Assessment of Genetic Diversity and Conservation in South African Indigenous Goat Ecotypes: A Review
Abstract
:Simple Summary
Abstract
1. Introduction
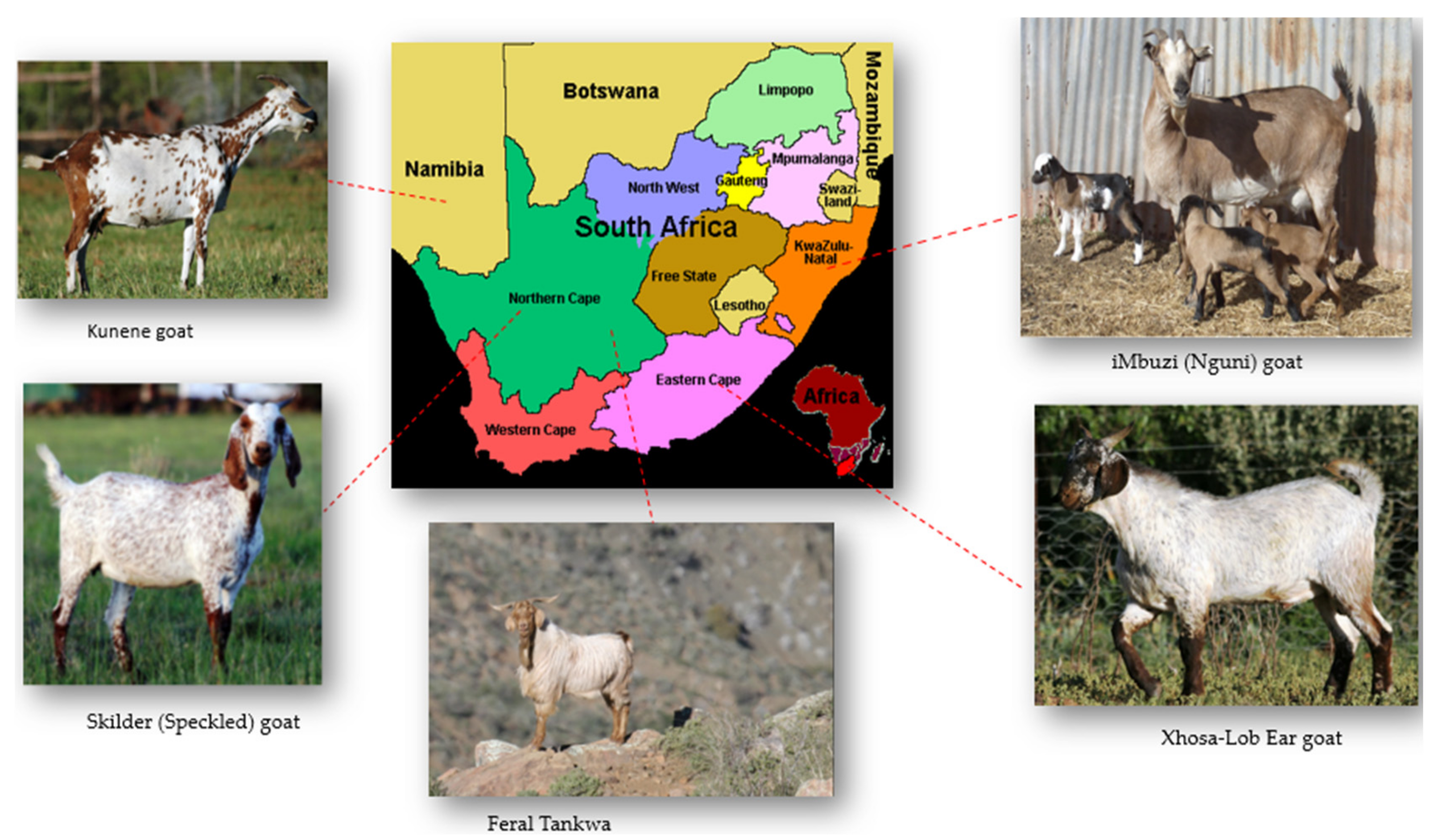
2. Production Potential of South Africa’s Indigenous Goat Ecotypes
3. The Availability of the Illumina Goat SNP BeadChip
4. Genetic Diversity Studies in Southern African Indigenous Goats
5. Adaptation of Indigenous Goat to Local Environments
6. Conservation Strategies for Goats
7. The Need to Conserve Indigenous Goat of South Africa
8. Natural Selection of Indigenous Goats
9. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Andre Mataveia, G.; Visser, C.; Sitoe, A. Smallholder Goat Production in Southern Africa: A Review. In Goat Science—Environment, Health and Economy; Kukovics, S., Ed.; IntechOpen: Rijeka, Croatia, 2021. [Google Scholar] [CrossRef]
- Department of Agriculture, Land Reform and Rural Development. A Profile of the South African Goat Market Value Chain 2018; Department of Agriculture, Forestry and Fisheries: Pretoria, South Africa, 2018; pp. 1–30.
