Genome-Wide Expression Patterns of Rhoptry Kinases during the Eimeria tenella Life-Cycle
Abstract
:1. Introduction
2. Materials and Methods
2.1. Ethics Statements
2.2. Parasite Strains and Propagation
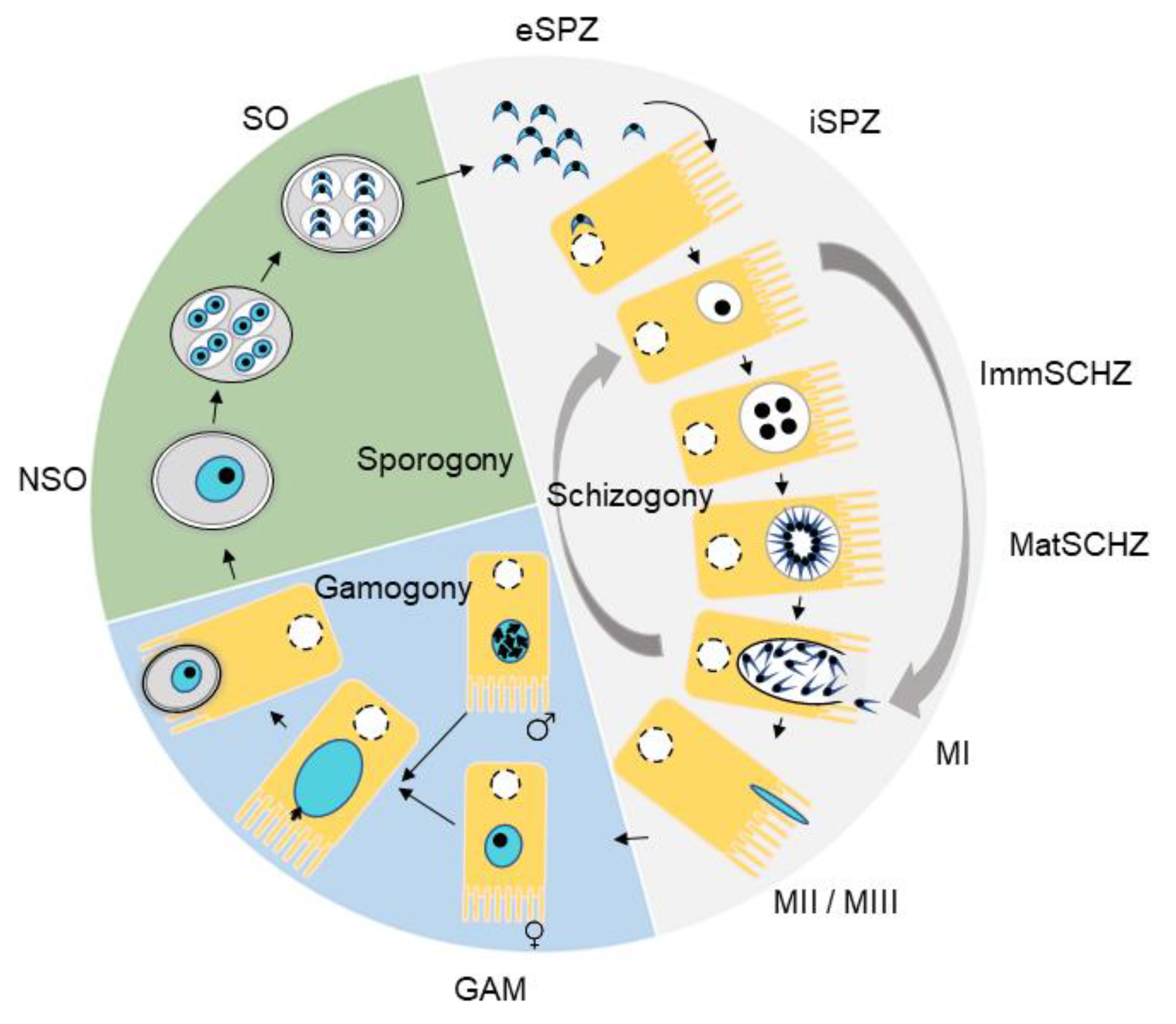
2.3. Purification of Developmental Stages
2.4. RNA Extraction
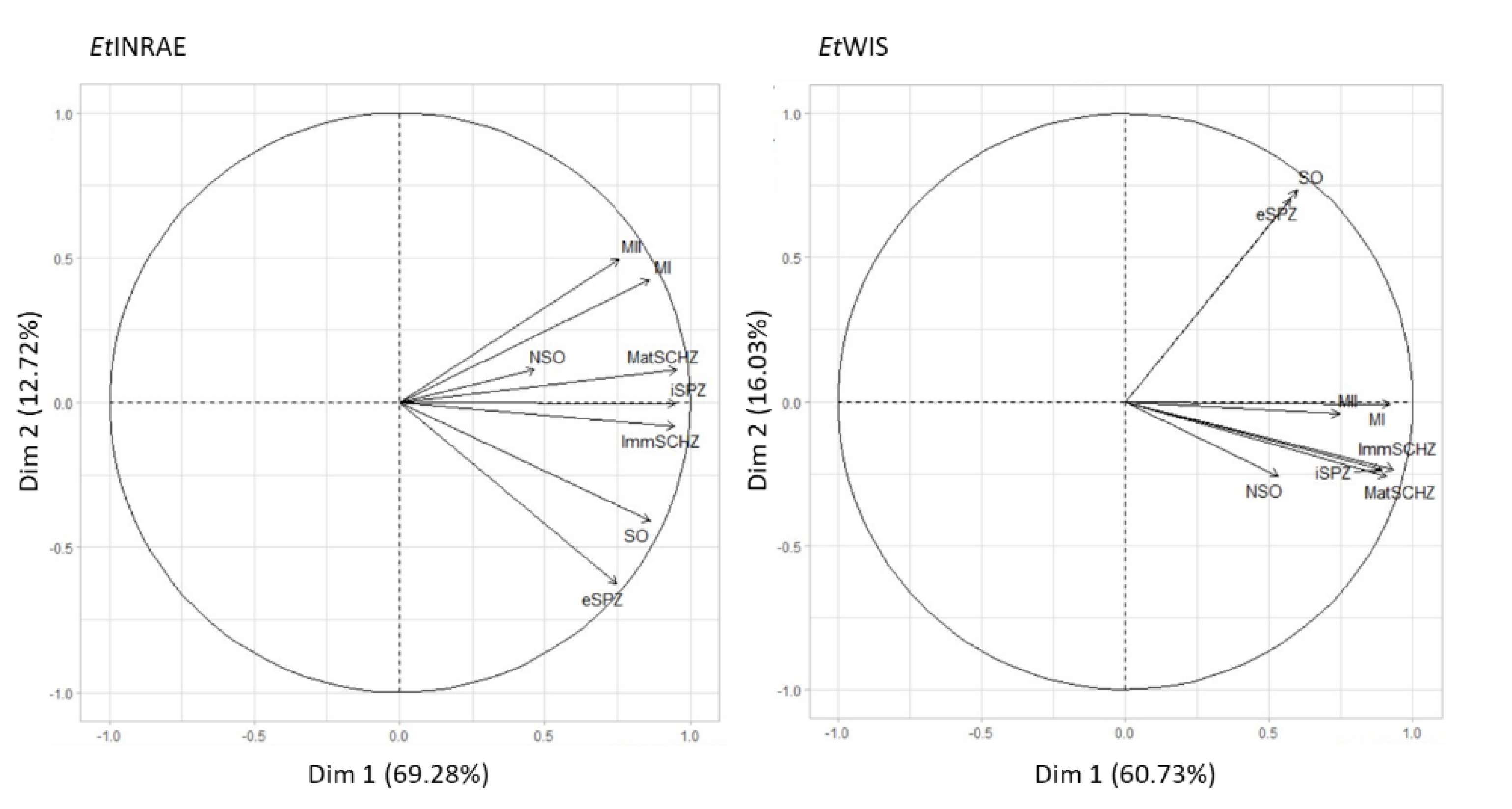
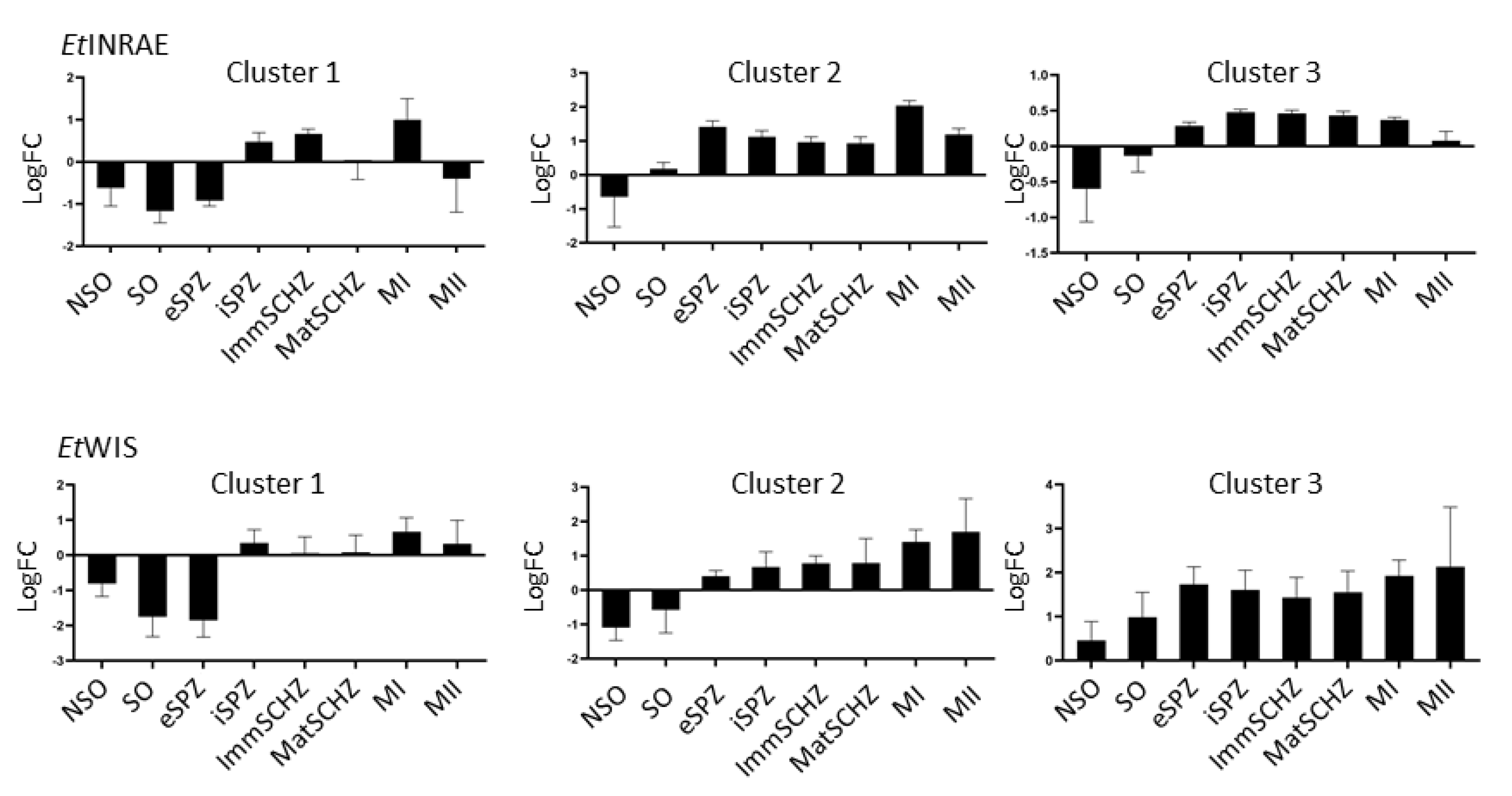
2.5. Gene Expression Profiling and Hierarchical Clustering
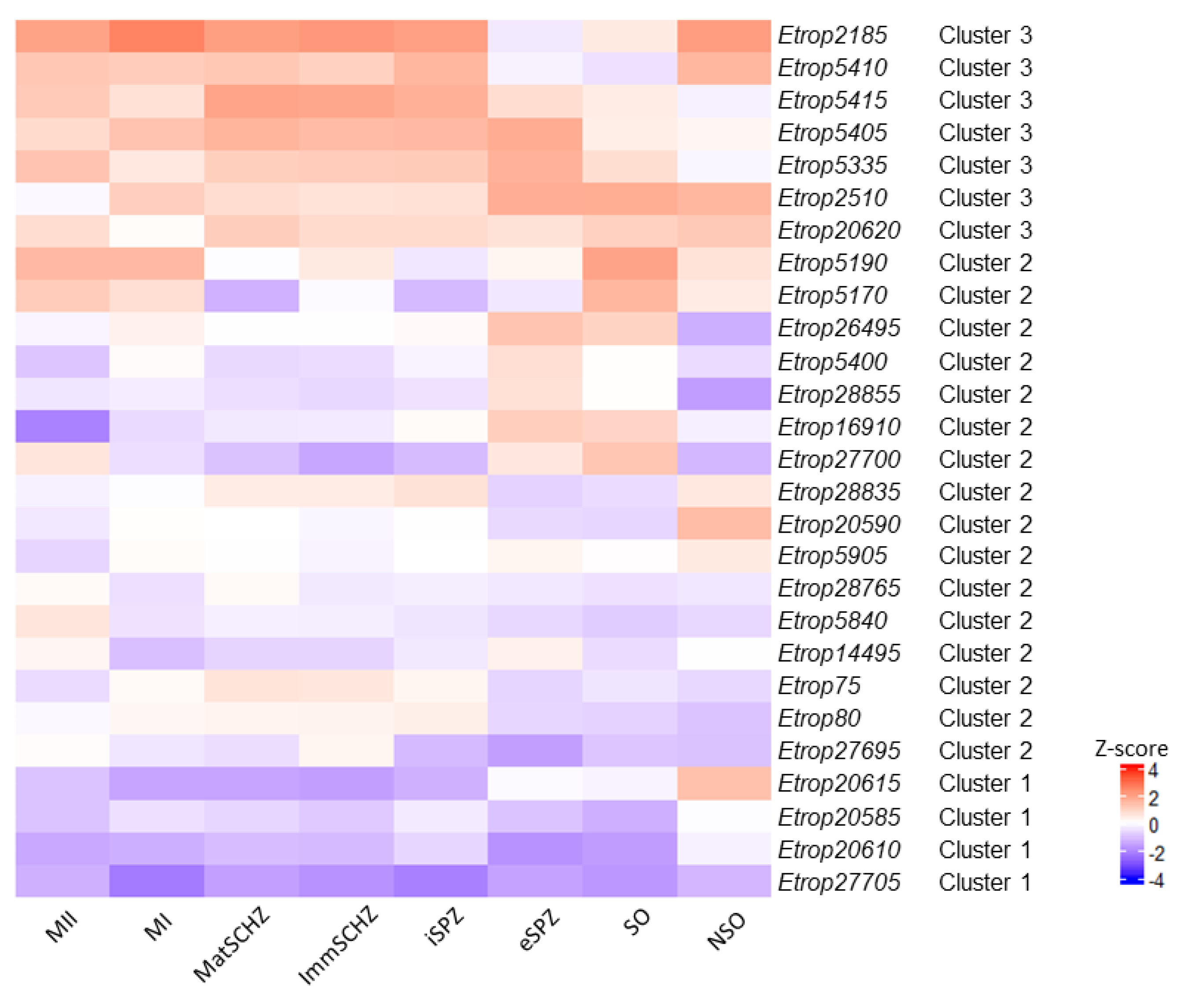
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Noack, S.; Chapman, H.D.; Selzer, P.M. Anticoccidial drugs of the livestock industry. Parasitol. Res. 2019, 118, 2009–2026. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Soutter, F.; Werling, D.; Tomley, F.M.; Blake, D.P. Poultry Coccidiosis: Design and Interpretation of Vaccine Studies. Front. Vet. Sci. 2020, 7, 7. [Google Scholar] [CrossRef] [Green Version]
- Madlala, T.; Okpeku, M.; Adeleke, M.A. Understanding the interactions between Eimeria infection and gut microbiota, towards the control of chicken coccidiosis: A review. Parasite 2021, 28, 48. [Google Scholar] [CrossRef] [PubMed]
