Glucose-6-Phosphate Dehydrogenase::6-Phosphogluconolactonase from the Parasite Giardia lamblia. A Molecular and Biochemical Perspective of a Fused Enzyme
Abstract
:1. Introduction
2. Materials and Methods
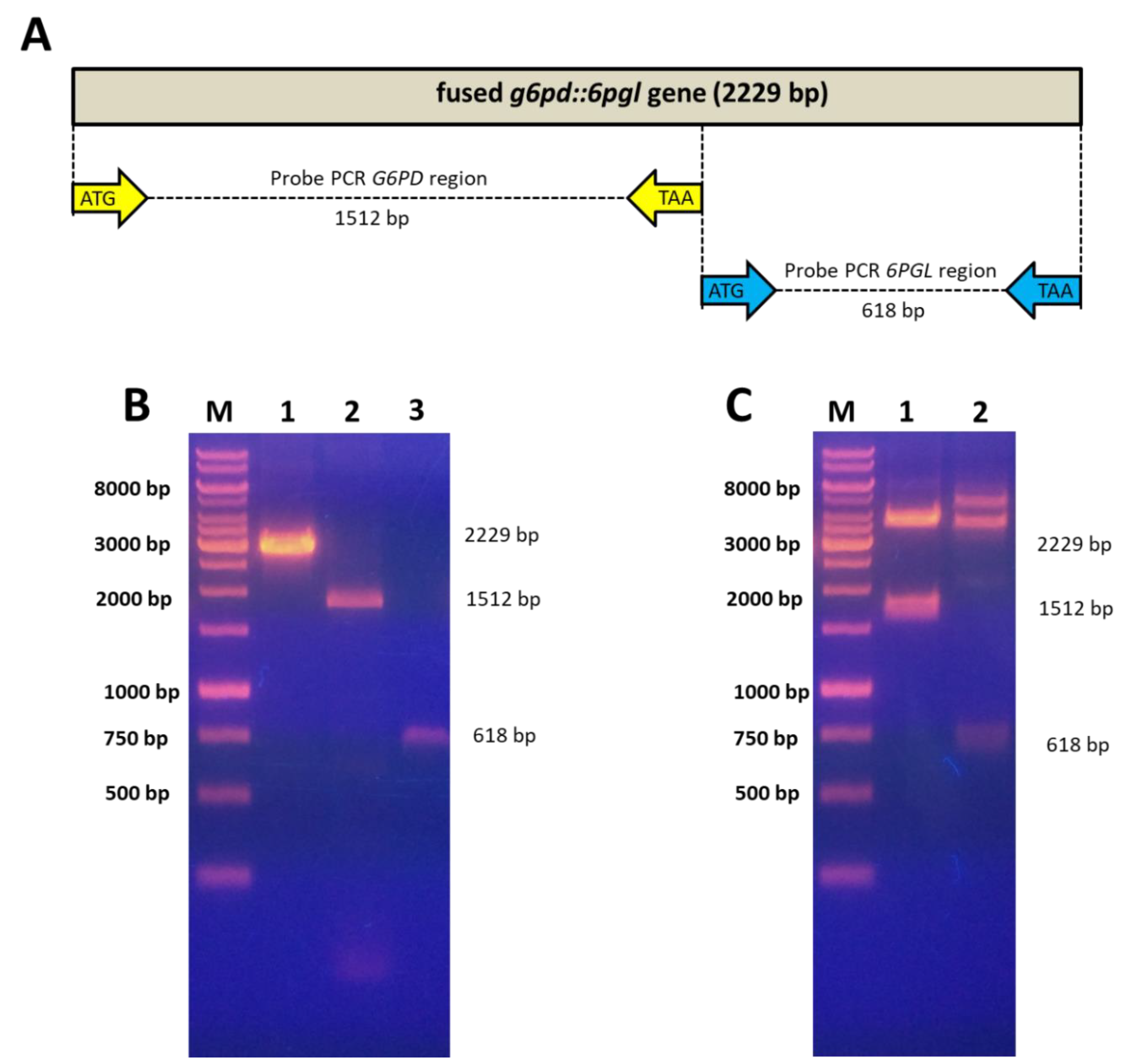
2.1. Cloning of the Individual g6pd and 6pgl Gene Regions of the Fused g6pd::6pgl Gene from Giardia lamblia
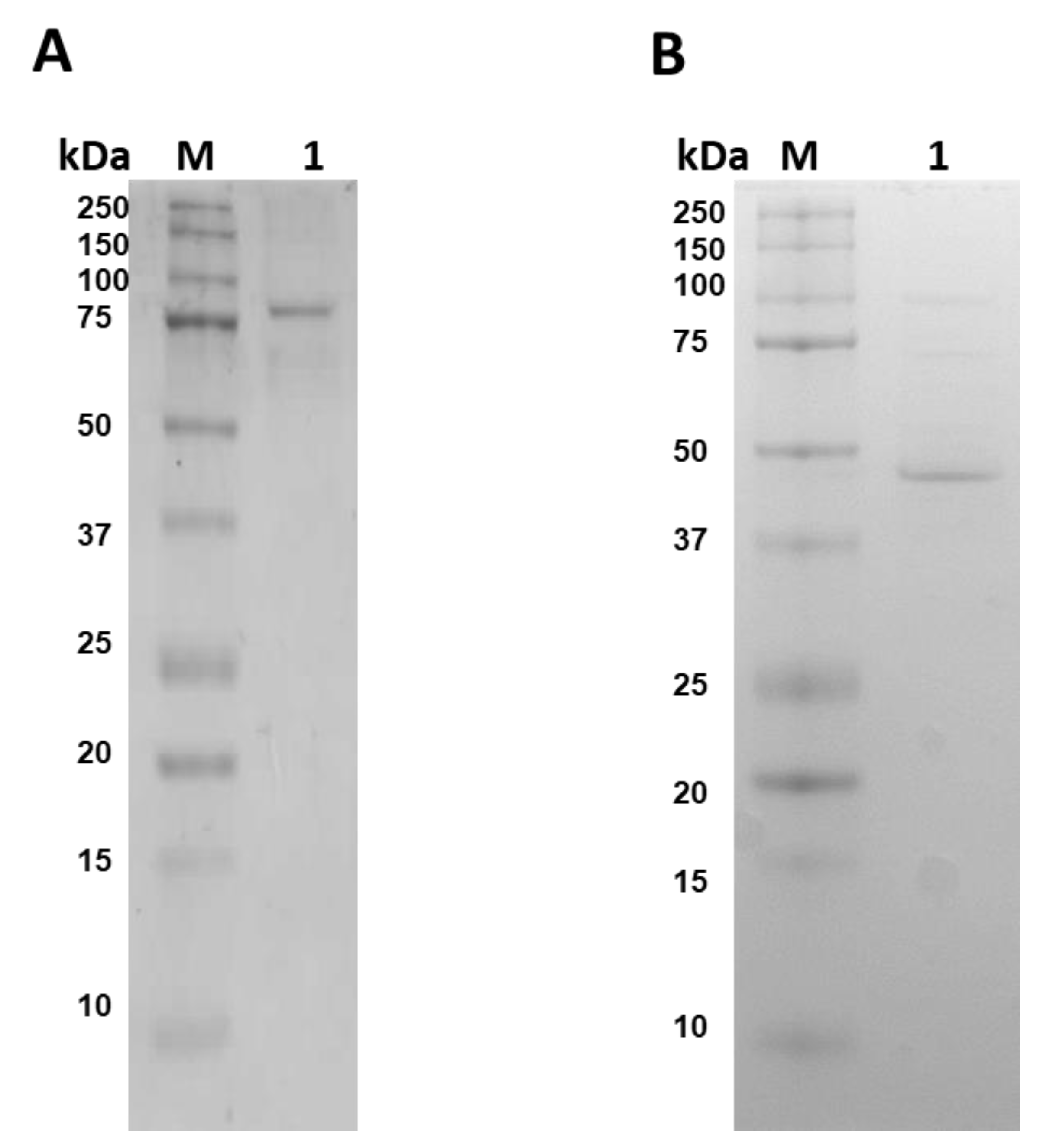
2.2. Expression and Purification of Fused G6PD::6PGL and Individual G6PD and 6PGL Proteins
2.3. Kinetic Characterization of the Domains G6PD, 6PGL of the Fused G6PD::6PGL, and the Individuals G6PD and 6PGL Proteins
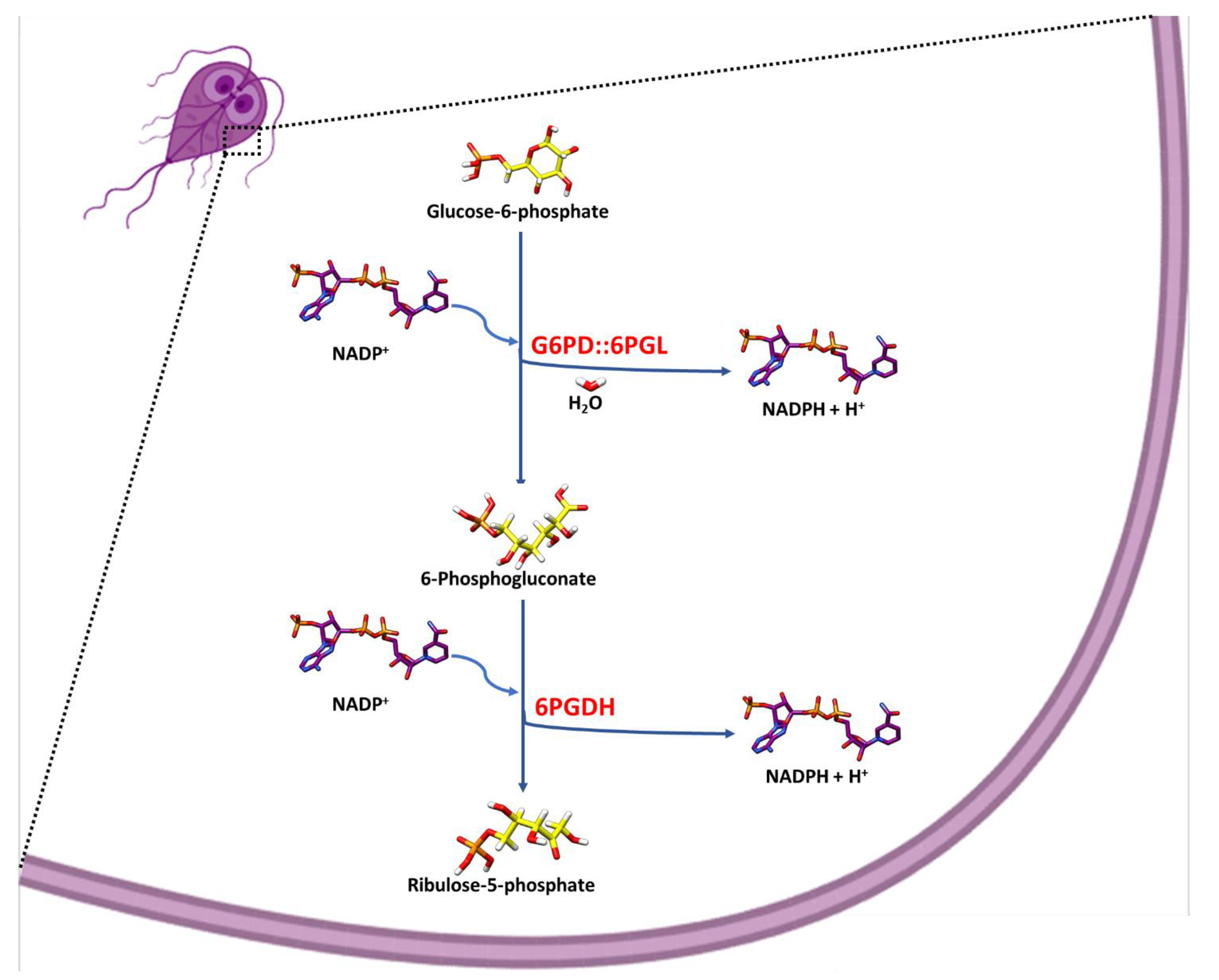
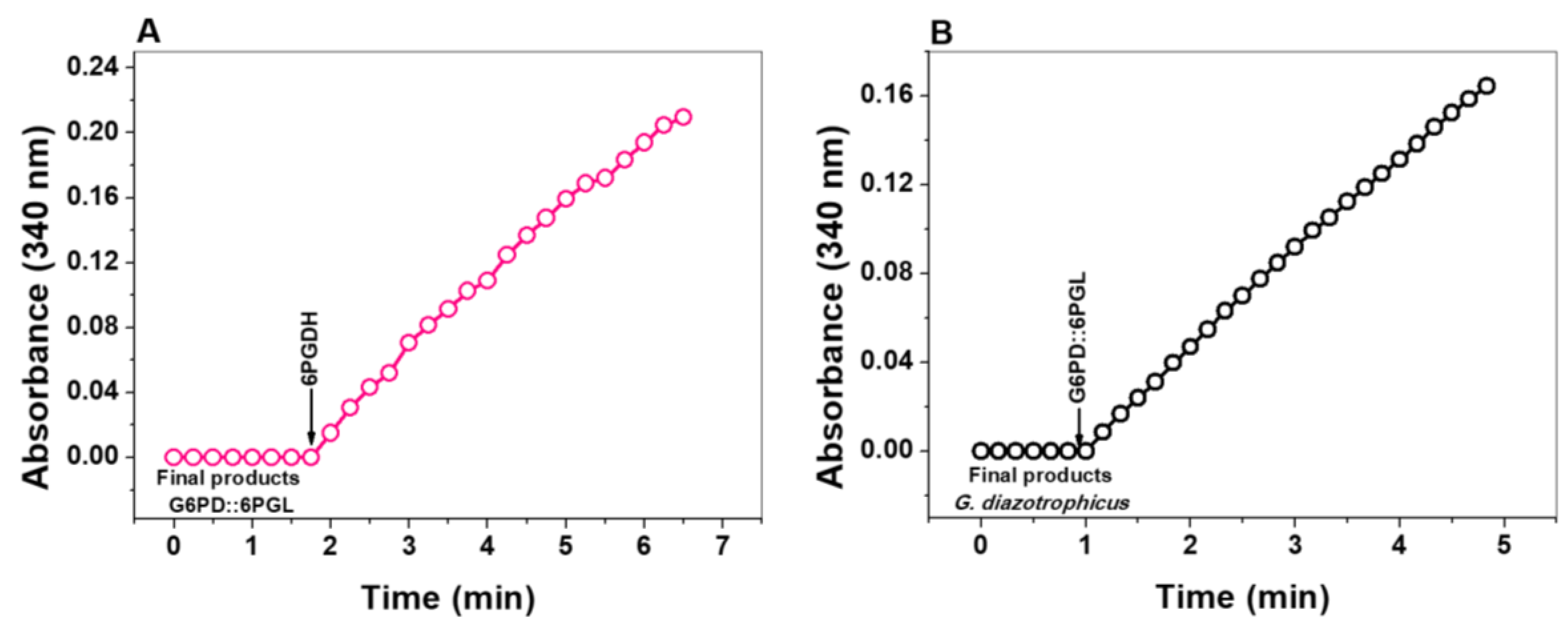
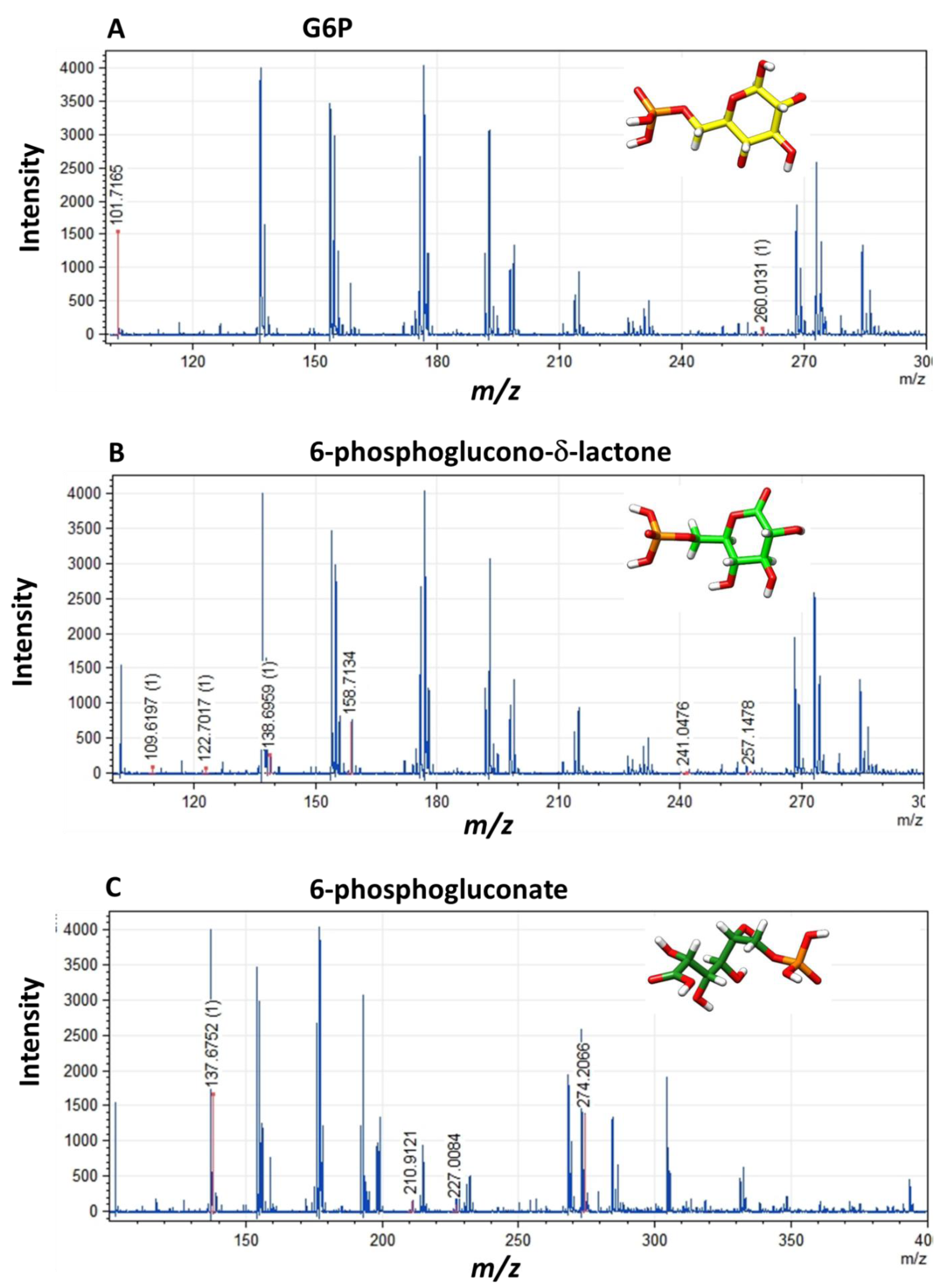
2.4. Identification of the Metabolites as Final Products of Fused G6PD::6PGL Enzyme
2.4.1. Spectrophotometric Method
2.4.2. MALDI-TOF Method
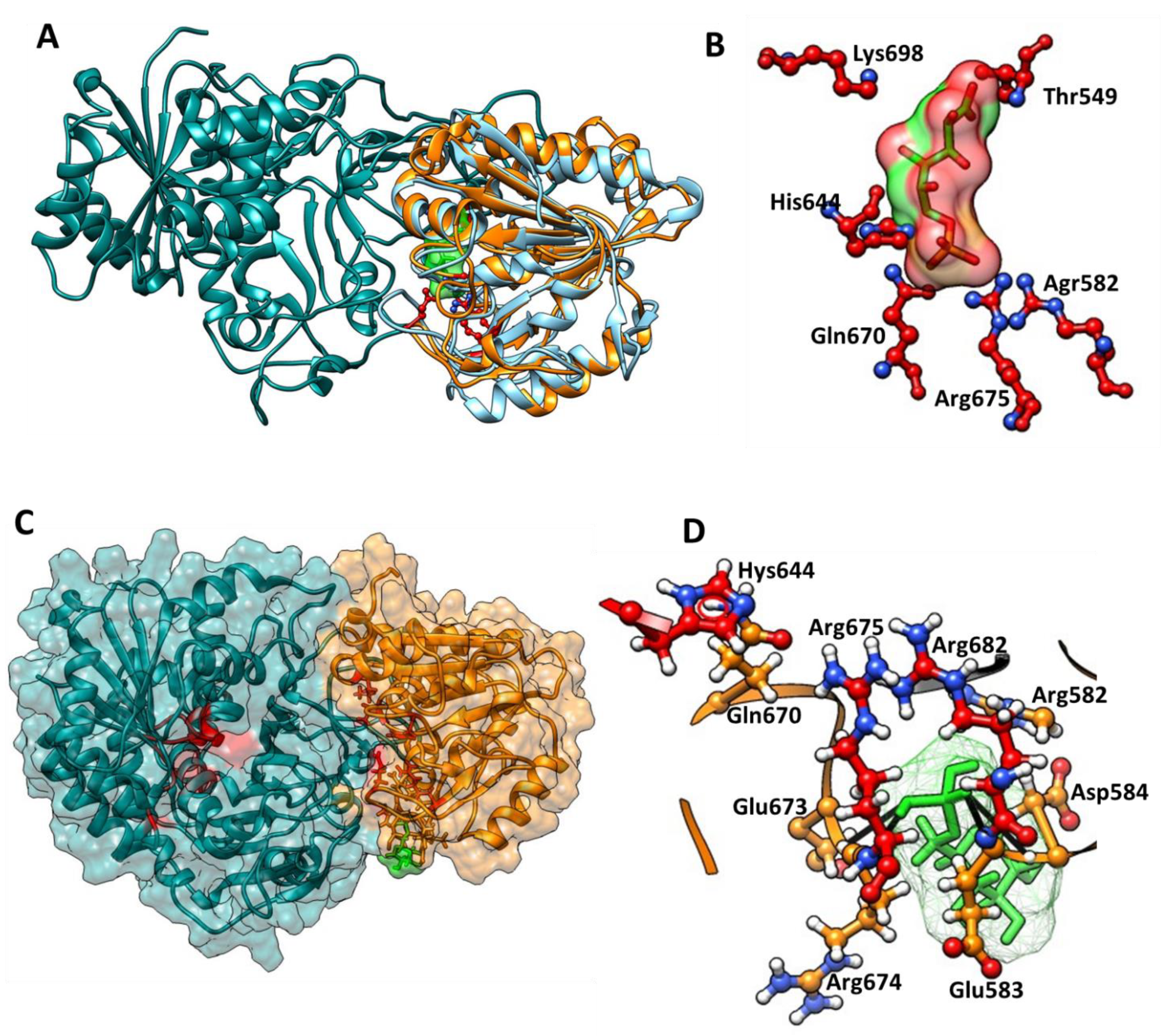
2.5. Prediction of the 6-Phosphoglucone-δ-lactone Substrate Binding Site
3. Results and Discussion
3.1. Amplification and Cloning of the Individual g6pd and 6pgl Gene Regions of the Fused g6pd::6pgl Gene from G. lamblia
3.2. Purification of Fused G6PD::6PGL and Individual G6PD and 6PGL Proteins
3.3. Kinetic Characterization of the Domains G6PD, 6PGL of the Fused G6PD::6PGL, and the Individual G6PD Protein
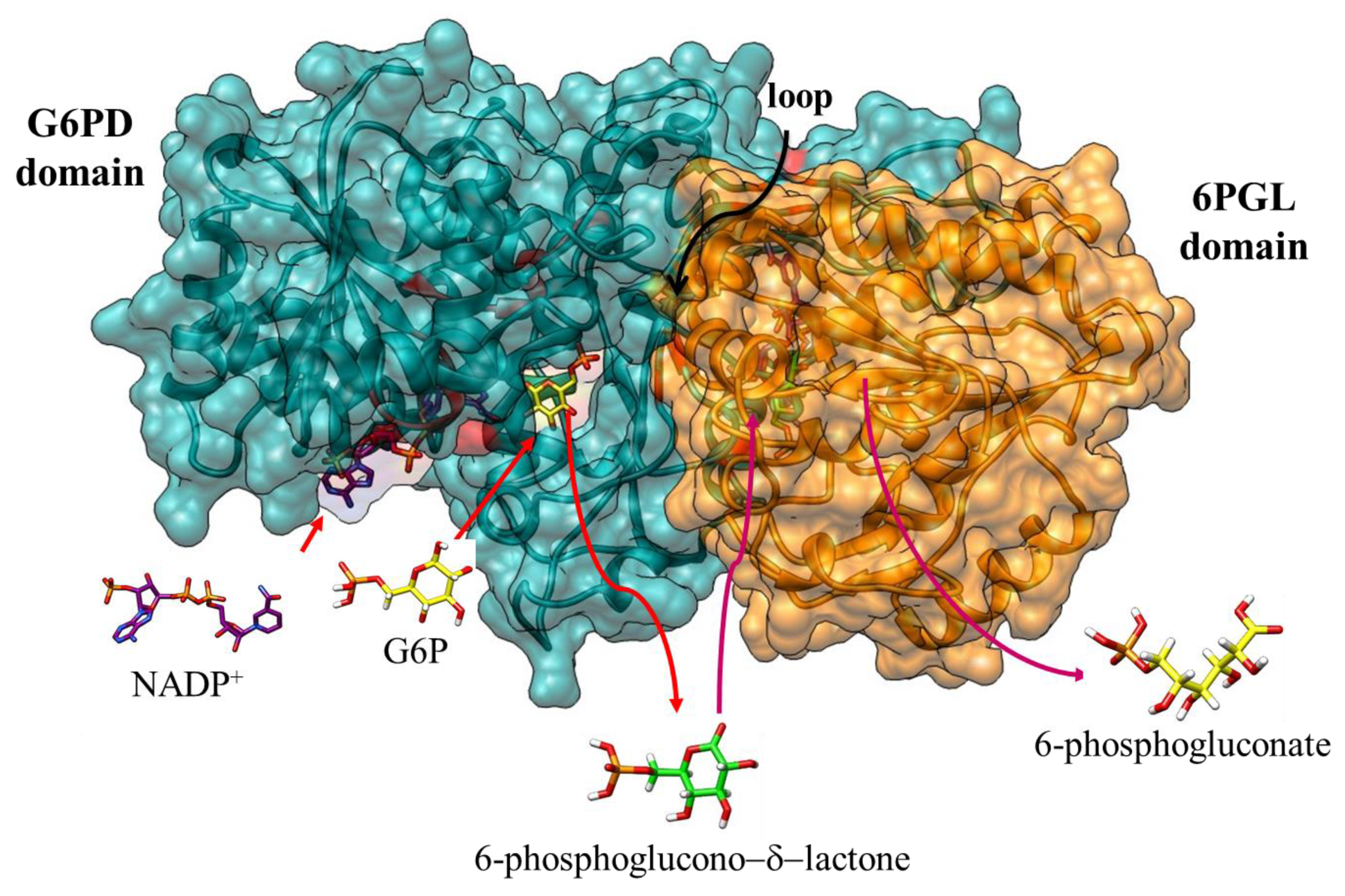
