The Manifesto of Pharmacoenosis: Merging HIV Pharmacology into Pathocoenosis and Syndemics in Developing Countries
Abstract
:1. Introduction
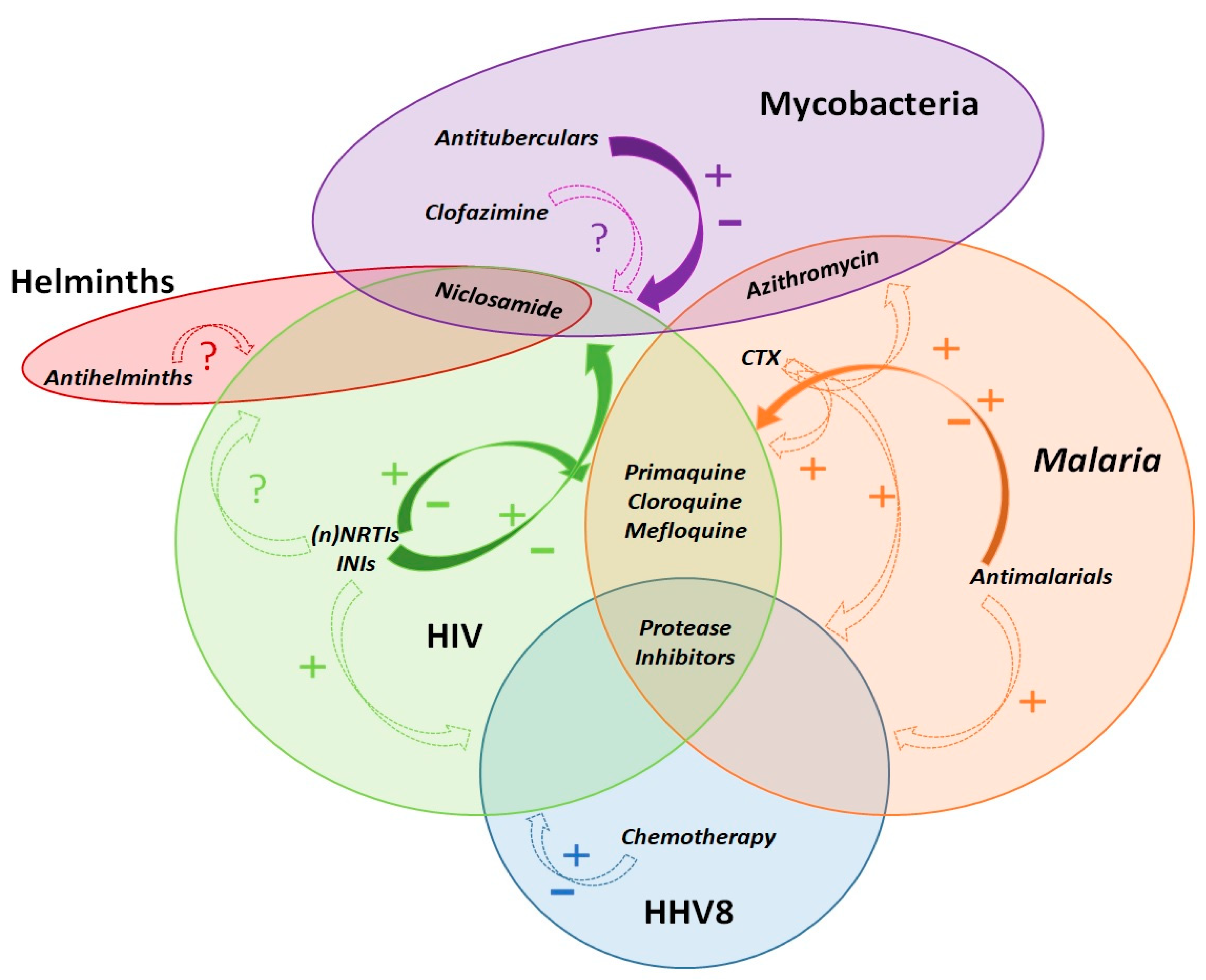
1.1. Pathocoenosis and Syndemic Theories
1.2. Pharmacoenosis
2. Malaria and HIV
2.1. PD Opportunities
2.2. PK and PG Opportunities
2.3. Companion Drugs
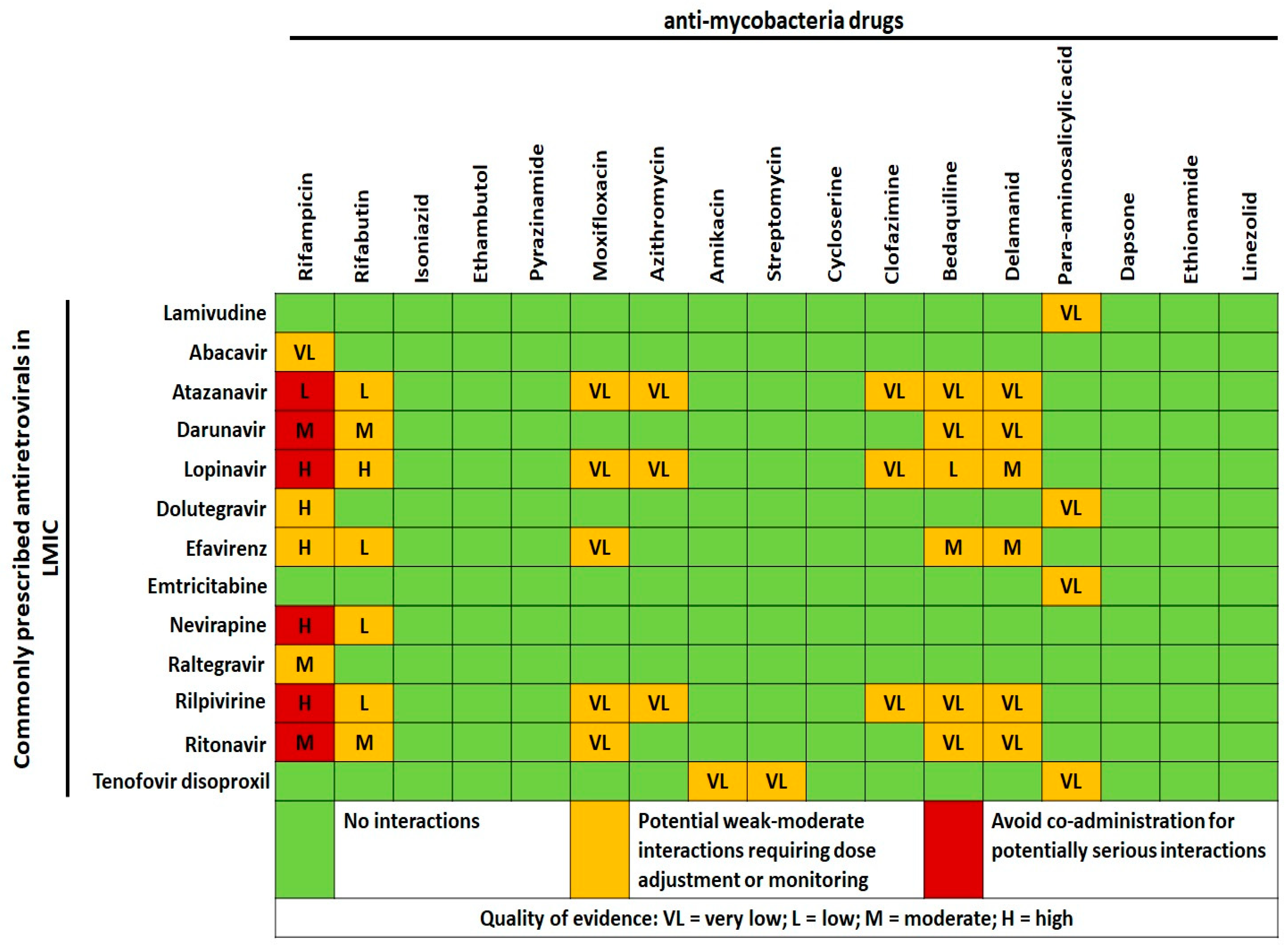
3. Mycobacteria and HIV
3.1. Tuberculosis and HIV
3.2. Nontuberculous Mycobacteria and HIV
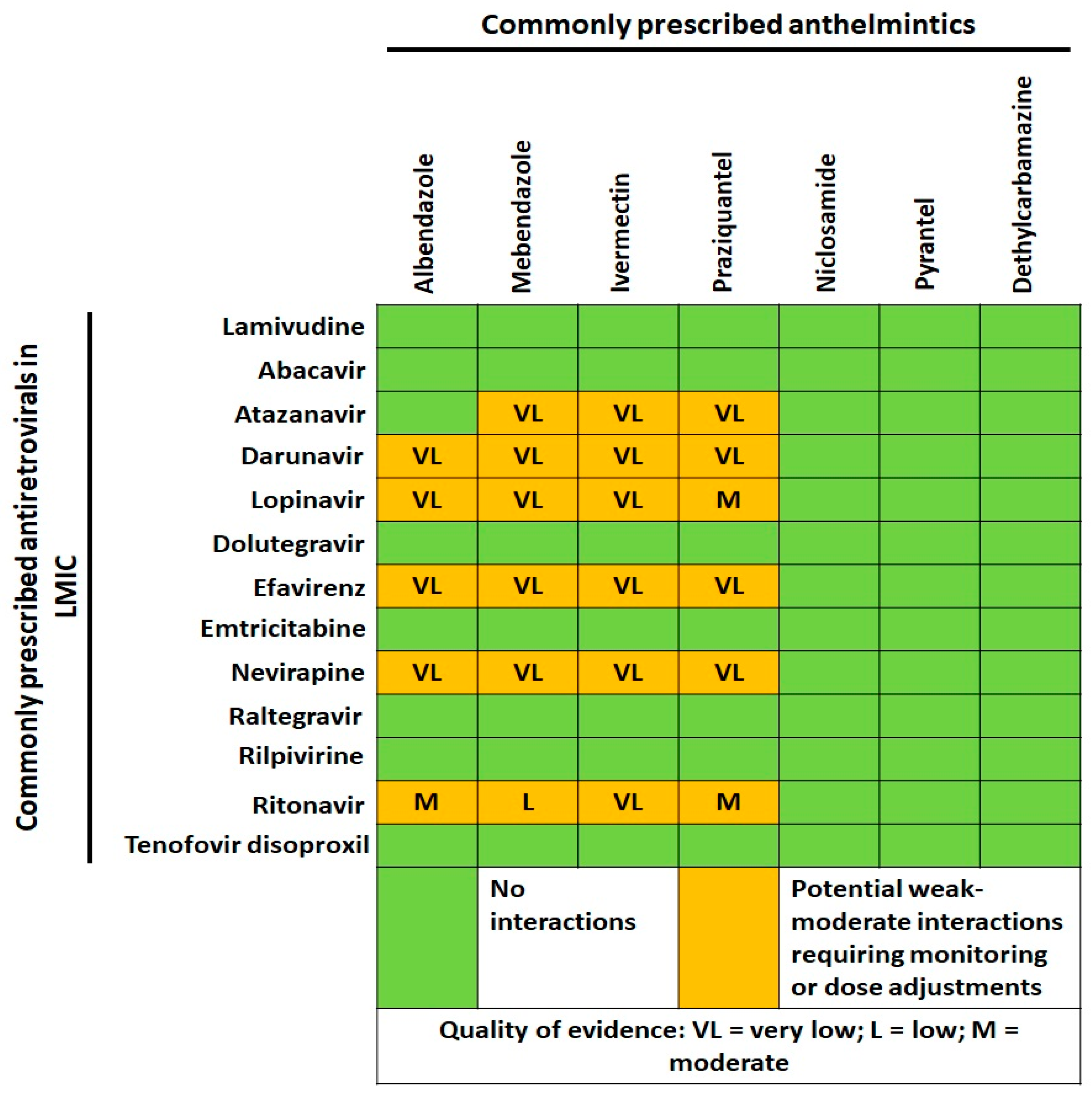
4. Helminths and HIV
5. Non-Communicable Diseases and HIV
6. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- McArdle, A.J.; Turkova, A.; Cunnington, A.J. When Do Co-Infections Matter? Curr. Opin. Infect. Dis. 2018, 31, 209–215. [Google Scholar] [CrossRef] [PubMed]
- Motta, I.; Trunfio, M.; Calcagno, A.; Pirriatore, V.; Scabini, S.; Palazzo, A.; Audagnotto, S.; Fatiguso, G.; Liberini, V.; Bellò, M.; et al. Undetectable Antimicrobial Plasma Concentrations in an HIV-Positive Patient with Protein-Losing Enteropathy and Chylothorax during Mycobacterium Genavense and Leishmania Abdominal Infections. J. Antimicrob. Chemother. 2018, 73, 546–548. [Google Scholar] [CrossRef] [PubMed]
- Trunfio, M.; Savoldi, A.; Viganò, O.; d’Arminio Monforte, A. Bacterial Coinfections in Dengue Virus Disease: What We Know and What Is Still Obscure about an Emerging Concern. Infection 2017, 45, 1–10. [Google Scholar] [CrossRef]
- Calcagno, A.; Ghisetti, V.; Burdino, E.; Trunfio, M.; Allice, T.; Boglione, L.; Bonora, S.; Di Perri, G. Co-Infection with Other Respiratory Pathogens in COVID-19 Patients. Clin. Microbiol. Infect. 2020, 27, 297–298. [Google Scholar] [CrossRef] [PubMed]
- Gourevitch, D. The Galenic Plague: A Breakdown of the Imperial Pathocoenosis. Pathocoenosis and Longue Durée. Hist. Philos. Life Sci. 2005, 27, 57–69. [Google Scholar] [PubMed]
- Faure, E. Malarial Pathocoenosis: Beneficial and Deleterious Interactions between Malaria and Other Human Diseases. Front. Physiol. 2014, 5, 441. [Google Scholar] [CrossRef] [Green Version]
- Singer, M.; Bulled, N.; Ostrach, B.; Mendenhall, E. Syndemics and the Biosocial Conception of Health. Lancet 2017, 389, 941–950. [Google Scholar] [CrossRef]
- Singer, M. Pathogen-Pathogen Interaction: A Syndemic Model of Complex Biosocial Processes in Disease. Virulence 2010, 1, 10–18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mendenhall, E. Syndemics: A New Path for Global Health Research. Lancet 2017, 389, 889–891. [Google Scholar] [CrossRef]
- Marr, J.S.; Cathey, J.T. The Yellow Fever Vaccine Misadventure of 1942. J. Public Health Manag. Pract. 2017, 23, 651–657. [Google Scholar] [CrossRef] [PubMed]
- Gürtler, L.G.; Eberle, J. Aspects on the History of Transmission and Favor of Distribution of Viruses by Iatrogenic Action: Perhaps an Example of a Paradigm of the Worldwide Spread of HIV. Med. Microbiol. Immunol. 2017, 206, 287–293. [Google Scholar] [CrossRef] [Green Version]
- Strickland, G.T. Liver Disease in Egypt: Hepatitis C Superseded Schistosomiasis as a Result of Iatrogenic and Biological Factors. Hepatology 2006, 43, 915–922. [Google Scholar] [CrossRef] [PubMed]
- Chitnis, A.; Rawls, D.; Moore, J. Origin of HIV Type 1 in Colonial French Equatorial Africa? AIDS Res. Hum. Retrovir. 2000, 16, 5–8. [Google Scholar] [CrossRef] [PubMed]
- Nel, L.H.; Le Roux, K.; Atlas, R.M. Meeting the Rabies Control Challenge in South Africa; American Society for Microbiology: Washington, DC, USA, 2009. [Google Scholar]
