Effect of Dietary Sugarcane Bagasse on Reproductive Performance, Constipation, and Gut Microbiota of Gestational Sows
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals, Experimental Design, and Sample Collection
2.2. Determination of Nutrients and Swelling Rates of Different Fiber Ingredients
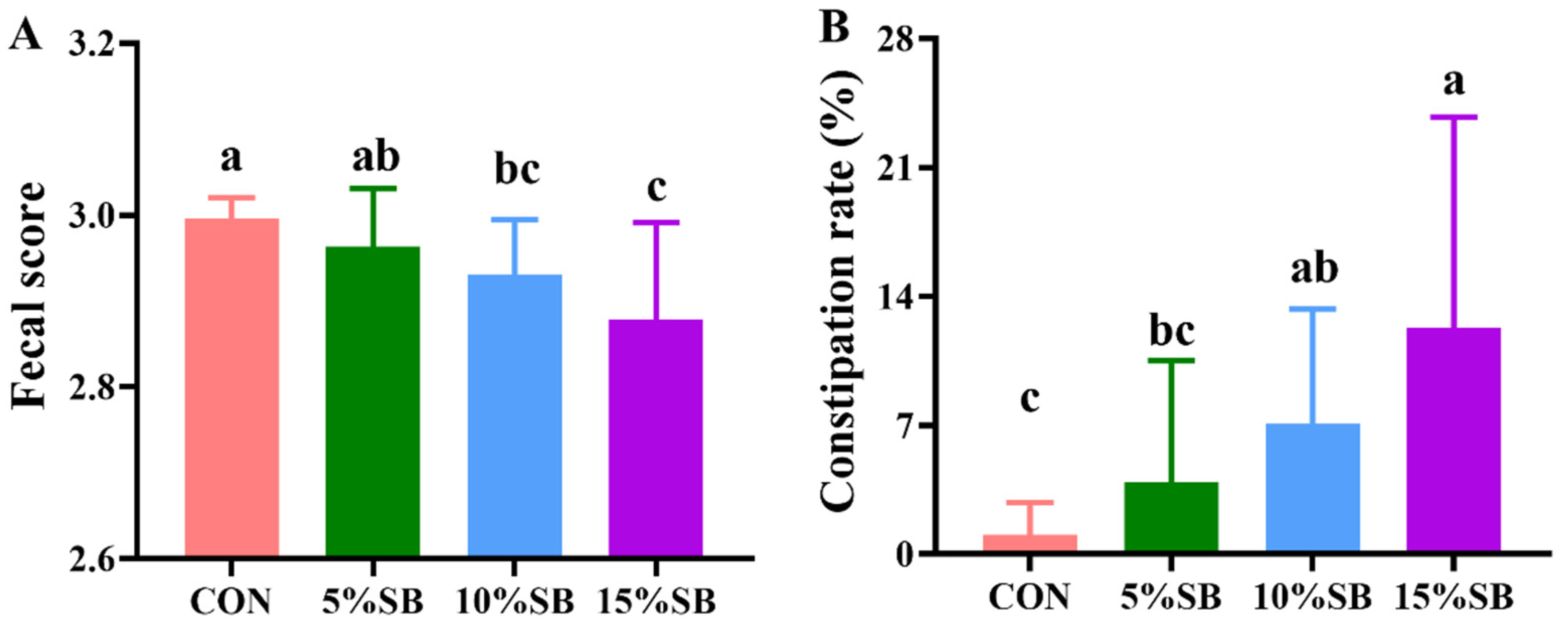
2.3. Determination of Fecal Score and Constipation Rate during Pregnancy
2.4. Determination of the Fecal SCFAs Profile
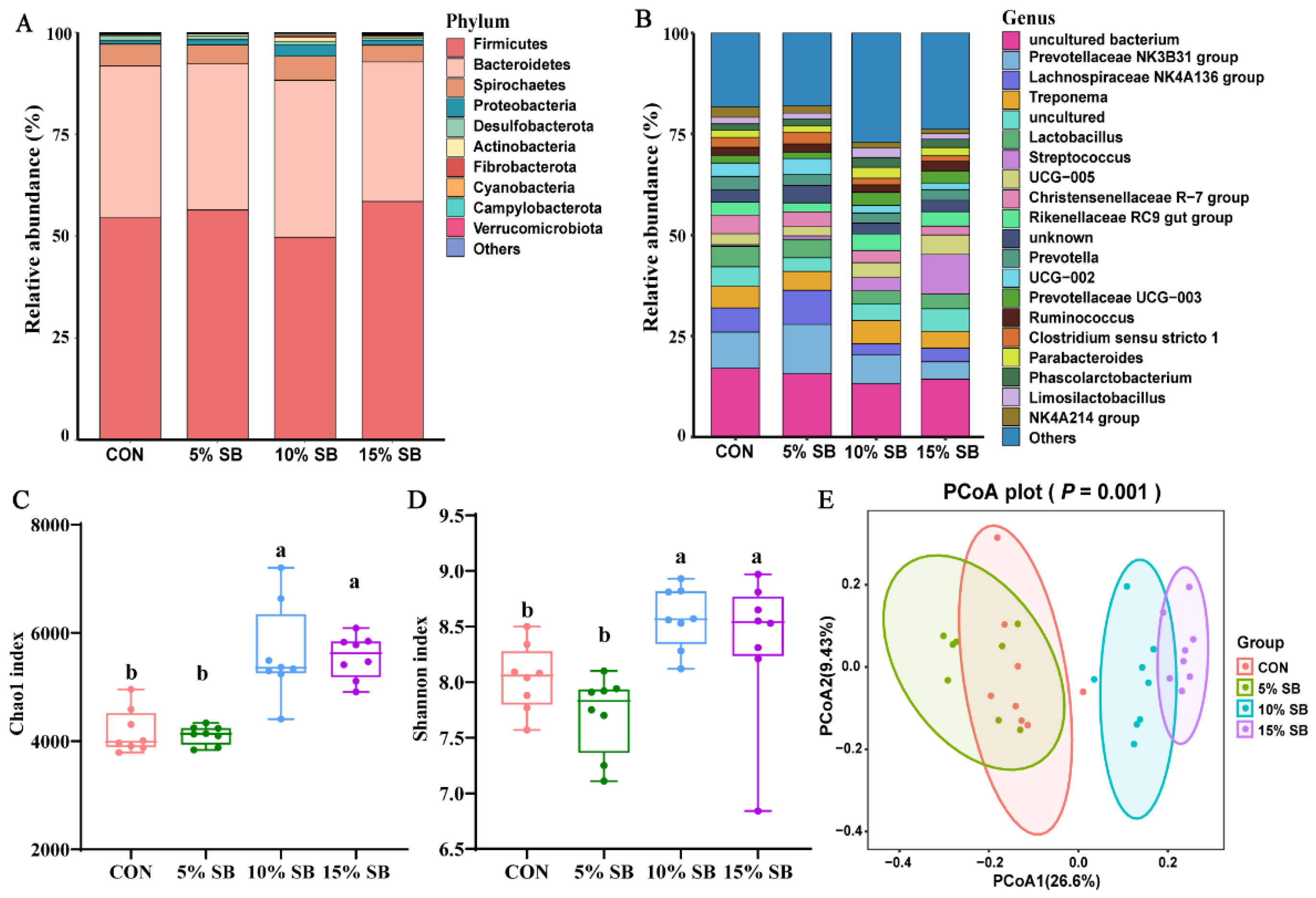
2.5. 16S rRNA Gene Sequencing and Microbiome Analysis
2.6. Statistical Analysis
3. Results
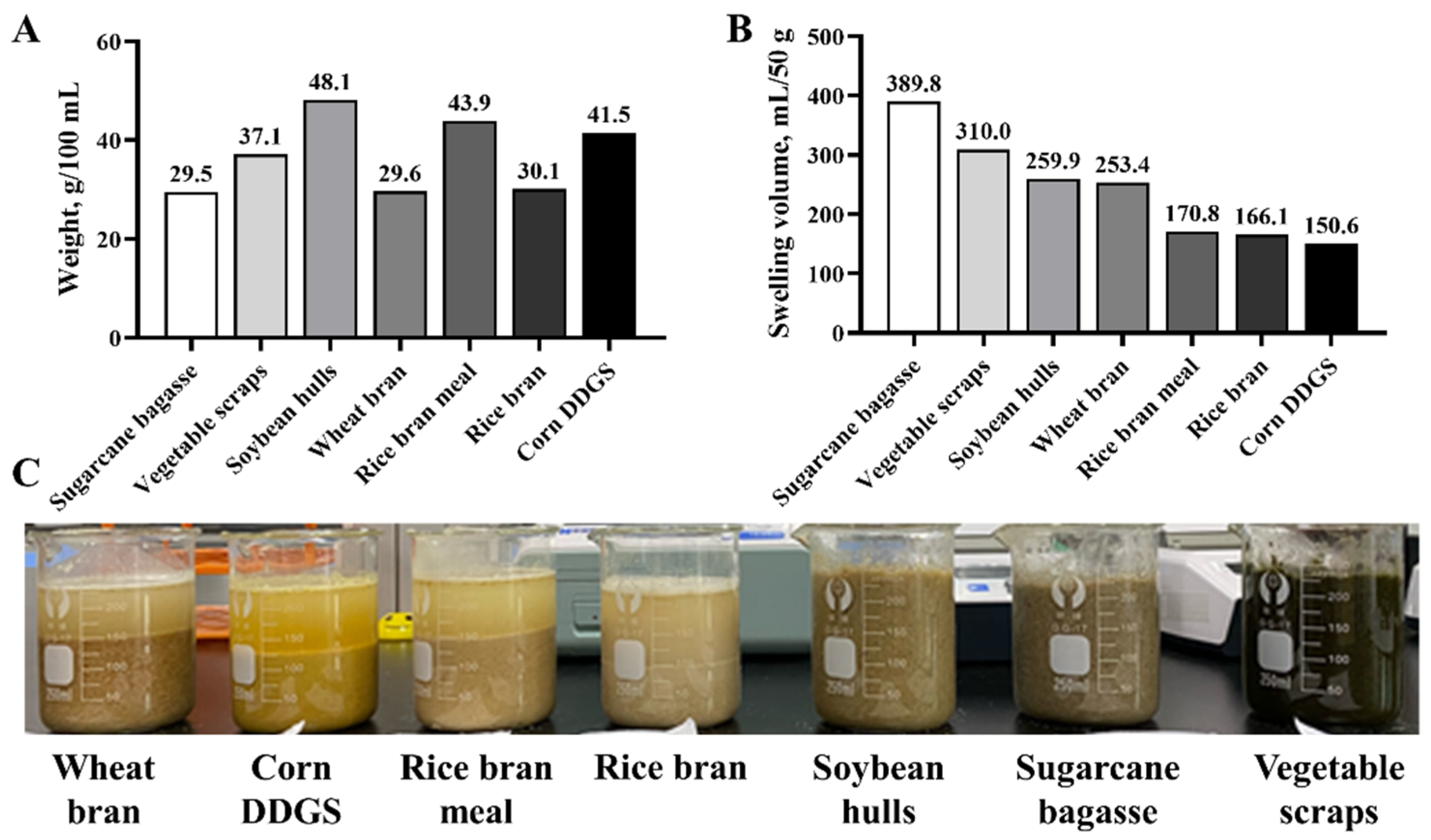
3.1. Nutrients of Different Fiber Raw Materials
3.2. Water Swelling Performance of Different Fiber Raw Materials
3.3. Reproductive Performance and Fecal Parameters of Sows
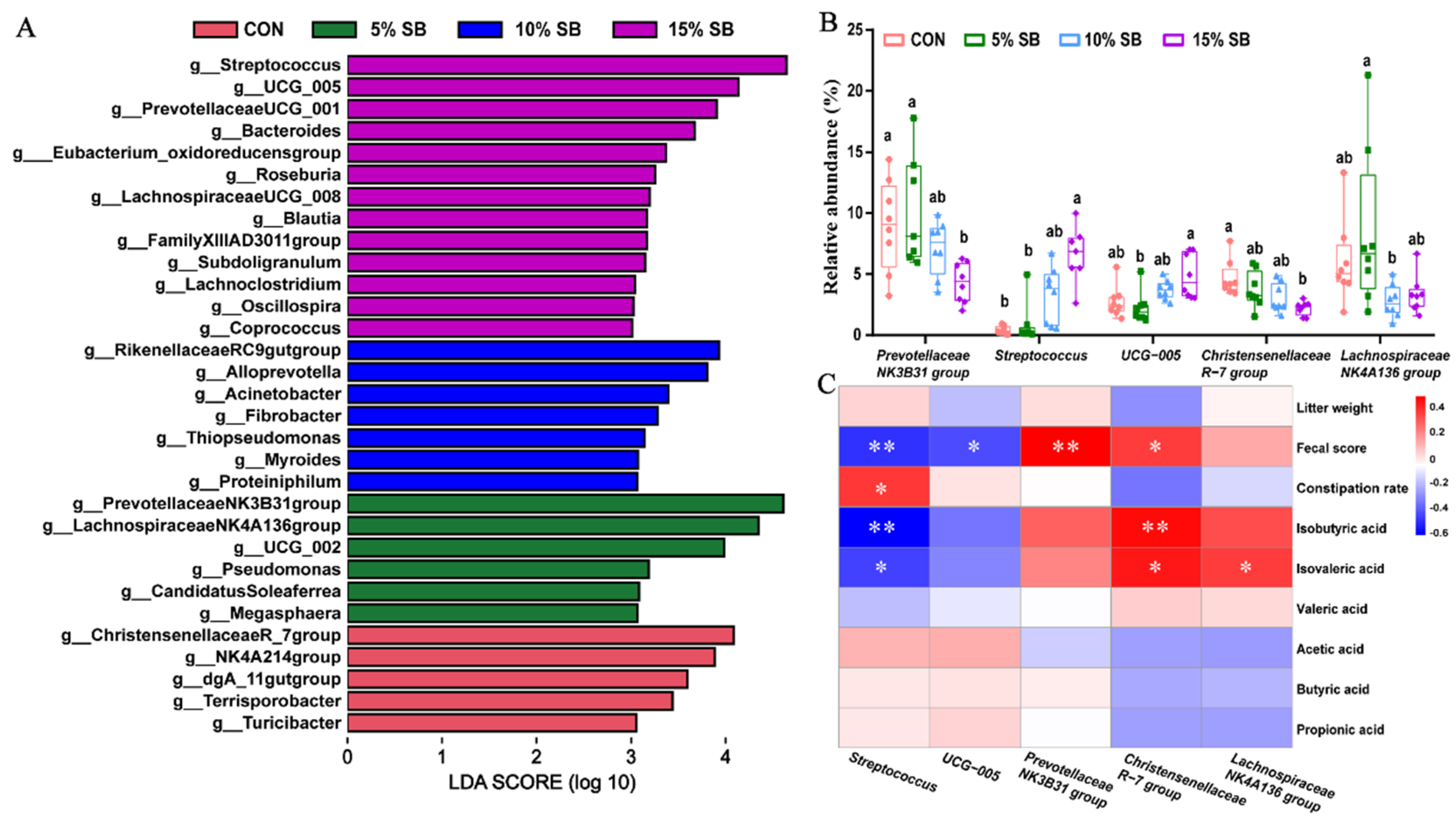
3.4. Short-Chain Fatty Acids Status
3.5. Gut Microbiota
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Fears, R.; ter Meulen, V.; von Braun, J. Global food and nutrition security needs more and new science. Sci. Adv. 2019, 5, eaba2946. [Google Scholar] [CrossRef]
- Beal, T.; Gardner, C.D.; Herrero, M.; Iannotti, L.L.; Merbold, L.; Nordhagen, S.; Mottet, A. Friend or foe? The role of animal-source foods in healthy and environmentally sustainable diets. J. Nutr. 2023, 153, 409–425. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.C.; Li, Y.K.; Lv, Z.Q.; Liu, H.; Zhao, J.B.; Noblet, J.; Wang, F.L.; Lai, C.H.; Li, D.F. Net energy of corn, soybean meal and rapeseed meal in growing pigs. J. Anim. Sci. Biotechnol. 2017, 8, 44. [Google Scholar] [CrossRef] [PubMed]
- Rakita, S.; Kokic, B.; Manoni, M.; Mazzoleni, S.; Lin, P.; Luciano, A.; Ottoboni, M.; Cheli, F.; Pinotti, L. Cold-pressed oilseed cakes as alternative and sustainable feed ingredients: A Review. Foods 2023, 12, 432. [Google Scholar] [CrossRef]
- Omar, A.E.; Al-Khalaifah, H.S.; Ismail, T.A.; Abd El-Aziz, R.M.; El-Mandrawy, S.A.M.; Shalaby, S.I.; Ibrahim, D. Performance, serum biochemical and immunological parameters, and digestive enzyme and intestinal barrier-related gene expression of broiler chickens fed fermented fava bean by-products as a substitute for conventional feed. Front. Vet. Sci. 2021, 8, 696841. [Google Scholar] [CrossRef]
