1. Introduction
Trichoderma is a versatile genus of fungi that has agricultural as well as industrial importance. It is one of the most widespread biological agents currently used in agriculture to control different plant diseases [
1,
2]. It is present in more than 60% of registered biological pesticides worldwide. No other beneficial fungus in the agriculture field has received as much combined attention from science and the commercial market [
2].
Trichoderma species can promote plant growth and productivity, help to manage pests and pathogens, alleviate abiotic stresses, biodegrade xenobiotic compounds, and produce industrially important metabolites. Using biocontrol agents is one of the sustainable approaches for crop cultivation with numerous benefits, including increased disease protection and yield, as well as reduced chemical impact on the environment [
3,
4,
5,
6,
7].
The development of new biocontrol agent products to control plant pathogens requires large scale screening of candidate antagonists, developing mass production protocols that optimize product quantity and quality, and devising a product formulation that preserves, aids product delivery and enhances bioactivity [
8,
9]. The establishment of robust technology is key for manufacturing microbial biopesticides. Formulation-based solutions related to challenges in terms of biocontrol agent stability, efficacy, and application have been addressed by evaluating the impact of formulation ingredients and processes on the physical characteristics, biological activity, storage stability and field efficacy of selected biocontrol agents [
10,
11,
12]. It is important in the manufacturing process to ensure protection of the active ingredients (conidia or mycelia of antagonist fungi) against conditions of extreme pH, low humidity, chemicals, and UV radiation. The development of a reliable biocontrol agent requires identification of a proper formulation to overcome environmental limitations and give the antagonist a competitive advantage over pathogens and other microflora. The formulations can be designed to include nutrients important for the biocontrol agent’s growth, osmoregulation and initial growth from dried biomass [
13,
14]. Moreover, the biocontrol agents must survive several processing steps, including harvesting, drying, formulation, storage, and delivery.
Developmental costs and technological challenges are major hindrances to the development of successful products [
15,
16]. Cost-effective large-scale production can be achieved through solid-state fermentation (SSF). Thus, increasing demands for bio-fungicide production to replace excessively used chemical pesticides have recently enhanced interest in SSF technology. SSF simulates the natural habitat of fungi and is, therefore, the preferred choice for these microorganisms to grow and produce useful value-added products [
17]. It is a cost-effective process widely used for the mass production of filamentous fungi, their enzymes and/or other metabolites on solid substrates with sufficient moisture but not in the free state [
18]. The raw materials used as an organic substrate for biomass production account for 35–40% of production costs [
19]. Therefore, the utilization of agro-industrial wastes that are cheap, easily available and support extensive growth of
Trichoderma is required for the production of value-added products. It provides avenues for the safe utilization of wastes while reducing the cost and environmental pollution load of waste disposal. In recent years, the global production, registration, and application of biological pesticides in agriculture as alternatives to chemicals have rapidly increased owing to public concerns about human health, food safety and the impact on the environment [
20,
21]. Recent literature surveys have shown that the number of
Trichoderma-containing products on the international market has grown exponentially, with more than 300 products now available [
22,
23]. Currently, there are eight registered products based on the genus
Trichoderma in Brazil [
24] and more than 250 commercial formulations in India [
25], whereas there is no registered product in Ethiopia. This highlights the lack of formulated products commercially available in Ethiopia. One developed product has been tested for the control of coffee wilt disease (CWD) caused by
Fusarium xylarioides and shown to be effective and efficient under greenhouse and field conditions in Ethiopia (unpublished data). Introducing biological control agents as part of a
F. xylarioides control strategy is highly desirable, especially because there is a lack of an efficient synthetic fungicide.
Different
Trichoderma species require specific culture conditions for maximum conidia productivity, and hence no defined medium is available for optimum conidia production. Mathematical modeling is a useful approach for optimizing culture conditions with fewer experiments than conventional methods. Experimental design can be regarded as a process by which certain factors are selected and deliberately varied in a controlled manner to obtain their effects on a response of interest, often followed by the analysis of the experimental results. Several modeling and optimization methodologies are available ranging from simple models like one factor at a time (OFAT) to complex statistical designs such as two-level fractional factorial design (FFD), Box–Behnken design (BBD), and response surface methodology (RSM) [
26,
27]. OFAT is a traditional method employed for screening substrates and growth factors. This method has several disadvantages, such as time consumption, huge resource requirements, less capable of finding true optimum levels due to the interactions among factors and a several-fold increase in the number of experiments. A better alternative to OFAT is FFD, which can be employed for screening significant factors at different levels, with advantages of a better yield, reproducible results and better design space for experimental trials [
26]. Moreover, statistically designed experiments could effectively solve such issues and minimize the error in determining the effect of factors and interaction between factors [
27,
28]. The design of the experiment (DOE) offers a reduced number of experiments and increased process efficiency [
29].