- Rowan-Nash, A.D.; Korry, B.J.; Mylonakis, E.; Belenky, P. Cross-Domain and Viral Interactions in the Microbiome. Microbiol. Mol. Biol. Rev. 2019, 83. [Google Scholar] [CrossRef] [Green Version]
- Watt, G.; Kantipong, P.; Burnouf, T.; Shikuma, C.; Philpott, S. Natural Scrub Typhus Antibody Suppresses HIV CXCR4(X4) Viruses. Infect. Dis. Rep. 2013, 5, 27–37. [Google Scholar] [CrossRef]
- Dolcini, G.L.; Solana, M.E.; Andreani, G.; Celentano, A.M.; Parodi, L.M.; Donato, A.M.; Elissondo, N.; Cappa, S.M.G.; Giavedoni, L.D.; Peralta, L.M. Trypanosoma Cruzi (Chagas’ Disease Agent) Reduces HIV-1 Replication in Human Placenta. Retrovirology 2008, 5, 53. [Google Scholar] [CrossRef] [Green Version]
- Downs, J.A.; Dupnik, K.M.; van Dam, G.J.; Urassa, M.; Lutonja, P.; Kornelis, D.; de Dood, C.J.; Hoekstra, P.; Kanjala, C.; Isingo, R.; et al. Effects of Schistosomiasis on Susceptibility to HIV-1 Infection and HIV-1 Viral Load at HIV-1 Seroconversion: A Nested Case-Control Study. PLoS Negl. Trop. Dis. 2017, 11, e0005968. [Google Scholar] [CrossRef]
- Dadras, O.; Alinaghi, S.A.S.; Karimi, A.; MohsseniPour, M.; Barzegary, A.; Vahedi, F.; Pashaei, Z.; Mirzapour, P.; Fakhfouri, A.; Zargari, G.; et al. Effects of COVID-19 Prevention Procedures on Other Common Infections: A Systematic Review. Eur. J. Med. Res. 2021, 26, 67. [Google Scholar] [CrossRef]
- Gerber, W.; Steyn, J.D.; Kotzé, A.F.; Hamman, J.H. Beneficial Pharmacokinetic Drug Interactions: A Tool to Improve the Bioavailability of Poorly Permeable Drugs. Pharmaceutics 2018, 10, 106. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Edwards, D.J. Beneficial Pharmacokinetic Drug Interactions. Adv. Pharmacoepidemiol. Drug Saf. 2012, 1, 1–5. [Google Scholar] [CrossRef] [Green Version]
- Kwenti, T.E. Malaria and HIV Coinfection in Sub-Saharan Africa: Prevalence, Impact, and Treatment Strategies. Res. Rep. Trop. Med. 2018, 9, 123–136. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Obebe, O.O.; Falohun, O.O. Epidemiology of Malaria among HIV/AIDS Patients in Sub-Saharan Africa: A Systematic Review and Meta-Analysis of Observational Studies. Acta Trop. 2021, 215, 105798. [Google Scholar] [CrossRef] [PubMed]
- Van Geertruyden, J.-P. Interactions between Malaria and Human Immunodeficiency Virus Anno 2014. Clin. Microbiol. Infect. 2014, 20, 278–285. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Conant, K.L.; Kaleeba, J.A.R. Dangerous Liaisons: Molecular Basis for a Syndemic Relationship between Kaposi’s Sarcoma and P. Falciparum Malaria. Front. Microbiol. 2013, 4, 35. [Google Scholar] [CrossRef] [Green Version]
- Mbachu, I.I.; Ejikunle, S.D.; Anolue, F.; Mbachu, C.N.; Dike, E.; Ejikem, E.; Okeudo, C. Relationship between Placenta Malaria and Mother to Child Transmission of HIV Infection in Pregnant Women in South East Nigeria. Malar. J. 2020, 19, 97. [Google Scholar] [CrossRef]
- Alemu, A.; Shiferaw, Y.; Addis, Z.; Mathewos, B.; Birhan, W. Effect of Malaria on HIV/AIDS Transmission and Progression. Parasites Vectors 2013, 6, 18. [Google Scholar] [CrossRef] [Green Version]
- Berg, A.; Patel, S.; Aukrust, P.; David, C.; Gonca, M.; Berg, E.S.; Dalen, I.; Langeland, N. Increased Severity and Mortality in Adults Co-Infected with Malaria and HIV in Maputo, Mozambique: A Prospective Cross-Sectional Study. PLoS ONE 2014, 9, e88257. [Google Scholar] [CrossRef] [Green Version]