- Kanengoni, A.T.; Chimonyo, M.; Ndimba, B.K.; Dzama, K. Potential of using maize cobs in pig diets: A Review. Asian-Australas. J. Anim. Sci. 2015, 28, 1669–1679. [Google Scholar] [CrossRef]
- Hassanein, H.A.M.; Maggiolino, A.; El-Fadel, M.A.; Palo, P.D.; El-Sanafawy, H.A.; Hussein, A.M.; Salem, A.Z.M. Inclusion of as an unconventional feed of Zaraibi dairy goats, and effects on milk production and offspring performance. Front. Vet. Sci. 2023, 10, 1101424. [Google Scholar] [CrossRef]
- Zou, S.; Sun, C.C.; Li, F.; Xie, Y.J.; Liang, T.; Yang, Y.Q.; Shi, B.M.; Ma, Q.Q.; Shi, Z.; Chai, S.; et al. Effect of gardenia pomace supplementation on growth performance, blood metabolites, immune and antioxidant indices, and meat quality in xiangcun pigs. Animals 2022, 12, 2280. [Google Scholar] [CrossRef]
- Xandé, X.; Archimède, H.; Gourdine, J.L.; Anais, C.; Renaudeau, D. Effects of the level of sugarcane molasses on growth and carcass performance of Caribbean growing pigs reared under a ground sugarcane stalks feeding system. Trop. Anim. Health Prod. 2010, 42, 13–20. [Google Scholar] [CrossRef]
- Theil, P.K.; Farmer, C.; Feyera, T. Review: Physiology and nutrition of late gestating and transition sows. J. Anim. Sci. 2022, 100, skac176. [Google Scholar] [CrossRef]
- Ha, S.H.; Choi, Y.H.; Mun, J.Y.; Park, S.R.; Kinara, E.; Park, H.J.; Hong, J.S.; Kim, Y.M.; Kim, J.S. Correlation between reproductive performance and sow body weight change during gestation. J. Anim. Sci. Technol. 2024, 66, 543–554. [Google Scholar] [CrossRef]
- Ma, T.; Huang, W.Q.; Li, Y.L.; Jin, H.; Kwok, L.Y.; Sun, Z.H.; Zhang, H.P. Probiotics alleviate constipation and inflammation in late gestating and lactating sows. Npj Biofilms Microbiomes 2023, 9, 70. [Google Scholar] [CrossRef]
- Lu, D.D.; Pi, Y.; Ye, H.; Wu, Y.J.; Bai, Y.; Lian, S.; Han, D.D.; Ni, D.J.; Zou, X.H.; Zhao, J.B.; et al. Consumption of dietary fiber with different physicochemical properties during late pregnancy alters the gut microbiota and relieves constipation in sow model. Nutrients 2022, 14, 2511. [Google Scholar] [CrossRef]
