Foraging Habitat Availability and the Non-Fish Diet Composition of the Grey Heron (Ardea cinerea) at Two Spatial Scales
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
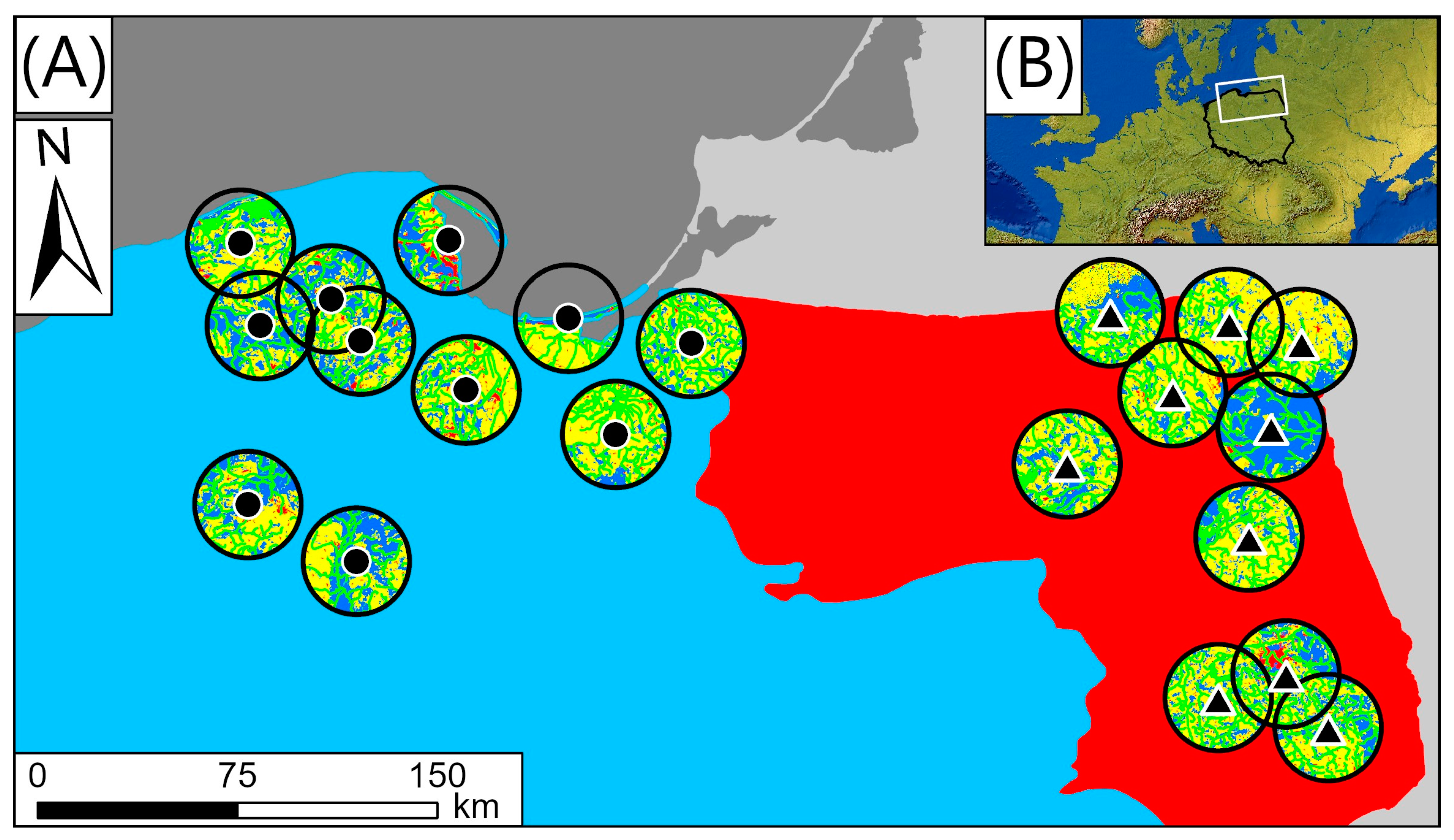
2.1. Study Area Regions
2.2. Data Collection and Analyses
2.3. Pellet Analyses
- (A)
- Fully/mostly aquatic: Acilius sp., Bivalvia, Carcinus maenas, Colymbetes fuscus, Corixidae, Dytiscus marginalis, Dytiscidae, Dytiscidae larvae, Haliplidae, Haliplus confinis, Ilyocoris cimicoides, Hydrophilidae, Naucoridae, Nepomorpha, Notonectidae, and Odonata;
- (B)
- Terrestrial: Carabidae, Curculionidae, Georissus sp., Graphosoma sp., Gryllotalpa gryllotalpa, Melolontha melolontha, Omophron limbatum, and Scarabeidae.
2.4. Habitat Composition Analyses
- (1)
- Hydrographic network: river/canals, water bodies (i.e., lakes, ponds), inland wetlands (e.g., peat bogs), and an area of the coastal zone (2 m wide; present within the foraging range of four studied colonies). Hydrographic networks serve as important foraging areas for Grey Herons during the breeding season [33], thus constituting an important landscape factor affecting the location of breeding colonies of this species [4,14,34];
- (2)
- Complex cultivation patterns, land principally occupied by agriculture, with significant areas of natural vegetation, non-irrigated arable land, pastures which are area of suboptimal foraging grounds;
- (3)
- Forests (all types combined), often serving as nesting habitats;
- (4)
- Urban zones (airports, construction sites, continuous urban fabric, discontinuous urban fabric, dumpsites, industrial or commercial units, mineral extract sites, port areas, road and rail networks, and sport and leisure facilities), which may be avoided by herons.
2.5. Statistical Analyses and Data Preparation
2.6. Prey–Habitat Relationship
2.7. Multivariate Analyses
- (1)
- Analysis of similarities (ANOSIM) to examine the significance of inter-regional spatial differences [50] in prey item compositions. This analysis is popularly used for testing ecological hypotheses about spatial differences, especially for detecting any environmental impacts on an organism’s assemblages [50]. We applied this method to test for any dissimilarities between regions in prey item contribution and test the impact of environmental variables. ANOSIM creates a value of R between −1 and +1, where 0 represents no impact. Higher positive R values stand for increasing differences between samples [50].