- ter Kuile, F.O.; Parise, M.E.; Verhoeff, F.H.; Udhayakumar, V.; Newman, R.D.; van Eijk, A.M.; Rogerson, S.J.; Steketee, R.W. The Burden of Co-Infection with Human Immunodeficiency Virus Type 1 and Malaria in Pregnant Women in Sub-Saharan Africa. Am. J. Trop. Med. Hyg. 2004, 71, 41–54. [Google Scholar] [CrossRef] [Green Version]
- Van Geertruyden, J.-P.; Menten, J.; Colebunders, R.; Korenromp, E.; D’Alessandro, U. The Impact of HIV-1 on the Malaria Parasite Biomass in Adults in Sub-Saharan Africa Contributes to the Emergence of Antimalarial Drug Resistance. Malar. J. 2008, 7, 134. [Google Scholar] [CrossRef] [Green Version]
- Abu-Raddad, L.J.; Patnaik, P.; Kublin, J.G. Dual Infection with HIV and Malaria Fuels the Spread of Both Diseases in Sub-Saharan Africa. Science 2006, 314, 1603–1606. [Google Scholar] [CrossRef]
- Zhan, X.-Y.; Wang, N.; Liu, G.; Qin, L.; Xu, W.; Zhao, S.; Qin, L.; Chen, X. Plasmodium Infection Reduces the Volume of the Viral Reservoir in SIV-Infected Rhesus Macaques Receiving Antiretroviral Therapy. Retrovirology 2014, 11, 112. [Google Scholar] [CrossRef] [PubMed]
- Organisation Mondiale de la Santé. Guidelines for the Treatment of Malaria; World Health Organization: Geneva, Switzerland, 2015. [Google Scholar]
- Machado, M.; Sanches-Vaz, M.; Cruz, J.P.; Mendes, A.M.; Prudêncio, M. Inhibition of Plasmodium Hepatic Infection by Antiretroviral Compounds. Front. Cell. Infect. Microbiol. 2017, 7, 329. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, X.; He, Z.; Chen, L.; Li, Y.; Li, Q.; Zhao, S.; Tao, Z.; Hu, W.; Qin, L.; Chen, X. Synergy of the Antiretroviral Protease Inhibitor Indinavir and Chloroquine against Malaria Parasites in Vitro and in Vivo. Parasitol. Res. 2011, 109, 1519–1524. [Google Scholar] [CrossRef] [PubMed]
- He, Z.; Chen, L.; You, J.; Qin, L.; Chen, X. In Vitro Interactions between Antiretroviral Protease Inhibitors and Artemisinin Endoperoxides against Plasmodium Falciparum. Int. J. Antimicrob. Agents 2010, 35, 191–193. [Google Scholar] [CrossRef] [PubMed]
- Hobbs, C.V.; Tanaka, T.Q.; Muratova, O.; Van Vliet, J.; Borkowsky, W.; Williamson, K.C.; Duffy, P.E. HIV Treatments Have Malaria Gametocyte Killing and Transmission Blocking Activity. J. Infect. Dis. 2013, 208, 139–148. [Google Scholar] [CrossRef] [Green Version]
- He, Z.; Chen, L.; You, J.; Qin, L.; Chen, X. Antiretroviral Protease Inhibitors Potentiate Chloroquine Antimalarial Activity in Malaria Parasites by Regulating Intracellular Glutathione Metabolism. Exp. Parasitol. 2009, 123, 122–127. [Google Scholar] [CrossRef]
- Abiodun, O.O.; Gbimadee, N.; Gbotosho, G.O. Lopinavir/Ritonavir Enhanced the Antimalarial Activity of Amodiaquine and Artesunate in a Mouse Model of Plasmodium Berghei. J. Chemother. 2016, 28, 482–486. [Google Scholar] [CrossRef] [PubMed]
- Martin, R.E.; Butterworth, A.S.; Gardiner, D.L.; Kirk, K.; McCarthy, J.S.; Skinner-Adams, T.S. Saquinavir Inhibits the Malaria Parasite’s Chloroquine Resistance Transporter. Antimicrob. Agents Chemother. 2012, 56, 2283–2289. [Google Scholar] [CrossRef] [Green Version]
- Al-Bari, M.A.A. Chloroquine Analogues in Drug Discovery: New Directions of Uses, Mechanisms of Actions and Toxic Manifestations from Malaria to Multifarious Diseases. J. Antimicrob. Chemother. 2015, 70, 1608–1621. [Google Scholar] [CrossRef] [Green Version]
- Mishra, L.C.; Bhattacharya, A.; Sharma, M.; Bhasin, V.K. HIV Protease Inhibitors, Indinavir or Nelfinavir, Augment Antimalarial Action of Artemisinin in Vitro. Am. J. Trop. Med. Hyg. 2010, 82, 148–150. [Google Scholar] [CrossRef]