- Yu, X.R.; Fu, C.S.; Cui, Z.C.; Chen, G.Y.; Xu, Y.L.; Yang, C.M. Inulin and isomalto-oligosaccharide alleviate constipation and improve reproductive performance by modulating motility-related hormones, short-chain fatty acids, and feces microflora in pregnant sows. J. Anim. Sci. 2021, 99, skab257. [Google Scholar] [CrossRef] [PubMed]
- Dimidi, E.; Christodoulides, S.; Scott, S.M.; Whelan, K. Mechanisms of action of probiotics and the gastrointestinal microbiota on gut motility and constipation. Adv. Nutr. 2017, 8, 484–494. [Google Scholar] [CrossRef] [PubMed]
- de Almeida, G.A.P.; Ferreira, M.D.; Silva, J.D.; Chagas, J.C.C.; Véras, A.S.C.; de Barros, L.J.A.; de Almeida, G.L.P. Sugarcane bagasse as exclusive roughage for dairy cows in smallholder livestock system. Asian-Australas. J. Anim. Sci. 2018, 31, 379–385. [Google Scholar] [CrossRef]
- Alokika, A.; Kumar, A.; Kumar, V.; Singh, B. Cellulosic and hemicellulosic fractions of sugarcane bagasse: Potential, challenges and future perspective. Int. J. Biol. Macromol. 2021, 169, 564–582. [Google Scholar] [CrossRef]
- Wang, S.Q.; Peng, Z.; Sun, H.; Han, Y.M.; Zhang, B.; Pineda, L.; Boerboom, G.; Sun, L.H.; Liu, Y.; Deng, Z.C. Evaluating the impact of an organic trace mineral mix on the redox homeostasis, immunity, and performance of sows and their offspring. Biol. Trace Elem. Res. 2024, 1–10. [Google Scholar] [CrossRef]
- Huang, W.; Ma, T.; Liu, Y.; Kwok, L.-Y.; Li, Y.; Jin, H.; Zhao, F.; Shen, X.; Shi, X.; Sun, Z.; et al. Spraying compound probiotics improves growth performance and immunity and modulates gut microbiota and blood metabolites of suckling piglets. Sci. China Life Sci. 2022, 66, 1092–1107. [Google Scholar] [CrossRef]
- Wang, D.; Kuang, Y.; Lv, Q.; Xie, W.; Xu, X.; Zhu, H.; Zhang, Y.; Cong, X.; Cheng, S.; Liu, Y. Selenium-enriched Cardamine violifolia protects against sepsis-induced intestinal injury by regulating mitochondrial fusion in weaned pigs. Sci. China Life Sci. 2023, 66, 2099–2111. [Google Scholar] [CrossRef]