Response surface methodology (RSM) is a collection of mathematical and statistical tools for designing experiments, developing models, evaluating the effects of factors and identifying optimum conditions of factors for desirable responses [
27]. It can be used to evaluate and predict interactions among different process parameters. The Box–Behnken design (BBD) is a second-order multivariate technique based on three-level partial factorial designs [
29]. It enables the estimation of parameters in a quadratic model and evaluation of the lack of fit of a model. This methodology was applied in the present study to identify the optimal growth conditions for maximizing conidia production from agro-industrial wastes. The optimization process involved three major steps: statistically designed experiments, estimation of the coefficients in a mathematical model, and prediction of the response to check the adequacy of the model [
28,
30]. Physical parameters such as the initial moisture content, cultivation time, and temperature greatly influence the SSF process [
31]. In this study, these three parameters were considered for the optimization of
Trichoderma species conidia production and to find interaction effects among these variables.
Therefore, the present study was undertaken to find suitable agro-industrial wastes for economical and high mass production of novel Trichoderma species under SSF by optimizing the culture conditions using a mathematical model and determining the viability of the formulated bio-product under different storage conditions.
4. Discussion
The use of agro-industrial waste for the production of value-added products is a good approach for developing low-cost carriers for the formulation of
Trichoderma-based bioproducts. It provides avenues for the safe utilization of wastes while reducing the cost and environmental pollution load of waste disposal. Intensive studies are needed to select fermentation substrates that provide large, stable and effective microbial populations for the formulation process [
47]. In this study, a broad range of organic materials locally available in Ethiopia was investigated for the growth and multiplication of
T. asperellum AU131 and
T. longibrachiatum AU158 in SSF to select suitable agriculture byproducts that favor the production of a high amount of conidia biomass with prolonged viability. Results of the current research showed that
T. asperellum AU131 and
T. longibrachiatum AU158 grew on all 14 solid substrates examined and abundantly sporulated on them, but the level of colonization and production of biomass differed among the growth media, most likely reflecting the different ingredients in the organic substrates and the food preference of the
Trichoderma species. The physicochemical features of organic substrates are known to greatly affect the fermentation process [
48].
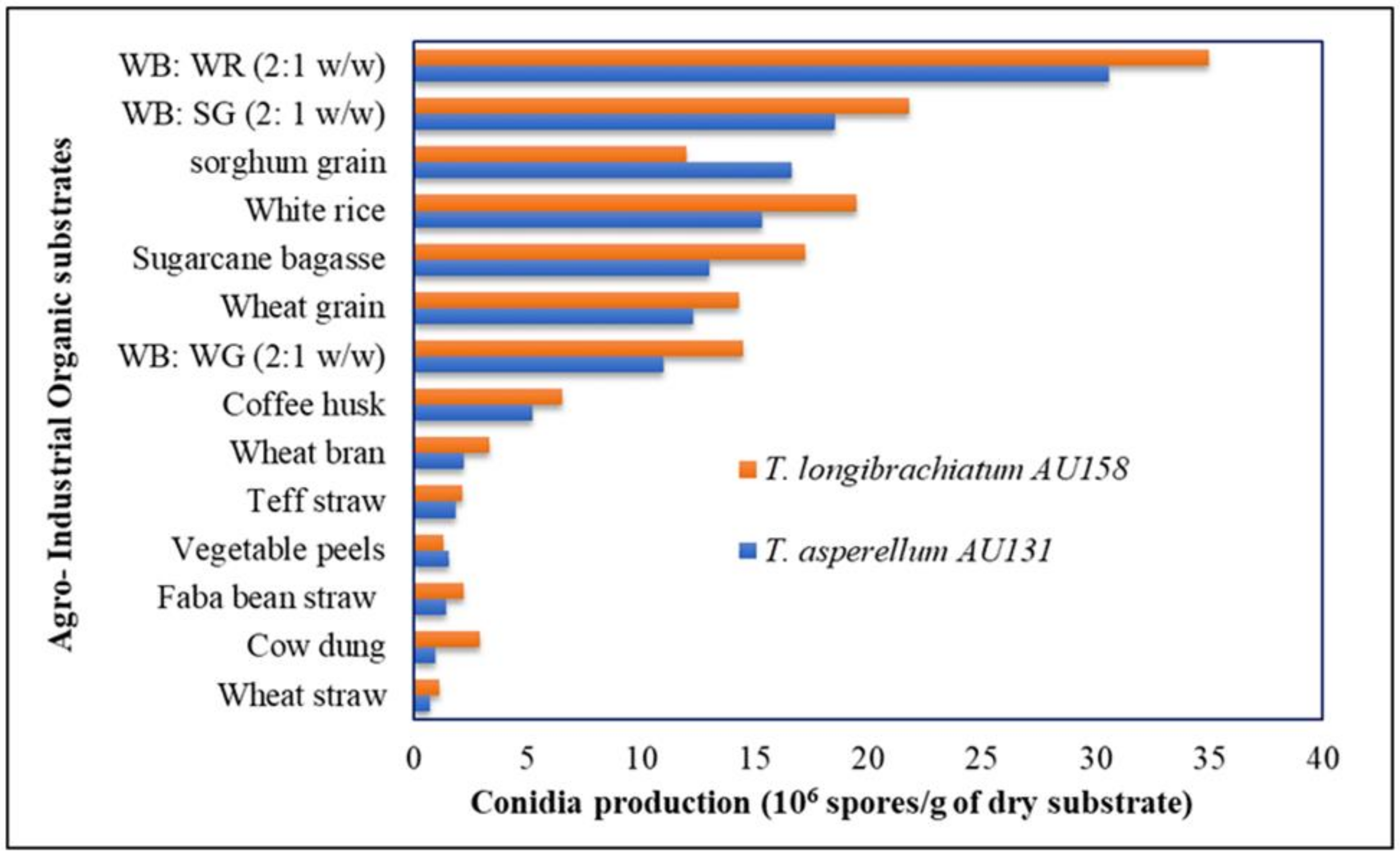
