Characterization of β-Glucans from Cereal and Microbial Sources and Their Roles in Feeds for Intestinal Health and Growth of Nursery Pigs
Abstract
:Simple Summary
Abstract
1. Introduction
2. Difference of Composition and Structure of β-Glucans Influence Viscosity of Digesta in GIT of Nursery Pigs
Structural and Compositional Difference of β-Glucans

3. Effects of Dietary β-Glucans on Intestinal Microbiota and Intestinal Health of Nursery Pigs
3.1. Effects of Cereal β-Glucans on Intestinal Health of Nursery Pigs
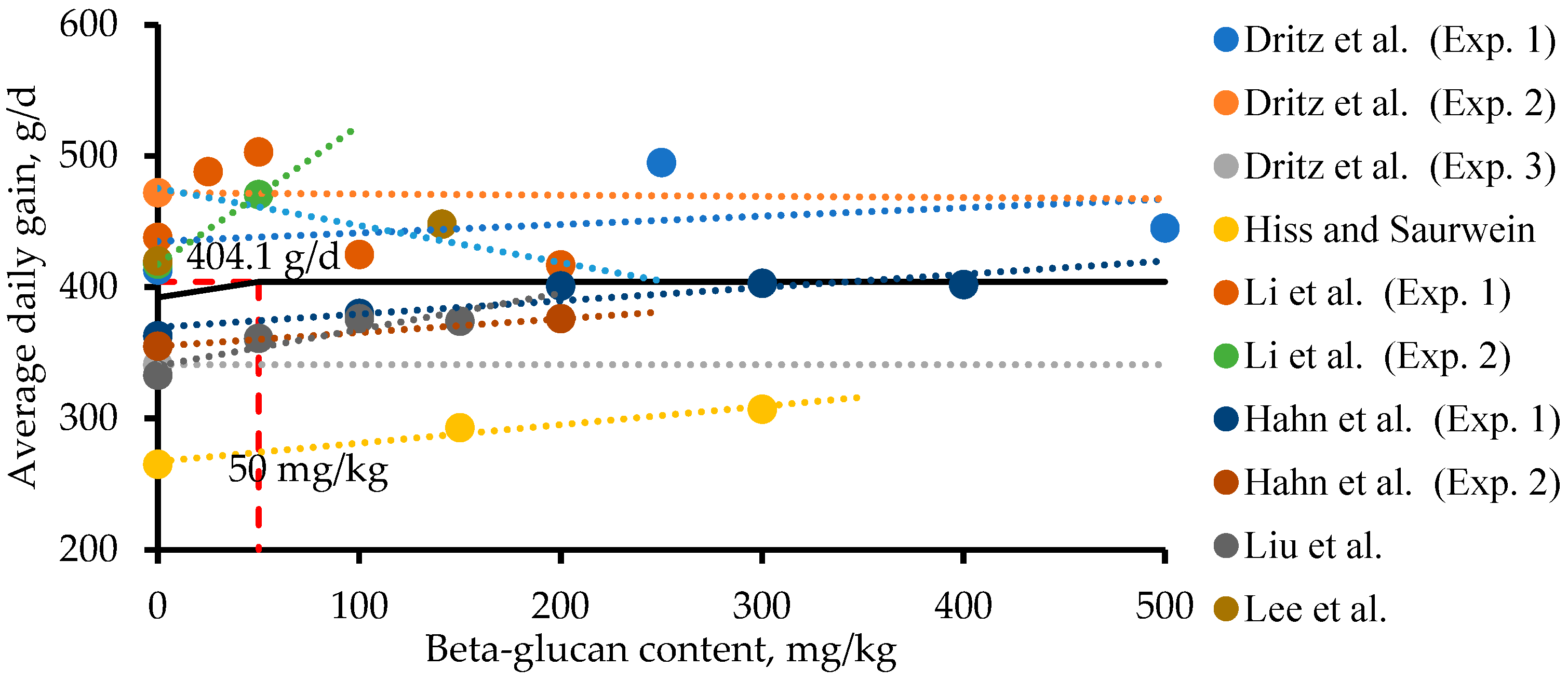
3.2. Effects of Microbial β-Glucans on Intestinal Health and Growth Performance of Nursery Pigs
3.2.1. Yeast (Saccharomyces cerevisiae)
| Item | Initial BW (kg) or Age (d) | Experimental Period (d) | β-Glucan Compound (mg/kg) | β-Glucan (mg/kg) | Results | Reference | ||
| Intestinal health | 8.0 kg | 28 | 500 | 141 | Increased jejunal goblet cells, tended to decrease diarrhea during d 0 to 14, tended to increase VH:CD, and tended to increase apparent ileal and total tract digestibility of energy | [54] | ||
| 6.4 kg | 35 | - | 100, 200, 300, and 400 | Linearly increased apparent total tract digestibility of nutrients | [55] | |||
| 5.8 kg | 21 | - | 50, 100, and 150 | Increased villus height and VH:CD on the jejunum | [61] | |||
| 15.3 kg | 28 | - | 250 | Decreased Enterobacteria spp. In ileum and proximal colon | [39] | |||
| Item | Initial BW (kg) or age (d) | Experimental period (d) | β-glucan compound (mg/kg) | β-glucan (mg/kg) | ADG (% change) | ADFI (% change) | G:F (% change) | Reference |
