www.climate.go.kr/home/ (accessed on 20 October 2018).
- Gill, H.K.; Garg, H. Pesticides: Environmental impacts and management strategies. In Pesticides-Toxic Aspects; Solenski, S., Larramenday, M.L., Eds.; InTech: Rijeka, Croatia, 2014; pp. 188–230. [Google Scholar] [CrossRef] [Green Version]
- Mahmood, I.; Imadi, S.R.; Shazadi, K.; Gul, A.; Hakeem, K.R. Effects of pesticides on environment, In Plant, Soil and Microbes, Hakeem, K.R.; Akhtar, M.S., Abdullah, S.N.A., Eds.; Springer International Publishing: Cham, Switzerland, 2016; pp. 253–269. [Google Scholar] [CrossRef]
- Nicolopoulou-Stamati, P.; Maipas, S.; Kotampasi, C.; Stamatis, P.; Hens, L. Chemical pesticides and human health: The urgent need for a new concept in agriculture. Front. Public Health 2016, 4, 148. [Google Scholar] [CrossRef] [Green Version]
- Ruiu, L. Insect pathogenic bacteria in integrated pest management. Insects 2015, 6, 352–367. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, Z.-Q.; Wei, J.-R.; Wang, X.-Y. Mass rearing and augmentative releases of the native parasitoid Chouioia cunea for biological control of the introduced fall webworm Hyphantria cunea in China. Biocontrol 2006, 51, 401–418. [Google Scholar] [CrossRef]
- Kramer, K.J.; Muthukrishnan, S. Insect chitinases: Molecular biology and potential use as biopesticides. Insect Biochem. Mol. Biol. 1997, 27, 887–900. [Google Scholar] [CrossRef]
- Berini, F.; Casartelli, M.; Montali, A.; Reguzzoni, M.; Tettamanti, G.; Marinelli, F. Metagenome-sourced microbial chitinases as potential insecticide proteins. Front. Microbiol. 2019, 10, 1358. [Google Scholar] [CrossRef] [PubMed]
- Koul, O. Microbial biopesticides: Opportunities and challenges. CAB Rev. Perspect. Agric. Veterinary. Sci. Nutr. Nat. Resour. 2012, 6, 56. [Google Scholar] [CrossRef] [Green Version]
- Ferré, J.; Van Rie, J. Biochemistry and genetics of insect resistance to Bacillus thuringiensis. Annu. Rev. Entomol. 2002, 47, 501–533. [Google Scholar] [CrossRef]
- Merzendorfer, H.; Zimoch, L. Chitin metabolism in insects: Structure, function and regulation of chitin synthases and chitinases. J. Exp. Biol. 2003, 206, 4393–4412. [Google Scholar] [CrossRef] [Green Version]
- Dong, Z.; Zhang, W.; Zhang, Y.; Zhang, X.; Zhao, P.; Xia, Q. Identification and characterization of novel chitin-binding proteins from the larval cuticle of silkworm, Bombyx mori. J. Proteome Res. 2016, 15, 1435–1445. [Google Scholar] [CrossRef]
- Harrison, R.L.; Bonning, B.C. Proteases as insecticidal agents. Toxins 2010, 2, 935–953. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Berini, F.; Katz, C.; Gruzdev, N.; Casartelli, M.; Tettamanti, G.; Marinelli, F. Microbial and viral chitinases: Attractive biopesticides for integrated pest management. Biotechnol. Adv. 2018, 36, 818–838. [Google Scholar] [CrossRef] [PubMed]
- Charnley, A.K. Fungal pathogens of insects: Cuticle degrading enzymes and toxins. Adv. Bot. Res. 2003, 40, 241–321. [Google Scholar] [CrossRef]
- Ali, S.; Huang, Z.; Ren, S. Production of cuticle degrading enzymes by Isaria fumosorosea and their evaluation as a biocontrol agent against diamondback moth. J. Pestic. Sci. 2010, 83, 361–370. [Google Scholar] [CrossRef]
