Variation in Sodic Soil Bacterial Communities Associated with Different Alkali Vegetation Types
Abstract
:1. Introduction
2. Materials and Methods
2.1. Description of Sampling Sites
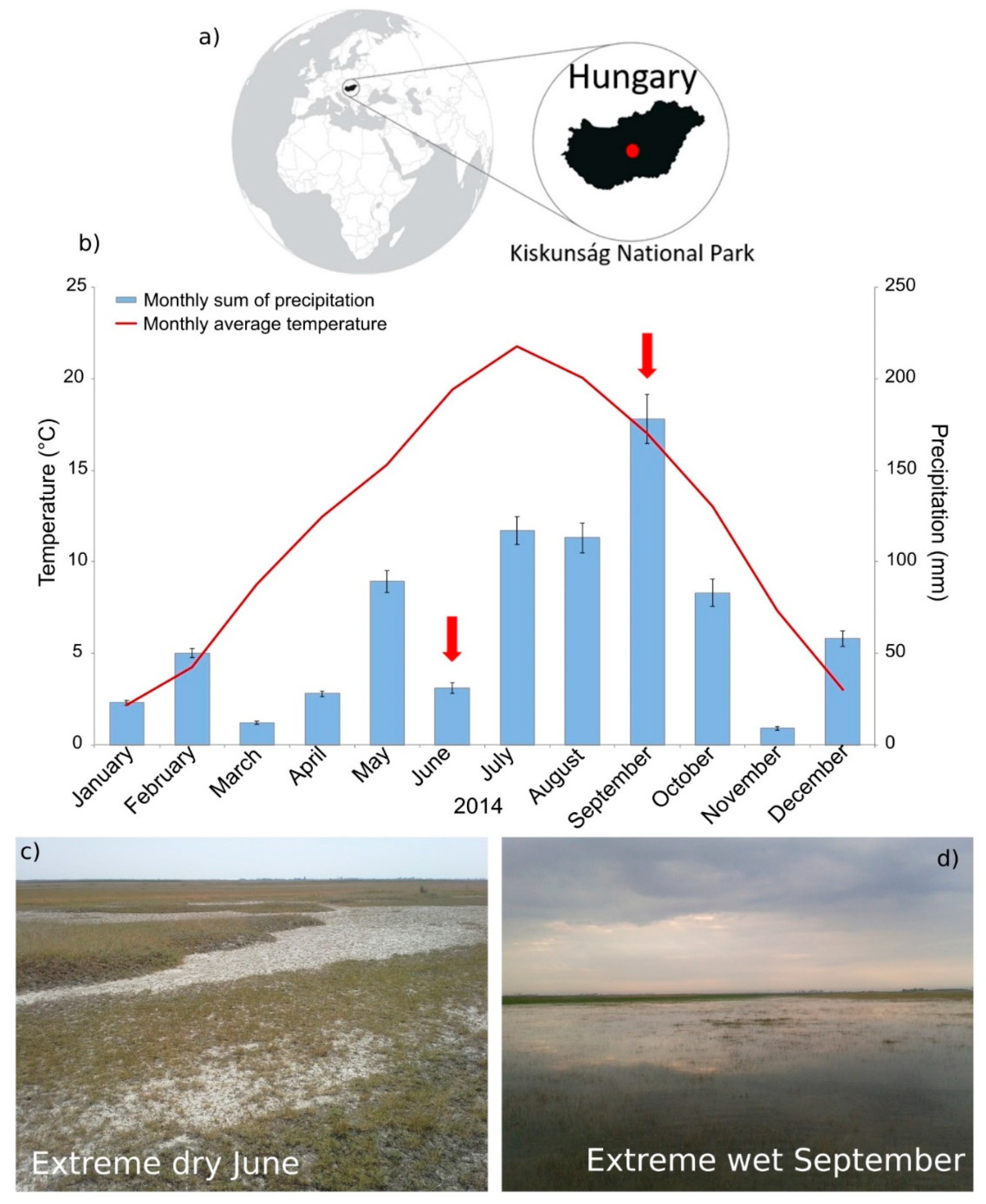
2.2. Weather Conditions, Sampling and Sample Processing
2.3. Physical and Chemical Characterization of the Soil Samples
2.4. Catabolic Activity Profiles
2.5. Cultivation-Based Bacteriological Examinations
2.6. Community DNA Extraction and Pyrosequencing
2.7. Statistical Analyses
3. Results
3.1. Physical and Chemical Characteristics of the Sodic Soils
3.2. Catabolic Activity Profiles
3.3. Diversity of Cultivated Bacterial Strains
3.4. Comparison of Sodic Grassland Soil Bacterial Communities by Pyrosequencing
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Flowers, T.J.; Muscolo, A. Introduction to the special issue: Halophytes in a changing world. AoB Plants 2015, 7, 1–5. [Google Scholar] [CrossRef]
- IUSS Working Group. International soil classification system for naming soils and creating legends for soil maps. In World Reference Base for Soil Resources; FAO: Rome, Italy, 2015; Available online: http://www.fao.org/3/i3794en/I3794en.pdf (accessed on 3 August 2021).
- Dagar, J.C.; Yadav, R.K.; Singh, A.; Singh, N.T. Historical Perspectives and Dynamics of Nature, Extent, Classification and Management of Salt-affected Soils and Waters. In Research Developments in Saline Agriculture; Dagar, J.C., Yadav, R.K., Sharma, P.C., Eds.; Springer: Singapore, 2019; pp. 3–52. [Google Scholar] [CrossRef]
- Mukhtar, S.; Malik, K.A.; Mehnaz, S. Microbiome of Halophytes: Diversity and Importance for Plant Health and Productivity. Microbiol. Biotechnol. Lett. 2019, 47, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Parida, A.K.; Das, A.B. Salt tolerance and salinity effects on plants: A review. Ecotoxicol. Environ. Saf. 2005, 60, 324–349. [Google Scholar] [CrossRef]
- Genitsaris, S.; Stefanidou, N.; Leontidou, K.; Matsi, T.; Karamanoli, K.; Mellidou, I. Bacterial Communities in the Rhizosphere and Phyllosphere of Halophytes and Drought-Tolerant Plants in Mediterranean Ecosystems. Microorganisms 2020, 8, 1708. [Google Scholar] [CrossRef]
- Mavi, M.S.; Marschner, P.; Chittleborough, D.J.; Cox, J.W.; Sanderman, J. Salinity and sodicity affect soil respiration and dissolved organic matter dynamics differentially in soils varying in texture. Soil Biol. Biochem. 2012, 45, 8–13. [Google Scholar] [CrossRef]