- Mdladla, K.; Dzomba, E.F.; Muchadeyi, F.C. Characterization of the Village Goat Production Systems in the Rural Communities of the Eastern Cape, KwaZulu-Natal, Limpopo and North West Provinces of South Africa. Trop. Anim. Health Prod. 2017, 49, 515–527. [Google Scholar] [CrossRef] [PubMed]
- Chokoe, T.C.; Mdladla-Hadebe, K.; Muchadeyi, F.; Dzomba, E.; Matelele, T.; Mphahlele, T.; Mpofu, T.J.; Nephawe, K.; Mtileni, B. Genetic Diversity of South African Indigenous Goat Population from Four Provinces Using Genome-Wide SNP Data. Sustainability 2020, 12, 361. [Google Scholar] [CrossRef]
- Campbell, Q.P. The Origin and Description of Southern Africa’ s Indigenous Goats. S. Afr. J. Anim. Sci. 2003, 4, 18–22. [Google Scholar]
- Monau, P.I. Phenotypic and Genetic Characterisation of Indigenous Tswana Goat in Botswana. Ph.D. Thesis, University of Pretoria, Pretoria, South Africa, 2018. [Google Scholar]
- Kotzé, A.; Grobler, J.; Van Marle-Köster, E.; Jonker, T.; Dalton, D. The Tankwa Karoo National Park Feral Goat Population: A Unique Genetic Resource. S. Afr. J. Anim. Sci. 2014, 44, 43. [Google Scholar] [CrossRef] [Green Version]
- Mdladla, K. Landscape Genomic Approach to Investigate Genetic Adaptation in South African Indigenous Goat Populations. Ph.D. Thesis, University of KwaZulu-Natal, Pietermaritzburg, South Africa, 2016. [Google Scholar]
- Mdladla, K.; Dzomba, E.F.; Huson, H.J.; Muchadeyi, F.C. Population Genomic Structure and Linkage Disequilibrium Analysis of South African Goat Breeds Using Genome-Wide SNP Data. Anim. Genet. 2016, 47, 471–482. [Google Scholar] [CrossRef]
- Mohlatlole, R.P.; Dzomba, E.F.; Muchadeyi, F.C. Addressing Production Challenges in Goat Production Systems of South Africa: The Genomics Approach. Small Rumin. Res. 2015, 131, 43–49. [Google Scholar] [CrossRef]
- Thobile, F.M.; Olivia, N.M.; Keabetswe, T.N.; Edgar, F.D.; Tlou, C.M.; Farai, C.M.; Khanyisile, H. Goat Farmers Production Objectives and Trait Preferences in the North West Province of South Africa: An Approach to Identify Selection Criteria for Community-Based Breeding Program. Int. J. Livest. Prod. 2021, 12, 64–75. [Google Scholar] [CrossRef]
- Visser, C.; van Marle-Köster, E. The Development and Genetic Improvement of South African Goats. In Goat Science; Kukovics, S., Ed.; IntechOpen: Rijeka, Croatia, 2018. [Google Scholar] [CrossRef] [Green Version]
- FAO. Recommended Microsatellite Markers; FAO: Rome, Italy, 2011; ISBN 9789251070321. [Google Scholar]
- Toro, M.A.; Fernández, J.; Caballero, A. Molecular Characterization of Breeds and Its Use in Conservation. Livest. Sci. 2009, 120, 174–195. [Google Scholar] [CrossRef]
- Frankham, R. Challenges and Opportunities of Genetic Approaches to Biological Conservation. Biol. Conserv. 2010, 143, 1919–1927. [Google Scholar] [CrossRef]
- Allendorf, F.W.; Hohenlohe, P.A.; Luikart, G. Genomics and the Future of Conservation Genetics. Nat. Rev. Genet. 2010, 11, 697–709. [Google Scholar] [CrossRef]
- Van Wyk, G.L.; Hoffman, L.C.; Strydom, P.E.; Frylinck, L. Southern African Large Frame Indigenous Veld Goat and Boer Goat Wether and Buck Tenderness and Colour of Six Muscles. Preprints 2021, 12, 382. [Google Scholar] [CrossRef]