| Growth performance | 4.9 kg | 28 | - | 250 | 19.9 ** | 23.2 ** | −1.1 | [58] |
| 500 | 7.7 | 11.6 | −1.1 | |||||
| 5.0 kg | 28 | - | 1000 | −1.9 | −7.1 ** | 2.6 | ||
| 1000 | 0 | −1.5 ** | 0 | |||||
| 28 d | 28 | - | 150 | 10.6 | 7.4 | 0 | [62] | |
| 300 | 15.8 | 15.4 * | 0 | |||||
| 8.7 3 kg | 28 | - | 25 | 11.4 | 7.5 | 3.1 | [10] | |
| 50 | 14.8 | 11.6 | 2.7 | |||||
| 100 | −3 | −4.6 | 0.6 | |||||
| 200 | −4.8 | −3.7 | −1.3 | |||||
| 8.2 kg | 28 | - | 50 | 12.7 ** | 11.5 ** | 1.3 | ||
| 6.4 4 kg | 35 | - | 100 | 4.7 | 3.2 | 1.6 | [55] | |
| 200 | 10.5 | 9 | 0 | |||||
| 300 | 11 | 6.8 | 3.2 | |||||
| 400 | 10.7 | 5.4 | 4.8 | |||||
| 6.2 kg | 35 | - | 200 | 5.9 | 1.8 | 4.2 | ||
| 5.8 kg | 21 | - | 50 | 8.4 ** | 2.4 | 5.9 | [61] | |
| 100 | 12.9 ** | 6.0 ** | 6.6 | |||||
| 150 | 12.3 ** | 8.8 | 3.2 | |||||
| 8.0 kg | 28 | 500 | 141 | 6.7 * | 2.8 | 3.8 | [54] | |
| 6.0 kg | 35 | 2000 | NA | 7.4 ** | 6.5 ** | 0.9 | [64] | |
| 6.0 kg | 48 | 2000 | NA | −5.5 | −6.9 | 1.6 | [65] | |
| Average % change: | 7.6 | 5.3 | 1.9 | |||||
3.2.2. Bacteria (Agrobacterium sp.)
3.2.3. Algae (Euglena gracilis, Laminaria digitata, and Laminaria hyperborea)
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Flis, M.; Sobotka, W.; Antoszkiewicz, Z. Fiber substrates in the nutrition of weaned piglets—A review. Ann. Anim. Sci. 2017, 17, 627–644. [Google Scholar] [CrossRef] [Green Version]
- Zheng, L.; Duarte, M.E.; Sevarolli Loftus, A.; Kim, S.W. Intestinal health of pigs upon weaning: Challenges and nutritional intervention. Front. Vet. Sci. 2021, 8, 628258. [Google Scholar] [CrossRef] [PubMed]
- Casewell, M.; Friis, C.; Marco, E.; McMullin, P.; Phillips, I. The European ban on growth-promoting antibiotics and emerging consequences for human and animal health. J. Antimicrob. Chemother. 2003, 52, 159–161. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, S.W.; Duarte, M.E. Understanding intestinal health in nursery pigs and the relevant nutritional strategies. Anim. Biosci. 2021, 34, 338. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Xing, J.; Li, D.; Wang, X.; Zhao, L.; Lv, S.; Huang, D. Effects of β-glucan extracted from Saccharomyces cerevisiae on humoral and cellular immunity in weaned piglets. Arch. Anim. Nutr. 2005, 59, 303–312. [Google Scholar] [CrossRef]
- Luo, J.; Liu, S.; Yu, B.; He, J.; Mao, X.; Cheng, L.; Chen, D. Beta-glucan from Agrobacterium sp. ZX09 improves growth performance and intestinal function in weaned piglets. J. Anim. Physiol. Anim. Nutr. 2019, 103, 1818–1827. [Google Scholar] [CrossRef]
- Suchecka, D.; Gromadzka-Ostrowska, J.; Żyła, E.; Harasym, J.; Oczkowski, M. Selected physiological activities and health promoting properties of cereal beta-glucans. A review. J. Anim. Feed Sci. 2017, 26, 183–191. [Google Scholar] [CrossRef]
- Volman, J.J.; Ramakers, J.D.; Plat, J. Dietary modulation of immune function by β-glucans. Physiol. Behav. 2008, 94, 276–284. [Google Scholar] [CrossRef] [PubMed]
- Kaur, R.; Sharma, M.; Ji, D.; Xu, M.; Agyei, D. Structural features, modification, and functionalities of beta-glucan. Fibers 2019, 8, 1. [Google Scholar] [CrossRef] [Green Version]
- Li, J.; Li, D.F.; Xing, J.J.; Cheng, Z.B.; Lai, C.H. Effects of β-glucan extracted from Saccharomyces cerevisiae on growth performance, and immunological and somatotropic responses of pigs challenged with Escherichia coli lipopolysaccharide. J. Anim. Sci. 2006, 84, 2374–2381. [Google Scholar] [CrossRef] [Green Version]