- Nathoo, S.; Serghides, L.; Kain, K.C. Effect of HIV-1 Antiretroviral Drugs on Cytoadherence and Phagocytic Clearance of Plasmodium Falciparum-Parasitised Erythrocytes. Lancet 2003, 362, 1039–1041. [Google Scholar] [CrossRef]
- Achan, J.; Kakuru, A.; Ikilezi, G.; Ruel, T.; Clark, T.D.; Nsanzabana, C.; Charlebois, E.; Aweeka, F.; Dorsey, G.; Rosenthal, P.J.; et al. Antiretroviral Agents and Prevention of Malaria in HIV-Infected Ugandan Children. N. Engl. J. Med. 2012, 367, 2110–2118. [Google Scholar] [CrossRef] [Green Version]
- Kasirye, R.P.; Grosskurth, H.; Munderi, P.; Levin, J.; Anywaine, Z.; Nunn, A.; Kamali, A.; Baisley, K. Effect of Antiretroviral Therapy on Malaria Incidence in HIV-Infected Ugandan Adults. AIDS 2017, 31, 577–582. [Google Scholar] [CrossRef] [PubMed]
- Plantone, D.; Koudriavtseva, T. Current and Future Use of Chloroquine and Hydroxychloroquine in Infectious, Immune, Neoplastic, and Neurological Diseases: A Mini-Review. Clin. Drug Investig. 2018, 38, 653–671. [Google Scholar] [CrossRef] [PubMed]
- Kiang, T.K.L.; Wilby, K.J.; Ensom, M.H.H. Clinical Pharmacokinetic Drug Interactions Associated with Artemisinin Derivatives and HIV-Antivirals. Clin. Pharmacokinet. 2014, 53, 141–153. [Google Scholar] [CrossRef] [PubMed]
- Calcagno, A.; Cusato, J.; D’Avolio, A.; Bonora, S. Genetic Polymorphisms Affecting the Pharmacokinetics of Antiretroviral Drugs. Clin. Pharmacokinet. 2017, 56, 355–369. [Google Scholar] [CrossRef]
- Abdullahi, S.T.; Soyinka, J.O.; Olagunju, A.; Bolarinwa, R.A.; Olarewaju, O.J.; Bakare-Odunola, M.T.; Winterberg, M.; Tarning, J.; Owen, A.; Khoo, S. Differential Impact of Nevirapine on Artemether-Lumefantrine Pharmacokinetics in Individuals Stratified by CYP2B6 c.516G>T Genotypes. Antimicrob. Agents Chemother. 2019, 64. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Walimbwa, S.I.; Lamorde, M.; Waitt, C.; Kaboggoza, J.; Else, L.; Byakika-Kibwika, P.; Amara, A.; Gini, J.; Winterberg, M.; Chiong, J.; et al. Drug Interactions between Dolutegravir and Artemether-Lumefantrine or Artesunate-Amodiaquine. Antimicrob. Agents Chemother. 2019, 63. [Google Scholar] [CrossRef] [Green Version]
- Kone, A.; van de Vegte-Bolmer, M.; Siebelink-Stoter, R.; van Gemert, G.-J.; Dara, A.; Niangaly, H.; Luty, A.; Doumbo, O.K.; Sauerwein, R.; Djimde, A.A. Sulfadoxine-Pyrimethamine Impairs Plasmodium Falciparum Gametocyte Infectivity and Anopheles Mosquito Survival. Int. J. Parasitol. 2010, 40, 1221–1228. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mbeye, N.; ter Kuile, F.O.; Davies, M.-A.; Phiri, K.; Egger, M.; Wandeler, G. Cotrimoxazole Prophylactic Treatment Prevents Malaria in Children in Sub-Saharan Africa: Systematic Review and Meta-Analysis. Trop. Med. Int. Health 2014, 19, 1057–1067. [Google Scholar] [CrossRef] [Green Version]
- Manyando, C.; Njunju, E.M.; D’Alessandro, U.; Van Geertruyden, J.-P. Safety and Efficacy of Co-Trimoxazole for Treatment and Prevention of Plasmodium Falciparum Malaria: A Systematic Review. PLoS ONE 2013, 8, e56916. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kasirye, R.P.; Baisley, K.; Munderi, P.; Levin, J.; Anywaine, Z.; Nunn, A.; Kamali, A.; Grosskurth, H. Incidence of Malaria by Cotrimoxazole Use in HIV-Infected Ugandan Adults on Antiretroviral Therapy: A Randomised, Placebo-Controlled Study. AIDS 2016, 30, 635–644. [Google Scholar] [CrossRef] [Green Version]
- WHO. Global Tuberculosis Report 2019. Available online: http://www.who.int/tb/publications/global_report/en/ (accessed on 9 August 2020).
