In Vitro Effects of Short-Term and Long-Term Heat Exposures on the Immune Response and Prostaglandin Biosynthesis in Bovine Endometrial Cells
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample
2.2. Primary Bovine Endometrial Cells Culture
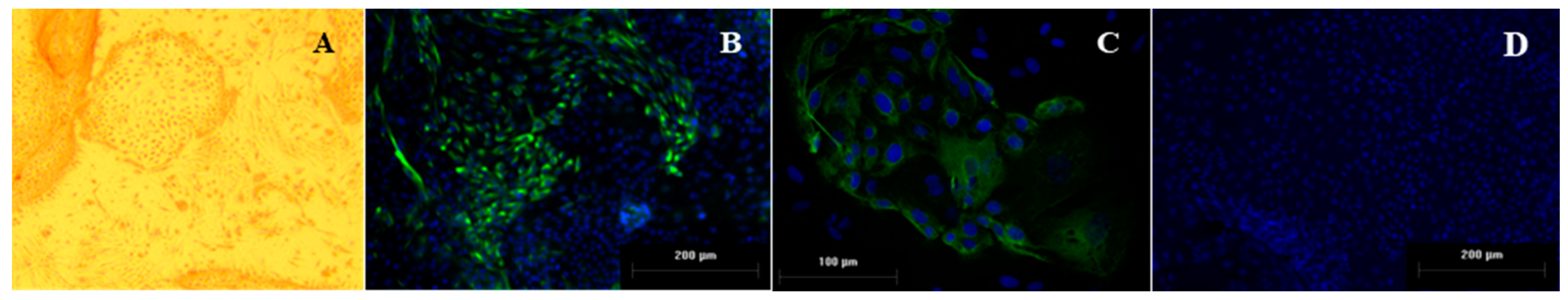
2.3. Characterization of Endometrial Culture System
2.4. Cell Culture Treatment with Heat Exposure and LPS
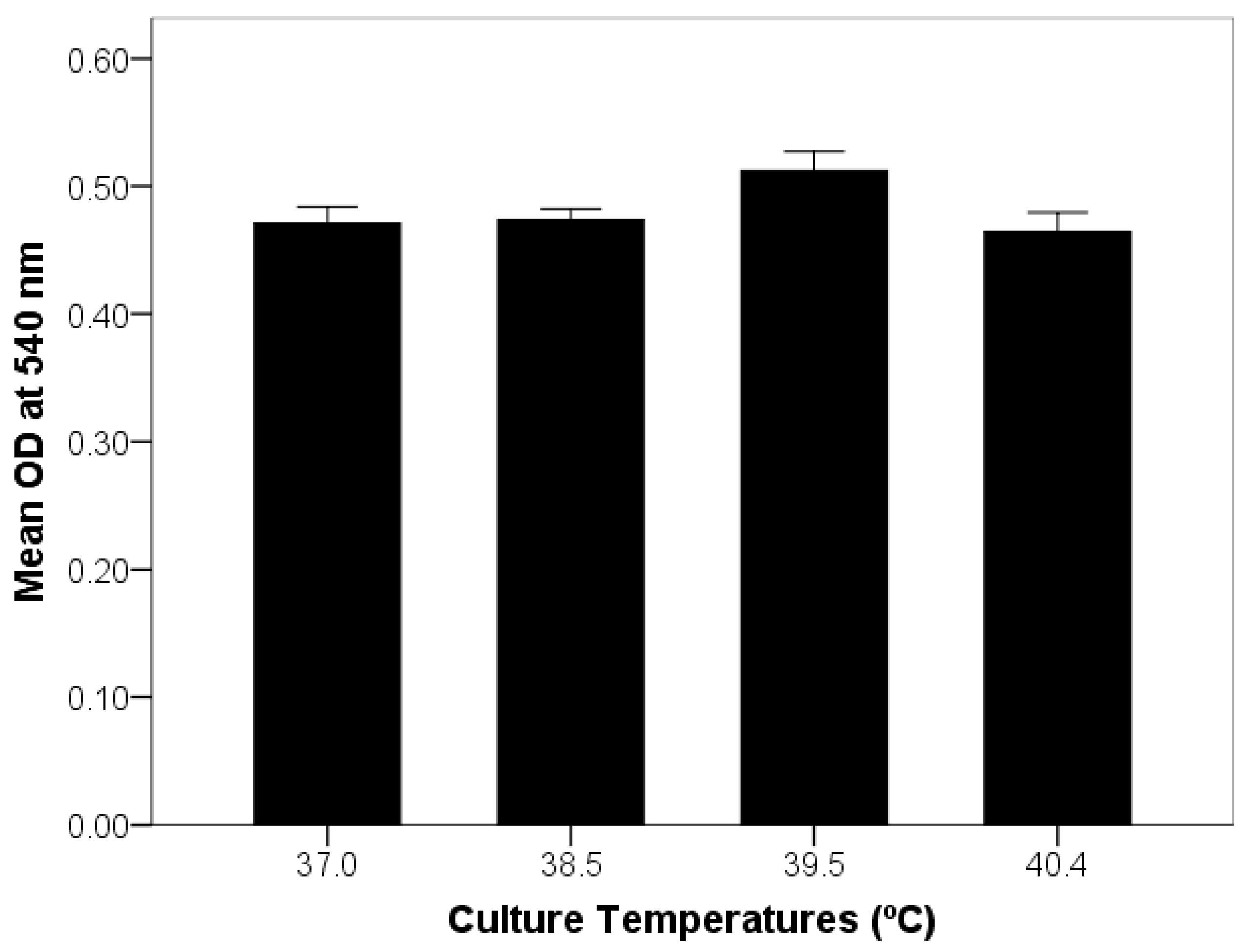
2.5. Optimization of Endometrial Cell Culture and Exposure to Elevated Temperature
2.6. Sample Collecting for Evaluation
2.7. Quantitative Real-Time PCR System
2.7.1. RNA Isolation and Reverse Transcription
2.7.2. Primer Sequences and Optimization of qPCR
2.7.3. Real-Time qPCR
2.8. Measurement of Secreted Protein Using ELISA for IL8 and PGF2α
2.9. Statistical Analysis
3. Results
3.1. Effect of Heat Exposure and LPS on Gene Response of Bovine Endometrial Cells
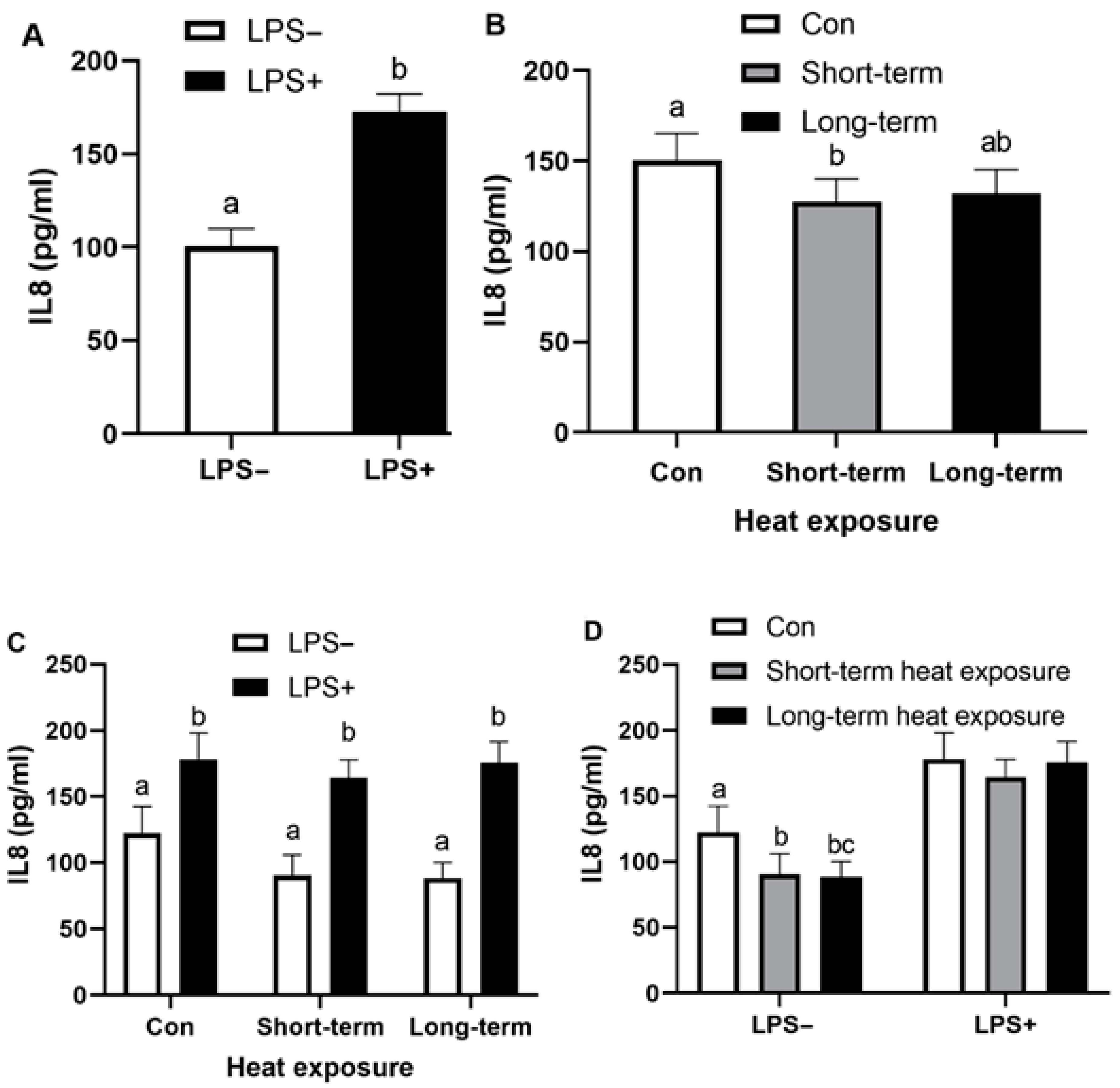
3.2. Effect of Heat Exposure and LPS on Secretory IL8 of Bovine Endometrial Cells
3.3. Effect of Heat Exposure and LPS on Secretory PGF2α of Bovine Endometrial Cells
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lucy, M.C. Reproductive loss in high-producing dairy cattle: Where will it end? J. Dairy Sci. 2001, 84, 1277–1293. [Google Scholar] [PubMed]
- Morton, J.M.; Trainer, W.P.; Mayer, D.G.; Jonsson, N.N. Effects of environmental heat on conception rates in lactating dairy cows: Critical periods of exposure. J. Dairy Sci. 2007, 90, 2271–2278. [Google Scholar] [PubMed]
- Bohmanova, J.; Misztal, I.; Cole, J.B. Temperature-humidity indices as indicators of milk production losses due to heat stress. J. Dairy Sci. 2007, 90, 1947–1956. [Google Scholar] [CrossRef] [PubMed]
- Schüller, L.K.; Burfeind, O.; Heuwieser, W. Impact of heat stress on conception rate of dairy cows in the moderate climate considering different temperature–humidity index thresholds, periods relative to breeding, and heat load indices. Theriogenology 2014, 81, 1050–1057. [Google Scholar] [CrossRef] [PubMed]
- Polsky, L.; von Keyserlingk, M.A.G. Invited review: Effects of heat stress on dairy cattle welfare. J. Dairy Sci. 2017, 100, 8645–8657. [Google Scholar]
- De Rensis, F.; Garcia-Ispierto, I.; López-Gatius, F. Seasonal heat stress: Clinical implications and hormone treatments for the fertility of dairy cows. Theriogenology 2015, 84, 659–666. [Google Scholar]
- Berman, A.; Folman, Y.; Kaim, M.; Mamen, M.; Herz, Z.; Wolfenson, D.; Arieli, A.; Graber, Y. Upper critical temperatures and forced ventilation effects for high-yielding dairy cows in a subtropical climate. J. Dairy Sci. 1985, 68, 1488–1495. [Google Scholar]
- Al-Katanani, Y.M.; Webb, D.W.; Hansen, P.J. Factors affecting seasonal variation in 90-day nonreturn rate to first service in lactating Holstein cows in a hot climate. J. Dairy Sci. 1999, 82, 2611–2616. [Google Scholar] [CrossRef]