- van Wyk, G.L.; Hoffman, L.C.; Strydom, P.E.; Frylinck, L. Differences in Meat Quality of Six Muscles Obtained from Southern African Large-Frame Indigenous Veld Goat and Boer Goat Wethers and Bucks. Animals 2022, 12, 382. [Google Scholar] [CrossRef] [PubMed]
- Morrison, J.W. A Guide to the Identification of the Natural African Indigenous Veld Goats of Southern Africa; Indigenous Veld Goat Breeders Society: Pretoria, South Africa, 2007. [Google Scholar]
- Dube, K.; Muchenje, V.; Mupangwa, J.F. Inbreeding Depression and Simulation of Production Potential of the Communally Raised Indigenous Xhosa Lop Eared Goats. Small Rumin. Res. 2016, 144, 164–169. [Google Scholar] [CrossRef]
- Menezes, L.M.; Sousa, W.H.; Cavalcanti-Filho, E.P.; Gama, L.T. Genetic Parameters for Reproduction and Growth Traits in Boer Goats in Brazil. Small Rumin. Res. 2016, 136, 247–256. [Google Scholar] [CrossRef]
- Norton, B.W. Improving goat production from village systems in tropical climates: An experience from southern Thailand. In Research and Training Strategies for Goat Production Systems in South Africa; Queensland University: Brisbane, Australia, 1998. [Google Scholar]
- Ncube, K.T.; Dzomba, E.F.; Rosen, B.D.; Schroeder, S.G.; Van Tassell, C.P.; Muchadeyi, F.C. Differential Gene Expression and Identification of Growth-Related Genes in the Pituitary Gland of South African Goats. Front. Genet. 2022, 13, 811193. [Google Scholar] [CrossRef]
- Mrode, R.; Tarekegn, G.M.; Mwacharo, J.M.; Djikeng, A. Invited Review: Genomic Selection for Small Ruminants in Developed Countries: How Applicable for the Rest of the World? Animal 2018, 12, 1333–1340. [Google Scholar] [CrossRef] [Green Version]
- Tosser-Klopp, G.; Bardou, P.; Bouchez, O.; Cabau, C.; Crooijmans, R.; Dong, Y.; Donnadieu-Tonon, C.; Eggen, A.; Heuven, H.C.M.; Jamli, S.; et al. Design and Characterization of a 52K SNP Chip for Goats. PLoS ONE 2014, 9, e86227. [Google Scholar] [CrossRef]
- Lashmar, S.F.; Visser, C.; Van Marle-Köster, E. Validation of the 50k Illumina Goat SNP Chip in the South African Angora Goat. S. Afr. J. Anim. Sci. 2015, 45, 56–59. [Google Scholar] [CrossRef] [Green Version]
- Dotsev, A.V.; Rodionov, A.N.; Kharzinova, V.R.; Petrov, S.N.; Medvedev, D.G.; Bagirov, V.A.; Brem, G.; Zinovieva, N.A. An Assessment of Applicability of Snp Chip Developed for Domestic Goats in Genetic Studies of Caucasian Tur (Capra caucasica). Diversity 2021, 13, 312. [Google Scholar] [CrossRef]
- Monau, P.I.; Visser, C.; Muchadeyi, F.C.; Okpeku, M.; Nsoso, S.J.; Van Marle-Köster, E. Population Structure of Indigenous Southern African Goats Based on the Illumina Goat50K SNP Panel. Trop. Anim. Health Prod. 2020, 52, 1795–1802. [Google Scholar] [CrossRef]
- Visser, C.; Hefer, C.A.; van Marle-Köster, E.; Kotze, A. Genetic Variation of Three Commercial and Three Indigenous Goat Populations in South Africa. S. Afr. J. Anim. Sci. 2004, 34, 24–27. [Google Scholar]