3.4. Identification of the Metabolites as Final Products of Fused G6PD::6PGL Enzyme
3.4.1. Enzymatic Methods
3.4.2. MALDI-TOF Method
3.5. Identification of the 6-Phosphoglucono-δ-lactone Substrate Binding Site on G6PD::6PGL from G. lamblia
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Morrison, H.G.; McArthur, A.G.; Gillin, F.D.; Aley, S.B.; Adam, R.D.; Olsen, G.J.; Best, A.A.; Cande, W.Z.; Chen, F.; Cipriano, M.J.; et al. Genomic minimalism in the early diverging intestinal parasite Giardia lamblia. Science 2007, 317, 1921–1926. [Google Scholar] [CrossRef] [Green Version]
- Cernikova, L.; Faso, C.; Hehl, A.B. Five facts about Giardia lamblia. PLoS Pathog. 2018, 14, e1007250. [Google Scholar] [CrossRef] [Green Version]
- Escobedo, A.A.; Hanevik, K.; Almirall, P.; Cimerman, S.; Alfonso, M. Management of chronic Giardia infection. Expert Rev. Anti-Infect. Ther. 2014, 12, 1143–1157. [Google Scholar] [CrossRef]
- Cedillo-Rivera, R.; Darby, J.M.; Enciso-Moreno, J.A.; Ortega-Pierres, G.; Ey, P.L. Genetic homogeneity of axenic isolates of Giardia intestinalis derived from acute and chronically infected individuals in Mexico. Parasitol. Res. 2003, 90, 119–123. [Google Scholar] [CrossRef] [PubMed]
- Berkman, D.S.; Lescano, A.G.; Gilman, R.H.; Lopez, S.L.; Black, M.M. Effects of stunting, diarrhoeal disease, and parasitic infection during infancy on cognition in late childhood: A follow-up study. Lancet 2002, 359, 564–571. [Google Scholar] [CrossRef]