- Doerig, C.; Billker, O.; Haystead, T.; Sharma, P.; Tobin, A.B.; Waters, N.C. Protein kinases of malaria parasites: An update. Trends Parasitol. 2008, 24, 570–577. [Google Scholar] [CrossRef] [PubMed]
- Hanks, S.K. Genomic analysis of the eukaryotic protein kinase superfamily: A perspective. Genome Biol. 2003, 4, 111. [Google Scholar] [CrossRef] [Green Version]
- Talevich, E.; Kannan, N. Structural and evolutionary adaptation of rhoptry kinases and pseudokinases, a family of coccidian virulence factors. BMC Evol. Biol. 2013, 13, 117. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peixoto, L.; Chen, F.; Harb, O.; Davis, P.H.; Beiting, D.P.; Brownback, C.S.; Ouloguem, D.; Roos, D.S. Integrative Genomic Approaches Highlight a Family of Parasite-Specific Kinases that Regulate Host Responses. Cell Host Microbe 2010, 8, 208–218. [Google Scholar] [CrossRef] [Green Version]
- Fox, B.A.; Rommereim, L.M.; Guevara, R.B.; Falla, A.; Triana, M.A.H.; Sun, Y.; Bzik, D.J. The Toxoplasma gondii Rhoptry Kinome Is Essential for Chronic Infection. mBio 2016, 7, e00193-16. [Google Scholar] [CrossRef] [Green Version]