- Suadsong, S. Alleviating heat stress leads to improved cow reproductive performance. In Milk Production—An Up-to-Date Overview of Animal Nutrition, Management and Health; Chaiyabutr, N., Ed.; InTech: Novi Sad, Croatia, 2012; pp. 309–330. [Google Scholar]
- Ratchamak, R.; Ratsiri, T.; Chumchai, R.; Boonkum, W.; Chankitisakul, V. Relationship of the temperature-humidity index (THI) with ovarian responses and embryo production in superovulated Thai-Holstein crossbreds under tropical climate conditions. Vet. Sci. 2021, 8, 270. [Google Scholar] [CrossRef]
- Sejian, V.; Bhatta, R.; Gaughan, J.B.; Dunshea, F.R.; Lacetera, N. Review: Adaptation of animals to heat stress. Animal 2018, 12, s431–s444. [Google Scholar]
- Roman-Ponce, H.; Thatcher, W.W.; Caton, D.; Barron, D.H.; Wilcox, C.J. Thermal stress effects on uterine blood flow in dairy cows. J. Anim. Sci. 1978, 46, 175–180. [Google Scholar] [CrossRef] [PubMed]
- Gwazdauskas, F.C.; Thatcher, W.W.; Wilcox, C.J. Physiological, environmental, and hormonal factors at insemination which may affect conception. J. Dairy Sci. 1973, 56, 873–877. [Google Scholar] [CrossRef]
- Rutledge, J.J. Use of embryo transfer and IVF to bypass effects of heat stress. Theriogenology 2001, 55, 105–111. [Google Scholar] [CrossRef]
- Ray, D.E.; Halbach, T.J.; Armstrong, D.V. Season and lactation number effects on milk production and reproduction of dairy cattle in Arizona. J. Dairy Sci. 1992, 75, 2976–2983. [Google Scholar] [CrossRef]
- Rivera, R.M.; Hansen, P.J. Development of cultured bovine embryos after exposure to high temperatures in the physiological range. Reproduction 2001, 121, 107–115. [Google Scholar] [CrossRef]
- Kasimanickam, R.; Kasimanickam, V. Impact of heat stress on embryonic development during first 16 days of gestation in dairy cows. Sci. Rep. 2021, 11, 14839. [Google Scholar] [CrossRef]
- Katagiri, S.; Moriyoshi, M. Alteration of the endometrial EGF profile as a potential mechanism connecting the alterations in the ovarian steroid hormone profile to embryonic loss in repeat breeders and high-producing cows. J. Reprod. Dev. 2013, 59, 415–420. [Google Scholar] [CrossRef]
- Kawano, K.; Yanagawa, Y.; Nagano, M.; Katagiri, S. Effects of heat stress on the endometrial epidermal growth factor profile and fertility in dairy cows. J. Reprod. Dev. 2022, 68, 144–151. [Google Scholar] [CrossRef]
- Swangchan-Uthai, T.; Chen, Q.; Kirton, S.E.; Fenwick, M.A.; Cheng, Z.; Patton, J.; Fouladi-Nashta, A.A.; Wathes, D.C. Influence of energy balance on the antimicrobial peptides S100A8 and S100A9 in the endometrium of the postpartum dairy cow. Reproduction 2013, 145, 527–539. [Google Scholar] [CrossRef]