- Canfora, L.; Papa, G.L.; Antisari, L.V.; Bazan, G.; Dazzi, C.; Benedetti, A. Spatial microbial community structure and biodiversity analysis in “extreme” hypersaline soils of a semiarid Mediterranean area. Appl. Soil Ecol. 2015, 93, 120–129. [Google Scholar] [CrossRef] [Green Version]
- Canfora, L.; Salvati, L.; Benedetti, A.; Francaviglia, R. Is soil microbial diversity affected by soil and groundwater salinity? Evidences from a coastal system in central Italy. Environ. Monit. Assess. 2017, 189, 319. [Google Scholar] [CrossRef]
- Ma, B.; Gong, J. A meta-analysis of the publicly available bacterial and archaeal sequence diversity in saline soils. World J. Microbiol. Biotechnol. 2013, 29, 2325–2334. [Google Scholar] [CrossRef] [PubMed]
- Xie, K.; Deng, Y.; Zhang, S.; Zhang, W.; Liu, J.; Xie, Y.; Zhang, X.; Huang, H. Prokaryotic Community Distribution along an Ecological Gradient of Salinity in Surface and Subsurface Saline Soils. Sci. Rep. 2017, 7, 13332. [Google Scholar] [CrossRef] [Green Version]
- Molnár, Z.; Borhidi, A. Hungarian alkali vegetation: Origins, landscape history, syntaxonomy, conservation. Phytocoenologia 2003, 33, 377–408. [Google Scholar] [CrossRef]
- Zalatnai, M.; Körmöczi, L.; Tóth, T. Community boundaries and edaphic factors in saline-sodic grassland communities along an elevation gradient. Tiscia 2007, 36, 7–15. [Google Scholar]
- Kelemen, A.; Török, P.; Valkó, O.; Migléc, T.; Tóthmérész, B. Mechanisms shaping plant biomass and species richness: Plant strategies and litter effect in alkali and loess grasslands. J. Veg. Sci. 2013, 24, 1195–1203. [Google Scholar] [CrossRef]
- Kuti, L.; Tóth, T.; Kerék, B. Fluctuation of the groundwater level, and its consequences in the soil-parent material-groundwater system of a sodic grassland. Agrokémia Talajtan 2002, 51, 253–262. [Google Scholar] [CrossRef]
- Ladányi, Z.; Blanka, V.; Deák, Á.J.; Rakonczai, J.; Mezősi, G. Assessment of soil and vegetation changes due to hydrologically driven desalinization process in an alkaline wetland, Hungary. Ecol. Complex. 2016, 25, 1–10. [Google Scholar] [CrossRef]
- Mádl-Szőnyi, J.; Tóth, J. A hydrogeological type section for the Duna-Tisza Interfluve, Hungary. Hydrogeol. J. 2009, 17, 961–980. [Google Scholar] [CrossRef]
- Anda, D.; Szabó, A.; Kovács-Bodor, P.; Makk, J.; Felföldi, T.; Ács, É.; Mádl-Szőnyi, J.; Borsodi, A.K. In situ modelling of biofilm formation in a hydrothermal spring cave. Sci. Rep. 2020, 10, 21733. [Google Scholar] [CrossRef]