- Jha, R.; Rossnagel, B.; Pieper, R.; Van Kessel, A.; Leterme, P. Barley and oat cultivars with diverse carbohydrate composition alter ileal and total tract nutrient digestibility and fermentation metabolites in weaned piglets. Animal 2010, 4, 724–731. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Skendi, A.; Biliaderis, C.G.; Lazaridou, A.; Izydorczyk, M.S. Structure and rheological properties of water soluble β-glucans from oat cultivars of Avena sativa and Avena bysantina. J. Cereal Sci. 2003, 38, 15–31. [Google Scholar] [CrossRef]
- Holtekjølen, A.K.; Vhile, S.G.; Sahlstrøm, S.; Knutsen, S.H.; Uhlen, A.K.; Åssveen, M.; Kjos, N.P. Changes in relative molecular weight distribution of soluble barley beta-glucan during passage through the small intestine of pigs. Livest. Sci. 2014, 168, 102–108. [Google Scholar] [CrossRef] [Green Version]
- Lambo, A.M.; Öste, R.; Nyman, M.E.L. Dietary fibre in fermented oat and barley β-glucan rich concentrates. Food Chem. 2005, 89, 283–293. [Google Scholar] [CrossRef]
- Wood, P.J. Oat and rye β-glucan: Properties and function. Cereal Chem. 2010, 87, 315–330. [Google Scholar] [CrossRef]
- Högberg, A.; Lindberg, J.E. Influence of cereal non-starch polysaccharides and enzyme supplementation on digestion site and gut environment in weaned piglets. Anim. Feed Sci. Technol. 2004, 116, 113–128. [Google Scholar] [CrossRef]
- MacArtain, P.; Gill, C.I.; Brooks, M.; Campbell, R.; Rowland, I.R. Nutritional value of edible seaweeds. Nutr. Rev. 2007, 65, 535–543. [Google Scholar] [CrossRef]
- Read, S.M.; Currie, G.; Bacic, A. Analysis of the structural heterogeneity of laminarin by electrospray-ionisation-mass spectrometry. Carbohydr. Res. 1996, 281, 187–201. [Google Scholar] [CrossRef]
- Manners, D.J.; Masson, A.J.; Patterson, J.C. The structure of a β-(1→3)-D-glucan from yeast cell walls. Biochem. J. 1973, 135, 19–30. [Google Scholar] [CrossRef]
- Du, B.; Meenu, M.; Liu, H.; Xu, B. A concise review on the molecular structure and function relationship of β-glucan. Int. J. Mol. Sci. 2019, 20, 4032. [Google Scholar] [CrossRef] [Green Version]
- Zhou, X.; Beltranena, E.; Zijlstra, R.T. Effect of feeding wheat- or barley-based diets with low or and high nutrient density on nutrient digestibility and growth performance in weaned pigs. Anim. Feed Sci. Technol. 2016, 218, 93–99. [Google Scholar] [CrossRef]
- Nasir, Z.; Wang, L.F.; Young, M.G.; Swift, M.L.; Beltranena, E.; Zijlstra, R.T. The effect of feeding barley on diet nutrient digestibility and growth performance of starter pigs. Anim. Feed Sci. Technol. 2015, 210, 287–294. [Google Scholar] [CrossRef]
- Che, T.M.; Perez, V.G.; Song, M.; Pettigrew, J.E. Effect of rice and other cereal grains on growth performance, pig removal, and antibiotic treatment of weaned pigs under commercial conditions1. J. Anim. Sci. 2012, 90, 4916–4924. [Google Scholar] [CrossRef] [Green Version]
- Dikeman, C.L.; Fahey, G.C., Jr. Viscosity as related to dietary fiber: A review. Crit. Rev. Food Sci. Nutr. 2006, 46, 649–663. [Google Scholar] [CrossRef] [PubMed]