Among the various agro-industrial organic substrates and cereals screened using the OFAT method, wheat bran combined with white rice (2:1
w/
w) was found to support maximum growth of
T. asperellum AU131 (3.2 × 10
7 spores/g) and
T. longibrachiatum AU158 (3.5 × 10
7 spores/g), significantly (
p ≤ 0.05) higher than for the other substrates. De la Cruz-Quiroz et al. [
35] used corn cob as a substrate and a plastic bag as a bioreactor for SSF of a
T. asperellum strain and showed that the sporulation rate was 1.4 × 10
9 conidia/g. Surprisingly, rice and wheat bran, which are widely used as organic substrates for the mass production of
Trichoderma species, enabled high growth of the biocontrol agents examined in our study. One of the most important criteria for selecting a substrate for SSF is the amount of sporulation of the target microorganism. Combination of wheat bran and rice (2: 1
w/w) was found to be a suitable raw material for maximum conidia production under SSF, most likely due to the presence of soluble oligosaccharides, nitrogen content, hemicellulose, starches and easily available celluloses, which significantly induce cellulase production [
49]. Wheat bran and rice-based substrates are the most common media for the growth and sporulation of many fungi [
50]. Both substrates are rich in cellulose, hemicellulose, and lignin and represent a good source of nutrients for the prolific growth and sporulation of
Trichoderma species [
51]. Degradation of cellulose helps
Trichoderma species to obtain nutrients effectively [
52]. Both
T. asperellum and
T. longibrachiatum have been found to produce cellulase enzymes [
53,
54,
55], which can effectively degrade the cellulosic content. It is well known that wheat bran is particularly suitable for SSF because of its porosity, allowing good water absorption, which is indispensable for carrying out microbial metabolism. Moreover, in terms of the volume produced, it is a major solid agro-industrial byproduct generated worldwide, including in Ethiopia [
56]. Wheat bran promotes fungal growth just as in the natural environmental conditions and requires no additional nutrients for the production of
Trichoderma spores [
18]. Sala et al. [
50] used rice husks in SSF of
T. harzianum and achieved final spore concentrations of up to 2.0 × 10
9 conidia g
−1 dry matter. Members belonging to the genus
Trichoderma are saprophytic fungi, which grow profusely on a wide range of organic substrates in nature [
57]. Most commercial products contain
Trichoderma conidia. Thus, the high productivity of SSF systems is important for the successful production of biocontrol agents [
58]. Other reports have also indicated that wheat bran is a suitable substrate for the growth of
T. harzianum,
T. viride,
T. koningii, T. asperellum, T. longibrachiatum, and
T. polysporum by SSF, supporting the findings of the current study. A lower percentage of lignin may also provide conditions for the easier uptake of cellulose and other inducers required for cellulase production (
Table 11).