- Tóth, T.; Szendrei, G. Types and distribution of salt affected soils in Hungary and the characterisation of the processes of salt accumulation. Topogr. Mineral. Hungariae 2006, 9, 7–20. [Google Scholar]
- Buzás, I. Manual for Soil and Agrochemical Analyses 2: Physico-Chemical and Chemical Analysis of Soils; Mezőgazdasági Kiadó: Budapest, Hungary, 1988. (In Hungarian) [Google Scholar]
- Campbell, C.D.; Chapman, S.J.; Cameron, C.M.; Davidson, M.S.; Potts, J.M. A rapid microtiter plate method to measure carbon dioxide evolved from carbon substrate amendments so as to determine the physiological profiles of soil microbial communities by using whole soil. Appl. Environ. Microbiol. 2003, 69, 3593–3599. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Borsodi, A.K.; Bárány, Á.; Krett, G.; Márialigeti, K.; Szili-Kovács, T. Diversity and ecological tolerance of bacteria isolated from the rhizosphere of halophyton plants living nearby Kiskunság soda ponds, Hungary. Acta Microbiol. Immunol. Hung. 2015, 62, 183–197. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yoon, S.H.; Ha, S.-M.; Kwon, S.; Lim, J.; Kim, Y.; Seo, H.; Chun, J. Introducing EzBioCloud: A taxonomically united database of 16S rRNA gene sequences and whole-genome assemblies. Int. J. Syst. Evol. Microbiol. 2017, 67, 1613–1617. [Google Scholar] [CrossRef]
- Kumar, S.; Stecher, G.; Li, M.; Knyaz, C.; Tamura, K. MEGA X: Molecular Evolutionary Genetics Analysis across computing platforms. Mol. Biol. Evol. 2018, 35, 1547–1549. [Google Scholar] [CrossRef]
- Nei, M.; Kumar, S. Molecular Evolution and Phylogenetics; Oxford University Press: Oxford, UK, 2000. [Google Scholar]
- Szabó, A.; Korponai, K.; Kerekes, C.; Somogyi, B.; Vörös, L.; Bartha, D.; Márialigeti, K.; Felföldi, T. Soda pans of the Pannonian steppe harbor unique bacterial communities adapted to multiple extreme conditions. Extremophiles 2017, 21, 639–649. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schloss, P.D.; Westcott, S.L.; Ryabin, T.; Hall, J.R.; Hartmann, M.; Hollister, E.B.; Lesniewski, R.A.; Oakley, B.B.; Parks, D.H.; Robinson, C.J.; et al. Introducing mothur: Open-source, platform-independent, community-supported software for describing and comparing microbial communities. Appl. Environ. Microbiol. 2009, 75, 7537–7541. [Google Scholar] [CrossRef] [Green Version]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2019; Available online: https://www.R-project.org/ (accessed on 3 August 2021).