- Owusu-Asiedu, A.; Patience, J.F.; Laarveld, B.; Van Kessel, A.G.; Simmins, P.H.; Zijlstra, R.T. Effects of guar gum and cellulose on digesta passage rate, ileal microbial populations, energy and protein digestibility, and performance of grower pigs. J. Anim. Sci. 2006, 84, 843–852. [Google Scholar] [CrossRef] [PubMed]
- Hooda, S.; Matte, J.J.; Vasanthan, T.; Zijlstra, R.T. Dietary oat β-glucan reduces peak net glucose flux and insulin production and modulates plasma incretin in portal-vein catheterized grower pigs. J. Nutr. 2010, 140, 1564–1569. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Willamil, J.; Badiola, I.; Devillard, E.; Geraert, P.A.; Torrallardona, D. Wheat-barley-rye-or corn-fed growing pigs respond differently to dietary supplementation with a carbohydrase complex. J. Anim. Sci. 2012, 90, 824–832. [Google Scholar] [CrossRef] [Green Version]
- Knudsen, K.E.B. Carbohydrate and lignin contents of plant materials used in animal feeding. Anim. Feed Sci. Technol. 1997, 67, 319–338. [Google Scholar] [CrossRef]
- Hopwood, D.E.; Pethick, D.W.; Pluske, J.R.; Hampson, D.J. Addition of pearl barley to a rice-based diet for newly weaned piglets increases the viscosity of the intestinal contents, reduces starch digestibility and exacerbates post-weaning colibacillosis. Br. J. Nutr. 2004, 92, 419–427. [Google Scholar] [CrossRef] [Green Version]
- Passos, A.A.; Park, I.; Ferket, P.; Von Heimendahl, E.; Kim, S.W. Effect of dietary supplementation of xylanase on apparent ileal digestibility of nutrients, viscosity of digesta, and intestinal morphology of growing pigs fed corn and soybean meal based diet. Anim. Nutr. 2015, 1, 19–23. [Google Scholar] [CrossRef]
- Chen, H.; Zhang, S.; Kim, S.W. Effects of supplemental xylanase on health of the small intestine in nursery pigs fed diets with corn distillers’ dried grains with solubles. J. Anim. Sci. 2020, 98, skaa185. [Google Scholar] [CrossRef]
- Medel, P.; Baucells, F.; Gracia, M.I.; De Blas, C.; Mateos, G.G. Processing of barley and enzyme supplementation in diets for young pigs. Anim. Feed Sci. Technol. 2002, 95, 113–122. [Google Scholar] [CrossRef]
- Schop, M.; Jansman, A.; de Vries, S.; Gerrits, W. Increased diet viscosity by oat β-glucans decreases the passage rate of liquids in the stomach and affects digesta physicochemical properties in growing pigs. Animal 2020, 14, 269–276. [Google Scholar] [CrossRef] [PubMed]
- Baker, J.T.; Duarte, M.E.; Holanda, D.M.; Kim, S.W. Friend or foe? Impacts of dietary xylans, xylooligosaccharides, and xylanases on intestinal health and growth performance of monogastric animals. Animals 2021, 11, 609. [Google Scholar] [CrossRef] [PubMed]
- Johansen, H.N.; Bach Knudsen, K.E.; Wood, P.J.; Fulcher, R.G. Physico-chemical properties and the degradation of oat bran polysaccharides in the gut of pigs. J. Sci. Food Agric. 1997, 73, 81–92. [Google Scholar] [CrossRef]
- Thacker, P.A.; Campbell, G.L.; Grootwassink, J. The effect of organic acids and enzyme supplementation on the performance of pigs fed barley-based diets. Can. J. Anim. Sci. 1992, 72, 395–402. [Google Scholar] [CrossRef]
- Bischoff, S.C. ‘Gut health’: A new objective in medicine? BMC Med. 2011, 9, 24. [Google Scholar] [CrossRef]
- Bach Knudsen, K.E.; Hedemann, M.S.; Lærke, H.N. The role of carbohydrates in intestinal health of pigs. Anim. Feed Sci. Technol. 2012, 173, 41–53. [Google Scholar] [CrossRef]