- Shankar, E.M.; Vignesh, R.; Ellegård, R.; Barathan, M.; Chong, Y.K.; Bador, M.K.; Rukumani, D.V.; Sabet, N.S.; Kamarulzaman, A.; Velu, V.; et al. HIV-Mycobacterium Tuberculosis Co-Infection: A “danger-Couple Model” of Disease Pathogenesis. Pathog. Dis. 2014, 70, 110–118. [Google Scholar] [CrossRef] [Green Version]
- Pawlowski, A.; Jansson, M.; Sköld, M.; Rottenberg, M.E.; Källenius, G. Tuberculosis and HIV Co-Infection. PLoS Pathog. 2012, 8, e1002464. [Google Scholar] [CrossRef] [PubMed]
- Toor, J.S.; Singh, S.; Sharma, A.; Arora, S.K. Mycobacterium Tuberculosis Modulates the Gene Interactions to Activate the HIV Replication and Faster Disease Progression in a Co-Infected Host. PLoS ONE 2014, 9, e106815. [Google Scholar] [CrossRef] [Green Version]
- Diedrich, C.R.; O’Hern, J.; Wilkinson, R.J. HIV-1 and the Mycobacterium Tuberculosis Granuloma: A Systematic Review and Meta-Analysis. Tuberculosis 2016, 98, 62–76. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Naqvi, K.F.; Endsley, J.J. Myeloid C-Type Lectin Receptors in Tuberculosis and HIV Immunity: Insights Into Co-Infection? Front. Cell. Infect. Microbiol. 2020, 10, 263. [Google Scholar] [CrossRef]
- Kwan, C.K.; Ernst, J.D. HIV and Tuberculosis: A Deadly Human Syndemic. Clin. Microbiol. Rev. 2011, 24, 351–376. [Google Scholar] [CrossRef] [Green Version]
- Daskapan, A.; Idrus, L.R.; Postma, M.J.; Wilffert, B.; Kosterink, J.G.W.; Stienstra, Y.; Touw, D.J.; Andersen, A.B.; Bekker, A.; Denti, P.; et al. A Systematic Review on the Effect of HIV Infection on the Pharmacokinetics of First-Line Tuberculosis Drugs. Clin. Pharmacokinet. 2019, 58, 747–766. [Google Scholar] [CrossRef] [Green Version]
- Motta, I.; Calcagno, A.; Bonora, S. Pharmacokinetics and Pharmacogenetics of Anti-Tubercular Drugs: A Tool for Treatment Optimization? Expert Opin. Drug Metab. Toxicol. 2018, 14, 59–82. [Google Scholar] [CrossRef]
- López-Cortés, L.F.; Ruiz-Valderas, R.; Viciana, P.; Alarcón-González, A.; Gómez-Mateos, J.; León-Jimenez, E.; Sarasanacenta, M.; López-Pua, Y.; Pachón, J. Pharmacokinetic Interactions between Efavirenz and Rifampicin in HIV-Infected Patients with Tuberculosis. Clin. Pharmacokinet. 2002, 41, 681–690. [Google Scholar] [CrossRef]
- Dandara, C.; Swart, M.; Mpeta, B.; Wonkam, A.; Masimirembwa, C. Cytochrome P450 Pharmacogenetics in African Populations: Implications for Public Health. Expert. Opin. Drug Metab. Toxicol. 2014, 10, 769–785. [Google Scholar] [CrossRef]
- Gounden, V.; van Niekerk, C.; Snyman, T.; George, J.A. Presence of the CYP2B6 516G> T Polymorphism, Increased Plasma Efavirenz Concentrations and Early Neuropsychiatric Side Effects in South African HIV-Infected Patients. AIDS Res. Ther. 2010, 7, 32. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kitabi, E.N.; Minzi, O.M.S.; Mugusi, S.; Sasi, P.; Janabi, M.; Mugusi, F.; Bertilsson, L.; Burhenne, J.; Aklillu, E. Long-Term Efavirenz Pharmacokinetics Is Comparable between Tanzanian HIV and HIV/Tuberculosis Patients with the Same CYP2B6*6 Genotype. Sci. Rep. 2018, 8, 16316. [Google Scholar] [CrossRef] [PubMed]
- Court, M.H.; Almutairi, F.E.; Greenblatt, D.J.; Hazarika, S.; Sheng, H.; Klein, K.; Zanger, U.M.; Bourgea, J.; Patten, C.J.; Kwara, A. Isoniazid Mediates the CYP2B6*6 Genotype-Dependent Interaction between Efavirenz and Antituberculosis Drug Therapy through Mechanism-Based Inactivation of CYP2A6. Antimicrob. Agents Chemother. 2014, 58, 4145–4152. [Google Scholar] [CrossRef] [Green Version]
- Kaboggoza, J.P.; Wang, X.; Neary, M.; Ayuso, P.; Sekaggya-Wiltshire, C.; Nakalema, S.; Owen, A.; McClure, M.; Lamorde, M.; Boffito, M. A Lower Dose of Efavirenz Can Be Coadministered with Rifampicin and Isoniazid in Tuberculosis Patients. Open Forum Infect. Dis. 2019, 6, ofz035. [Google Scholar] [CrossRef]
- O’Donnell, M.R.; Padayatchi, N.; Metcalfe, J.Z. Elucidating the Role of Clofazimine for the Treatment of Tuberculosis. Int. J. Tuberc. Lung Dis. 2016, 20, 52–57. [Google Scholar] [CrossRef]
- WHO. Consolidated Guidelines on Tuberculosis, Module 4: Treatment—Drug-Resistant Tuberculosis Treatment. Available online: https://www.who.int/publications-detail-redirect/9789240007048 (accessed on 9 August 2020).