- Anderson, M.J. A new method for non-parametric multivariate analysis of variance. Austral. Ecol. 2001, 26, 32–46. [Google Scholar] [CrossRef]
- Oksanen, J.; Blanchet, F.G.; Kindt, R.; Legendre, P.; O’Hara, R.B.; Simpson, G.L.; Solymos, P.; Stevens, M.H.; Wagner, H. Vegan: Community Ecology Package, R Package Version 2.2-0. 2014. Available online: http://CRAN.Rproject.org/package=vegan (accessed on 3 August 2021).
- Anderson, M.J. PERMDISP: A Fortran Computer Program for Permutational Analysis of Multivariate Dispersions (for Any Two-Factor ANOVA Design) Using Permutation Tests; Department of Statistics, University of Auckland: Auckland, New Zealand, 2014. [Google Scholar]
- Wickham, H. ggplot2: Elegant Graphics for Data Analysis; Springer: New York, NY, USA, 2016; Available online: https://ggplot2.tidyverse.org (accessed on 3 August 2021).
- Hammer, Ø.; Harper, D.A.T.; Ryan, P.D. PAST: Paleontological statistics software package for education and data analysis. Paleontol. Electron. 2001, 4, 1–9. [Google Scholar]
- Peres-Neto, P.R.; Legendre, P.; Dray, S.; Borcard, D. Variation partitioning of species data matrices: Estimation and comparison of fractions. Ecology 2006, 87, 2614–2625. [Google Scholar] [CrossRef]
- Andronov, E.E.; Petrova, S.N.; Pinaev, A.G.; Pershina, E.V.; Rakhimgalieva, S.Z.; Akhmedenov, K.M.; Gorobets, A.V.; Sergaliev, N.K. Analysis of the structure of microbial community in soils with different degrees of salinization using T-RFLP and real-time PCR techniques. Eurasian. Soil. Sci. 2012, 45, 147–156. [Google Scholar] [CrossRef]
- Canfora, L.; Bacci, G.; Pinzari, F.; Lo Papa, G.; Dazzi, C.; Benedetti, A. Salinity and bacterial diversity: To what extent does the concentration of salt affect the bacterial community in a saline soil ? PLoS ONE 2014, 9, e106662. [Google Scholar] [CrossRef] [Green Version]
- Ding, G.-C.; Piceno, Y.M.; Heuer, H.; Weinert, N.; Dohrmann, A.B.; Carrillo, A.; Andersen, G.L.; Castellanos, T.; Tebbe, C.C.; Smalla, K. Changes of soil bacterial diversity as a consequence of agricultural land use in a semi-arid ecosystem. PLoS ONE 2013, 8, e59497. [Google Scholar] [CrossRef] [Green Version]
- Paul, D.; Lade, H. Plant-growth-promoting rhizobacteria to improve crop growth in saline soils: A review. Agron. Sustain. Dev. 2014, 34, 737–752. [Google Scholar] [CrossRef]
- Rath, K.M.; Rousk, J. Salt effects on the soil microbial decomposer community and their role in organic carbon cycling: A review. Soil. Biol. Biochem. 2015, 81, 108–123. [Google Scholar] [CrossRef]
- Shrivastava, P.; Kumar, R. Soil salinity: A serious environmental issue and plant growth promoting bacteria as one of the tools for its alleviation. Saudi J. Biol. Sci. 2015, 22, 123–131. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zahran, H.H. Diversity, adaptation and activity of the bacterial flora in saline environments. Biol. Fertil. Soils 1997, 25, 211–223. [Google Scholar] [CrossRef]