- (2)
- Non–metric multidimensional scaling (nMDS) to summarize patterns of food composition of the diet of the Grey Heron among colonies based on an ordination of pairwise site dissimilarities [51,52] using the Euclidean distance as a measure of dissimilarity and convex hulls. nMDS is an ordination technique using an algorithm that creates the best combination of variables explaining observed differences or similarities among groups [53]. After the ordination procedure conducted by nMDS, prey items and environmental variables were represented on a biplot based on the correlation coefficients between their values and the coordinates of the virtual assemblages of the nMDS axes [54]. The importance of differences among variables was based on calculated coefficients of determination (R2) for each of two axes. We conducted this analysis since the stress value was acceptable (<15) [55].
- (3)
- Similarity Percentage (SIMPER) analysis was used to recognize prey items contributing the most to inter-regional differences. In this technique, the general significance of the difference is commonly assessed by ANOSIM. This difference is based on the observed dissimilarity measured in Euclidean distance. For this analysis, we considered cases with a contribution of 10% ≤ of observed differences [55,56].
3. Results
3.1. Inter-Regional Differences in Diet Composition
3.2. Difference in Diet Composition between Coastal and Inland Colonies in the N Region
3.3. Inter-Regional Differences in Habitats
3.4. Prey–Habitat Relationship
3.5. Multivariate Analyses
3.5.1. ANOSIM
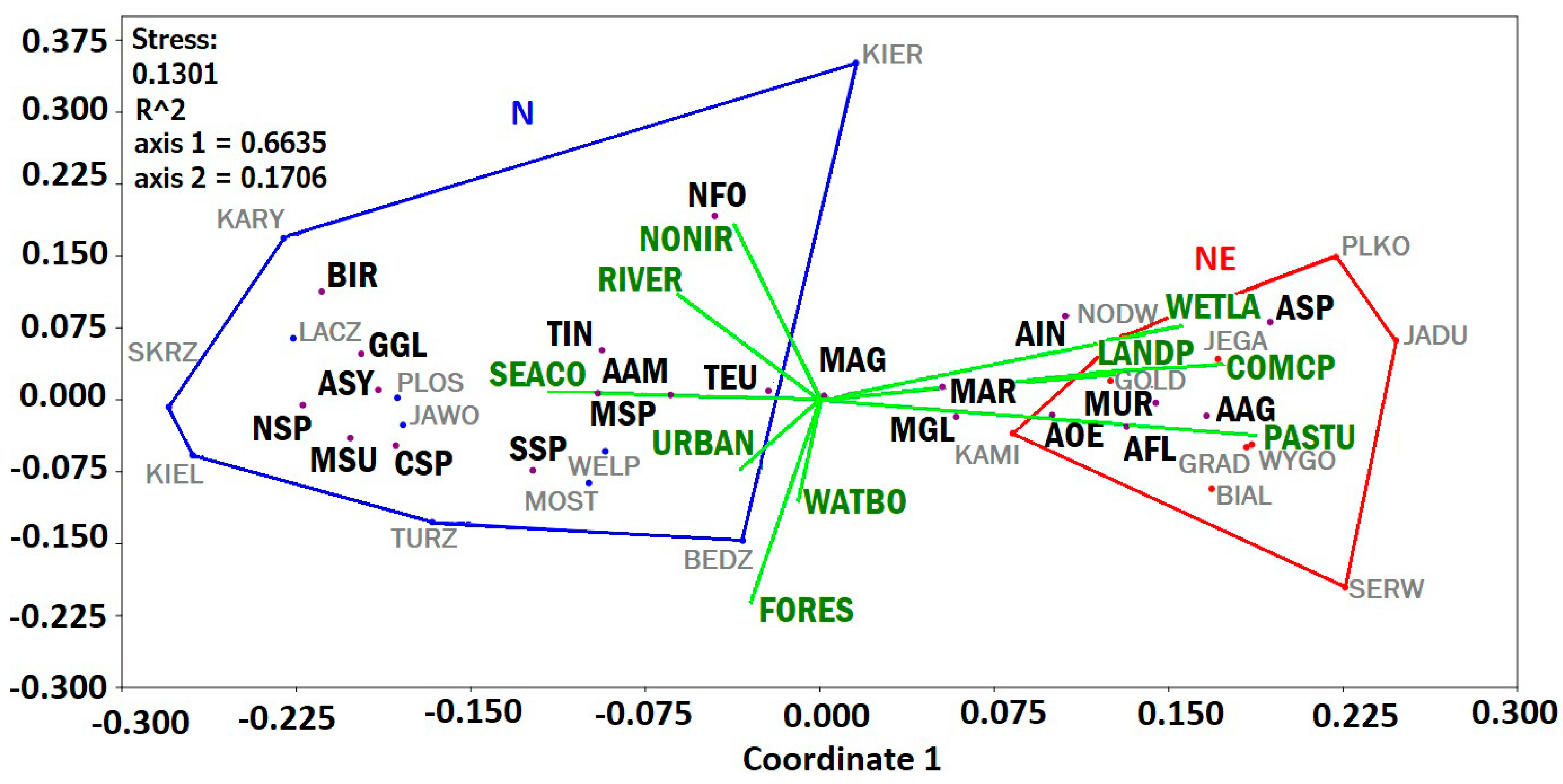
3.5.2. nMDS
3.5.3. SIMPER
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Abookire, A.A.; Duffy-Anderson, J.T.; Jump, C.M. Habitat associations and diet of young-of-the-year Pacific cod (Gadus macrocephalus) near Kodiak, Alaska. Mar. Biol. 2007, 150, 713–726. [Google Scholar] [CrossRef]
- Cramp, S. The Complete Birds of the Western Palearctic; CD-ROM, Oxford University Press: Oxford, UK, 1998. [Google Scholar]
- Jakubas, D.; Mioduszewska, A. Diet composition and food consumption of the grey heron (Ardea cinerea) from breeding colonies in northern Poland. Eur. J. Wildl. Res. 2005, 51, 191–198. [Google Scholar] [CrossRef]
- Manikowska-Ślepowrońska, B.; Lazarus, M.; Żółkoś, K.; Zbyryt, A.; Kitowski, I.; Jakubas, D. Influence of landscape features on the location of grey heron Ardea cinerea colonies in Poland. CR Biol. 2016, 339, 507–516. [Google Scholar] [CrossRef] [PubMed]
- Draulans, D.; Perremans, K.; Vessem, J.; Pollet, M. Analysis of pellets of the Grey heron, Ardea cinerea, from colonies in Belgium. J. Zool. 1987, 211, 695–708. [Google Scholar] [CrossRef]
- Jakubas, D.; Manikowska, B. The response of Grey Herons Ardea cinerea to changes in prey abundance. Bird Study 2011, 58, 487–494. [Google Scholar] [CrossRef]
- Hewson, R.; Hancox, M. Prey remains in grey heron pellets from north-east Scotland. Bird Study 1979, 26, 29–32. [Google Scholar] [CrossRef]
- Draulans, D.; Van Vessem, J. Age-related differences in the use of time and space by radio-tagged grey herons (Ardea cinerea) in winter. J. Anim. Ecol. 1985, 54, 771–780. [Google Scholar] [CrossRef]
- Gregory, S.N. The foraging ecology and feeding behaviour of the grey heron (Ardea cinerea) in the Camargue, S. France. Ph.D. Thesis, Durham University, Durham, UK, 1990. [Google Scholar]
- Jakubas, D. The response of the grey heron to a rapid increase of the round goby. Waterbirds 2004, 27, 304–307. [Google Scholar] [CrossRef]
- Brigham, C.J. Chitin and chitosan: Sustainable, medically relevant biomaterials. Int. J. Biotechnol. Wellness Ind. 2017, 6, 41–47. [Google Scholar] [CrossRef]
- Charnov, E.L. Optimal foraging, the marginal value theorem. Theor. Popul. Biol. 1976, 9, 129–136. [Google Scholar] [CrossRef]