- Ihara, F.; Nishikawa, Y. Toxoplasma gondii manipulates host cell signaling pathways via its secreted effector molecules. Parasitol. Int. 2021, 83, 102368. [Google Scholar] [CrossRef]
- Schwarz, J.A.; Fouts, A.E.; Cummings, C.A.; Ferguson, D.; Boothroyd, J.C. A novel rhoptry protein in Toxoplasma gondii bradyzoites and merozoites. Mol. Biochem. Parasitol. 2005, 144, 159–166. [Google Scholar] [CrossRef]
- Behnke, M.S.; Zhang, T.P.; Dubey, J.P.; Sibley, L.D. Toxoplasma gondii merozoite gene expression analysis with comparison to the life cycle discloses a unique expression state during enteric development. BMC Genom. 2014, 15, 350. [Google Scholar] [CrossRef] [Green Version]
- Ramakrishnan, C.; Smith, N.C. Recent achievements and doors opened for coccidian parasite research and development through transcriptomics of enteric sexual stages. Mol. Biochem. Parasitol. 2021, 243, 111373. [Google Scholar] [CrossRef] [PubMed]
- Talevich, E.; Mirza, A.; Kannan, N. Structural and evolutionary divergence of eukaryotic protein kinases in Apicomplexa. BMC Evol. Biol. 2011, 11, 321. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reid, A.; Blake, D.; Ansari, H.R.; Billington, K.; Browne, H.; Bryant, J.; Dunn, M.; Hung, S.S.; Kawahara, F.; Miranda-Saavedra, D.; et al. Genomic analysis of the causative agents of coccidiosis in domestic chickens. Genome Res. 2014, 24, 1676–1685. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oakes, R.D.; Kurian, D.; Bromley, E.; Ward, C.; Lal, K.; Blake, D.; Reid, A.; Pain, A.; Sinden, R.E.; Wastling, J.M.; et al. The rhoptry proteome of Eimeria tenella sporozoites. Int. J. Parasitol. 2013, 43, 181–188. [Google Scholar] [CrossRef] [PubMed]
- Diallo, M.A.; Sausset, A.; Gnahoui-David, A.; Silva, A.R.E.; Brionne, A.; Le Vern, Y.; Bussière, F.I.; Tottey, J.; Lacroix-Lamandé, S.; Laurent, F.; et al. Eimeria tenella ROP kinase EtROP1 induces G0/G1 cell cycle arrest and inhibits host cell apoptosis. Cell. Microbiol. 2019, 21, e13027. [Google Scholar] [CrossRef] [Green Version]
- Doran, D.J.; Vetterling, J.M.; Augustine, P.C. Eimeria-tenella—in-vivo and in-vitro comparison of wisconsin, weybridge, and beltsville strains. Proc. Helminthol. Soc. Wash. 1974, 41, 77–80. [Google Scholar]
- Shirley, M.W. Eimeria species and strains of chicken. Biotechnology-Guidelines Techniques Coccidiosis Research; Eur. Commission DGXII: Luxembourg, 1995; pp. 1–24. [Google Scholar]
- Walker, R.A.; Sharman, P.A.; Miller, C.M.; Lippuner, C.; Okoniewski, M.; Eichenberger, R.M.; Ramakrishnan, C.; Brossier, F.; Deplazes, P.; Hehl, A.B.; et al. RNA Seq analysis of the Eimeria tenella gametocyte transcriptome reveals clues about the molecular basis for sexual reproduction and oocyst biogenesis. BMC Genom. 2015, 16, 94. [Google Scholar] [CrossRef] [Green Version]
- Walker, R.; Slapetova, I.; Slapeta, J.; Miller, C.M.; Smith, N.C. The Glycosylation Pathway of Eimeria tenella Is Upregulated during Gametocyte Development and May Play a Role in Oocyst Wall Formation. Eukaryot. Cell 2010, 9, 127–135. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- R Core Team. R: A Language and Environment for Statistical Computing; R foundation for Statistical Computing: Vienna, Austria, 2020; Available online: https://www.R-project.org/ (accessed on 31 March 2021).