- Foldi, J.; Kulcsar, M.; Pecsi, A.; Huyghe, B.; de Sa, C.; Lohuis, J.A.; Cox, P.; Huszenicza, G. Bacterial complications of postpartum uterine involution in cattle. Anim. Reprod. Sci. 2006, 96, 265–281. [Google Scholar] [CrossRef]
- Williams, E.J.; Fischer, D.P.; Noakes, D.E.; England, G.C.; Rycroft, A.; Dobson, H.; Sheldon, I.M. The relationship between uterinepathogen growth density and ovarian function in the postpartum dairy cow. Theriogenology 2007, 68, 549–559. [Google Scholar] [CrossRef]
- Sheldon, I.M.; Cronin, J.; Goetze, L.; Donofrio, G.; Schuberth, H.J. Defining postpartum uterine disease and the mechanisms of infection and immunity in the female reproductive tract in cattle. Biol. Reprod. 2009, 81, 1025–1032. [Google Scholar] [CrossRef]
- Herath, S.; Dobson, H.; Bryant, C.E.; Sheldon, I.M. Use of the cow as a large animal model of uterine infection and immunity. J. Reprod. Immunol. 2006, 69, 13–22. [Google Scholar] [CrossRef]
- Peter, A.T.; Bosu, W.T.; Gilbert, R.O. Absorption of Escherichia coli endotoxin (lipopolysaccharide) from the uteri of postpartum dairy cows. Theriogenology 1990, 33, 1011–1014. [Google Scholar] [CrossRef]
- Chapwanya, A.; Meade, K.G.; Foley, C.; Narciandi, F.; Evans, A.C.; Doherty, M.L.; Callanan, J.J.; O’Farrelly, C. The postpartum endometrial inflammatory response: A normal physiological event with potential implications for bovine fertility. Reprod. Fertil. Dev. 2012, 24, 1028–1039. [Google Scholar] [CrossRef]
- Fu, Y.; Liu, B.; Feng, X.; Liu, Z.; Liang, D.; Li, F.; Li, D.; Cao, Y.; Feng, S.; Zhang, X.; et al. Lipopolysaccharide increases Toll-like receptor 4 and downstream Toll-like receptor signaling molecules expression in bovine endometrial epithelial cells. Vet. Immunol. Immunopathol. 2013, 151, 20–27. [Google Scholar] [CrossRef]
- Deng, Y.; Liu, B.; Fu, C.; Gao, L.; Shen, Y.; Liu, K.; Li, Q.; Cao, J.; Mao, W. Lipopolysaccharide stimulates bovine endometrium explants through Toll-like receptor 4 signaling and PGE2 synthesis. Prostaglandins Leukot. Essent. Fatty Acids 2021, 168, 102272. [Google Scholar] [CrossRef]
- Swangchan-Uthai, T.; Lavender, C.R.; Cheng, Z.; Fouladi-Nashta, A.A.; Wathes, D.C. Time course of defense mechanisms in bovine endometrium in response to lipopolysaccharide. Biol. Reprod. 2012, 87, 135. [Google Scholar] [CrossRef]
- Gilbert, R.O. The effects of endometritis on the establishment of pregnancy in cattle. Reprod. Fertil. Dev. 2012, 24, 252–257. [Google Scholar] [CrossRef]
- Fusco, R.; Cordaro, M.; Siracusa, R.; Peritore, A.F.; D’Amico, R.; Licata, P.; Crupi, R.; Gugliandolo, E. Effects of hydroxytyrosol against lipopolysaccharide-induced inflammation and oxidative stress in bovine mammary epithelial cells: A natural therapeutic tool for bovine mastitis. Antioxidants 2020, 9, 693. [Google Scholar] [CrossRef]