- Qin, X.; Tang, J.C.; Li, D.S.; Zhang, Q.M. Effect of salinity on the bioremediation of petroleum hydrocarbons in a saline-alkaline soil. Lett. Appl. Microbiol. 2012, 55, 210–217. [Google Scholar] [CrossRef]
- Tóth, T. Medium-term vegetation dynamics and their association with edaphic conditions in two Hungarian saline grassland communities. Grassl. Sci. 2010, 56, 13–18. [Google Scholar] [CrossRef]
- Bárány, Á.; Szili-Kovács, T.; Krett, G.; Füzy, A.; Márialigeti, K.; Borsodi, A. Metabolic activity and genetic diversity of microbial communities inhabiting the rhizosphere of halophyton plants. Acta Microbiol. Immunol. Hung. 2014, 61, 347–361. [Google Scholar] [CrossRef]
- Yuan, B.C.; Li, Z.Z.; Liu, H.; Gao, M.; Zhang, Y.Y. Microbial biomass and activity in salt affected soils under arid conditions. Appl. Soil Ecol. 2007, 35, 319–328. [Google Scholar] [CrossRef]
- Bérard, A.; Bouchet, T.; Sévenier, G.; Pablo, A.L.; Gros, R. Resilience of soil microbial communities impacted by severe drought and high temperature in the context of Mediterranean heat waves. Eur. J. Soil Biol. 2011, 47, 333–342. [Google Scholar] [CrossRef]
- Borsodi, A.K.; Márialigeti, K.; Szabó, G.; Palatinszky, M.; Pollák, B.; Kéki, Z.; Kovács, A.L.; Schumann, P.; Tóth, E.M.T. Bacillus aurantiacus sp. nov., an alkaliphilic and moderately halophilic bacterium isolated from Hungarian soda lakes. Int. J. Syst. Evol. Microbiol. 2008, 58, 845–851. [Google Scholar] [CrossRef] [Green Version]
- Borsodi, A.K.; Pollák, B.; Kéki, Z.; Rusznyák, A.; Kovács, A.L.; Spröer, C.; Schumann, P.; Márialigeti, K.; Tóth, E.M. Bacillus alkalisediminis sp. nov., an alkaliphilic and moderately halophilic bacterium isolated from sediment of extremely shallow soda ponds. Int. J. Syst. Evol. Microbiol. 2011, 61, 1880–1886. [Google Scholar] [CrossRef] [Green Version]
- Prashar, P.; Kapoor, N.; Sachdeva, S. Rhizosphere: Its structure, bacterial diversity and significance. Rev. Environ. Sci. Biotechnol. 2014, 13, 63–77. [Google Scholar] [CrossRef]
- Rivadeneyra, M.A.; Delgado, R.; Delgado, G.; del Moral, A.; Ferrer, M.R.; Ramos-Cormenzana, A. Precipitation of carbonates by Bacillus sp. isolated from saline soils. Geomicrobiol. J. 1993, 11, 175–184. [Google Scholar] [CrossRef]
- Trujillo-Cabrera, Y.; Ponce-Mendoza, A.; Vásquez-Murrieta, M.S.; Rivera-Orduña, F.N.; Wang, E.T. Diverse cellulolytic bacteria isolated from the high humus, alkaline-saline chinampa soils. Ann. Microbiol. 2013, 63, 779–792. [Google Scholar] [CrossRef]