- Pyke, G.H. Optimal foraging theory: A critical review. Annu. Rev. Ecol. Evol. Syst. 1984, 15, 523–575. [Google Scholar] [CrossRef]
- Manikowska-Ślepowrońska, B.; Ślepowroński, K.; Jakubas, D. Grey Heron Ardea cinerea productivity in relation to habitat features and different spatial scales. Pol. J. Ecol. 2016, 64, 384–398. [Google Scholar]
- Dimalexis, A.; Pyrovetsi, M.; Sgardelis, S. Foraging ecology of the grey heron (Ardea cinerea), great egret (Ardea alba) and little egret (Egretta garzetta) in response to habitat, at 2 Greek wetlands. Colon Waterbird 1997, 20, 261–272. [Google Scholar] [CrossRef]
- Hodkinson, I.D.; Jackson, J.K. Terrestrial and aquatic invertebrates as bioindicators for environmental monitoring, with particular reference to mountain ecosystems. Environ. Manag. 2005, 35, 649–666. [Google Scholar] [CrossRef] [PubMed]
- Rychling, A. Regionalizacja fizykogeograficzna. In Geografia Fizyczna Polski; Red Rychling, A., Ostaszewska, K., Eds.; PWN: Warszawa, Poland, 2006. [Google Scholar]
- Solon, J.; Borzyszkowski, J.; Bidłasik, M.; Richling, A.; Badora, K.; Balon, J.; Brzezińska-Wójcik, T.; Chabudziński, Ł.; Dobrowolski, R.; Grzegorczyk, I.; et al. Physico-geographical mesoregions of Poland: Verification and adjustment of boundaries on the basis of contemporary spatial data. Geogr. Pol. 2018, 91, 143–170. [Google Scholar] [CrossRef]
- Fac–Beneda, J. Changes in the Area and Depth of the Depression in Żuławy Elbląskie; Peribalticum VIII. Gdańskie Towarzystwo Naukowe, Wydz. V Nauk o Ziemi: Gdańsk, Poland, 2000. [Google Scholar]
- Moskalewicz, D.; Woźniak, P.P. Vistula River delta-plain—A region of fluvial, coastal, and land reclamation impact on landscape development. In Landscapes and Landforms of Poland; Springer International Publishing: Cham, Switzerland, 2024; pp. 725–740. [Google Scholar]
- Kondracki, J. Geografia Regionalna Polski; PWN: Warszawa, Poland, 2002. [Google Scholar]
- Manikowska-Ślepowrońska, B.; Lazarus, M.; Żółkoś, K.; Jakubas, D. Determinants of the re-occupation and size of Grey Heron Ardea cinerea breeding colonies in northern Poland. Ecol. Res. 2015, 30, 879–888. [Google Scholar] [CrossRef]
- Ellenberg, H. Vegetation Ecology of Central Europe, 4th ed.; Cambridge University Press: Cambridge, UK, 1988. [Google Scholar]
- Pucek, Z. Klucz do Oznaczania Ssaków Polski; PWN: Warszawa, Poland, 1984. [Google Scholar]
- Teerink, B.J. Hair of West-European Mammals; Cambridge University Press: Cambridge, UK, 2003. [Google Scholar]
- Dziurdziuk, B. Klucz do oznaczania włosów ssaków Polski. Acta Zool. Cracov 1973, 18, 73–92. [Google Scholar]
- Choate, P.M. Illustrated Key to Florida Species of Tiger Beetles-(Coleoptera: Cicindelidae); Entomology and Nematology; University of Florida: Gainesville, FL, USA, 2003. [Google Scholar]
- Tończyk, G.; Siciński, T. Klucz do Oznaczania Makrobezkręgowców Bentosowych dla Potrzeb Oceny Stanu Ekologicznego Wód Powierzchniowych; Biblioteka Monitoringu Środowiska: Warszawa, Poland, 2013. [Google Scholar]
- Vinokurov, A.A. [On the food digestion role in heron Ardea purpurea]. Moskovskogo Obshchestva Ispytatelei Prirody. Bull. Otdel. Biol. Mosk. 1960, 65, 10. [Google Scholar]
- Available online: https://clc.gios.gov.pl/doc/clc/CLC_Legend_EN.pdf (accessed on 26 February 2023).
- Büttner, G. CORINE land cover and land cover change products. In Land Use and Land Cover Mapping in Europe: Practices & Trends; Springer: Berlin/Heidelberg, Germany, 2014; pp. 55–74. [Google Scholar]
- European Environment Agency. The European Topic Centre on Inland, Coastal and Marine Waters. Version 13 2013. Available online: https://eea.europa.eu/data-and-maps/data/waterbaserivers-9 (accessed on 10 March 2022).