- Williams, E.J.; Fischer, D.P.; Pfeiffer, D.U.; England, G.C.; Noakes, D.E.; Dobson, H.; Sheldon, I.M. Clinical evaluation of postpartum vaginal mucus reflects uterine bacterial infection and the immune response in cattle. Theriogenology 2005, 63, 102–117. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.; Mao, W.; Liu, B.; Li, T.; Wang, X.; Pei, L.; Cao, J.; Wang, F. Prostaglandin E2 promotes Staphylococcus aureus infection via EP4 receptor in bovine endometrium. Microb. Pathog. 2021, 158, 105019. [Google Scholar] [CrossRef] [PubMed]
- Mavangira, V.; Sordillo, L.M. Role of lipid mediators in the regulation of oxidative stress and inflammatory responses in dairy cattle. Res. Vet. Sci. 2018, 116, 4–14. [Google Scholar] [CrossRef] [PubMed]
- Chapwanya, A.; Meade, K.G.; Doherty, M.L.; Callanan, J.J.; Mee, J.F.; O’Farrelly, C. Histopatho-logical and molecular evaluation of Holstein-Friesian cows postpartum: Toward an improved understanding of uterine innate immunity. Theriogenology 2009, 71, 1396–1407. [Google Scholar] [CrossRef]
- Gilbert, R.O.; Shin, S.T.; Guard, C.L.; Erb, H.N.; Frajblat, M. Prevalence of endometritis and its effects on reproductive performance of dairy cows. Theriogenology 2005, 64, 1879–1888. [Google Scholar] [CrossRef]
- Ireland, J.J.; Murphee, R.L.; Coulson, P.B. Accuracy of predicting stages of bovine estrous cycle by gross appearance of the corpus luteum. J. Dairy Sci. 1980, 63, 155–160. [Google Scholar] [CrossRef]
- Swangchan-Uthai, T.; Walsh, S.W.; Alexander, S.L.; Cheng, Z.; Crowe, M.A.; Evans, A.C.; Wathes, D.C. Comparison of mRNA for IGFs and their binding proteins in the oviduct during the peri-oestrous period between dairy heifers and lactating cows. Reproduction 2011, 142, 457–465. [Google Scholar] [CrossRef]
- Cheng, Z.; Oguejiofor, C.F.; Swangchan-Uthai, T.; Carr, S.; Wathes, D.C. Relationships between circulating urea concentrations and endometrial function in postpartum dairy cows. Animals 2015, 5, 748–773. [Google Scholar] [CrossRef]
- Vandesompele, J.; De Preter, K.; Pattyn, F.; Poppe, B.; Van Roy, N.; De Paepe, A.; Speleman, F. Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes. Genome Biol. 2002, 3, RESEARCH0034. [Google Scholar] [CrossRef]
- Wathes, D.C.; Swangchan-Uthai, T.; Oguejiofor, C.F.; Cheng, Z. Energy balance, immune function and fertility in the postpartum dairy cow. Cattle Pract. 2013, 21, 129–137. [Google Scholar]
- Tanikawa, M.; Kim, T.S.; Okuda, K.; Ryoo, Z.Y.; Park, S.B.; Shin, J.H.; Park, C.K.; Lee, D.S. Cell-type specificity of interleukins 1alpha and 1beta on prostaglandin and plasminogen activator production in bovine endometrial cells. Anim. Reprod. Sci. 2009, 114, 32–42. [Google Scholar] [CrossRef]