- Fguira, L.F.-B.; Fotso, S.; Ameur-Mehdi, R.B.; Mellouli, L.; Laatsch, H. Purification and structure elucidation of antifungal and antibacterial activities of newly isolated Streptomyces sp. strain US80. Res. Microbiol. 2005, 156, 341–347. [Google Scholar] [CrossRef]
- Sajid, I.; Yao, C.B.F.F.; Shaaban, K.A.; Hasnain, S.; Laatsch, H. Antifungal and antibacterial activities of indigenous Streptomyces isolates from saline farmlands: Prescreening, ribotyping and metabolic diversity. World J. Microbiol. Biotechnol. 2009, 25, 601–610. [Google Scholar] [CrossRef]
- Sadeghi, A.; Karimi, E.; Dahaji, P.A.; Javid, M.G.; Dalvand, Y.; Askari, H. Plant growth promoting activity of an auxin and siderophore producing isolate of Streptomyces under saline soil conditions. World J. Microbiol. Biotechnol. 2012, 28, 1503–1509. [Google Scholar] [CrossRef]
- Peng, M.; Jia, H.; Wang, Q. The effect of land use on bacterial communities in saline-alkali soil. Curr. Microbiol. 2017, 74, 325–333. [Google Scholar] [CrossRef]
- Švec, P.; Černohlávková, J.; Busse, H.-J.; Vojtková, H.; Pantůček, R.; Cnockaert, M.; Mašlaňová, I.; Králová, S.; Vandamme, P.; Sedláček, I. Classification of strain CCM 4446 T as Rhodococcus degradans sp. nov. Int. J. Syst. Evol. Microbiol. 2015, 65, 4381–4387. [Google Scholar] [CrossRef]
- Xu, J.; He, J.; Wang, Z.-C.; Wang, K.; Li, W.-J.; Tang, S.-K.; Li, S.-P. Rhodococcus qingshengii sp. nov., a carbendazim- degrading bacterium. Int. J. Syst. Evol. Microbiol. 2007, 57, 2754–2757. [Google Scholar] [CrossRef]
- Woo, S.-G.; Cui, Y.; Kang, M.-S.; Jin, L.; Kim, K.K.; Lee, S.-T.; Lee, M.; Park, J. Georgenia daeguensis sp. nov., isolated from 4-chlorophenol enrichment culture. Int. J. Syst. Evol. Microbiol. 2012, 62, 1703–1709. [Google Scholar] [CrossRef] [Green Version]
- Fierer, N.; Schimel, J.P.; Holden, P.A. Influence of drying-rewetting frequency on soil bacterial community structure. Microb. Ecol. 2003, 45, 63–71. [Google Scholar] [CrossRef]
- Barnard, R.L.; Osborne, C.A.; Firestone, M.K. Responses of soil bacterial and fungal communities to extreme desiccation and rewetting. ISME J. 2013, 7, 2229–2241. [Google Scholar] [CrossRef] [PubMed]
- McIlroy, S.; Seviour, R.J. Elucidating further phylogenetic diversity among the Defluviicoccus-related glycogen-accumulating organisms in activated sludge. Environ. Microbiol. Rep. 2009, 1, 563–568. [Google Scholar] [CrossRef]
- Takeuchi, M.; Sakane, T.; Yanagi, M.; Yamasato, K.; Hamana, K.; Yokota, A. Taxonomic study of bacteria isolated from plants: Proposal of Sphingomonas rosa sp. nov., Sphingomonas pruni sp. nov., Sphingomonas asaccharolytica sp. nov., and Sphingomonas mali sp. nov. Int. J. Syst. Bacteriol. 1995, 45, 334–341. [Google Scholar] [CrossRef] [Green Version]