- Ritland, K. A Marker-Based Method for Inferences about Quantitative Inheritance in Natural Populations. Evolution 1996, 50, 1062–1073. [Google Scholar] [CrossRef] [PubMed]
- Edea, Z.; Dadi, H.; Kim, S.; Dessie, T.; Lee, T.; Kim, H.; Kim, J.; Kim, K. Genetic Diversity, Population Structure and Relationships in Indigenous Cattle Populations of Ethiopia and Korean Hanwoo Breeds Using SNP Markers. Front. Genet. 2013, 4, 35. [Google Scholar] [CrossRef] [Green Version]
- Pareek, C.S.; Smoczyński, R.; Kadarmideen, H.N.; Dziuba, P.; Błaszczyk, P.; Sikora, M.; Walendzik, P.; Grzybowski, T.; Pierzchała, M.; Horbańczuk, J.; et al. Single Nucleotide Polymorphism Discovery in Bovine Pituitary Gland Using RNA-Seq Technology. PLoS ONE 2016, 11, e0161370. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Monau, P.I.; Visser, C.; Nsoso, S.J.; Van Marle-Köster, E. Phenotypic and Genetic Characterization of Indigenous Tswana Goats. S. Afr. J. Anim. Sci. 2019, 48, 925. [Google Scholar] [CrossRef]
- Chokoe, T.C.; Matelele, T.C.; Maqhashu, A.; Ramukhithi, F.V.; Mphahlele, T.D.; Mpofu, T.J.; Nephawe, K.A.; Mtileni, B. Phenotypic Diversity of South African Indigenous Goat Population in Selected Rural Areas. Am. J. Anim. Vet. Sci. 2020, 15, 59–66. [Google Scholar] [CrossRef]
- Toro, M.A.; Caballero, A. Characterization and Conservation of Genetic Diversity in Subdivided Populations. Philos. Trans. R. Soc. B Biol. Sci. 2005, 360, 1367–1378. [Google Scholar] [CrossRef] [Green Version]
- Weir, B.S.; Cockerham, C.C. Estimating F-Statistics for the Analysis of Population Structure. Evolution 1984, 38, 1358–1370. [Google Scholar] [CrossRef]
- Els, J.F.; Kotze, A.; Swart, H. Genetic Diversity of Indigenous Goats in Namibia Using Microsatellite Markers: Preliminary Results. S. Afr. J. Anim. Sci. 2004, 34, 65–67. [Google Scholar]
- Garrine, C.M.L.P. Genetic Characterization of Indigenous Goat Populations of Mozambique. Master’s Thesis, University of Pretoria, Pretoria, South Africa, 2007. [Google Scholar]
- Maletsanake, D.; Nsoso, S.J.; Kgwatalala, P.M. Genetic Variation from 12 Microsatellite Makers in an Indigenous Tswana Goat Flock in South-Eastern Botswana. Livest. Res. Rural Dev. 2013, 25, 1–6. [Google Scholar]
- Shapiro, B.J.; Alm, E.J. Comparing Patterns of Natural Selection across Species Using Selective Signatures. PLoS Genet. 2008, 4, e23. [Google Scholar] [CrossRef]
- Onzima, R.B.; Upadhyay, M.R.; Mukiibi, R.; Kanis, E.; Groenen, M.A.M.; Crooijmans, R.P.M.A. Genome-Wide Population Structure and Admixture Analysis Reveals Weak Differentiation among Ugandan Goat Breeds. Anim. Genet. 2018, 49, 59–70. [Google Scholar] [CrossRef] [PubMed]
- Waineina, R.W.; Ngeno, K.; Otieno, T.O.; Ilatsia, E.D. Genetic Diversity and Population Structure among Goat Genotypes in Kenya. bioRxiv 2020. [Google Scholar] [CrossRef]
- Zheng, Z.; Wang, X.; Li, M.; Li, Y.; Yang, Z.; Wang, X.; Pan, X.; Gong, M.; Zhang, Y.; Guo, Y.; et al. The Origin of Domestication Genes in Goats. Sci. Adv. 2020, 6, eaaz5216. [Google Scholar] [CrossRef]
- Kaiser, S.; Hennessy, M.B.; Sachser, N. Domestication Affects the Structure, Development and Stability of Biobehavioural Profiles. Front. Zool. 2015, 12, S19. [Google Scholar] [CrossRef] [Green Version]