- Cronin, J.G.; Turner, M.L.; Goetze, L.; Bryant, C.E.; Sheldon, I.M. Toll-like receptor 4 and MYD88-dependent signaling mechanisms of the innate immune system are essential for the response to lipopolysaccharide by epithelial and stromal cells of the bovine endometrium. Biol. Reprod. 2012, 86, 51. [Google Scholar] [CrossRef]
- Chotimanukul, S.; Swangchan-Uthai, T.; Suwimonteerabutr, J.; Techakumphu, M. Effect of TLR4 antagonist on immune responses of bovine endometrial cells. Thai J. Vet. Med. 2017, 47, 87–96. [Google Scholar]
- Thompson, J.A.; Magee, D.D.; Tomaszewski, M.A.; Wilks, D.L.; Fourdraine, R.H. Management of summer infertility in Texas Holstein dairy cattle. Theriogenology 1996, 46, 547–558. [Google Scholar] [CrossRef]
- Nabenishi, H.; Ohta, H.; Nishimoto, T.; Morita, T.; Ashizawa, K.; Tsuzuki, Y. Effect of the temperature-humidity index on body temperature and conception rate of lactating dairy cows in southwestern Japan. J. Reprod. Dev. 2011, 57, 450–456. [Google Scholar] [CrossRef]
- Abdelnour, S.A.; Abd El-Hack, M.E.; Khafaga, A.F.; Arif, M.; Taha, A.E.; Noreldin, A.E. Stress biomarkers and proteomics alteration to thermal stress in ruminants: A review. J. Therm. Biol. 2019, 79, 120–134. [Google Scholar] [CrossRef]
- Bai, H.; Ukita, H.; Kawahara, M.; Mitani, T.; Furukawa, E.; Yanagawa, Y.; Yabuuchi, N.; Kim, H.; Takahashi, M. Effect of summer heat stress on gene expression in bovine uterine endometrial tissues. Anim. Sci. J. 2020, 91, e13474. [Google Scholar] [CrossRef]
- Zerbe, H.; Schuberth, H.J.; Engelke, F.; Frank, J.; Klug, E.; Leibold, W. Development and comparison of in vivo and in vitro models for endometritis in cows and mares. Theriogenology 2003, 60, 209–223. [Google Scholar] [CrossRef]
- Christoffersen, M.; Baagoe, C.D.; Jacobsen, S.; Bojesen, A.M.; Petersen, M.R.; Lehn-Jensen, H. Evaluation of the systemic acute phase response and endometrial gene expression of serum amyloid A and pro- and anti-inflammatory cytokines in mares with experimentally induced endometritis. Vet. Immumol. Immunopathol. 2010, 138, 95–105. [Google Scholar] [CrossRef]
- Ghasemi, F.; Gonzalez-Cano, P.; Griebel, P.J.; Palmer, C. Proinflammatory cytokine gene expression in endometrial cytobrush samples harvested from cows with and without subclinical endometritis. Theriogenology 2012, 78, 1538–1547. [Google Scholar] [CrossRef]
- Karstrup, C.C.; Agerholm, J.S.; Jensen, T.K.; Swaro, L.; Klitgaard, K.; Rasmussen, E.L.; Krogh, K.M.; Pedersen, H.G. Presence and localization of bacteria in the bovine endometrium postpartum using fluorescence in situ hybridization. Theriogenology 2017, 92, 167–175. [Google Scholar] [CrossRef]
- Sheldon, I.M.; Cronin, J.G.; Healey, G.D.; Gabler, C.; Heuwieser, W.; Streyl, D.; Bromfield, J.J.; Miyamoto, A.; Fergani, C.; Dobson, H. Innate immunity and inflammation of the bovine female reproductive tract in health and disease. Reproduction 2014, 148, R41–R51. [Google Scholar] [CrossRef] [PubMed]