In the present study, the maximum conidia production was obtained on WB: WR (2:1
w/
w), WB: SG (2:1
w/
w), sorghum grain, white rice, and sugarcane bagasse, suggesting a feasible approach for using different agro-industrial wastes for the biomass production of these biocontrol agents. The results showed that most of the screened organic substrates could be used to produce a high quantity and quality of
T. asperellum AU131 and
T. longibrachiatum AU158 inoculum at low cost. However, other organic substrates screened in this study did not support maximum conidia production of either
Trichoderma species. Thus, the production of a large number of highly viable conidia was influenced by the type of substrate and presence of a high lignin content (
Table 11). Lignin is closely bound to cellulose and hemicellulose, and its functions are to provide rigidity and cohesion to the material cell wall, to confer water impermeability to xylem vessels and to form a physicochemical barrier against microbial attack [
59,
60].
Sachdev et al. [
61] reported that sugarcane bagasse + spent tea leaves was the best substrate for the growth of
T. ressei, whereas for
T. viride and
T. koningii, spent tea leaves + wheat bran and for
T. asperellum, wheat straw + wheat bran were found to be suitable substrates for
T. longibrachiatum. It is not clear which substrate was best for
T. longibrachiatum. Analogously, the maximum growth of
T. asperellum on rice bran was recorded as 10.80 × 10
8 CFU/g, whereas on sugarcane bagasse only 3.73 × 10
8 CFU/g was documented after 20 days of incubation [
62]. Sargin et al. [
63] tested various inexpensive agricultural co-products, including wheat bran, sawdust, rice straw, hazelnut shell, grape marc and cottonseed cake for propagule production of a
T. harzianum strain and reported that the maximum micro propagule count was achieved with a wheat bran malt sprout mixture. Rayhane et al. [
64] studied a fermentation process for enzymes and conidia with a
T. asperellum strain and scaled up the process from a flask and glass column to a bioreactor. The current study showed that most of the screened organic substrates can be used to produce a high quantity and quality of
T. asperellum AU131 and
T. longibrachiatum AU158 inoculum at low cost. In general, these findings suggest that the mass production of
Trichoderma on different substrates is species-specific according to the different ability to utilize carbon and nitrogen as a source of nutrition.
Table 11.
Chemical composition of agro-industrial substrates used for SSF.
Table 11.
Chemical composition of agro-industrial substrates used for SSF.
| Substrate | Chemical Composition (% w/w) | References |
|---|
| Cellulose | Hemicellulose | Lignin | Ash | Moisture |
|---|
| Sugarcane bagasse | 30.2 | 56.7 | 13.4 | 1.9 | 4.8 | [51] |
| Wheat straw | 32.9 | 24 | 8.9 | 6.7 | 7 | [65] |
| Coffee husks | 23.77 | 16.7 | 86 | 5.4 | – | [66] |
| Potato peel waste | 2.2 | – | – | 7.7 | 9.9 | [67] |
| Orange peel | 9.21 | 10.5 | 0.84 | 3.5 | 11.9 | [68] |
| Wheat bran | 10.9 | 39 | 5.08 | 6.3 | 12.5 | [69] |
| White rice | 0.49 | 2.85 | 0.10 | 3.5 | 6.4 | [70] |
Among the six growth factors screened using FFD, incubation temperature, moisture content, inoculum concentration and incubation period were found to be the most significant in affecting the conidia production of both
Trichoderma species (
p < 0.05) (
Table 8 and
Table 9). Individual and combined effects of all the factors in the optimization process were explained by RSM. RSM is beneficial for evaluating multiple parameters and their interactions with a reduced number of experimental trials and aids the improvement, development, and optimization of processes [
70].