- Bertolini, F.; Servin, B.; Talenti, A.; Rochat, E.; Kim, E.S.; Oget, C.; Palhière, I.; Crisà, A.; Catillo, G.; Steri, R.; et al. Signatures of Selection and Environmental Adaptation across the Goat Genome Post—Domestication. Genet. Sel. Evol. 2018, 50, 57. [Google Scholar] [CrossRef]
- Gregory, T.R. Artificial Selection and Domestication: Modern Lessons from Darwin’s Enduring Analogy. Evol. Educ. Outreach 2008, 2, 5–27. [Google Scholar] [CrossRef] [Green Version]
- Mirkena, T.; Duguma, G.; Haile, A.; Tibbo, M.; Okeyo, A.M.; Wurzinger, M.; Sölkner, J. Genetics of Adaptation in Domestic Farm Animals: A Review. Livest. Sci. 2010, 132, 1–12. [Google Scholar] [CrossRef]
- Rege, J.E.O.; Lipner, M.E. African Animal Genetic Resources: Their Characterisation, Conservation And Utilisation. In Proceedings of the Ethiopia International Livestock Centre for Africa, Addis Ababa, Ethiopia, 19–21 February 1992. [Google Scholar]
- Al-Dawood, A. Towards Heat Stress Management in Small Ruminants—A Review. Ann. Anim. Sci. 2017, 17, 59–88. [Google Scholar] [CrossRef] [Green Version]
- Sarangi, S.; Subhashree Sarangi, C. A41—Adaptability of Goats to Heat Stress—A Review. Pharma Innov. J. 2018, 7, 1114–1126. [Google Scholar]
- Berihulay, H.; Abied, A.; He, X.; Jiang, L.; Ma, Y. Adaptation Mechanisms of Small Ruminants to Environmental Heat Stress. Animals 2019, 9, 75. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Idowu, P.A.; Adelabu, O.A. An Investigation of Coat Colour Distribution of West African Dwarf Goats. J. Agric. Sci. 2018, 10, 228. [Google Scholar] [CrossRef] [Green Version]
- Koluman Darcan, N.; Silanikove, N. The Advantages of Goats for Future Adaptation to Climate Change: A Conceptual Overview. Small Rumin. Res. 2018, 163, 34–38. [Google Scholar] [CrossRef]
- Kaliber, M.; Koluman, N.; Silanikove, N. Physiological and Behavioral Basis for the Successful Adaptation of Goats to Severe Water Restriction under Hot Environmental Conditions. Animal 2016, 10, 82–88. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Russello, M.; Amato, G.; DeSalle, R.; Knapp, M. Conservation Genetics and Genomics. Genes 2020, 11, 318. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mdladla, K.; Dzomba, E.F.; Muchadeyi, F.C. The Potential of Landscape Genomics Approach in the Characterization of Adaptive Genetic Diversity in Indigenous Goat Genetic Resources: A South African Perspective. Small Rumin. Res. 2017, 150, 87–92. [Google Scholar] [CrossRef]
- Monau, P.; Raphaka, K.; Zvinorova-Chimboza, P.; Gondwe, T. Sustainable Utilization of Indigenous Goats in Southern Africa. Diversity 2020, 12, 20. [Google Scholar] [CrossRef] [Green Version]
- Li, R.; Li, Y.; Fang, X.; Yang, H.; Wang, J.; Kristiansen, K.; Wang, J. SNP Detection for Massively Parallel Whole-Genome Resequencing. Genome Res. 2009, 19, 1124–1132. [Google Scholar] [CrossRef] [Green Version]
- Angeloni, F.; Wagemaker, N.; Vergeer, P.; Ouborg, J. Genomic Toolboxes for Conservation Biologists. Evol. Appl. 2012, 5, 130–143. [Google Scholar] [CrossRef]