- Sweeney, T.; Collins, C.B.; Reilly, P.; Pierce, K.M.; Ryan, M.; O’doherty, J.V. Effect of purified β-glucans derived from Laminaria digitata, Laminaria hyperborea and Saccharomyces cerevisiae on piglet performance, selected bacterial populations, volatile fatty acids and pro-inflammatory cytokines in the gastrointestinal tract of pigs. Br. J. Nutr. 2012, 108, 1226–1234. [Google Scholar] [CrossRef] [Green Version]
- Reilly, P.; Sweeney, T.; O’Shea, C.; Pierce, K.M.; Figat, S.; Smith, A.G.; Gahan, D.A.; O’Doherty, J.V. The effect of cereal-derived beta-glucans and exogenous enzyme supplementation on intestinal microflora, nutrient digestibility, mineral metabolism and volatile fatty acid concentrations in finisher pigs. Anim. Feed Sci. Technol. 2010, 158, 165–176. [Google Scholar] [CrossRef]
- Ewaschuk, J.B.; Johnson, I.R.; Madsen, K.L.; Vasanthan, T.; Ball, R.; Field, C.J. Barley-derived β-glucans increases gut permeability, ex vivo epithelial cell binding to E. coli, and naïve T-cell proportions in weanling pigs1,2. J. Anim. Sci. 2012, 90, 2652–2662. [Google Scholar] [CrossRef]
- Metzler-Zebeli, B.U.; Zijlstra, R.T.; Mosenthin, R.; Gänzle, M.G. Dietary calcium phosphate content and oat β-glucan influence gastrointestinal microbiota, butyrate-producing bacteria and butyrate fermentation in weaned pigs. FEMS Microbiol. Ecol. 2011, 75, 402–413. [Google Scholar] [CrossRef]
- Liu, Y.; Espinosa, C.D.; Abelilla, J.J.; Casas, G.A.; Lagos, L.V.; Lee, S.A.; Kwon, W.B.; Mathai, J.K.; Navarro, D.M.; Jaworski, N.W. Non-antibiotic feed additives in diets for pigs: A review. Anim. Nutr. 2018, 4, 113–125. [Google Scholar] [CrossRef] [PubMed]
- Metzler-Zebeli, B.U.; Zebeli, Q. Cereal β-glucan alters nutrient digestibility and microbial activity in the intestinal tract of pigs, and lower manure ammonia emission: A meta-analysis. J. Anim. Sci. 2013, 91, 3188–3199. [Google Scholar] [CrossRef]
- McDonald, D.E.; Pethick, D.W.; Mullan, B.P.; Hampson, D.J. Increasing viscosity of the intestinal contents alters small intestinal structure and intestinal growth, and stimulates proliferation of enterotoxigenic Escherichia coli in newly-weaned pigs. Br. J. Nutr. 2001, 86, 487–498. [Google Scholar] [CrossRef] [Green Version]
- Hu, C.H.; Xiao, K.; Luan, Z.S.; Song, J. Early weaning increases intestinal permeability, alters expression of cytokine and tight junction proteins, and activates mitogen-activated protein kinases in pigs1. J. Anim. Sci. 2013, 91, 1094–1101. [Google Scholar] [CrossRef] [Green Version]
- Moeser, A.J.; Pohl, C.S.; Rajput, M. Weaning stress and gastrointestinal barrier development: Implications for lifelong gut health in pigs. Anim. Nutr. 2017, 3, 313–321. [Google Scholar] [CrossRef]
- Luo, J.; Chen, D.; Mao, X.; He, J.; Yu, B.; Cheng, L.; Zeng, D. Purified β-glucans of different molecular weights enhance growth performance of LPS-challenged piglets via improved gut barrier function and microbiota. Animals 2019, 9, 602. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, K.; Ehrlich, A.; Perng, V.; Chase, J.A.; Raybould, H.; Li, X.; Atwill, E.R.; Whelan, R.; Sokale, A.; Liu, Y. Algae-derived β-glucan enhanced gut health and immune responses of weaned pigs experimentally infected with a pathogenic E. coli. Anim. Feed Sci. Technol. 2019, 248, 114–125. [Google Scholar] [CrossRef]
- Drummond, R.A.; Brown, G.D. The role of Dectin-1 in the host defence against fungal infections. Curr. Opin. Microbiol. 2011, 14, 392–399. [Google Scholar] [CrossRef] [PubMed]