Our results demonstrated its applicability for predicting the growth rate of T. asperellum AU131 and T. longibrachiatum AU158 under SSF conditions. The results of the statistical analysis indicated that the effects of temperature, initial moisture content of the substrate, inoculum size and incubation time were highly significant (p ≤ 0.05). These growth factors were identified as the most influential among physicochemical parameters. Overall, the results suggested that biomass production is markedly affected by these parameters and slight changes in their respective values can affect the process significantly.
As indicated by the ANOVA results in
Table 9 and
Table 10, the
F-values of 10.38 (
T. asperellum AU131) and 12.01 (
T. longibrachiatum AU158) indicate that the model was significant at
p ≤ 0.05, and the probability that the
F-values were due to noise was only 5%. According to Ferreira et al. [
71], comparison between the residual and pure error represents the lack of fit. The
F-value obtained from the lack of fit was 0.186, indicating that the lack of fit was insignificant (
p > 0.05) for
T. asperellum AU131. A non-significant “lack of fit” is acceptable, and therefore the number of experiments was deemed sufficient to evaluate the effects of variables on the response [
72]. On the other hand, the regression equation obtained indicated an
R2 value of 0.9301 for
T. asperellum AU31 and 0.939 for
T. longibrachiatum AU158. These values demonstrate that the quadratic model was highly significant and could explain about 93% and 93.9%, respectively, of the variability in conidia production by the species. Previous studies have reported ANOVA with high
R2 of 0.9978–0.9070 and
p < 0.05 [
45,
73].
The temperature of the SSF culture medium dramatically affected the
Trichoderma species and conidia production. Both
Trichoderma species showed an optimal temperature of 25 °C for maximum conidia production (
Figure 6a,b). This result is comparable with the results reported by Singh et al. [
74], where 25–30 °C was observed as the optimum temperature range for fast growth of
Trichoderma species. In contrast to the present study, Mohiddin et al. [
75] reported that 10 °C was the most favorable temperature for supporting maximum growth of
T. harzianum compared to 20 °C and 30 °C. The optimal temperature might be the parameter with the highest dependence on the specific strain. Several studies using different substrates have shown an optimal production temperature of 25–28 °C using different
Trichoderma species [
50,
76], although conidia production decreased considerably at >30 °C, as also observed in the present study. At higher temperatures, microbial growth is affected and shows less conidia production due to alterations in membrane structure and protein degradation [
77]. At lower temperatures, the growth rate of
Trichoderma species is slow and takes a longer time for conidia production. All these results indicate that temperature is the most important parameter in terms of significance affecting SSF performance, as suggested by Mishra and Khan [
31] and Singh et al. [
78]. It is known that extremely high or low temperatures denature already synthesized enzymes and other enzymes needed for microbial metabolic activities [
79].
The moisture content also determines the growth rate and other physiological activities of
Trichoderma species. The initial moisture content was found to be significant for conidia production of both
Trichoderma species analyzed: a lower initial moisture content resulted in higher conidia production. In SSF, microbial growth and product formation occur on the surface of solid particles. The statistical analysis of this study indicated that the optimum moisture content was 50% and 66.1% for
T. asperellum AU131 and
T. longibrachiatum AU158, respectively (
Figure 6a,b). Santa et al. [
80] analyzed the effect of initial moisture and reported an optimal value of 65%, which is similar to that obtained in this study for
T. longibrachiatum AU158. Even though fungal strains can grow in a wide range of moisture content varying from 40% to 80%, the optimal moisture content may be more dependent on the exact substrate used than on the fungal strain, as suggested by Manpreet et al. [
81]. At the same time, the optimum moisture content used in our study was higher than that reported by Mishra et al. [
76] (51–54%), which might be due to configuration differences between the reactors used, i.e., packed-bed reactors [
76] vs. Erlenmeyer flasks (in this study). Gervais and Molin [
82] observed that increased moisture increases aerial mycelial growth of
Trichoderma species, lowers oxygen transfer and decreases substrate porosity.