- Eudy, B.J.; Odle, J.; Lin, X.; Maltecca, C.; Walter, K.R.; McNulty, N.P.; Fellner, V.; Jacobi, S.K. Dietary prebiotic oligosaccharides and arachidonate alter the fecal microbiota and mucosal lipid composition of suckling pigs. J. Nutr. 2023, 153, 2249–2262. [Google Scholar] [CrossRef] [PubMed]
- He, C.; Xu, S.; Li, Z.; Yu, Z.; Levesque, C.; Zhang, Y.; Wang, Z.; Shi, C.; Wang, F.; Liu, H. Determination and prediction of the net energy content of wheat bran for pregnant sow. Arch. Anim. Nutr. 2024, 1–18, online ahead of print. [Google Scholar] [CrossRef]
- Latimer, G.W. (Ed.) Official Methods of Analysis of AOAC International, 22nd ed.; AOAC Publications: New York, NY, USA, 2023. [Google Scholar]
- Hua, M.; Lu, J.; Qu, D.; Liu, C.; Zhang, L.; Li, S.; Chen, J.; Sun, Y. Structure, physicochemical properties and adsorption function of insoluble dietary fiber from ginseng residue: A potential functional ingredient. Food Chem. 2019, 286, 522–529. [Google Scholar] [CrossRef]
- Lan, R.X.; Li, T.S.; Kim, I. Effects of xylanase supplementation on growth performance, nutrient digestibility, blood parameters, fecal microbiota, fecal score and fecal noxious gas emission of weaning pigs fed corn-soybean meal-based diet. Anim. Sci. J. 2017, 88, 1398–1405. [Google Scholar] [CrossRef]
- Li, Y.; He, J.; Zhang, L.; Liu, H.; Cao, M.; Lin, Y.; Xu, S.; Che, L.; Fang, Z.; Feng, B.; et al. Improvement of insulin sensitivity by dietary fiber consumption during late pregnant sows is associated with gut microbiota regulation of tryptophan metabolism. Anim. Microbiome 2024, 6, 34. [Google Scholar] [CrossRef] [PubMed]
- Deng, Z.C.; Yang, J.C.; Huang, Y.X.; Zhao, L.; Zheng, J.S.; Xu, Q.B.; Guan, L.L.; Sun, L.H. Translocation of gut microbes to epididymal white adipose tissue drives lipid metabolism disorder under heat stress. Sci. China Life Sci. 2023, 66, 2877–2895. [Google Scholar] [CrossRef]
- Deng, Z.C.; Wang, J.; Wang, J.; Yan, Y.Q.; Huang, Y.X.; Chen, C.Q.; Sun, L.h.; Liu, M. Tannic acid extracted from gallnut improves intestinal health with regulation of redox homeostasis and gut microbiota of weaned piglets. Anim. Res. One Health 2024, 2, 16–27. [Google Scholar] [CrossRef]