- Babicki, S.; Arndt, D.; Marcu, A.; Liang, Y.; Grant, J.R.; Maciejewski, A.; Wishart, D.S. Heatmapper: Web-enabled heat mapping for all. Nucleic Acids Res. 2016, 44, W147–W153. [Google Scholar] [CrossRef]
- Camejo, A.; Gold, D.A.; Lu, D.; McFetridge, K.; Julien, L.; Yang, N.; Jensen, K.D.C.; Saeij, J.P. Identification of three novel Toxoplasma gondii rhoptry proteins. Int. J. Parasitol. 2014, 44, 147–160. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, E.W.; Nadipuram, S.M.; Tetlow, A.L.; Barshop, W.D.; Liu, P.T.; Wohlschlegel, J.A.; Bradley, P.J. The Rhoptry Pseudokinase ROP54 Modulates Toxoplasma gondii Virulence and Host GBP2 Loading. mSphere 2016, 1, e00045-16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tokunaga, N.; Nozaki, M.; Tachibana, M.; Baba, M.; Matsuoka, K.; Tsuboi, T.; Torii, M.; Ishino, T. Expression and Localization Profiles of Rhoptry Proteins in Plasmodium berghei Sporozoites. Front. Cell. Infect. Microbiol. 2019, 9, 316. [Google Scholar] [CrossRef] [PubMed]
- Ben Chaabene, R.; Lentini, G.; Soldati-Favre, D. Biogenesis and discharge of the rhoptries: Key organelles for entry and hijack of host cells by the Apicomplexa. Mol. Microbiol. 2021, 115, 453–465. [Google Scholar] [CrossRef] [PubMed]
- Hakimi, M.-A.; Olias, P.; Sibley, L.D. Toxoplasma Effectors Targeting Host Signaling and Transcription. Clin. Microbiol. Rev. 2017, 30, 615–645. [Google Scholar] [CrossRef] [Green Version]
- Behnke, M.; Fentress, S.J.; Mashayekhi, M.; Li, L.X.; Taylor, G.A.; Sibley, L.D. The Polymorphic Pseudokinase ROP5 Controls Virulence in Toxoplasma gondii by Regulating the Active Kinase ROP. PLoS Pathog. 2012, 8, e1002992. [Google Scholar] [CrossRef]
- El Hajj, H.; Lebrun, M.; Fourmaux, M.N.; Vial, H.; Dubremetz, J.F. Inverted topology of the Toxoplasma gondii ROP5 rhoptry protein provides new insights into the association of the ROP2 protein family with the parasitophorous vacuole membrane. Cell. Microbiol. 2007, 9, 54–64. [Google Scholar] [CrossRef]
- Etheridge, R.D.; Alaganan, A.; Tang, K.; Lou, H.J.; Turk, B.E.; Sibley, L.D. The Toxoplasma Pseudokinase ROP5 Forms Complexes with ROP18 and ROP17 Kinases that Synergize to Control Acute Virulence in Mice. Cell Host Microbe 2014, 15, 537–550. [Google Scholar] [CrossRef] [Green Version]
- Niedelman, W.; Gold, D.A.; Rosowski, E.; Sprokholt, J.K.; Lim, D.; Arenas, A.; Melo, M.; Spooner, E.; Yaffe, M.B.; Saeij, J.P.J. The Rhoptry Proteins ROP18 and ROP5 Mediate Toxoplasma gondii Evasion of the Murine, But Not the Human, Interferon-Gamma Response. PLoS Pathog. 2012, 8, e1002784. [Google Scholar] [CrossRef] [Green Version]
- Novaes, J.; Rangel, L.T.L.D.; Ferro, M.; Abe, R.Y.; Manha, A.P.D.S.; De Mello, J.C.M.; Varuzza, L.; Durham, A.M.; Madeira, A.M.B.N.; Gruber, A. A comparative transcriptome analysis reveals expression profiles conserved across three Eimeria spp. of domestic fowl and associated with multiple developmental stages. Int. J. Parasitol. 2012, 42, 39–48. [Google Scholar] [CrossRef]