- Duffieux, F.; Van Roy, J.; Michels, P.A.; Opperdoes, F.R. Molecular characterization of the first two enzymes of the pentose-phosphate pathway of Trypanosoma brucei. Glucose-6-phosphate dehydrogenase and 6-phosphogluconolactonase. J. Biol. Chem. 2000, 275, 27559–27565. [Google Scholar] [CrossRef] [Green Version]
- Stover, N.A.; Dixon, T.A.; Cavalcanti, A.R. Multiple independent fusions of glucose-6-phosphate dehydrogenase with enzymes in the pentose phosphate pathway. PLoS ONE 2011, 6, e22269. [Google Scholar] [CrossRef] [Green Version]
- Morales-Luna, L.; Serrano-Posada, H.; Gonzalez-Valdez, A.; Ortega-Cuellar, D.; Vanoye-Carlo, A.; Hernandez-Ochoa, B.; Sierra-Palacios, E.; Rufino-Gonzalez, Y.; Castillo-Rodriguez, R.A.; Perez de la Cruz, V.; et al. Biochemical Characterization and Structural Modeling of Fused Glucose-6-Phosphate Dehydrogenase-Phosphogluconolactonase from Giardia lamblia. Int. J. Mol. Sci. 2018, 19, 2518. [Google Scholar] [CrossRef] [Green Version]
- Morales-Luna, L.; González-Valdez, A.; Sixto-López, Y.; Correa-Basurto, J.; Hernández-Ochoa, B.; Cárdenas-Rodríguez, N.; Castillo-Rodríguez, R.A.; Ortega-Cuellar, D.; Arreguin-Espinosa, R.; Pérez de la Cruz, V. Identification of the NADP+ structural binding site and coenzyme effect on the fused G6PD:: 6PGL protein from Giardia lamblia. Biomolecules 2020, 10, 46. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gómez-Manzo, S.; Terrón-Hernández, J.; la Mora-De la Mora, D.; González-Valdez, A.; Marcial-Quino, J.; García-Torres, I.; Vanoye-Carlo, A.; López-Velázquez, G.; Hernández-Alcántara, G.; Oria-Hernández, J. The stability of G6PD is affected by mutations with different clinical phenotypes. Int. J. Mol. Sci. 2014, 15, 21179–21201. [Google Scholar] [CrossRef] [Green Version]
- Gómez-Manzo, S.; Marcial-Quino, J.; Vanoye-Carlo, A.; Enríquez-Flores, S.; la Mora-De la Mora, D.; González-Valdez, A.; García-Torres, I.; Martínez-Rosas, V.; Sierra-Palacios, E.; Lazcano-Pérez, F. Mutations of glucose-6-phosphate dehydrogenase Durham, Santa-Maria and A+ variants are associated with loss functional and structural stability of the protein. Int. J. Mol. Sci. 2015, 16, 28657–28668. [Google Scholar] [CrossRef]
- Morales-Luna, L.; Hernandez-Ochoa, B.; Martinez-Rosas, V.; Gonzalez-Valdez, A.; Cardenas-Rodriguez, N.; Enriquez-Flores, S.; Marcial-Quino, J.; Gomez-Manzo, S. Cloning, purification, and characterization of the 6-phosphogluconate dehydrogenase (6 PGDH) from Giardia lamblia. Mol. Biochem. Parasitol. 2021, 244, 111383. [Google Scholar] [CrossRef]