- Naik, T.; Sharda, M.C.P.L.; Virbhadra, K.; Pandit, A. High-quality single amplicon sequencing method for illumina MiSeq platform using pool of ‘N’ (0-10) spacer-linked target specific primers without PhiX spike-in. BMC Genom. 2023, 24, 141. [Google Scholar] [CrossRef]
- Cao, K.X.; Deng, Z.C.; Liu, M.; Huang, Y.X.; Yang, J.C.; Sun, L.H. Heat stress impairs male reproductive system with potential disruption of retinol metabolism and microbial balance in the testis of mice. J. Nutr. 2023, 153, 3373–3381. [Google Scholar] [CrossRef]
- Yang, J.C.; Huang, Y.X.; Sun, H.; Liu, M.; Zhao, L.; Sun, L.H. Selenium deficiency dysregulates one-carbon metabolism in nutritional muscular dystrophy of chicks. J. Nutr. 2023, 153, 47–55. [Google Scholar] [CrossRef] [PubMed]
- Yan, Y.Q.; Liu, M.; Xu, Z.J.; Xu, Z.J.; Huang, Y.X.; Li, X.M.; Chen, C.J.; Zuo, G.; Yang, J.C.; Lei, X.G.; et al. Optimum doses and forms of selenium maintaining reproductive health via regulating homeostasis of gut microbiota and testicular redox, inflammation, cell proliferation, and apoptosis in roosters. J. Nutr. 2024, 154, 369–380. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Liu, M.; Sun, H.; Yang, J.C.; Huang, Y.X.; Huang, J.Q.; Lei, X.; Sun, L.H. Selenium deficiency-induced multiple tissue damage with dysregulation of immune and redox homeostasis in broiler chicks under heat stress. Sci. China Life Sci. 2023, 66, 2056–2069. [Google Scholar] [CrossRef] [PubMed]
- Jo, H.; Kim, B.G. Effects of dietary fiber in gestating sow diets—A review. Anim. Biosci. 2023, 36, 1619–1631. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Ma, L.T.; Zhang, L.L.; Liu, N.A.; Li, Z.Q.; Zhang, F.; Liu, X.; Ma, X.K. Dietary inulin regulated gut microbiota and improved neonatal health in a pregnant sow model. Front. Nutr. 2021, 8, 716723. [Google Scholar] [CrossRef] [PubMed]
- So, S.R.; Cherdthong, A.; Wanapat, M. Improving sugarcane bagasse quality as ruminant feed with, cellulase, and molasses. J. Anim. Sci. Technol. 2020, 62, 648–658. [Google Scholar] [CrossRef]