- Singh, D.K.; Dwivedi, V.P.; Ranganathan, A.; Bishai, W.R.; Van Kaer, L.; Das, G. Blockade of the Kv1.3 K+ Channel Enhances BCG Vaccine Efficacy by Expanding Central Memory T Lymphocytes. J. Infect. Dis. 2016, 214, 1456–1464. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, H.; Liu, J.; Xu, E.; Tu, G.; Guo, M.; Liang, S.; Xiong, H. Human Immunodeficiency Virus Protein Tat Induces Oligodendrocyte Injury by Enhancing Outward K+ Current Conducted by KV1.3. Neurobiol. Dis. 2017, 97, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Chen, W.; Mook, R.A.; Premont, R.T.; Wang, J. Niclosamide: Beyond an Antihelminthic Drug. Cell Signal 2018, 41, 89–96. [Google Scholar] [CrossRef]
- Sun, Z.; Zhang, Y. Antituberculosis Activity of Certain Antifungal and Antihelmintic Drugs. Tuber. Lung Dis. 1999, 79, 319–320. [Google Scholar] [CrossRef]
- Berube, B.J.; Castro, L.; Russell, D.; Ovechkina, Y.; Parish, T. Novel Screen to Assess Bactericidal Activity of Compounds Against Non-Replicating Mycobacterium Abscessus. Front. Microbiol. 2018, 9, 2417. [Google Scholar] [CrossRef] [Green Version]
- Fan, X.; Xu, J.; Files, M.; Cirillo, J.D.; Endsley, J.J.; Zhou, J.; Endsley, M.A. Dual Activity of Niclosamide to Suppress Replication of Integrated HIV-1 and Mycobacterium Tuberculosis (Beijing). Tuberculosis 2019, 116S, S28–S33. [Google Scholar] [CrossRef]
- Adjemian, J.; Daniel-Wayman, S.; Ricotta, E.; Prevots, D.R. Epidemiology of Nontuberculous Mycobacteriosis. Semin. Respir. Crit. Care Med. 2018, 39, 325–335. [Google Scholar] [CrossRef] [PubMed]
- Hoefsloot, W.; van Ingen, J.; Andrejak, C.; Angeby, K.; Bauriaud, R.; Bemer, P.; Beylis, N.; Boeree, M.J.; Cacho, J.; Chihota, V.; et al. The Geographic Diversity of Nontuberculous Mycobacteria Isolated from Pulmonary Samples: An NTM-NET Collaborative Study. Eur. Respir. J. 2013, 42, 1604–1613. [Google Scholar] [CrossRef]
- Bonard, D.; Messou, E.; Seyler, C.; Vincent, V.; Gabillard, D.; Anglaret, X. High Incidence of Atypical Mycobacteriosis in African HIV-Infected Adults with Low CD4 Cell Counts: A 6-Year Cohort Study in Côte d’Ivoire. AIDS 2004, 18, 1961–1964. [Google Scholar] [CrossRef] [PubMed]
- Nyamogoba, H.D.; Mbuthia, G.; Mining, S.; Kikuvi, G.; Biegon, R.; Mpoke, S.; Menya, D.; Waiyaki, P.G. HIV Co-Infection with Tuberculous and Non-Tuberculous Mycobacteria in Western Kenya: Challenges in the Diagnosis and Management. Afr. Health Sci. 2012, 12, 305–311. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aliyu, G.; El-Kamary, S.S.; Abimiku, A.; Brown, C.; Tracy, K.; Hungerford, L.; Blattner, W. Prevalence of Non-Tuberculous Mycobacterial Infections among Tuberculosis Suspects in Nigeria. PLoS ONE 2013, 8, e63170. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Okoi, C.; Anderson, S.T.B.; Antonio, M.; Mulwa, S.N.; Gehre, F.; Adetifa, I.M.O. Non-Tuberculous Mycobacteria Isolated from Pulmonary Samples in Sub-Saharan Africa—A Systematic Review and Meta Analyses. Sci. Rep. 2017, 7, 12002. [Google Scholar] [CrossRef] [Green Version]
- Yangco, B.G.; Buchacz, K.; Baker, R.; Palella, F.J.; Armon, C.; Brooks, J.T. HIV Outpatient Study Investigators. Is Primary Mycobacterium Avium Complex Prophylaxis Necessary in Patients with CD4 <50 Cells/ΜL Who Are Virologically Suppressed on CART? AIDS Patient Care STDS 2014, 28, 280–283. [Google Scholar] [CrossRef] [Green Version]
- Rosenthal, P.J. Azithromycin for Malaria? Am. J. Trop. Med. Hyg. 2016, 95, 2–4. [Google Scholar] [CrossRef] [Green Version]
- Keenan, J.D.; Bailey, R.L.; West, S.K.; Arzika, A.M.; Hart, J.; Weaver, J.; Kalua, K.; Mrango, Z.; Ray, K.J.; Cook, C.; et al. Azithromycin to Reduce Childhood Mortality in Sub-Saharan Africa. N. Engl. J. Med. 2018, 378, 1583–1592. [Google Scholar] [CrossRef]
- Ferrand, R.A.; McHugh, G.; Rehman, A.M.; Mujuru, H.; Simms, V.; Majonga, E.D.; Nicol, M.P.; Flaegstad, T.; Gutteberg, T.J.; Gonzalez-Martinez, C.; et al. Effect of Once-Weekly Azithromycin vs Placebo in Children With HIV-Associated Chronic Lung Disease: The BREATHE Randomized Clinical Trial. JAMA Netw. Open 2020, 3, e2028484. [Google Scholar] [CrossRef]
- Jones, J.; Weiss, K.; Mermin, J.; Dietz, P.; Rosenberg, E.S.; Gift, T.L.; Chesson, H.; Sullivan, P.S.; Lyles, C.; Bernstein, K.T.; et al. Proportion of Incident Human Immunodeficiency Virus Cases Among Men Who Have Sex With Men Attributable to Gonorrhea and Chlamydia: A Modeling Analysis. Sex Transm. Dis. 2019, 46, 357–363. [Google Scholar] [CrossRef]
- Yang, C.-J.; Tang, H.-J.; Chang, S.-Y.; Hsieh, S.-M.; Lee, K.-Y.; Lee, Y.-T.; Sheng, W.-H.; Yang, S.-P.; Hung, C.-C.; Chang, S.-C. Comparison of Serological Responses to Single-Dose Azithromycin (2 g) versus Benzathine Penicillin G in the Treatment of Early Syphilis in HIV-Infected Patients in an Area of Low Prevalence of Macrolide-Resistant Treponema Pallidum Infection. J. Antimicrob. Chemother. 2016, 71, 775–782. [Google Scholar] [CrossRef] [Green Version]