- Hoffmann, I.; Scherf, B. Animal Genetic Resources—Time to Worry? Livest. Rep. 2006, 57–76. [Google Scholar]
- Gandini, G.; Oldenbroek, K. Strategies for moving from conservation to utilization. In Utilisation and Conservation of Farm Animal Genetic Resources; Oldenbroek, K., Ed.; Wageningen Academic Publishers: Wageningen, The Netherlands, 2007; pp. 29–54. [Google Scholar]
- Dzama, K. Is the Livestock Sector in Southern Africa Prepared for Climate Change; South African Institute of International Affairs: Johannesburg, South Africa, 2016; pp. 1–4. [Google Scholar]
- Nyamushamba, G.B.; Mapiye, C.; Tada, O.; Halimani, T.E.; Muchenje, V. Conservation of Indigenous Cattle Genetic Resources in Southern Africa’s Smallholder Areas: Turning Threats into Opportunities—A Review. Asian Australas. J. Anim. Sci. 2017, 30, 603–621. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hoban, S.M.; Campbell, C.D.; Silva, J.M.; Ekblom, R.; Funk, W.C.; Garner, B.A.; Godoy, J.A.; Kershaw, F.; MacDonald, A.J.; Mergeay, J.; et al. Genetic diversity is considered important but interpreted narrowly in country reports to the Convention on Biological Diversity: Current actions and indicators are insufficient. Biol. Conserv. 2021, 261, 109233. [Google Scholar] [CrossRef]
- Hanotte, O.; Jianlin, H. Genetic Characterization of Livestock Populations and Its Use in Conservation Decision Making. In Proceedings of the Workshop on the Role of Biotechnology, Turin, Italy, 5–7 March 2005; pp. 131–136. [Google Scholar]
- National Plan for Conservation and Sustainable Use of Farm Animal Genetic Resources; Department of Agriculture, Land Reform and Rural Development: Pretoria: South Africa, 2016; p. 26.
- Zhang, M.; Peng, W.F.; Hu, X.J.; Zhao, Y.X.; Lv, F.H.; Yang, J. Global Genomic Diversity and Conservation Priorities for Domestic Animals Are Associated with the Economies of Their Regions of Origin. Sci. Rep. 2018, 8, 11677. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boettcher, P.J.; Tixier-Boichard, M.; Toro, M.A.; Simianer, H.; Eding, H.; Gandini, G.; Joost, S.; Garcia, D.; Colli, L.; Ajmone-Marsan, P. Objectives, Criteria and Methods for Using Molecular Genetic Data in Priority Setting for Conservation of Animal Genetic Resources. Anim. Genet. 2010, 41, 64–77. [Google Scholar] [CrossRef] [PubMed]
- Gwaze, F.R.; Chimonyo, M.; Dzama, K. Communal Goat Production in Southern Africa: A Review. Trop. Anim. Health Prod. 2009, 41, 1157–1168. [Google Scholar] [CrossRef] [PubMed]
- Mueller, J.P.; Rischkowsky, B.; Haile, A.; Philipsson, J.; Mwai, O.; Besbes, B.; Valle Zárate, A.; Tibbo, M.; Mirkena, T.; Duguma, G.; et al. Community-Based Livestock Breeding Programmes: Essentials and Examples. J. Anim. Breed. Genet. 2015, 132, 155–168. [Google Scholar] [CrossRef]
- Reist-Marti, S.B.; Simianer, H.; Gibson, J.; Hanotte, O.; Rege, J.E.O. Weitzman’s Approach and Conservation of Breed Diversity: An Application to African Cattle Breeds. Conserv. Biol. 2003, 17, 1299–1311. [Google Scholar] [CrossRef] [Green Version]