- Huntley, N.F.; Nyachoti, C.M.; Patience, J.F. Lipopolysaccharide immune stimulation but not β-mannanase supplementation affects maintenance energy requirements in young weaned pigs. J. Anim. Sci. Biotechnol. 2018, 9, 47. [Google Scholar] [CrossRef] [Green Version]
- Van der Meer, Y.; Jansman, A.J.M.; Gerrits, W.J.J. Low sanitary conditions increase energy expenditure for maintenance and decrease incremental protein efficiency in growing pigs. Animal 2020, 14, 1811–1820. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.B.; Piao, X.S.; Kim, S.W.; Wang, L.; Liu, P.; Yoon, I.; Zhen, Y.G. Effects of yeast culture supplementation on growth performance, intestinal health, and immune response of nursery pigs. J. Anim. Sci. 2009, 87, 2614–2624. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, J.J.; Kyoung, H.; Cho, J.H.; Choe, J.; Kim, Y.; Liu, Y.; Kang, J.; Lee, H.; Kim, H.B.; Song, M. Dietary yeast cell wall improves growth performance and prevents of diarrhea of weaned pigs by enhancing gut health and anti-Inflammatory immune responses. Animals 2021, 11, 2269. [Google Scholar] [CrossRef] [PubMed]
- Hahn, T.-W.; Lohakare, J.D.; Lee, S.L.; Moon, W.K.; Chae, B.J. Effects of supplementation of β-glucans on growth performance, nutrient digestibility, and immunity in weanling pigs. J. Anim. Sci. 2006, 84, 1422–1428. [Google Scholar] [CrossRef] [PubMed]
- Blecha, F.; Reddy, D.N.; Chitko-McKown, C.G.; McVey, D.S.; Chengappa, M.M.; Goodband, R.D.; Nelssen, J.L. Influence of recombinant bovine interleukin-1β and interleukin-2 in pigs vaccinated and challenged with Streptococcus suis. Vet. Immunol. Immunopathol. 1995, 44, 329–346. [Google Scholar] [CrossRef]
- Zhang, B.; Guo, Y.; Wang, Z. The modulating effect of β-1, 3/1, 6-glucan supplementation in the diet on performance and immunological responses of broiler chickens. Asian Australas. J. Anim. Sci. 2008, 21, 237–244. [Google Scholar] [CrossRef]
- Dritz, S.S.; Shi, J.; Kielian, T.L.; Goodband, R.D.; Nelssen, J.L.; Tokach, M.D.; Chengappa, M.M.; Smith, J.E.; Blecha, F. Influence of dietary β-glucan on growth performance, nonspecific immunity, and resistance to Streptococcus suis infection in weanling pigs. J. Anim. Sci. 1995, 73, 3341–3350. [Google Scholar] [CrossRef]
- Duarte, M.E.; Stahl, C.H.; Kim, S.W. Intestinal damages by F18+ Escherichia coli and its amelioration with an antibacterial bacitracin fed to nursery pigs. Antioxidants 2023, 12, 1040. [Google Scholar] [CrossRef]
- Young, S.-H.; Ye, J.; Frazer, D.G.; Shi, X.; Castranova, V. Molecular mechanism of tumor necrosis factor-α production in 1→3- β-glucan (zymosan)-activated macrophages. J. Biol. Chem. 2001, 276, 20781–20787. [Google Scholar] [CrossRef] [Green Version]
- Liu, G.; Yu, L.; Martínez, Y.; Ren, W.; Ni, H.; Abdullah Al-Dhabi, N.; Duraipandiyan, V.; Yin, Y. Dietary Saccharomyces cerevisiae cell wall extract supplementation alleviates oxidative stress and modulates serum amino acids profiles in weaned piglets. Oxid. Med. Cell. Longev. 2017, 2017, 3967439. [Google Scholar] [CrossRef] [Green Version]