- Marion, L. Territorial feeding and colonial breeding are not mutually exclusive: The case of the Grey Heron (Ardea cinerea). J. Anim. Ecol. 1989, 58, 693–710. [Google Scholar] [CrossRef]
- Boisteau, B.; Marion, L. Landscape influence on the Grey Herons colonies distribution. CR Biol. 2006, 329, 208–216. [Google Scholar] [CrossRef]
- Boisteau, B. Rôle de la Structure du Paysage Hydrographique Dans la Distribution Spatiale des Colonies de Hérons Cendrés (Ardea cinerea); Mémoire du DEA Eco-Éthologie Évolutive: Rennes, France, 2002; pp. 1–27. [Google Scholar]
- Boisteau, B.; Marion, L. Habitat use by the Grey Heron (Ardea cinerea) in eastern France. CR Biol. 2007, 330, 629–634. [Google Scholar] [CrossRef] [PubMed]
- Environmental Systems Research Institute (ESRI). ArcGIS Desktop; ESRI: Redlands, CA, USA, 2024. [Google Scholar]
- Available online: https://scihub.copernicus.eu (accessed on 26 February 2023).
- Vermote, E.F.; Vermeulen, A. Atmospheric correction algorithm spectral reflectances (MOD09). ATBD Version 1999, 4, 1–107. [Google Scholar]
- Song, C.; Woodcock, C.E.; Seto, K.C.; Lenney, M.P.; Macomber, S.A. Classification and change detection using Landsat TM data: When and how to correct atmospheric effects? Remote Sens. Environ. 2001, 75, 230–244. [Google Scholar] [CrossRef]
- Congedo, L. Semi-automatic classification plugin documentation. Release 2016, 4, 29. [Google Scholar]
- Available online: https://gisgeography.com/sentinel-2-bands-combinations (accessed on 26 February 2023).
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2023; Available online: https://www.R-project.org/ (accessed on 15 April 2023).
- Hervé, M.; Hervé, M.M. Package ‘RVAideMemoire’. Available online: https://cran.r-project.org/web/packages/RVAideMemoire/index.html (accessed on 3 November 2023).
- Lüdecke, D.; Ben-Shachar, M.S.; Patil, I.; Waggoner, P.; Makowski, D. Performance: An R package for assessment, comparison and testing of statistical models. J. Open Source Softw. 2021, 6, 3139. [Google Scholar] [CrossRef]
- Bozdogan, H. Model selection and Akaike’s information criterion (AIC): The general theory and its analytical extensions. Psychometrika 1987, 52, 345–370. [Google Scholar] [CrossRef]
- Barton, K. MuMIn: Multi-Model Inference. R Package Version 1.7. 2. Available online: http://CRAN.R-project.org/package=MuMIn (accessed on 3 November 2023).
- Venables, W.N.; Ripley, B.D. Modern Applied Statistics with S, 4th ed.; Springer: New York, NY, USA, 2002; ISBN 0-387-95457-0. Available online: https://www.stats.ox.ac.uk/pub/MASS4/ (accessed on 3 November 2023).
- Lesnoff, M.; Lancelot, R. aod: Analysis of Overdispersed Data. R Package Version 1.3.3. Available online: https://cran.r-project.org/package=aod (accessed on 3 November 2023).
- Chapman, M.G.; Underwood, A.J. Ecological patterns in multivariate assemblages information and interpretation of negative values in ANOSIM tests. Mar. Ecol. Prog. Ser. 1999, 180, 257–265. [Google Scholar] [CrossRef]
- Field, J.G.; Clarke, K.R.; Warwick, R.M. A practical strategy for analyzing multispecies distribution patterns. Mar. Ecol. Prog. Ser. 1982, 8, 37–52. [Google Scholar] [CrossRef]
- Mueter, F.J.; Norcross, B.L. Linking community structure of small demersal fishes around Kodiak Island, Alaska, to environmental variables. Mar. Ecol. Prog. Ser. 1999, 190, 37–51. [Google Scholar] [CrossRef]
- Prentice, I.C. Non-metric ordination methods in ecology. J. Ecol. 1977, 65, 85–94. [Google Scholar] [CrossRef]
- Lee, D.Y.; Lee, D.S.; Bae, M.J.; Hwang, S.J.; Noh, S.Y.; Moon, J.S.; Park, Y.S. Distribution patterns of odonate assemblages in relation to environmental variables in streams of South Korea. Insects 2018, 9, 152. [Google Scholar] [CrossRef] [PubMed]
- Clarke, K.R. Non-parametric multivariate analyses of changes in community structure. Aust. J. Ecol. 1993, 18, 117–143. [Google Scholar] [CrossRef]
- Hammer, Ø.; Harper, D.A.; Ryan, P.D. PAST: Paleontological statistics software package for education and data analysis. Palaeontol. Electron. 2001, 4, 9. [Google Scholar]
- Marquiss, M.; Leitch, A.F. The diet of Grey Herons Ardea cinerea breeding at Loch Leven, Scotland, and the importance of their predation on ducklings. Ibis 1990, 132, 535–549. [Google Scholar] [CrossRef]
- Fasola, M.; Rosa, P.; Canova, L. The diets of squacco herons, little egrets, night, purple and grey herons in their Italian breeding ranges. Rev. d’Ecologie Terre Vie 1993, 48, 35–45. [Google Scholar] [CrossRef]
- Chenchouni, H. Variation in White Stork (Ciconia ciconia) diet along a climatic gradient and across rural-to-urban landscapes in North Africa. Int. J. Biometeorol. 2017, 61, 549–564. [Google Scholar] [CrossRef]
- Adams, C.E.; Mitchell, J. The response of a grey heron Ardea cinerea breeding colony to rapid change in prey species. Bird Study 1995, 42, 44–49. [Google Scholar] [CrossRef]
- Ditsche, P.; Summers, A.P. Aquatic versus terrestrial attachment: Water makes a difference. Beilstein J. Nanotechnol. 2014, 5, 2424–2439. [Google Scholar] [CrossRef]
- Zub, K.; Jędrzejewska, B.; Jędrzejewski, W.; Bartoń, K.A. Cyclic voles and shrews and non-cyclic mice in a marginal grassland within European temperate forest. Acta Theriol. 2012, 57, 205–216. [Google Scholar] [CrossRef]
- Fischer, C.; Schröder, B. Predicting spatial and temporal habitat use of rodents in a highly intensive agricultural area. Agric. Ecosyst. Environ. 2014, 189, 145–153. [Google Scholar] [CrossRef]
- Available online: https://iucnredlist.org (accessed on 3 July 2023).