- Ying-Hui, L.; Li-Sheng, W.; Xiao-Fei, G.; Hao, X.; Yun-Hai, Z.; Jian-Ping, D.; Zi-Jun, Z.; Xiao-Rong, Z. Assessment of Genetic Diversity among the Twelve Chinese Meat Goat Breeds Using Weitzman Approach. J. Anim. Plant Sci. 2014, 24, 986–990. [Google Scholar]
- Ruane, J. A Critical Review of the Value of Genetic Distance Studies in Conservation of Animal Genetic Resources. J. Anim. Breed. Genet. 1999, 116, 317–323. [Google Scholar] [CrossRef]
- Ruane, J. A Framework for Prioritizing Domestic Animal Breeds for Conservation Purposes at the National Level: A Norwegian Case Study. Conserv. Biol. 2000, 14, 1385–1393. [Google Scholar] [CrossRef]
- Bellon, M.R.; Dulloo, E.; Sardos, J.; Thormann, I.; Burdon, J.J. In situ conservation-harnessing natural and human-derived evolutionary forces to ensure future crop adaptation. Evol Appl. 2017, 10, 965–977. [Google Scholar] [CrossRef] [PubMed]
- Mdladla, K.; Dzomba, E.F.; Muchadeyi, F.C. Landscape Genomics and Pathway Analysis to Understand Genetic Adaptation of South African Indigenous Goat Populations. Heredity 2018, 120, 369–378. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Groeneveld, L.F.; Lenstra, J.A.; Eding, H.; Toro, M.A.; Scherf, B.; Pilling, D.; Negrini, R.; Finlay, E.K.; Jianlin, H.; Groeneveld, E.; et al. Genetic Diversity in Farm Animals—A Review. Anim. Genet. 2010, 41, 6–31. [Google Scholar] [CrossRef] [Green Version]
- Gwaze, F.R.; Chimonyo, M.; Dzama, K. Effect of Season and Age on Blood Minerals, Liver Enzyme Levels, and Faecal Egg Counts in Nguni Goats of South Africa. Czech J. Anim. Sci. 2012, 57, 443–453. [Google Scholar] [CrossRef] [Green Version]
- Onzima, R.B.; Upadhyay, M.R.; Doekes, H.P.; Brito, L.F.; Bosse, M.; Kanis, E.; Groenen, M.A.M.; Crooijmans, R.P.M.A. Genome-Wide Characterization of Selection Signatures and Runs of Homozygosity in Ugandan Goat Breeds. Front. Genet. 2018, 9, 318. [Google Scholar] [CrossRef] [PubMed]
- Qanbari, S.; Simianer, H. Mapping Signatures of Positive Selection in the Genome of Livestock. Livest. Sci. 2014, 166, 133–143. [Google Scholar] [CrossRef]
- Kim, E.; Elbeltagy, A.R.; Aboul-naga, A.M.; Rischkowsky, B.; Sayre, B.; Mwacharo, J.M.; Rothschild, M.F. Multiple Genomic Signatures of Selection in Goats and Sheep Indigenous to a Hot Arid Environment. Heredity 2016, 116, 255–264. [Google Scholar] [CrossRef]
- Talenti, A.; Bertolini, F.; Pagnacco, G.; Pilla, F.; Ajmone-Marsan, P.; Rothschild, M.F.; Crepaldi, P. The Valdostana Goat: A Genome-Wide Investigation of the Distinctiveness of Its Selective Sweep Regions. Mamm. Genome 2017, 28, 114–128. [Google Scholar] [CrossRef] [Green Version]
- Burren, A.; Neuditschko, M.; Signer-Hasler, H.; Frischknecht, M.; Reber, I.; Menzi, F.; Drögemüller, C.; Flury, C. Genetic Diversity Analyses Reveal First Insights into Breed-Specific Selection Signatures within Swiss Goat Breeds. Anim. Genet. 2016, 47, 727–739. [Google Scholar] [CrossRef]
- Brito, L.F.; Kijas, J.W.; Ventura, R.V.; Sargolzaei, M.; Porto-Neto, L.R.; Cánovas, A.; Feng, Z.; Jafarikia, M.; Schenkel, F.S. Genetic Diversity and Signatures of Selection in Various Goat Breeds Revealed by Genome-Wide SNP Markers. BMC Genom. 2017, 18, 229. [Google Scholar] [CrossRef]