- Leys, N.M.E.J.; Ryngaert, A.; Bastiaens, L.; Verstraete, W.; Top, E.M.; Springael, D. Occurrence and phylogenetic diversity of Sphingomonas strains in soils contaminated with polycyclic aromatic hydrocarbons. Appl. Environ. Microbiol. 2004, 70, 1944–1955. [Google Scholar] [CrossRef] [Green Version]
- Niederberger, T.D.; McDonald, I.R.; Hacker, A.L.; Soo, R.M.; Barrett, J.E.; Wall, D.H.; Cary, S.C. Microbial community composition in soils of Northern Victoria Land, Antarctica. Environ. Microbiol. 2008, 10, 1713–1724. [Google Scholar] [CrossRef] [PubMed]
- Wilhelm, R.C.; Niederberger, T.D.; Greer, C.; Whyte, L.G. Microbial diversity of active layer and permafrost in an acidic wetland from the Canadian High Arctic. Can. J. Microbiol. 2011, 57, 303–315. [Google Scholar] [CrossRef] [Green Version]
- Zhang, H.; Sekiguchi, Y.; Hanada, S.; Hugenholtz, P.; Kim, H.; Kamagata, Y.; Nakamura, K. Gemmatimonas aurantiaca gen. nov., sp. nov., a Gram-negative, aerobic, polyphosphate-accumulating micro-organism, the first cultured representative of the new bacterial phylum Gemmatimonadetes phyl. nov. Int. J. Syst. Evol. Microbiol. 2003, 53, 1155–1163. [Google Scholar] [CrossRef]
- Yang, H.; Hu, J.; Long, X.; Liu, Z.; Rengel, Z. Salinity altered root distribution and increased diversity of bacterial communities in the rhizosphere soil of Jerusalem artichoke. Sci. Rep. 2016, 6, 20687. [Google Scholar] [CrossRef]
- Sullivan, R.F.; Holtman, M.A.; Zylstra, G.J.; White, J.F.; Kobayashi, D.Y. Taxonomic positioning of two biological control agents for plant diseases as Lysobacter enzymogenes based on phylogenetic analysis of 16S rDNA, fatty acid composition and phenotypic characteristics. J. Appl. Microbiol. 2003, 94, 1079–1086. [Google Scholar] [CrossRef]





| Bare Spot | Puccinellia Sward | Artemisia Alkali Steppe | Achillea Alkali Steppe | |
|---|---|---|---|---|
| Sample Identifier | AL-06; AL-09 | AP-06; AP-09 | AA-06; AA-09 | AF-06; AF09 |
| Geographic coordinates | 47°05′11.6″ N, 19°05′54.4″ E | 47°05′08.5″ N, 19°06′07.1″ E | 47°05′09.4″ N, 19°06′02.6″ E | 47°05′11.4″ N, 19°05′53.1″ E |
| Natural plant association | Lepidio crassifolii—Champhorosmetum annuae | Lepidio crassifolii—Puccinellietum limosae | Artemisio santonici—Festucetum pseudovinae | Achilleo setaceae—Festucetum pseudovinae |
| Plant coverage | 16% | 68% | 42% | 90% |
| Dominant plant species | Lepidium crassifolium, Champhorosma annua | Puccinellia limosa | Artemisia maritima, Plantago maritima, Festuca pseudovina | Festuca pseudovina, Achillea setacea |
| Other plant species (accompanying/sporadic) | Puccinellia limosa | Phragmites australis, Lepidium crassifolium, Plantago maritima | Podospermum canum, Puccinellia limosa, Agropyron repens | Cerastium pumilum, Plantago lanceolate, Koeleria cristata, Agropyron repens, Lotus corniculatus, Thymus pannonicus, Potentilla argentea, Trifolium repens |