- Brzeziński, M.; Jedlikowski, J.; Komar, E. Space use, habitat selection and daily activity of water voles Arvicola amphibius co-occurring with the invasive American mink Neovison vison. Folia Zool. 2019, 68, 21–28. [Google Scholar] [CrossRef]
- Stojak, J.; Tarnowska, E. Polish suture zone as the goblet of truth in post-glacial history of mammals in Europe. Mammal Res. 2019, 64, 463–475. [Google Scholar] [CrossRef]

| Species | Scientific Name | Code |
|---|---|---|
| striped field mouse | Apodemus agrarius | AAG |
| European water vole | Arvicola amphibius | AAM |
| yellow-necked mouse | Apodemus flavicollis | AFL |
| aquatic invertebrates | - | AIN |
| tundra vole | Alexandromys oeconomus | AOE |
| species of genus Apodemus | Apodemus sp. | ASP |
| wood mouse | Apodemus sylvaticus | ASY |
| bird taxa | - | BIR |
| species of genus Crocidura | Crocidura sp. | CSP |
| European edible dormouse | Glis glis | GGL |
| short-tailed field vole | Microtus agrestis | MAG |
| common vole | Microtus arvalis | MAR |
| bank vole | Myodes glareolus | MGL |
| voles | Microtus sp. | MSP |
| European pine vole | Microtus subterraneus | MSU |
| species from family of Muridae | Muridae | MUR |
| Eurasian water shrew | Neomys fodiens | NFO |
| species of genus Neomys | Neomys sp. | NSP |
| species of genus Sorex | Sorex sp. | SSP |
| European mole | Talpa europaea | TEU |
| terrestrial invertebrates | - | TIN |
| Code (Habitat) | Short Characteristics |
|---|---|
| COMCP (Complex cultivation patterns) | Juxtaposition of small parcels of annual crops, city gardens, pastures, fallow lands, and/or permanent crops somewhere with scattered houses. |
| FORES (Forests) | Broad-leaved, coniferous, and mixed forests combined. |