- Hiss, S.; Sauerwein, H. Influence of dietary β-glucan on growth performance, lymphocyte proliferation, specific immune response and haptoglobin plasma concentrations in pigs. J. Anim. Physiol. Anim. Nutr. 2003, 87, 2–11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Choi, H.; Kim, B.G. A low-fiber diet requires a longer adaptation period before collecting feces of pigs compared with a high-fiber diet in digestibility experiments using the inert marker method. Anim. Feed Sci. Technol. 2019, 256, 114254. [Google Scholar] [CrossRef]
- Sun, Y.; Park, I.; Guo, J.; Weaver, A.C.; Kim, S.W. Impacts of low level aflatoxin in feed and the use of modified yeast cell wall extract on growth and health of nursery pigs. Anim. Nutr. 2015, 1, 177–183. [Google Scholar] [CrossRef]
- Kim, S.W.; Holanda, D.M.; Gao, X.; Park, I.; Yiannikouris, A. Efficacy of a yeast cell wall extract to mitigate the effect of naturally co-occurring mycotoxins contaminating feed ingredients fed to young pigs: Impact on gut health, microbiome, and growth. Toxins 2019, 11, 633. [Google Scholar] [CrossRef] [Green Version]
- Wu, Y.; Li, X.; Liu, H.; Du, Y.; Zhou, J.; Zou, L.; Xiong, X.; Huang, H.; Tan, Z.; Yin, Y. A water-soluble β-glucan improves growth performance by altering gut microbiome and health in weaned pigs. Anim. Nutr. 2021, 7, 1345–1351. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Luo, Y.; Yu, B.; Zheng, P.; Yu, J.; Huang, Z.; Mao, X.; Luo, J.; Yan, H.; He, J. Effect of β-glucan supplementation on growth performance and intestinal epithelium functions in weaned pigs challenged by enterotoxigenic Escherichia coli. Antibiotics 2022, 11, 519. [Google Scholar] [CrossRef]
- Zhou, Y.; Luo, Y.; Yu, B.; Zheng, P.; Yu, J.; Huang, Z.; Mao, X.; Luo, J.; Yan, H.; He, J. Agrobacterium sp. ZX09 β-glucan attenuates Enterotoxigenic Escherichia coli-induced disruption of intestinal epithelium in weaned pigs. Int. J. Mol. Sci. 2022, 23, 10290. [Google Scholar] [CrossRef]
- Hwang, Y.H.; Park, B.K.; Lim, J.H.; Kim, M.S.; Song, I.B.; Park, S.C.; Jung, H.K.; Hong, J.H.; Yun, H.I. Effects of β-glucan from Paenibacillus polymyxa and L-theanine on growth performance and immunomodulation in weanling piglets. Asian Australas. J. Anim. Sci. 2008, 21, 1753–1759. [Google Scholar] [CrossRef]
- Gahan, D.A.; Lynch, M.B.; Callan, J.J.; O’sullivan, J.T.; O’Doherty, J.V. Performance of weanling piglets offered low-, medium-or high-lactose diets supplemented with a seaweed extract from Laminaria spp. Animal 2009, 3, 24–31. [Google Scholar] [CrossRef] [Green Version]
- Heim, G.; Sweeney, T.; O’Shea, C.J.; Doyle, D.N.; O’Doherty, J.V. Effect of maternal supplementation with seaweed extracts on growth performance and aspects of gastrointestinal health of newly weaned piglets after challenge with enterotoxigenic Escherichia coli K88. Br. J. Nutr. 2014, 112, 1955–1965. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zou, T.; Yang, J.; Guo, X.; He, Q.; Wang, Z.; You, J. Dietary seaweed-derived polysaccharides improve growth performance of weaned pigs through maintaining intestinal barrier function and modulating gut microbial populations. J. Anim. Sci. Biotechnol. 2021, 12, 28. [Google Scholar] [CrossRef] [PubMed]
| Item | Initial BW (kg) or Age (d) | Experimental Period (d) | β-Glucan Compound (mg/kg) | β-Glucan (mg/kg) | Results | Reference | ||