| Reference | Indigenous Goats | Village Management (kg) | Improved Management (kg) |
|---|---|---|---|
| [21] | Birth weight (kg) | N/A | 1.7 |
| Weight at: 3 months | 6.8 | 9.2 | |
| Weight at: 6 months | 10.0 | 12.4 | |
| Weight at: 12 months | 13.0 | 20.0 | |
| Weight at: 18 months | 17.3 | 24.1 | |
| Weight at: 24 months | 21.5 | 29.5 |
| Reference | Breed | Production System | Trial | 12 W (kg) | 24 W (kg) | 36 W (kg) |
|---|---|---|---|---|---|---|
| [22] | 1 | 9 | 22.5 | 32 | ||
| Village ecotypes | Intensive | 2 | 10.5 | 19.5 | 31 | |
| 3 | 16.4 | 24 | 33 | |||
| Mean ± SD | - | 11.97 ± 3.91 | 22.00 ± 2.29 | 32 ± 1.00 | ||
| 1 | 7 | 16 | 22.5 | |||
| Village ecotypes | Extensive | 2 | 13 | 20.5 | 28 | |
| 3 | 13 | 15 | 27.5 | |||
| Mean ± SD | - | 11.00 ± 3.46 | 17.17 ± 2.93 | 26 ± 3.04 |
| Country | Populations | Markers | Marker Density | Ho | HE | Reference |
|---|---|---|---|---|---|---|
| South Africa | Indigenous goats | Microsatellite | 10 Microsatellite loci | 0.64–0.6981 | 0.631–0.673 | [28] |
| Communal indigenous goat populations | SNP Markers | The Illumina Goats 50K SNP BeadChip | 0.39–0.42 | 0.38–0.40 | [4] | |
| Nguni, Tswana, Venda, Xhosa, Zulu, and Tankwa | SNP Markers | The Illumina Goat 50K SNP BeadChip | 0.35–0.41 | 0.33–0.40 | [9] | |
| Namibia | Ovambo Caprivi Kunene Kavango | Microsatellite | 18 Microsatellite loci | - | 0.623 | [36] |
| Mozambique | Pafuri, Tete, Maputo | Microsatellite | 17 microsatellites loci | 0.553 | 0.620 | [37] |
| Botswana | Tswana goats | Microsatellite | 12 microsatellite loci | 0.121 | 0.162 | [38] |
| Tswana goats | SNP Markers | The Illumina Goat 50K SNP BeadChip | 0.419 | 0.423 | [32] |
| Genes | Fst | Chromosome | Gene Function |
|---|---|---|---|
| UHRF2 | 0.72 | 8 | Ubiquitin-like with PHD and ring finger domains 2 |
| GLDC | 0.69 | 8 | Glycine decarboxylase |
| NDST3 | 0.68 | 6 | N-deacetylase and N-sulfotransferase 3 |
| CFAP61 | 0.68 | 13 | Cilia and flagella associated protein 61 |
| CUBN | 0.67 | 13 | Cubilin |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Magoro, A.M.; Mtileni, B.; Hadebe, K.; Zwane, A. Assessment of Genetic Diversity and Conservation in South African Indigenous Goat Ecotypes: A Review. Animals 2022, 12, 3353. https://doi.org/10.3390/ani12233353
Magoro AM, Mtileni B, Hadebe K, Zwane A. Assessment of Genetic Diversity and Conservation in South African Indigenous Goat Ecotypes: A Review. Animals. 2022; 12(23):3353. https://doi.org/10.3390/ani12233353
Chicago/Turabian StyleMagoro, Aletta Matshidiso, Bohani Mtileni, Khanyisile Hadebe, and Avhashoni Zwane. 2022. "Assessment of Genetic Diversity and Conservation in South African Indigenous Goat Ecotypes: A Review" Animals 12, no. 23: 3353. https://doi.org/10.3390/ani12233353
APA StyleMagoro, A. M., Mtileni, B., Hadebe, K., & Zwane, A. (2022). Assessment of Genetic Diversity and Conservation in South African Indigenous Goat Ecotypes: A Review. Animals, 12(23), 3353. https://doi.org/10.3390/ani12233353