| Bare Spot | Puccinellia Sward | Artemisia Alkali Steppe | Achillea Alkali Steppe | |||||
|---|---|---|---|---|---|---|---|---|
| Sample Identifier | AL-06 | AL-09 | AP-06 | AP-09 | AA-06 | AA-09 | AF-06 | AF-09 |
| pHH2O | 10.3 ± 0.1 | 10.3 ± 0.1 | 8.9 ± 0.6 | 9.5 ± 0.2 | 8.3 ± 0.3 | 9.9 ± 0.1 | 7.4 ± 0.2 | 8.0 ± 0.2 |
| EC | 3800 ± 834 | 2147 ± 114 | 1196 ± 220 | 1131 ± 46.5 | 562 ± 202 | 592 ± 28.7 | 325 ± 35.7 | 284 ± 22.9 |
| Salt | 0.55 ± 0.23 | 0.25 ± 0.02 | 0.14 ± 0.03 | 0.13 ± 0.01 | 0.05 ± 0.04 | 0.13 ± 0.02 | <0.02 | <0.02 |
| Corg | 0.4 ± 0.1 | 0.3 ± 0.0 | 1.3 ± 0.3 | 0.6 ± 0.1 | 1.7 ± 0.3 | 1.1 ± 0.2 | 2.6 ± 0.2 | 1.6 ± 0.5 |
| CaCO3 | 22.3 ± 4.1 | 20.9 ± 3.0 | 23.2 ± 0.7 | 22.5 ± 1.0 | 12.5 ± 4.2 | 12.5 ± 0.9 | 15.9 ± 2.9 | 15.6 ± 2.2 |
| Sample Identifier | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| AL-06 | AL-09 | AP-06 | AP-09 | AA-06 | AA-09 | AF-06 | AF-09 | ||
| Total high-quality sequences | 4493 | 3571 | 4580 | 2658 | 3144 | 5958 | 3350 | 2066 | |
| Good’s coverage | 99.89% | 99.69% | 99.80% | 99.74% | 99.87% | 99.97% | 99.70% | 99.18% | |
| Number of OTUs | 366 | 382 | 417 | 520 | 669 | 624 | 707 | 618 | |
| Species richness | Chao1 | 429.1 | 447.3 | 497.2 | 543.1 | 721.0 | 855.0 | 775.1 | 618.3 |
| ACE | 431.0 | 451.2 | 500.8 | 545.6 | 722.3 | 855.7 | 778.5 | 624.5 | |
| Diversity index | Inverse Simpson | 55.9 | 86.9 | 17.2 | 69.1 | 242.7 | 173.6 | 235.3 | 265.7 |
| Number of identified | phyla | 17 | 20 | 25 | 22 | 23 | 23 | 19 | 24 |
| orders | 93 | 86 | 101 | 90 | 99 | 100 | 102 | 97 | |
| genera | 141 | 126 | 174 | 161 | 204 | 216 | 219 | 194 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Borsodi, A.K.; Mucsi, M.; Krett, G.; Szabó, A.; Felföldi, T.; Szili-Kovács, T. Variation in Sodic Soil Bacterial Communities Associated with Different Alkali Vegetation Types. Microorganisms 2021, 9, 1673. https://doi.org/10.3390/microorganisms9081673
Borsodi AK, Mucsi M, Krett G, Szabó A, Felföldi T, Szili-Kovács T. Variation in Sodic Soil Bacterial Communities Associated with Different Alkali Vegetation Types. Microorganisms. 2021; 9(8):1673. https://doi.org/10.3390/microorganisms9081673
Chicago/Turabian StyleBorsodi, Andrea K., Márton Mucsi, Gergely Krett, Attila Szabó, Tamás Felföldi, and Tibor Szili-Kovács. 2021. "Variation in Sodic Soil Bacterial Communities Associated with Different Alkali Vegetation Types" Microorganisms 9, no. 8: 1673. https://doi.org/10.3390/microorganisms9081673
APA StyleBorsodi, A. K., Mucsi, M., Krett, G., Szabó, A., Felföldi, T., & Szili-Kovács, T. (2021). Variation in Sodic Soil Bacterial Communities Associated with Different Alkali Vegetation Types. Microorganisms, 9(8), 1673. https://doi.org/10.3390/microorganisms9081673