- Dai, F.W.; Lin, T.; Huang, X.; Shi, X.L.; Yang, Y.J.; Nong, X.; Zuo, J.J.; Liu, H. Effects from supplementary feeding of bamboo powder in perinatal period on farrowing process, serum biochemical indexes, and fecal microbes of sows and offspring piglets. Front. Microbiol. 2023, 14, 1139625. [Google Scholar] [CrossRef]
- Do, S.; Jang, J.C.; Lee, G.I.; Kim, Y.Y. The role of dietary fiber in improving pig welfare. Animals 2023, 13, 879. [Google Scholar] [CrossRef]
- Qin, F.; Wei, W.Y.; Gao, J.J.; Jiang, X.M.; Che, L.Q.; Fang, Z.F.; Lin, Y.; Feng, B.; Zhuo, Y.; Hua, L.; et al. Effect of dietary fiber on reproductive performance, intestinal microorganisms and immunity of the sow: A Review. Microorganisms 2023, 11, 2292. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, L.J.; Liu, H.Y.; Yang, Y.; He, J.Q.; Cao, M.; Yang, M.; Zhong, W.; Lin, Y.; Zhuo, Y.; et al. Effects of the ratio of insoluble fiber to soluble fiber in gestation diets on sow performance and offspring intestinal development. Animals 2019, 9, 422. [Google Scholar] [CrossRef]
- Renteria-Flores, J.A.; Johnston, L.J.; Shurson, G.C.; Moser, R.L.; Webel, S.K. Effect of soluble and insoluble dietary fiber on embryo survival and sow performance. J. Anim. Sci. 2008, 86, 2576–2584. [Google Scholar] [CrossRef]
- Huang, S.B.; Cui, Z.J.; Hao, X.Y.; Cheng, C.H.; Chen, J.Z.; Wu, D.Y.; Luo, H.F.; Deng, J.P.; Tan, C.Q. Dietary fibers with low hydration properties exacerbate diarrhea and impair intestinal health and nutrient digestibility in weaned piglets. J. Anim. Sci. Biotechnol. 2022, 13, 142. [Google Scholar] [CrossRef]
- Guan, Z.W.; Yu, E.Z.; Feng, Q. Soluble dietary fiber, one of the most important nutrients for the gut microbiota. Molecules 2021, 26, 6802. [Google Scholar] [CrossRef] [PubMed]
- Zijlstra, R.T.; Jha, R.; Woodward, A.D.; Fouhse, J.; van Kempen, T.A.T.G. Starch and fiber properties affect their kinetics of digestion and thereby digestive physiology in pigs. J. Anim. Sci. 2012, 90, 49–58. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Yang, M.; Zhang, L.J.; Mao, Z.Y.; Lin, Y.; Xu, S.Y.; Fang, Z.F.; Che, L.Q.; Feng, B.; Li, J.; et al. Dietary fiber supplementation in gestating sow diet improved fetal growth and placental development and function through serotonin signaling pathway. Front. Vet. Sci. 2022, 9, 831703. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.Y.; Wang, Y.B.; Yang, G.; Zhang, Q.H.; Meng, L.B.; Xin, Y.; Jiang, X. The role of short-chain fatty acids in intestinal barrier function, inflammation, oxidative stress, and colonic carcinogenesis. Pharmacol. Res. 2021, 165, 105420. [Google Scholar] [CrossRef] [PubMed]
- Ndou, S.P.; Kiarie, E.; de Lange, C.F.; Nyachoti, C.M. Interactive effects of dietary fiber and lipid types modulate the predicted production and absorption of cecal and colorectal short-chain fatty acids in growing pigs. J. Nutr. 2024, 154, 2042–2052. [Google Scholar] [CrossRef]
- Igudesman, D.; Crandell, J.L.; Corbin, K.D.; Hooper, J.; Thomas, J.M.; Bulik, C.M.; Pence, B.W.; Pratley, R.E.; Kosorok, M.R.; Maahs, D.M.; et al. Associations of dietary intake with the intestinal microbiota and short-chain fatty acids among young adults with type 1 diabetes and overweight or obesity. J. Nutr. 2023, 153, 1178–1188. [Google Scholar] [CrossRef]
- Lammers-Jannink, K.C.M.; Pellikaan, W.F.; de Vries, S.; Stigter, E.C.A.; Gerrits, W.J.J. Standardisation of the C:N ratio in ileal digesta changes relationships among fermentation end-products during in vitro hindgut fermentation in pigs. Animal 2023, 17, 101026. [Google Scholar] [CrossRef]
- Jha, R.; Berrocoso, J.F.D. Dietary fiber and protein fermentation in the intestine of swine and their interactive effects on gut health and on the environment: A review. Anim. Feed Sci. Technol. 2016, 212, 18–26. [Google Scholar] [CrossRef]
- Wu, Y.; Liu, X.; Zou, Y.; Zhang, X.; Wang, Z.; Hu, J.; Han, D.; Zhao, J.; Dai, Z.; Wang, J. Lactobacillus amylovorus promotes lactose utilization in small intestine and enhances intestinal barrier function in intrauterine growth restricted piglets. J. Nutr. 2024, 154, 535–542. [Google Scholar] [CrossRef]