- Ramirez-Nava, E.J.; Ortega-Cuellar, D.; Gonzalez-Valdez, A.; Castillo-Rodriguez, R.A.; Ponce-Soto, G.Y.; Hernandez-Ochoa, B.; Cardenas-Rodriguez, N.; Martinez-Rosas, V.; Morales-Luna, L.; Serrano-Posada, H.; et al. Molecular Cloning and Exploration of the Biochemical and Functional Analysis of Recombinant Glucose-6-Phosphate Dehydrogenase from Gluconoacetobacter diazotrophicus PAL5. Int. J. Mol. Sci. 2019, 20, 5279. [Google Scholar] [CrossRef]
- Duclert-Savatier, N.; Poggi, L.; Miclet, E.; Lopes, P.; Ouazzani, J.; Chevalier, N.; Nilges, M.; Delarue, M.; Stoven, V. Insights into the enzymatic mechanism of 6-phosphogluconolactonase from Trypanosoma brucei using structural data and molecular dynamics simulation. J. Mol. Biol. 2009, 388, 1009–1021. [Google Scholar] [CrossRef] [PubMed]
- Grosdidier, A.; Zoete, V.; Michielin, O. SwissDock, a protein-small molecule docking web service based on EADock DSS. Nucleic Acids Res. 2011, 39, W270–W277. [Google Scholar] [CrossRef] [Green Version]
- Williams, C.J.; Headd, J.J.; Moriarty, N.W.; Prisant, M.G.; Videau, L.L.; Deis, L.N.; Verma, V.; Keedy, D.A.; Hintze, B.J.; Chen, V.B.; et al. MolProbity: More and better reference data for improved all-atom structure validation. Protein Sci. 2018, 27, 293–315. [Google Scholar] [CrossRef] [PubMed]
- Krieger, E.; Joo, K.; Lee, J.; Lee, J.; Raman, S.; Thompson, J.; Tyka, M.; Baker, D.; Karplus, K. Improving physical realism, stereochemistry, and side-chain accuracy in homology modeling: Four approaches that performed well in CASP8. Proteins Struct. Funct. Bioinform. 2009, 77, 114–122. [Google Scholar] [CrossRef] [Green Version]