| Intestinal health Bacteria (Agrobacterium sp.) | 21 d | 28 | - | 50 | Increased villus height, decreased crypt depth, and increased VH:CD after LPS challenge; increased mRNA abundance representing intestinal permeability (Z0-1, occludin, claudin, and MUC1 and 2), and decreased malondialdehyde in the jejunal mucosa after LPS challenge | [48] | ||
| 7.0 kg | 28 | - | 50, 100, and 200 | Linearly increased IL-10 and linearly decreased TNF-α level of jejunal mucosa | [6] | |||
| 100 | Increased MUC1 and 2 to β-actin mRNA ratio | [6] | ||||||
| 6.1 kg | 21 | - | 200 | Increased VH:CD in jejunum and increased mRNA abundance of an intestinal permeability parameter (occludin) | [66] | |||
| 6.1 kg | 21 | 500 | 300 | Increased VH:CD in jejunum, increased mRNA abundance of intestinal permeability parameter in jejunum (Z0-1, claudin-1, and MUC2), and increased Lactobacillus spp. and propionic acid in cecum digesta after ETEC challenge | [67] | |||
| Decreased malondialdehyde, TNF-α, and IL-6 in jejunum after ETEC challenge | [68] | |||||||
| Item | Initial BW (kg) or age (d) | Experimental period (d) | β-glucan compound (mg/kg) | β-glucan (mg/kg) | ADG (% change) | ADFI (% change) | G:F (% change) | Reference |
| Growth performance | 21 d | 21 | - | 50 | 21.6 ** | 11.0 ** | 9.2 | [48] |
| Bacteria (Agrobacterium sp.) | 50 | 14.1 | 8.2 | 6.6 | ||||
| 7.0 3 kg | 28 | - | 25 | 2.5 | 2.8 | −0.6 | [6] | |
| 50 | 10.4 | 8.0 | 2.4 | [6] | ||||
| 100 | 15.7 | 10.2 | 4.9 | |||||
| 200 | −0.9 | 1.0 | −2.3 | |||||
| 6.1 kg | 21 | - | 200 | 17.6 | 6.9 | 4.3 | [66] | |
| Average % change | 11.6 | 6.9 | 3.5 | |||||
| Bacteria (Paenibacillus polymyxa) | 5.6 kg | 28 | 400 | 5.8 * | −0.8 | 6.6 | [69] | |
| Item | Initial BW (kg) or Age (d) | Experimental Period (d) | β-Glucan (mg/kg) | Results | Reference |
|---|---|---|---|---|---|
| Algae (Euglena gracilis) | 7.7 kg | 17 | 54 | Increased mRNA abundance representing intestinal permeability (claudin, occludin, and MUC2) in jejunal mucosa on d 12 | [65] |
| 108 | Increased mRNA abundance representing intestinal permeability (dectin) in jejunal mucosa on d 5 and 12. Decreased transcellular permeability. | ||||
| Algae (Laminaria digitata) | 15.3 kg | 28 | 250 | Decreased Enterobacteria spp. in ileum and proximal colon; increased acetic acid and decreased propionic acid in ileum | [39] |
| Algae (Laminaria hyperborea) | 15.3 kg | 28 | 250 | Decreased Enterobacteria spp. in ileum and proximal colon; decreased total volatile fatty acid in the ileum |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Choi, H.; Kim, S.W. Characterization of β-Glucans from Cereal and Microbial Sources and Their Roles in Feeds for Intestinal Health and Growth of Nursery Pigs. Animals 2023, 13, 2236. https://doi.org/10.3390/ani13132236
Choi H, Kim SW. Characterization of β-Glucans from Cereal and Microbial Sources and Their Roles in Feeds for Intestinal Health and Growth of Nursery Pigs. Animals. 2023; 13(13):2236. https://doi.org/10.3390/ani13132236
Chicago/Turabian StyleChoi, Hyunjun, and Sung Woo Kim. 2023. "Characterization of β-Glucans from Cereal and Microbial Sources and Their Roles in Feeds for Intestinal Health and Growth of Nursery Pigs" Animals 13, no. 13: 2236. https://doi.org/10.3390/ani13132236
APA StyleChoi, H., & Kim, S. W. (2023). Characterization of β-Glucans from Cereal and Microbial Sources and Their Roles in Feeds for Intestinal Health and Growth of Nursery Pigs. Animals, 13(13), 2236. https://doi.org/10.3390/ani13132236