A low moisture content in the substrate can reduce nutrient solubility and increase the surface tension of the water layer, hampering fungal growth. If the moisture content is too high, porosity and gas exchange in the substrate are reduced, reducing the efficiency of SSF [
18]. This behavior was evidenced in the results presented in
Table 5 and
Table 6, which show that the lowest initial moisture content (65% and 50%) yielded the highest conidia production (run 9) for
T. asperellum AU131 and
T. longibrachiatum AU158 (run 1), respectively. These results agree with Wahid et al. [
83] and Aita et al. [
84], who investigated optimization of conidia production by SSF with
Trichoderma ressei. In another study, initial moisture contents from 55 to 65% were evaluated and the best results were obtained with the lowest initial moisture [
85]. Therefore, the optimization of initial moisture content in the substrate is essential to maintain the physicochemical characteristics of the substrate and ensure process productivity.
In the present study,
T. asperellum AU131 was found to be still in the growth phase after 21 days of incubation, indicating that further increasing the incubation time could result in more conidia production. The maximum conidia production (8.6 log
10CFU/g dry substrate) was predicted to be after 27.8 days of incubation using optimal point prediction analysis under similar conditions of temperature and moisture. This finding agrees with the results of Sachdev et al. [
61], who reported the maximum spore density after 31 days of incubation.
Lastly, an increase in inoculum size from 5% led to a progressive increase in conidia production, reaching the highest value at 10% for
T. longibrachiatum AU158. The effect of inoculum size on conidia production was also studied by Hamrouni et al. [
86], who reported that maximum conidia production was obtained using a 5% inoculum. A smaller inoculum may not be adequate for growth initiation and may delay the lag phase as well as enzyme synthesis [
35], whereas a high inoculum size shortens the lag phase but may also increase competition for limiting nutrients due to overcrowded growth of the organism per unit substrate [
87]. Therefore, the use of an appropriate inoculum size or dosage is required for healthier fungal propagation and conidia production.
The formulated wettable powder retained the viability of
T. asperellum AU131 and
T. longibrachiatum AU158 for a long time. However, there was a general drop in the number of CFUs with the time of storage at 4 and 25 °C, with a rapid decrease occurring at room temperature. High conidia viability is an important aspect of the economics of the production of a
Trichoderma bio-inoculum, and temperature and moisture are important factors that determine the longevity of a formulation. Although bio-fungicide formulations are dependent on the type of organism being used, the ultimate goal is to ensure that the agent is delivered in a form that is viable, virulent, and has required inoculum potential to be effective in the field [
88]. Counting the spores/conidia in the bioproduct does not necessarily reflect the conidia viability as formulations may undergo several stages of processing, including temperature and moisture variation, and storage periods [
89]. Thus, CFU/g is a more appropriate measure of conidia viability, as indicated in this study.
In general, despite the similarity of patterns, the viability and population of the antagonist in the talc-based formulation tended to be less affected by storage at 4 °C than at room temperature. The reduction in the shelf life of fungal biocontrol agents during storage is consistent with the findings of other researchers, who reported negative effects of high temperatures and long-term storage on the viability of inocula of
Trichoderma species [
90,
91]. Development and preparation of powdery formulations is important for practical application of antagonistic
Trichoderma species because they can be easily applied as soil and seed treatment for controlling plant diseases under field conditions. Woo et al. [
22] reviewed the current applications of
Trichoderma containing products in agriculture and concluded that 55.3% of
Trichoderma formulations are commercialized as wettable powders.
Moreover, the carrier selected should effectively deliver the promising inoculum to the plants. In this study, talc powder was selected because it is chemically defined, less prone to contamination and is internationally accepted [
16]. Talc-based formulations of both
Trichoderma species supplemented with CMC, glycerol and Tween 20 were found to have a stable shelf life of up to six months (
Figure 7). Wraight et al. [
92] reported that the incorporation of additives in the conidial formulation can have a positive influence on conidial fitness. Addition of glycerol helps to maintain a high moisture content in the formulation and protect the viable propagules from reduced water activity during the shelf life [
44]. CMC is an additive that is readily available and has a comparatively steady batch quality since it is a semi-synthetic polymer [
93]. In the present study, we concluded that the formulated bioproducts resulted in the retention of viable propagules above 10
6 conidia/g, which is the minimum requirement for biopesticide application in agriculture. Our previous findings concerning bio-efficacy studies of these bioagents against CWD caused by
F. xylarioides showed that the bio-fungicides applied under field conditions reduced CWD incidence and severity by 70% and 32% (unpublished data).