- Pettersen, E.F.; Goddard, T.D.; Huang, C.C.; Couch, G.S.; Greenblatt, D.M.; Meng, E.C.; Ferrin, T.E. UCSF Chimera--a visualization system for exploratory research and analysis. J. Comput. Chem. 2004, 25, 1605–1612. [Google Scholar] [CrossRef] [Green Version]
- Jortzik, E.; Mailu, B.M.; Preuss, J.; Fischer, M.; Bode, L.; Rahlfs, S.; Becker, K. Glucose-6-phosphate dehydrogenase-6-phosphogluconolactonase: A unique bifunctional enzyme from Plasmodium falciparum. Biochem. J. 2011, 436, 641–650. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Haeussler, K.; Berneburg, I.; Jortzik, E.; Hahn, J.; Rahbari, M.; Schulz, N.; Preuss, J.; Zapol’skii, V.A.; Bode, L.; Pinkerton, A.B.; et al. Glucose 6-phosphate dehydrogenase 6-phosphogluconolactonase: Characterization of the Plasmodium vivax enzyme and inhibitor studies. Malar. J. 2019, 18, 22. [Google Scholar] [CrossRef]
- d Moore, B. Bifunctional and moonlighting enzymes: Lighting the way to regulatory control. Trends Plant Sci. 2004, 9, 221–228. [Google Scholar] [CrossRef] [PubMed]

| Kinetic Parameters | G6PD::6PGL | Individual Proteins | ||
|---|---|---|---|---|
| G6PD | 6PGL | G6PD | 6PGL | |
| Km G6P (µM) | 18.1 | − | 31.8 | n.d |
| Km NADP+ (µM) | 13.9 | − | 26.7 | n.d |
| Km Lactone (µM) | − | 51.5 | − | n.d |
| Kcat (s−1) | 31.8 | 31.8 | 0.05 | n.d |