| LANDP (Land principally occupied by agriculture, with significant areas of natural vegetation) | Land occupied by irrigated cultivated parcels interspersed with significant areas of natural or semi-natural vegetation, e.g., forests, shrubs, vineyards, orchards, or plantations. |
| NONIR (Non-irrigated arable land) | Land occupied mainly by rainfed cultivated parcels with a crop rotation system. |
| PASTU (Pastures) | Dense grass cover of floral composition, dominated by Poaceae, not under a rotation system. Areas under intense human disturbance are included, mainly for grazing, but the folder may be harvested mechanically. Areas sparsely covered in vegetation or transitional woodland-shrub included. |
| RIVER (Rivers) | Natural or artificial water courses serve as water drainage channels. Includes canals and rivers that have been canalized. |
| SEACO (Seacoast) | Area of coastal zone of the Baltic Sea (2 m wide). |
| URBAN (Urban zones) | Land covered by structures and the transport network (roads, motorways, and railways, including associated installations, e.g., stations), buildings, artificially surfaced areas (e.g., asphalt), infrastructure of port areas, marinas, airports, dump sites, mineral extraction sites, green urban areas, and industrial fabric structures. |
| WATBO (Water bodies) | Natural or artificial stretches of water, also with low floating aquatic vegetation, archipelago of lakes inside land areas, coastal lagoons, and fish farms. |
| WETLA (Inland wetlands) | Low-lying land (inland wetlands, marshes, swamps) usually flooded in winter and more or less saturated by water all year round. Covered by a specific low ligneous, semi-ligneous, or herbaceous vegetation. |
| Prey Item | Regional Prevalence | Counts for Regions | Difference N vs. NE | ||
|---|---|---|---|---|---|
| N | NE | Test Value | p | ||