- Gonzalez-Martinez, C.; Kranzer, K.; McHugh, G.; Corbett, E.L.; Mujuru, H.; Nicol, M.P.; Rowland-Jones, S.; Rehman, A.M.; Gutteberg, T.J.; Flaegstad, T.; et al. Azithromycin versus Placebo for the Treatment of HIV-Associated Chronic Lung Disease in Children and Adolescents (BREATHE Trial): Study Protocol for a Randomised Controlled Trial. Trials 2017, 18, 1–18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kimani, J.; Phiri, K.; Kamiza, S.; Duparc, S.; Ayoub, A.; Rojo, R.; Robbins, J.; Orrico, R.; Vandenbroucke, P. Efficacy and Safety of Azithromycin-Chloroquine versus Sulfadoxine-Pyrimethamine for Intermittent Preventive Treatment of Plasmodium Falciparum Malaria Infection in Pregnant Women in Africa: An Open-Label, Randomized Trial. PLoS ONE 2016, 11, e0157045. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Akinyotu, O.; Bello, F.; Abdus-Salam, R.; Arowojolu, A. A Randomized Controlled Trial of Azithromycin and Sulphadoxine-Pyrimethamine as Prophylaxis against Malaria in Pregnancy among Human Immunodeficiency Virus-Positive Women. Trans. R. Soc. Trop. Med. Hyg. 2019, 113, 463–470. [Google Scholar] [CrossRef] [PubMed]
- Siddharthan, T.; Grigsby, M.R.; Goodman, D.; Chowdhury, M.; Rubinstein, A.; Irazola, V.; Gutierrez, L.; Miranda, J.J.; Bernabe-Ortiz, A.; Alam, D.; et al. Association between Household Air Pollution Exposure and Chronic Obstructive Pulmonary Disease Outcomes in 13 Low- and Middle-Income Country Settings. Am. J. Respir. Crit. Care Med. 2018, 197, 611–620. [Google Scholar] [CrossRef]
- Hotez, P.J.; Fenwick, A.; Savioli, L.; Molyneux, D.H. Rescuing the Bottom Billion through Control of Neglected Tropical Diseases. Lancet 2009, 373, 1570–1575. [Google Scholar] [CrossRef]
- Hotez, P.J.; Kamath, A. Neglected Tropical Diseases in Sub-Saharan Africa: Review of Their Prevalence, Distribution, and Disease Burden. PLoS Negl. Trop. Dis. 2009, 3, e412. [Google Scholar] [CrossRef] [Green Version]
- Means, A.R.; Burns, P.; Sinclair, D.; Walson, J.L. Antihelminthics in Helminth-Endemic Areas: Effects on HIV Disease Progression. Cochrane Database Syst. Rev. 2016, 4, CD006419. [Google Scholar] [CrossRef] [Green Version]
- Brown, M.; Kizza, M.; Watera, C.; Quigley, M.A.; Rowland, S.; Hughes, P.; Whitworth, J.A.G.; Elliott, A.M. Helminth Infection Is Not Associated with Faster Progression of HIV Disease in Coinfected Adults in Uganda. J. Infect. Dis. 2004, 190, 1869–1879. [Google Scholar] [CrossRef] [Green Version]
- Ndeffo Mbah, M.L.; Gilbert, J.A.; Galvani, A.P. Evaluating the Potential Impact of Mass Praziquantel Administration for HIV Prevention in Schistosoma Haematobium High-Risk Communities. Epidemics 2014, 7, 22–27. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gallagher, M.; Malhotra, I.; Mungai, P.L.; Wamachi, A.N.; Kioko, J.M.; Ouma, J.H.; Muchiri, E.; King, C.L. The Effects of Maternal Helminth and Malaria Infections on Mother-to-Child HIV Transmission. AIDS 2005, 19, 1849–1855. [Google Scholar] [CrossRef] [PubMed]
- Siegel, M.O.; Simon, G.L. Is Human Immunodeficiency Virus Infection a Risk Factor for Strongyloides Stercoralis Hyperinfection and Dissemination. PLoS Negl. Trop. Dis. 2012, 6, e1581. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Slater, H.C.; Foy, B.D.; Kobylinski, K.; Chaccour, C.; Watson, O.J.; Hellewell, J.; Aljayyoussi, G.; Bousema, T.; Burrows, J.; D’Alessandro, U.; et al. Ivermectin as a Novel Complementary Malaria Control Tool to Reduce Incidence and Prevalence: A Modelling Study. Lancet Infect. Dis. 2020, 20, 498–508. [Google Scholar] [CrossRef]
- Ballent, M.; Maté, L.; Virkel, G.; Sallovitz, J.; Viviani, P.; Lanusse, C.; Lifschitz, A. Intestinal Drug Transport: Ex Vivo Evaluation of the Interactions between ABC Transporters and Anthelmintic Molecules. J. Vet. Pharmacol. Ther. 2014, 37, 332–337. [Google Scholar] [CrossRef]
- Lespine, A.; Ménez, C.; Bourguinat, C.; Prichard, R.K. P-Glycoproteins and Other Multidrug Resistance Transporters in the Pharmacology of Anthelmintics: Prospects for Reversing Transport-Dependent Anthelmintic Resistance. Int. J. Parasitol. Drugs Drug Resist. 2012, 2, 58–75. [Google Scholar] [CrossRef] [PubMed]
- Kigen, G.; Edwards, G. Drug-Transporter Mediated Interactions between Anthelminthic and Antiretroviral Drugs across the Caco-2 Cell Monolayers. BMC Pharmacol. Toxicol. 2017, 18, 20. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wagstaff, K.M.; Sivakumaran, H.; Heaton, S.M.; Harrich, D.; Jans, D.A. Ivermectin Is a Specific Inhibitor of Importin α/β-Mediated Nuclear Import Able to Inhibit Replication of HIV-1 and Dengue Virus. Biochem. J. 2012, 443, 851–856. [Google Scholar] [CrossRef] [Green Version]
- Gekonge, B.; Bardin, M.C.; Montaner, L.J. Short Communication: Nitazoxanide Inhibits HIV Viral Replication in Monocyte-Derived Macrophages. AIDS Res. Hum. Retroviruses 2015, 31, 237–241. [Google Scholar] [CrossRef] [Green Version]
- Trabattoni, D.; Gnudi, F.; Ibba, S.V.; Saulle, I.; Agostini, S.; Masetti, M.; Biasin, M.; Rossignol, J.-F.; Clerici, M. Thiazolides Elicit Anti-Viral Innate Immunity and Reduce HIV Replication. Sci. Rep. 2016, 6, 27148. [Google Scholar] [CrossRef] [Green Version]
- Hautala, T.J.; Perelygina, L.; Vuorinen, T.; Hautala, N.M.; Hägg, P.M.; Bode, M.K.; Rusanen, H.T.; Renko, M.H.; Glumoff, V.; Schwab, N.; et al. Nitazoxanide May Modify the Course of Progressive Multifocal Leukoencephalopathy. J. Clin. Immunol. 2018, 38, 4–6. [Google Scholar] [CrossRef] [Green Version]