| Name Substrates/Products | Elemental Formula | Molecular Weight | Expected m/z | Obtained m/z |
|---|---|---|---|---|
| Glucose 6-phosphate | C6H13O9P | 260.14 | 101, 102, 132, 194, 209, 211, 226, 256, 260 | 260.01, 101.71 |
| 6-phosphoglucono−δ−lactone | C6H11O9P | 258.12 | 96, 98, 109, 122, 131, 138, 159, 171, 241, 257, 275 | 257.14, 241.04, 158.71, 138.69, 122.70, 109.61 |
| 6-phosphogluconate | C6H12O10P | 275.12 | 104, 114, 125, 131, 137, 144, 153, 211, 227, 274 | 274.20, 227.00, 210.91, 237.67 |
| NADPH | C21H30N7O17P3 | 745.4 | 123, 158, 166, 195, 257, 272, 287, 335, 415, 426, 471, 505, 520, 592, 728 | 158, 166, 272, 287, 335 |
| NADP+ | C21H29N7O17P3 | 744.41 | 122, 164, 194, 223, 246, 260, 272, 333, 426, 469, 505, 547, 562, 578, 580, 713, 725 | 194, 246, 260, 272, 333 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Morales-Luna, L.; González-Valdez, A.; Hernández-Ochoa, B.; Arreguin-Espinosa, R.; Ortega-Cuellar, D.; Castillo-Rodríguez, R.A.; Martínez-Rosas, V.; Cárdenas-Rodríguez, N.; Enríquez-Flores, S.; Canseco-Ávila, L.M.; et al. Glucose-6-Phosphate Dehydrogenase::6-Phosphogluconolactonase from the Parasite Giardia lamblia. A Molecular and Biochemical Perspective of a Fused Enzyme. Microorganisms 2021, 9, 1678. https://doi.org/10.3390/microorganisms9081678