- Xu, Y.; Zhang, Y.; Li, Y.; Li, G.; Liu, D.; Zhao, M.; Cai, N. Growth promotion of Yunnan pine early seedlings in response to foliar application of IAA and IBA. Int. J. Mol. Sci. 2012, 13, 6507–6520. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kwon, J.-H.; Won, S.-J.; Moon, J.-H.; Kim, C.-W.; Ahn, Y.-S. Control of fungal diseases and increase in yields of a cultivated jujube fruit (Zizyphus jujuba Miller var. inermis Rehder) orchard by employing Lysobacter antibioticus HS124. Forests 2019, 10, 1146. [Google Scholar] [CrossRef] [Green Version]
- Moon, J.-H.; Won, S.-J.; Maung, C.E.H.; Choi, J.-H.; Choi, S.-I.; Ajuna, H.B.; Ahn, Y.S. Bacillus velezensis CE 100 inhibits root rot diseases (Phytophthora spp.) and promotes growth of Japanese cypress (Chamaecyparis obtusa Endlicher) seedlings. Microorganisms 2021, 9, 821. [Google Scholar] [CrossRef]
- Al-haidary, H.K.M.A.; Abd AL-hassan, S. Foliar application of IAA at different growth stages and their influenced on growth and productivity of bread Wheat (Triticum aestivum L.). J. Phys. Conf. Ser. 2019, 1294, 092029. [Google Scholar] [CrossRef]
- Kang, S.-J.; Lee, Y.-S.; Lee, S.-Y.; Yun, G.-Y.; Hong, S.-H.; Park, Y.-S.; Kim, I.-S.; Park, R.-D.; Kim, K.-Y. Biological control of diamondback moth (Plutella xylostella L.) by Lysobacter antibioticus HS124. Korean J. Soil. Sci. Fert. 2010, 43, 659–666. [Google Scholar]
- Lingappa, Y. Chitin media for selective isolation and culture of actinomycetes. Phytopathology 1962, 52, 317–323. [Google Scholar] [CrossRef]
- Ghorbel-Frikha, B.; Sellami-Kamoun, A.; Fakhfakh, N.; Haddar, A.; Manni, L.; Nasri, M. Production and purification of a calcium-dependent protease from Bacillus cereus BG1. J. Ind. Microbiol. Biotechnol. 2005, 32, 186–194. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Rahman, A.; Sitepu, I.R.; Tang, S.-Y.; Hashidoko, Y. Salkowski’s reagent test as a primary screening index for functionalities of rhizobacteria isolated from wild dipterocarp saplings growing naturally on medium-strongly acidic tropical peat soil. Biosci. Biotechnol. Biochem. 2010, 74, 2202–2208. [Google Scholar] [CrossRef] [Green Version]
- Nakahara, Y.; Watanabe, M.; Fujita, A.; Kanamori, Y.; Tanaka, D.; Iwata, K.-I.; Furuki, T.; Sakurai, M.; Kikawada, T.; Okuda, T. Effects of dehydration rate on physiological responses and survival after rehydration in larvae of the anhydrobiotic chironomid. J. Insect Physiol. 2008, 54, 1220–1225. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, K.; Sugawara, N.; Suzuki, M.; Uchiyama, T.; Katouno, F.; Nikaidou, N.; Watanabe, T. Chitinases A, B and C1 of Serratia marcescens 2170 produced by recombinant Escherichia coli: Enzymatic properties and synergism on chitin degradation. Biosci. Biotechnol. Biochem. 2002, 66, 1075–1083. [Google Scholar] [CrossRef]
- Han, Y.; Taylor, E.B.; Luthe, D. Maize Endochitinase Expression in Response to Fall Armyworm Herbivory. J. Chem. Ecol. 2021, 1–18. [Google Scholar] [CrossRef]