| European water vole (AAM) | N | 97 | 43 | 10.086 | 0.001 |
| Microtus sp. (MSP) | N | 56 | 21 | 9.899 | 0.002 |
| aquatic invertebrates (AIN) | NE | 18 | 45 | 11.372 | 0.001 |
| terrestrial invertebrates (TIN) | N | 39 | 12 | 10.264 | 0.001 |
| tundra vole (AOE) | NE | 4 | 15 | - | 0.015 |
| yellow-necked mouse (AFL) | NE | 2 | 9 | - | 0.03 |
| Habitat | Area [%] | Difference | ||
|---|---|---|---|---|
| N | NE | G | p | |
| COMCP | 1.6 | 3.7 | 102.75 | <0.0001 |
| FORES | 34.0 | 29.9 | 25.05 | <0.0001 |
| LANDP | 4.8 | 10.6 | 298.96 | <0.0001 |
| NONIR | 44.0 | 35.2 | 105.05 | <0.0001 |
| PASTU | 7.8 | 12.4 | 145.2 | <0.0001 |
| RIVER | 0.4 | 0.3 | 3.1985 | 0.07 |
| SEACO | 0.05 | 0 | Not analyzed | - |
| URBAN | 4.4 | 2.25 | 19.717 | <0.0001 |
| WATBO | 2.5 | 2.2 | 1.1409 | 0.2855 |
| WETLA | 0.5 | 2.48 | 187.86 | <0.0001 |
| Model | df | logLik | AICc | ΔAICc | Weight |
| AAM~COMCP + PASTU | 3 | −43.370 | 94.2 | 0.00 | 0.618 |
| AAM~COMCP + PASTU + SEACO | 4 | −42.305 | 95.1 | 0.96 | 0.382 |
| Model | df | logLik | AICc | Delta | Weight |
|---|---|---|---|---|---|
| MSP~FORES + NONIR | 3 | −42.747 | 92.9 | 0.00 | 1.000 |
| Model | df | logLik | AICc | Delta | Weight |
|---|---|---|---|---|---|
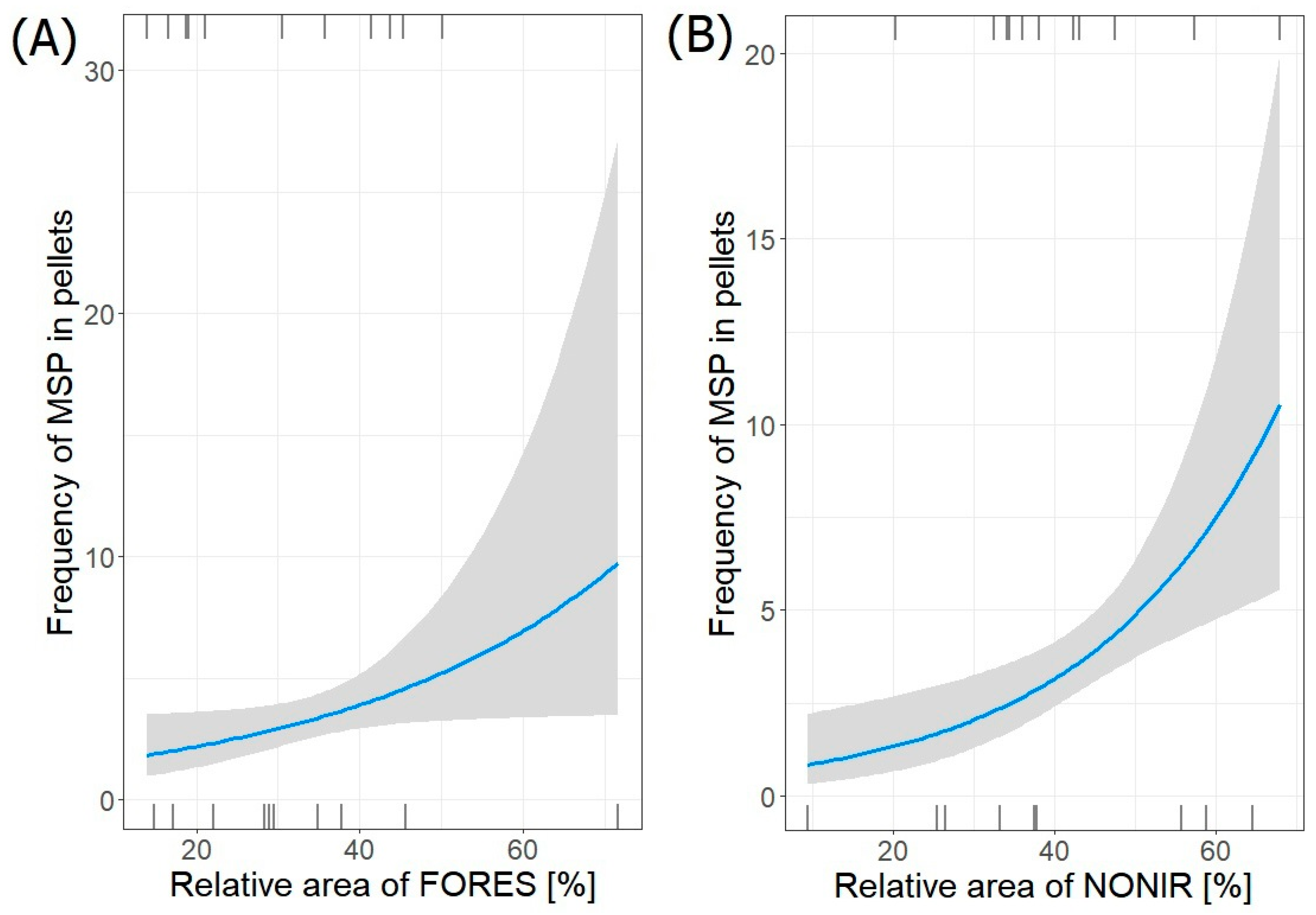
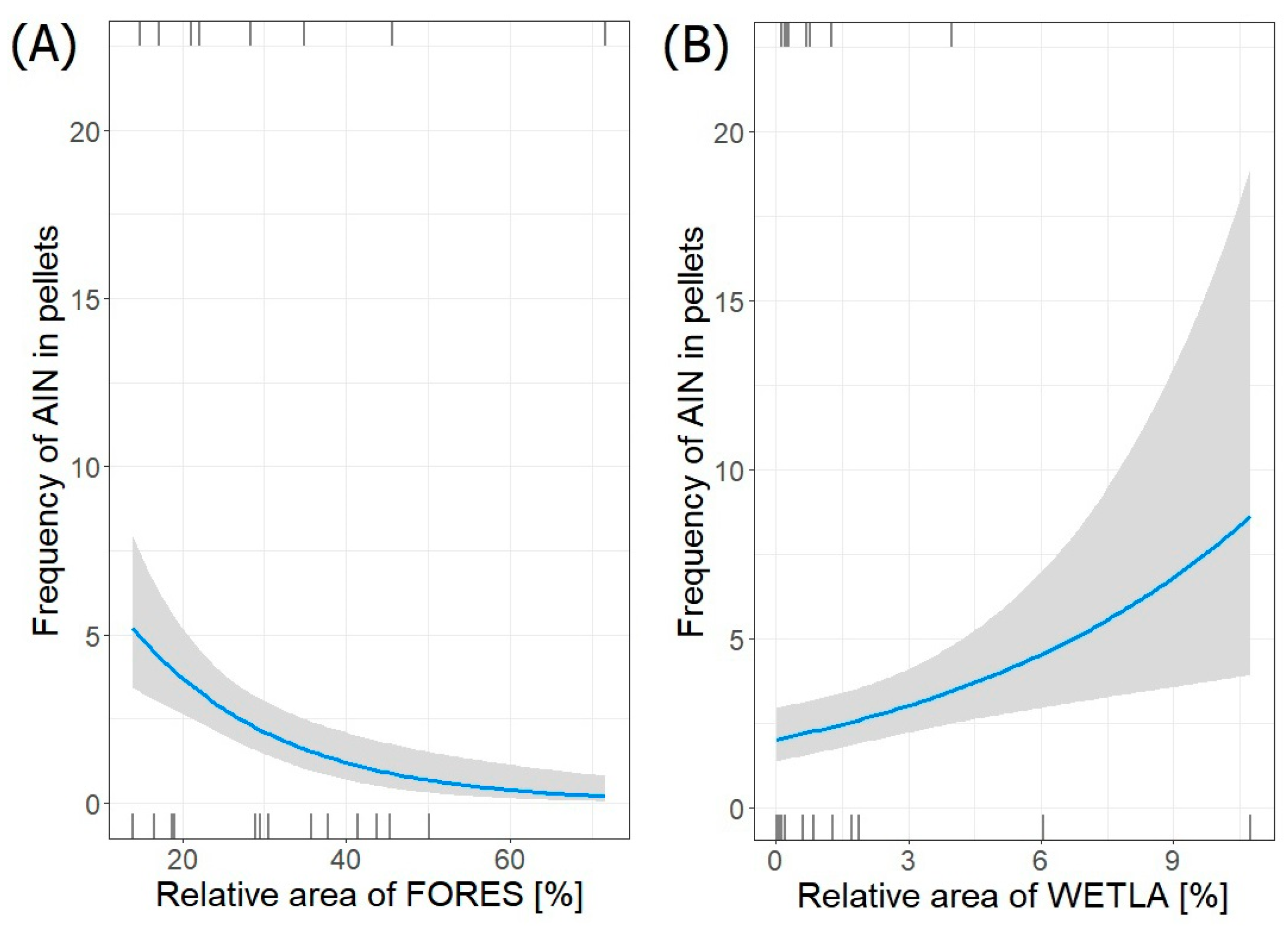
| AIN~FORES + WETLA | 3 | −40.792 | 89 | 0.00 | 1.000 |
| Model | df | logLik | AICc | Delta | Weight |
|---|---|---|---|---|---|
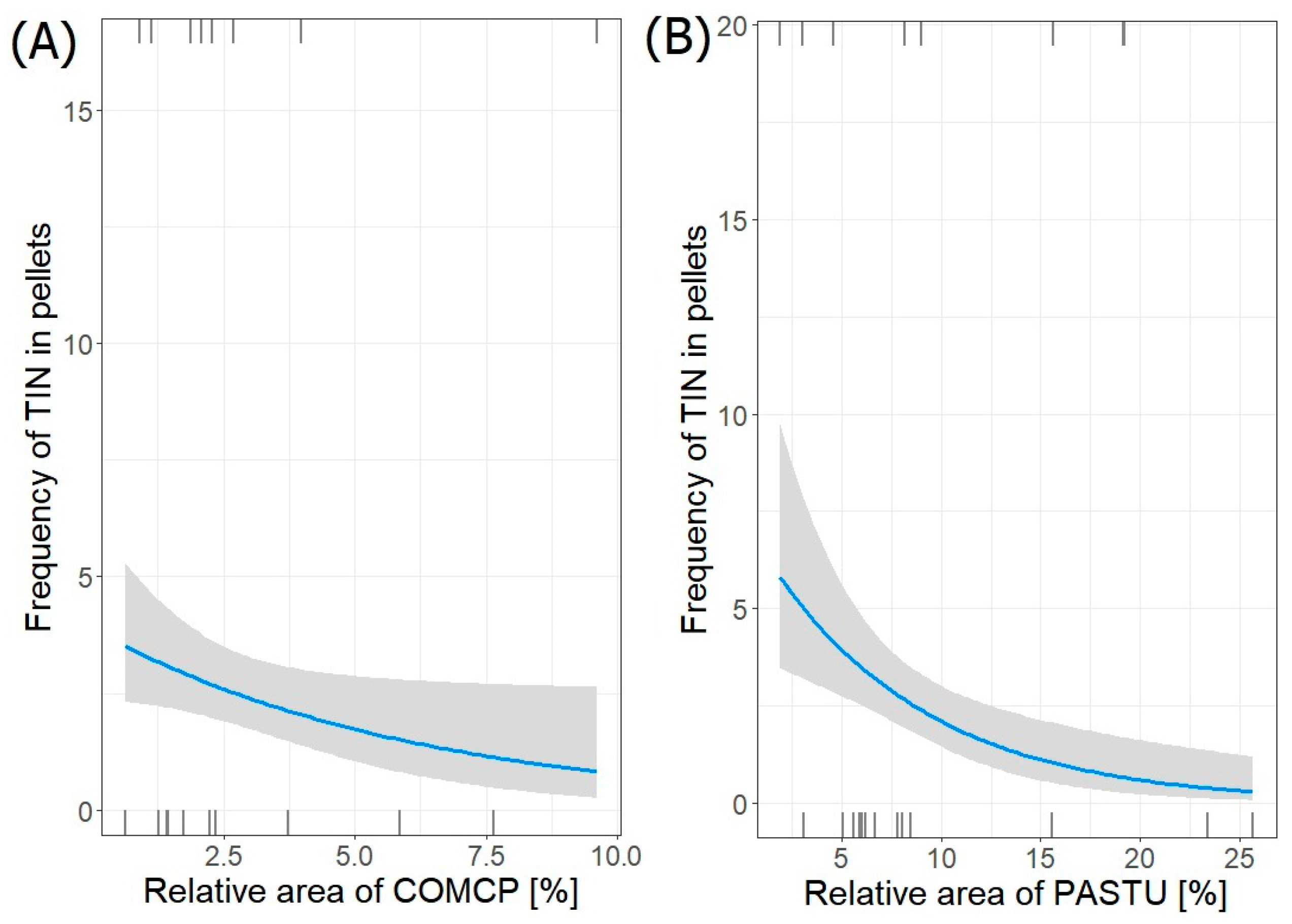
| TIN~COMCP + PASTU | 3 | −35.889 | 79.2 | 0.00 | 1.000 |
| Model | df | logLik | AICc | Delta | Weight |
|---|---|---|---|---|---|
| MGL~NONIR | 2 | −31.987 | 68.6 | 0.00 | 1.000 |
| Model | df | logLik | AICc | Delta | Weight |
|---|---|---|---|---|---|
| MAG~URBAN | 2 | −32.108 | 68.9 | 0.00 | 0.237 |
| MAG~INT | 1 | −33.371 | 69.0 | 0.07 | 0.229 |