- Sakai, S.; Hatabu, T.; Yamamoto, Y.; Kimura, K. Alteration of chemokine production in bovine endometrial epithelial and stromal cells under heat stress conditions. Physiol. Rep. 2020, 8, e14640. [Google Scholar] [CrossRef]
- Passey, R.J.; Williams, E.; Lichanska, A.M.; Wells, C.; Hu, S.; Geczy, C.L.; Little, M.H.; Hume, D.A. A null mutation in the inflammation-associated S100 protein S100A8 causes early resorption of the mouse embryo. J. Immunol. 1999, 163, 2209–2216. [Google Scholar] [PubMed]
- Ryan, D.P.; Blakewood, E.G.; Lynn, J.W.; Munyakazi, L.; Godke, R.A. Effect of heat-stress on bovine embryo development in vitro. J. Anim. Sci. 1992, 70, 3490–3497. [Google Scholar] [CrossRef] [PubMed]
- Sakai, S.; Hagihara, N.; Kuse, M.; Kimora, K.; Okuda, K. Heat stress affects prostaglandin synthesis in bovine endometrial cells. J. Reprod. Dev. 2018, 64, 311–317. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McCracken, J.A.; Custer, E.E.; Lamsa, J.C. Luteolysis: A neuro- endocrine-mediated event. Physiol. Rev. 1999, 79, 263–323. [Google Scholar] [CrossRef]
- Mateus, L.; Lopes da Costa, L.; Diniz, P.; Ziecik, A.J. Relationship between endotoxin and prostaglandin (PGE2 and PGFM) concentrations and ovarian function in dairy cows with puerperal endometritis. Anim. Reprod. Sci. 2003, 76, 143–154. [Google Scholar] [CrossRef]
- Namgaladze, D.; Snodgrass, R.G.; Angioni, C.; Grossmann, N.; Dehne, N.; Geisslinger, G.; Brüne, B. AMP-activated protein kinase suppresses arachidonate 15-lipoxygenase expression in interleukin 4-polarized human macrophages. J. Biol. Chem. 2015, 290, 24484–24494. [Google Scholar] [CrossRef]
- Singh, N.K.; Rao, G.N. Emerging role of 12/15-Lipoxygenase (ALOX15) in human pathologies. Prog. Lipid Res. 2019, 73, 28–45. [Google Scholar] [CrossRef]
- Uderhardt, S.; Herrmann, M.; Oskolkova, O.V.; Aschermann, S.; Bicker, W.; Ipseiz, N.; Sarter, K.; Frey, B.; Rothe, T.; Voll, R.; et al. 12/15-lipoxygenase orchestrates the clearance of apoptotic cells and maintains immunologic tolerance. Immunity 2012, 36, 834–846. [Google Scholar] [CrossRef] [PubMed]
- Hersberger, M. Potential role of the lipoxygenase derived lipid mediators in atherosclerosis: Leukotrienes, lipoxins and resolvins. Clin. Chem. Lab. Med. 2010, 48, 1063–1073. [Google Scholar] [CrossRef] [PubMed]
- Serhan, C.N.; Hamberg, M.; Samuelsson, B. Lipoxins: Novel series of biologically active compounds formed from arachidonic acid in human leukocytes. Proc. Natl. Acad. Sci. USA 1984, 81, 5335–5339. [Google Scholar] [CrossRef] [PubMed]
- Binder, R.J. Functions of heat shock proteins in pathways of the innate and adaptive immune system. J. Immunol. 2014, 193, 5765–5771. [Google Scholar] [CrossRef] [PubMed]