- Chen, W.; Qu, M.; Zhou, Y.; Yang, Q. Structural analysis of group II chitinase (ChtII) catalysis completes the puzzle of chitin hydrolysis in insects. J. Biol. Chem. 2018, 293, 2652–2660. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Anten, N.P.R.; Ackerly, D.D.A. new method of growth analysis for plants that experience periodic losses of leaf mass. Funct. Ecol. 2001, 15, 804–811. [Google Scholar] [CrossRef]
- Wan, S.; Xia, J.; Liu, W.; Niu, S. Photosynthetic overcompensation under nocturnal warming enhances grassland carbon sequestration. Ecology 2009, 90, 2700–2710. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Adamowski, M.; Friml, J. PIN-dependent auxin transport: Action, regulation and evolution. Plant Cell 2015, 27, 20–32. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zažímalová, E.; Murphy, A.S.; Yang, H.; Hoyerová, K.; Hošek, P. Auxin transporters—why so many? Cold Spring Harb. Perspect. Biol. 2010, 2, a001552. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Swarup, R.; Péret, B. AUX/LAX family of auxin influx carriers—an overview. Front. Plant Sci. 2012, 3, 225. [Google Scholar] [CrossRef] [PubMed] [Green Version]

| Treatment | Tree Growth (cm) | Tree Biomass (kg) | |||
|---|---|---|---|---|---|
| Root Collar Diameter | Height | Leaf Dry Weight | Stem Dry Weight | Total | |
| Control | 4.0 ± 0.1 * | 444.4 ± 13.0 * | 0.0 ± 0.0 * | 0.9 ± 0.1 * | 0.9 ± 0.1 * |
| Lysobacterantibioticus HS124 | 6.7 ± 0.2 * | 604.4 ± 21.9 * | 0.4 ± 0.0 * | 4.5 ± 0.3 * | 4.9 ± 0.3 * |
| p-value | 0.001 | 0.004 | <0.001 | <0.001 | <0.001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moon, J.-H.; Won, S.-J.; Maung, C.E.H.; Choi, J.-H.; Choi, S.-I.; Ajuna, H.B.; Ahn, Y.S.; Jo, Y.H. The Role of Lysobacter antibioticus HS124 on the Control of Fall Webworm (Hyphantria cunea Drury) and Growth Promotion of Canadian Poplar (Populus canadensis Moench) at Saemangeum Reclaimed Land in Korea. Microorganisms 2021, 9, 1580. https://doi.org/10.3390/microorganisms9081580
Moon J-H, Won S-J, Maung CEH, Choi J-H, Choi S-I, Ajuna HB, Ahn YS, Jo YH. The Role of Lysobacter antibioticus HS124 on the Control of Fall Webworm (Hyphantria cunea Drury) and Growth Promotion of Canadian Poplar (Populus canadensis Moench) at Saemangeum Reclaimed Land in Korea. Microorganisms. 2021; 9(8):1580. https://doi.org/10.3390/microorganisms9081580
Chicago/Turabian StyleMoon, Jae-Hyun, Sang-Jae Won, Chaw Ei Htwe Maung, Jae-Hyeok Choi, Su-In Choi, Henry B. Ajuna, Young Sang Ahn, and Yong Hun Jo. 2021. "The Role of Lysobacter antibioticus HS124 on the Control of Fall Webworm (Hyphantria cunea Drury) and Growth Promotion of Canadian Poplar (Populus canadensis Moench) at Saemangeum Reclaimed Land in Korea" Microorganisms 9, no. 8: 1580. https://doi.org/10.3390/microorganisms9081580
APA StyleMoon, J.-H., Won, S.-J., Maung, C. E. H., Choi, J.-H., Choi, S.-I., Ajuna, H. B., Ahn, Y. S., & Jo, Y. H. (2021). The Role of Lysobacter antibioticus HS124 on the Control of Fall Webworm (Hyphantria cunea Drury) and Growth Promotion of Canadian Poplar (Populus canadensis Moench) at Saemangeum Reclaimed Land in Korea. Microorganisms, 9(8), 1580. https://doi.org/10.3390/microorganisms9081580