Morales-Luna L, González-Valdez A, Hernández-Ochoa B, Arreguin-Espinosa R, Ortega-Cuellar D, Castillo-Rodríguez RA, Martínez-Rosas V, Cárdenas-Rodríguez N, Enríquez-Flores S, Canseco-Ávila LM, et al. Glucose-6-Phosphate Dehydrogenase::6-Phosphogluconolactonase from the Parasite Giardia lamblia. A Molecular and Biochemical Perspective of a Fused Enzyme. Microorganisms. 2021; 9(8):1678. https://doi.org/10.3390/microorganisms9081678
Chicago/Turabian StyleMorales-Luna, Laura, Abigail González-Valdez, Beatriz Hernández-Ochoa, Roberto Arreguin-Espinosa, Daniel Ortega-Cuellar, Rosa Angélica Castillo-Rodríguez, Víctor Martínez-Rosas, Noemi Cárdenas-Rodríguez, Sergio Enríquez-Flores, Luis Miguel Canseco-Ávila, and et al. 2021. "Glucose-6-Phosphate Dehydrogenase::6-Phosphogluconolactonase from the Parasite Giardia lamblia. A Molecular and Biochemical Perspective of a Fused Enzyme" Microorganisms 9, no. 8: 1678. https://doi.org/10.3390/microorganisms9081678
APA StyleMorales-Luna, L., González-Valdez, A., Hernández-Ochoa, B., Arreguin-Espinosa, R., Ortega-Cuellar, D., Castillo-Rodríguez, R. A., Martínez-Rosas, V., Cárdenas-Rodríguez, N., Enríquez-Flores, S., Canseco-Ávila, L. M., Cruz, V. P. d. l., Gómez-Chávez, F., & Gómez-Manzo, S. (2021). Glucose-6-Phosphate Dehydrogenase::6-Phosphogluconolactonase from the Parasite Giardia lamblia. A Molecular and Biochemical Perspective of a Fused Enzyme. Microorganisms, 9(8), 1678. https://doi.org/10.3390/microorganisms9081678