- Putney, D.J.; Malayer, J.R.; Gross, T.S.; Thatcher, W.W.; Hansen, P.J.; Drost, M. Heat stress-induced alterations in the synthesis and secretion of proteins and prostaglandins by cultured bovine conceptuses and uterine endometrium. Biol. Reprod. 1988, 39, 717–728. [Google Scholar] [CrossRef] [PubMed]
- Siripug, T.; Katawatin, S. Histological study of skin in Holstein-Friesian Crossbred with different genotype of HSP70-2 gene. Khon Kaen Agric. J. 2015, 43, 359–364. [Google Scholar]
- Igono, M.O.; Johnson, H.D. Physiological stress index of lactating dairy cows based on diurnal pattern of rectal temperature. J. Interdiscip. Cycle Res. 1990, 21, 303–320. [Google Scholar] [CrossRef]
- Hansen, P.J.; Arechiga, C.F. Strategies for managing reproduction in the heat-stressed dairy cow. J. Anim. Sci. 1999, 77 (Suppl. 2), 36–50. [Google Scholar] [CrossRef]
- Kadzere, C.T.; Murphy, M.R.; Silanikove, N.; Maltz, E. Heat stress in lactating dairy cows: A review. Livest. Prod. Sci. 2002, 77, 59–91. [Google Scholar] [CrossRef]
- Fathoni, A.; Boonkum, W.; Chankitisakul, V.; Duangjinda, M. An appropriate genetic approach for improving reproductive traits in crossbred Thai-Holstein Cattle under heat stress conditions. Vet. Sci. 2022, 9, 163. [Google Scholar] [CrossRef]

| Gene #. | Primer Sequence (5′ → 3′) | GenBank Accession | Product Length (bp) | Annealing Temp (°C) | R2 | E (%) |
|---|---|---|---|---|---|---|
| ALOX12 | F: TCTCTACGCCTGTGATGCTTTA | NM_001192336.1 | 202 | 55 | 0.986 | 99.63 |
| R: GAGTTGATTCTGGGACTGGAAG | ||||||
| HSPA5 | F: GGTATTGAAACTGTGGGAGGTG | NM_001075148 | 162 | 62.5 | 0.924 | 100.2 |
| R: AAGGTGATTGTCTTTCGTCAGG | ||||||
| IL1B | F: CTTGGGTATCAAGGACAAGAAT | NM_174093.1 | 207 | 62.5 | 0.991 | 99.7 |
| R: TCGATTTGAGAAGTGCTGATGT | ||||||
| IL8 | F: CCTAGTCTTGCCCTTATTATGC | NM_173925.2 | 232 | 62.5 | 0.998 | 101.6 |
| R:ACCAGTAGAAAGAACTGTGAACAT | ||||||
| S100A8 | F: TGCCATTAACTCCCTGATTGAC | NM_001113725.1 | 179 | 53 | 0.997 | 98.35 |
| R: TAATTCCACCATCCTGATTGAT | ||||||
| PTGES | F: TGGTCATCAAAATGTACGTGGT | NM_174443.2 | 201 | 59.5 | 0.976 | 99.9 |
| R: AGTAGACAAAGCCCAGGAACAG | ||||||
| AKR1B1 | F: GTCTCCAACTTCAACCATCTCC | NM_001012519.1 | 250 | 59.5 | 0.979 | 102.1 |
| R: TGTACTTGTCTGCAATCGCTTT | ||||||
| RN18S1 | F: CGGCGACGACCCATTCGAAC | NR_036642.1 | 99 | 51 | 0.959 | 99.9 |
| R: GAATCGAACCCTGATTCCCCGTC | ||||||
| ACTB | F: CATCGCGGACAGGATGCAGAAA | NM_173979.3 | 158 | 59.5 | 0.946 | 98.2 |
| R: CCTGCTTGCTGATCCACATCTGCT |
| Gene Symbol/ | LPS | Expression | Heat Exposure (mRNA Expression) | ||
|---|---|---|---|---|---|
| Protein | Control | Short-Term | Long-Term | ||
| ALOX12 | − | 27094 ± 3449 | 28675 ± 5812 | 28693 ± 7056 | 23913 ± 5345 |
| + | 51313 ± 7287 * | 45365 ± 10268 * | 56026 ± 13745 * | 52549 ± 14390 * | |
| Combined effect + | 37020 ± 6026 | 42360 ± 8075 | 38231 ± 8079 | ||