- Ivan, E.; Crowther, N.J.; Rucogoza, A.T.; Osuwat, L.O.; Munyazesa, E.; Mutimura, E.; Njunwa, K.J.; Zambezi, K.J.B.; Grobusch, M.P. Malaria and Helminthic Co-Infection among HIV-Positive Pregnant Women: Prevalence and Effects of Antiretroviral Therapy. Acta Trop. 2012, 124, 179–184. [Google Scholar] [CrossRef] [PubMed]
- Nugent, R.; Bertram, M.Y.; Jan, S.; Niessen, L.W.; Sassi, F.; Jamison, D.T.; Pier, E.G.; Beaglehole, R. Investing in Non-Communicable Disease Prevention and Management to Advance the Sustainable Development Goals. Lancet 2018, 391, 2029–2035. [Google Scholar] [CrossRef]
- Campbell, N.R.C.; Bovet, P.; Schutte, A.E.; Lemogoum, D.; Nkwescheu, A.S. High Blood Pressure in Sub-Saharan Africa: Why Prevention, Detection, and Control Are Urgent and Important. J. Clin. Hypertens. 2015, 17, 663–667. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Andrade, S.E.; Gurwitz, J.H.; Field, T.S.; Kelleher, M.; Majumdar, S.R.; Reed, G.; Black, R. Hypertension Management: The Care Gap between Clinical Guidelines and Clinical Practice. Am. J. Manag. Care 2004, 10, 481–486. [Google Scholar]
- Peyriere, H.; Eiden, C.; Macia, J.-C.; Reynes, J. Antihypertensive Drugs in Patients Treated with Antiretrovirals. Ann. Pharmacother. 2012, 46, 703–709. [Google Scholar] [CrossRef] [PubMed]
- Pushpakom, S.P.; Adaikalakoteswari, A.; Owen, A.; Back, D.J.; Tripathi, G.; Kumar, S.; McTernan, P.; Pirmohamed, M. Telmisartan Reverses Antiretroviral-Induced Adipocyte Toxicity and Insulin Resistance in Vitro. Diab. Vasc. Dis. Res. 2018, 15, 233–242. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rai, P.; Lederman, R.; Haque, S.; Rehman, S.; Kumar, V.; Sataranatrajan, K.; Malhotra, A.; Kasinath, B.S.; Singhal, P.C. Renin Angiotensin System Modulates MTOR Pathway through AT2R in HIVAN. Exp. Mol. Pathol. 2014, 96, 431–437. [Google Scholar] [CrossRef] [Green Version]
- Kalayjian, R.C.; Wu, K.; Evans, S.; Clifford, D.B.; Pallaki, M.; Currier, J.S.; Smurzynski, M. Proteinuria Is Associated with Neurocognitive Impairment in Antiretroviral Therapy Treated HIV-Infected Individuals. J. Acquir. Immune Defic. Syndr. 2014, 67, 30–35. [Google Scholar] [CrossRef] [Green Version]
- Erlandson, K.M.; Kitch, D.; Wester, C.W.; Kalayjian, R.C.; Overton, E.T.; Castillo-Mancilla, J.; Koletar, S.L.; Benson, C.A.; Campbell, T.B.; Robertson, K.; et al. The Impact of Statin and Angiotensin-Converting Enzyme Inhibitor/Angiotensin Receptor Blocker Therapy on Cognitive Function in Adults With Human Immunodeficiency Virus Infection. Clin. Infect. Dis. 2017, 65, 2042–2049. [Google Scholar] [CrossRef] [Green Version]
- Mosepele, M.; Molefe-Baikai, O.J.; Grinspoon, S.K.; Triant, V.A. Benefits and Risks of Statin Therapy in the HIV-Infected Population. Curr. Infect. Dis. Rep. 2018, 20, 20. [Google Scholar] [CrossRef]
- Calza, L.; Colangeli, V.; Borderi, M.; Manfredi, R.; Marconi, L.; Bon, I.; Re, M.C.; Viale, P. Rosuvastatin and Atorvastatin Preserve Renal Function in HIV-1-Infected Patients with Chronic Kidney Disease and Hyperlipidaemia. HIV Clin. Trials. 2018, 19, 120–128. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, M.; Montenont, E.; Hu, L.; Nardi, M.A.; Valdes, V.; Merolla, M.; Gettenberg, G.; Cavanagh, K.; Aberg, J.A.; Bhardwaj, N.; et al. Aspirin Attenuates Platelet Activation and Immune Activation in HIV-1-Infected Subjects on Antiretroviral Therapy: A Pilot Study. J. Acquir. Immune Defic. Syndr. 2013, 63, 280–288. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Falcinelli, E.; Francisci, D.; Schiaroli, E.; Minuz, P.; Orsini, S.; Malincarne, L.; Sebastiano, M.; Mezzasoma, A.M.; Pasticci, M.B.; Guglielmini, G.; et al. Effect of Aspirin Treatment on Abacavir-Associated Platelet Hyperreactivity in HIV-Infected Patients. Int. J. Cardiol. 2018, 263, 118–124. [Google Scholar] [CrossRef] [PubMed]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Trunfio, M.; Scabini, S.; Mornese Pinna, S.; Rugge, W.; Alcantarini, C.; Pirriatore, V.; Di Perri, G.; Bonora, S.; Castelnuovo, B.; Calcagno, A. The Manifesto of Pharmacoenosis: Merging HIV Pharmacology into Pathocoenosis and Syndemics in Developing Countries. Microorganisms 2021, 9, 1648. https://doi.org/10.3390/microorganisms9081648
Trunfio M, Scabini S, Mornese Pinna S, Rugge W, Alcantarini C, Pirriatore V, Di Perri G, Bonora S, Castelnuovo B, Calcagno A. The Manifesto of Pharmacoenosis: Merging HIV Pharmacology into Pathocoenosis and Syndemics in Developing Countries. Microorganisms. 2021; 9(8):1648. https://doi.org/10.3390/microorganisms9081648
Chicago/Turabian StyleTrunfio, Mattia, Silvia Scabini, Simone Mornese Pinna, Walter Rugge, Chiara Alcantarini, Veronica Pirriatore, Giovanni Di Perri, Stefano Bonora, Barbara Castelnuovo, and Andrea Calcagno. 2021. "The Manifesto of Pharmacoenosis: Merging HIV Pharmacology into Pathocoenosis and Syndemics in Developing Countries" Microorganisms 9, no. 8: 1648. https://doi.org/10.3390/microorganisms9081648
APA StyleTrunfio, M., Scabini, S., Mornese Pinna, S., Rugge, W., Alcantarini, C., Pirriatore, V., Di Perri, G., Bonora, S., Castelnuovo, B., & Calcagno, A. (2021). The Manifesto of Pharmacoenosis: Merging HIV Pharmacology into Pathocoenosis and Syndemics in Developing Countries. Microorganisms, 9(8), 1648. https://doi.org/10.3390/microorganisms9081648