- Sharma, J.; Rodriguez, P.; Roy, P.; Guiton, P.S. Transcriptional ups and downs: Patterns of gene expression in the life cycle of Toxoplasma gondii. Microbes Infect. 2020, 22, 525–533. [Google Scholar] [CrossRef]
- de Venevelles, P.; Chich, J.F.; Faigle, W.; Loew, D.; Labbé, M.; Girard-Misguich, F.; Péry, P. Towards a reference map of Eimeria tenella sporozoite proteins by two-dimensional electrophoresis and mass spectrometry. Int. J. Parasitol. 2004, 34, 1321–1331. [Google Scholar] [CrossRef]
- de Venevelles, P.; Chich, J.F.; Faigle, W.; Lombard, B.; Loew, D.; Péry, P.; Labbé, M. Study of proteins associated with the Eimeria tenella refractile body by a proteomic approach. Int. J. Parasitol. 2006, 36, 1399–1407. [Google Scholar] [CrossRef] [PubMed]
- Lal, K.; Bromley, E.; Oakes, R.; Prieto, J.H.; Sanderson, S.J.; Kurian, D.; Hunt, L.; Yates, J.R.; Wastling, J.M.; Sinden, R.E.; et al. Proteomic comparison of four Eimeria tenella life-cycle stages: Unsporulated oocyst, sporulated oocyst, sporozoite and second-generation merozoite. Proteomics 2009, 9, 4566–4576. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thabet, A.; Honscha, W.; Daugschies, A.; Bangoura, B. Quantitative proteomic studies in resistance mechanisms of Eimeria tenella against polyether ionophores. Parasitol. Res. 2017, 116, 1553–1559. [Google Scholar] [CrossRef] [PubMed]
- Li, X.-Y.; Liu, L.-L.; Zhang, M.; Zhang, L.-F.; Wang, X.-Y.; Wang, M.; Zhang, K.-Y.; Liu, Y.-C.; Wang, C.-M.; Xue, F.-Q.; et al. Proteomic analysis of the second-generation merozoites of Eimeria tenella under nitromezuril and ethanamizuril stress. Parasites Vectors 2019, 12, 592. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shen, X.-J.; Li, T.; Fu, J.-J.; Zhang, K.-Y.; Wang, X.-Y.; Liu, Y.-C.; Zhang, H.-J.; Fan, C.; Fei, C.-Z.; Xue, F.-Q. Proteomic analysis of the effect of diclazuril on second-generation merozoites of Eimeria tenella. Parasitol. Res. 2013, 113, 903–909. [Google Scholar] [CrossRef] [PubMed]
- Shirley, M.W.; Harvey, D.A. A Genetic Linkage Map of the Apicomplexan Protozoan Parasite Eimeria tenella. Genome Res. 2000, 10, 1587–1593. [Google Scholar] [CrossRef] [Green Version]
- Matsubayashi, M.; Kawahara, F.; Hatta, T.; Yamagishi, J.; Miyoshi, T.; Anisuzzaman; Sasai, K.; Isobe, T.; Kita, K.; Tsuji, N. Transcriptional profiles of virulent and precocious strains of Eimeria tenella at sporozoite stage; novel biological insight into attenuated asexual development. Infect. Genet. Evol. 2016, 40, 54–62. [Google Scholar] [CrossRef]