| HSPA5 | − | 1142994 ± 97170 | 1333116 ± 211903 | 973895 ± 156725 | 1121972 ± 121398 |
| + | 1393799 ± 102820 | 1516190 ± 236571 | 1374429 ± 134236 | 1290779 ± 156742 | |
| Combined effect + | 1424653 ± 156477 | 1174162 ± 109208 | 1206375 ± 98534 | ||
| IL1B | − | 3416 ± 919 | 4138 ± 2478 | 3283 ± 953 | 2773 ± 763 |
| + | 9853 ± 2915 * | 10559 ± 4430 | 8658 ± 3296 | 10341 ± 7100 * | |
| Combined effect + | 7349 ± 2571 | 5970 ± 1769 | 6721 ± 1582 | ||
| IL8 | − | 84364 ± 26922 | 76294 ± 27655 | 61850 ± 16574 | 114946 ± 75776 |
| + | 189605 ± 38673 * | 216197 ± 75931 * | 116970 ± 27697 | 235649 ± 83705 * | |
| Combined effect + | 146246 ± 42123 | 89410 ± 16798 | 175297 ± 56630 | ||
| S100A8 | − | 8204 ± 1441 | 11769 ± 3163 a | 9595 ± 2383 a | 3248 ± 757 b |
| + | 22952 ± 4626 * | 31561 ± 11748 * | 15243 ± 3582 | 22053 ± 6418 * | |
| Combined effect + | 21665 ± 6297 a | 12419 ± 2185 b | 12651 ± 3719 b | ||
| PTGES | − | 34703 ± 3097 | 28112 ± 4580 | 37523 ± 5786 | 38473 ± 5582 |
| + | 46914 ± 4294 * | 43547 ± 10126 * | 51730 ± 7728 | 45464 ± 3227 * | |
| Combined effect + | 35829 ± 5668 | 44626 ± 4948 | 41969 ± 3236 | ||
| AKR1B1 | − | 40722 ± 3621 | 31479 ± 1946 a | 40621 ± 6400 a,b | 50067 ± 8044 b |
| + | 31284 ± 1895 * | 28818 ± 3224 | 33273 ± 3596 | 31759 ± 3168 * | |
| Combined effect + | 30148 ± 1862 | 36947 ± 3671 | 40913 ± 4639 | ||
| IL8 | − | 100.6 ± 9.4 | 122.4 ± 20.2 a | 90.7 ± 15.2 b | 88.6 ± 11.6 b |
| (pg/mL) | + | 172.8 ± 9.4 * | 178.3 ± 19.7 * | 164.4 ± 13.5 * | 175.7 ± 16.1 * |
| Combined effect + | 150.4 ± 15.0 a | 127.5 ± 12.6 b | 132.1 ± 13.3 a,b | ||
| PGF2α | − | 18224 ± 3174 | 28436 ± 7454 a | 15663 ± 4768 b | 10574 ± 1578 b |
| (pg/mL) | + | 39060 ± 7193 * | 51854 ± 16261 | 37856 ± 13075 * | 27473 ± 5597 * |
| Combined effect + | 40145 ± 9082 a | 26759 ± 7188 b | 19024 ± 3345 b | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chotimanukul, S.; Suwimonteerabutr, J.; Techakumphu, M.; Swangchan-Uthai, T. In Vitro Effects of Short-Term and Long-Term Heat Exposures on the Immune Response and Prostaglandin Biosynthesis in Bovine Endometrial Cells. Animals 2022, 12, 2359. https://doi.org/10.3390/ani12182359
Chotimanukul S, Suwimonteerabutr J, Techakumphu M, Swangchan-Uthai T. In Vitro Effects of Short-Term and Long-Term Heat Exposures on the Immune Response and Prostaglandin Biosynthesis in Bovine Endometrial Cells. Animals. 2022; 12(18):2359. https://doi.org/10.3390/ani12182359
Chicago/Turabian StyleChotimanukul, Sroisuda, Junpen Suwimonteerabutr, Mongkol Techakumphu, and Theerawat Swangchan-Uthai. 2022. "In Vitro Effects of Short-Term and Long-Term Heat Exposures on the Immune Response and Prostaglandin Biosynthesis in Bovine Endometrial Cells" Animals 12, no. 18: 2359. https://doi.org/10.3390/ani12182359