| MAG~SEACO | 2 | −32.219 | 69.1 | 0.22 | 0.212 |
| MAG~LANDP | 2 | −32.412 | 69.5 | 0.61 | 0.175 |
| MAG~PASTU + SEACO | 3 | −31.206 | 69.8 | 0.94 | 0.148 |
| Model | df | logLik | AICc | Delta | Weight |
|---|---|---|---|---|---|
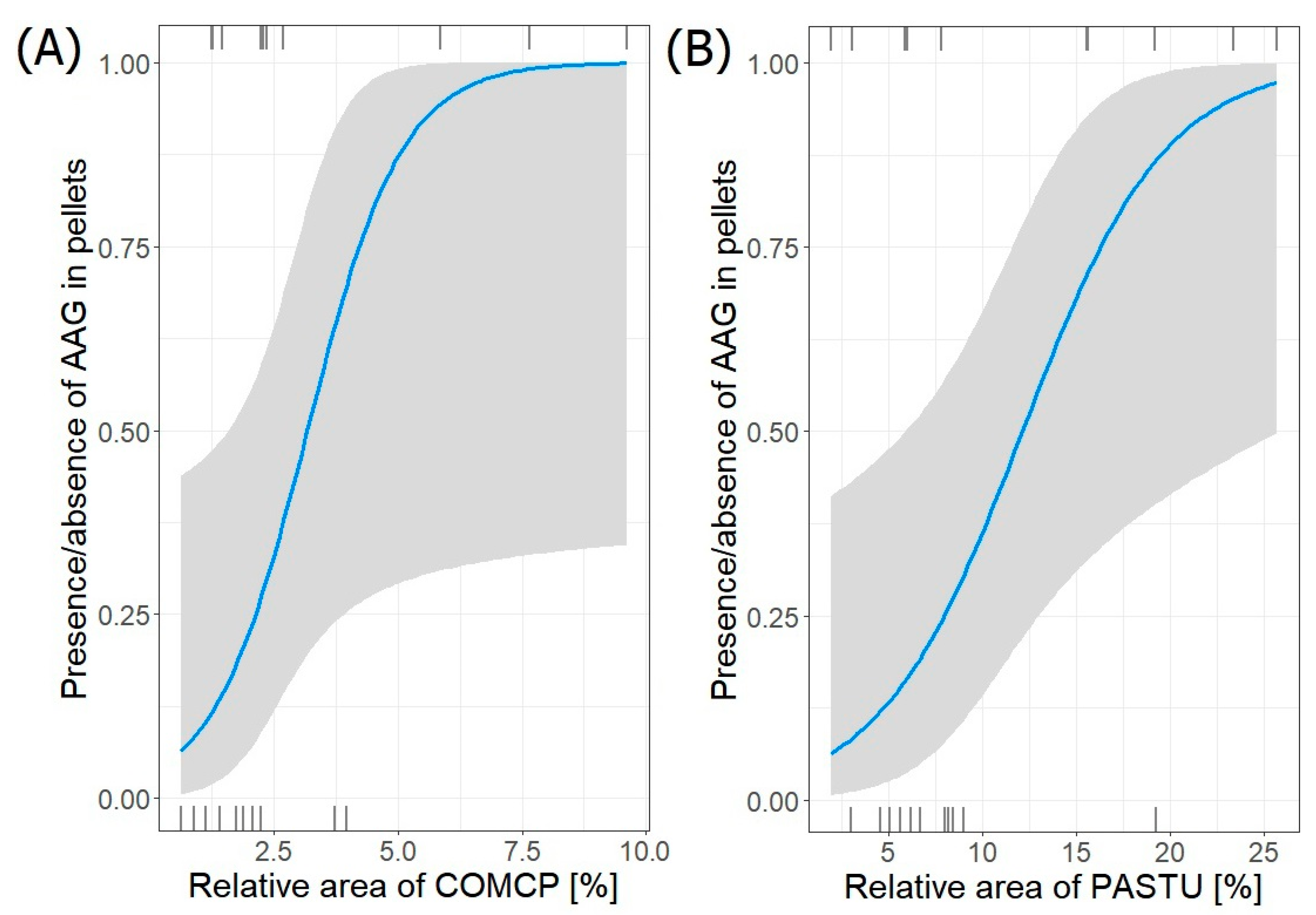
| AAG~COMCP + PASTU | 3 | −8.387 | 24.2 | 0.00 | 0.321 |
| AAG~LANDP + PASTU | 3 | −8.606 | 24.6 | 0.44 | 0.258 |
| AAG~COMCP + SEACO | 3 | −8.743 | 24.9 | 0.71 | 0.225 |
| AAG~SEACO + WETLA | 3 | −8.881 | 25.2 | 0.99 | 0.196 |
| Taxon (Code) | Av. Dissim. | Contrib. % | Cumulative % | Mean N | Mean NE |
|---|---|---|---|---|---|
| water vole (AAM) | 24.79 | 21.05 | 21.05 | 8.82 | 4.3 |
| aquatic invertebrates (AIN) | 21.01 | 17.84 | 38.89 | 1.64 | 4.5 |
| striped field mouse (AAG) | 19.5 | 16.56 | 55.45 | 0 | 4.3 |
| Microtus sp. (MSP) | 16.55 | 14.06 | 69.5 | 5.09 | 2.1 |
| terrestrial invertebrates (TIN) | 11.78 | 10 | 79.51 | 3.55 | 1.2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cieślińska, K.; Manikowska-Ślepowrońska, B.; Zbyryt, A.; Jakubas, D. Foraging Habitat Availability and the Non-Fish Diet Composition of the Grey Heron (Ardea cinerea) at Two Spatial Scales. Animals 2024, 14, 2461. https://doi.org/10.3390/ani14172461
Cieślińska K, Manikowska-Ślepowrońska B, Zbyryt A, Jakubas D. Foraging Habitat Availability and the Non-Fish Diet Composition of the Grey Heron (Ardea cinerea) at Two Spatial Scales. Animals. 2024; 14(17):2461. https://doi.org/10.3390/ani14172461
Chicago/Turabian StyleCieślińska, Karolina, Brygida Manikowska-Ślepowrońska, Adam Zbyryt, and Dariusz Jakubas. 2024. "Foraging Habitat Availability and the Non-Fish Diet Composition of the Grey Heron (Ardea cinerea) at Two Spatial Scales" Animals 14, no. 17: 2461. https://doi.org/10.3390/ani14172461