- Dereeper, A.; Guignon, V.; Blanc, G.; Audic, S.; Buffet, S.; Chevenet, F.; Dufayard, J.-F.; Guindon, S.; Lefort, V.; Lescot, M.; et al. Phylogeny.fr: Robust phylogenetic analysis for the non-specialist. Nucleic Acids Res. 2008, 36, W465–W469. [Google Scholar] [CrossRef]

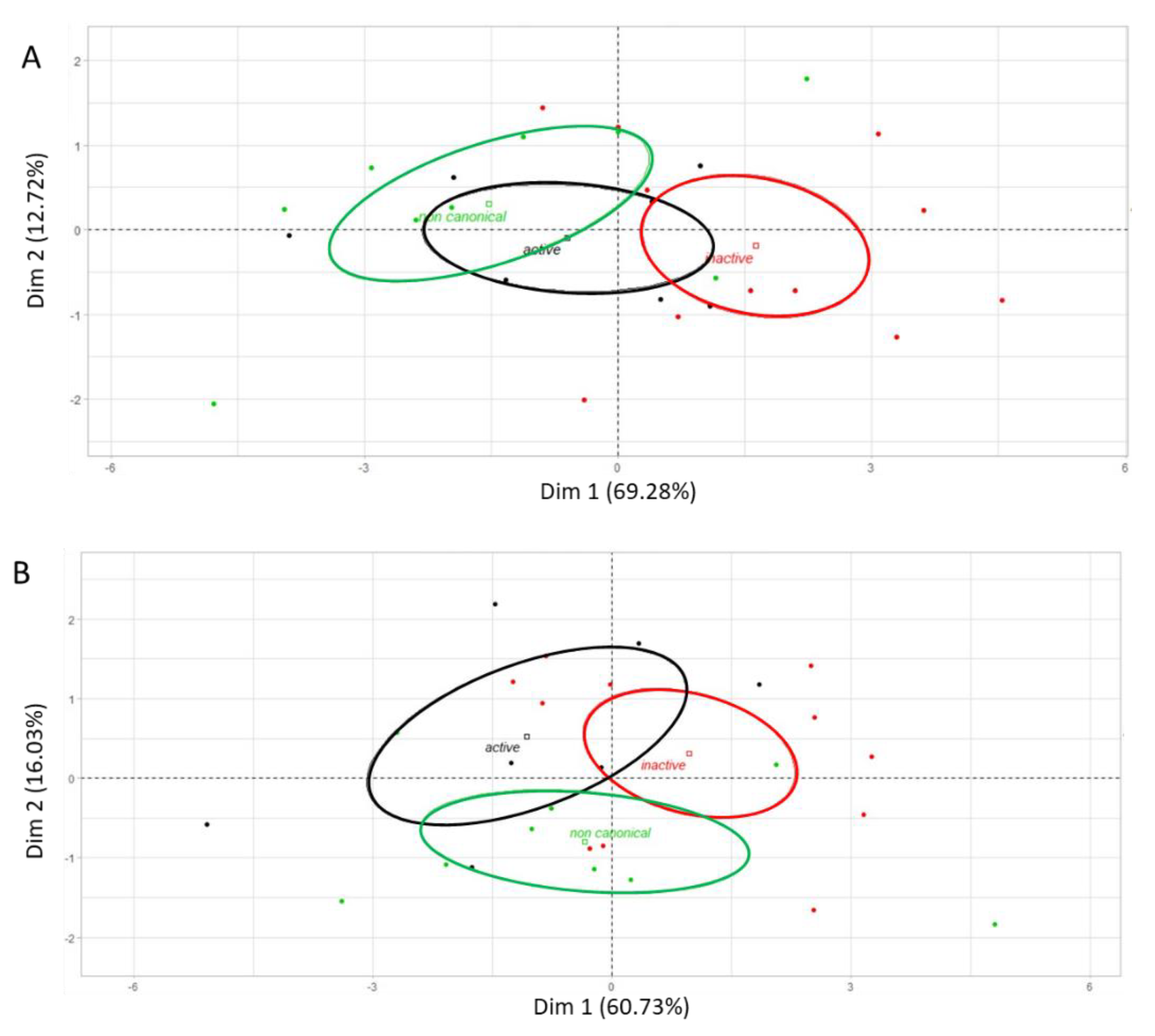


| Activity | Gene ID | Gene Symbol | Phylogeny 1 |
|---|---|---|---|
| active | ETH_00005190 | Etrop5190 | ROPK unique |
| active | ETH_00005905 | Etrop5905 | TgROP35 |
| active | ETH_00014495 | Etrop14495 | TgROP21 |
| active | ETH_00026495 | Etrop26495 | TgROP35 |
| active | ETH_00027695 | Etrop27695 | Eten1 |
| active | ETH_00027700 | Etrop27700 | Eten1 |
| active | ETH_00027705 | Etrop27705 | Eten1 |
| inactive | ETH_00000075 | Etrop75 | Eten4 |
| inactive | ETH_00000080 | Etrop80 | Eten4 |
| inactive | ETH_00002510 | Etrop2510 | Eten6 |
| inactive | ETH_00005170 | Etrop5170 | ROPK unique |
| inactive | ETH_00005335 | Etrop5335 | ROPK unique |
| inactive | ETH_00005400 | Etrop5400 | Eten5 |
| inactive | ETH_00005405 | Etrop5405 | Eten5 |
| inactive | ETH_00005410 | Etrop5410 | Eten5 |
| inactive | ETH_00005415 | Etrop5415 | Eten5 |
| inactive | ETH_00016910 | Etrop16910 | ROPK unique |
| inactive | ETH_00028835 | Etrop28835 | ROPK unique |
| non-canonical | ETH_00005840 | Etrop5840 | Eten3 |
| non-canonical | ETH_00020585 | Etrop20585 | Eten3 |
| non-canonical | ETH_00020590 | Etrop20590 | Eten3 |
| non-canonical | ETH_00020610 | Etrop20610 | Eten3 |
| non-canonical | ETH_00020615 | Etrop20615 | Eten3 |
| non-canonical | ETH_00020620 | Etrop20620 | Eten3 |
| non-canonical | ETH_00021185 | Etrop21185 | Eten3 |
| non-canonical | ETH_00028765 | Etrop28765 | Eten2a |
| non-canonical | ETH_00028855 | Etrop28855 | Eten2b |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ribeiro E Silva, A.; Sausset, A.; Bussière, F.I.; Laurent, F.; Lacroix-Lamandé, S.; Silvestre, A. Genome-Wide Expression Patterns of Rhoptry Kinases during the Eimeria tenella Life-Cycle. Microorganisms 2021, 9, 1621. https://doi.org/10.3390/microorganisms9081621
Ribeiro E Silva A, Sausset A, Bussière FI, Laurent F, Lacroix-Lamandé S, Silvestre A. Genome-Wide Expression Patterns of Rhoptry Kinases during the Eimeria tenella Life-Cycle. Microorganisms. 2021; 9(8):1621. https://doi.org/10.3390/microorganisms9081621
Chicago/Turabian StyleRibeiro E Silva, Adeline, Alix Sausset, Françoise I. Bussière, Fabrice Laurent, Sonia Lacroix-Lamandé, and Anne Silvestre. 2021. "Genome-Wide Expression Patterns of Rhoptry Kinases during the Eimeria tenella Life-Cycle" Microorganisms 9, no. 8: 1621. https://doi.org/10.3390/microorganisms9081621
APA StyleRibeiro E Silva, A., Sausset, A., Bussière, F. I., Laurent, F., Lacroix-Lamandé, S., & Silvestre, A. (2021). Genome-Wide Expression Patterns of Rhoptry Kinases during the Eimeria tenella Life-Cycle. Microorganisms, 9(8), 1621. https://doi.org/10.3390/microorganisms9081621