- Dong, Z.L.; Liu, S.; Deng, Q.Q.; Li, G.Y.; Tang, Y.L.; Wu, X.; Wan, D.; Yin, Y.L. Role of iron in host-microbiota interaction and its effects on intestinal mucosal growth and immune plasticity in a piglet model. Sci. China Life Sci. 2023, 66, 2086–2098. [Google Scholar] [CrossRef] [PubMed]
- Fan, L.; Xia, Y.; Wang, Y.; Han, D.; Liu, Y.; Li, J.; Fu, J.; Wang, L.; Gan, Z.; Liu, B.; et al. Gut microbiota bridges dietary nutrients and host immunity. Sci. China Life Sci. 2023, 66, 2466–2514. [Google Scholar] [CrossRef]
- Wang, D.D.; Tang, G.F.; Zhao, L.C.; Wang, M.Y.; Chen, L.Y.; Zhao, C.C.; Liang, Z.Q.; Chen, J.; Cao, Y.C.; Yao, J.H. Potential roles of the rectum keystone microbiota in modulating the microbial community and growth performance in goat model. J. Anim. Sci. Biotechnol. 2023, 14, 55. [Google Scholar] [CrossRef] [PubMed]
- Yu, M.; Gao, T.; Liu, Z.; Diao, X.P. Effects of dietary supplementation with high fiber (Stevia Residue) on the fecal flora of pregnant sows. Animals 2020, 10, 2247. [Google Scholar] [CrossRef]
- Tang, X.P.; Zhang, K.; Xiong, K.N. Fecal microbial changes in response to finishing pigs directly fed with fermented feed. Front. Vet. Sci. 2022, 9, 894909. [Google Scholar] [CrossRef]
- Medawar, E.; Haange, S.B.; Rolle-Kampczyk, U.; Engelmann, B.; Dietrich, A.; Thieleking, R.; Wiegank, C.; Fries, C.; Horstmann, A.; Villringer, A.; et al. Gut microbiota link dietary fiber intake and short-chain fatty acid metabolism with eating behavior. Transl. Psychiatry 2021, 11, 500. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.F.; Tsai, T.C.; Deng, F.L.; Wei, X.Y.; Chai, J.M.; Knapp, J.; Apple, J.; Maxwell, C.V.; Lee, J.A.; Li, Y.; et al. Longitudinal investigation of the swine gut microbiome from birth to market reveals stage and growth performance associated bacteria. Microbiome 2019, 7, 109. [Google Scholar] [CrossRef]
- Zhong, J.G.; Lan, W.T.; Feng, Y.Q.; Li, Y.H.; Shen, Y.Y.; Gong, J.H.; Zou, Z.; Hou, X.H. Associations between dysbiosis gut microbiota and changes of neurotransmitters and short-chain fatty acids in valproic acid model rats. Front. Physiol. 2023, 14, 1077821. [Google Scholar] [CrossRef]
- Jeong, J.J.; Jin, Y.J.; Ganesan, R.; Park, H.J.; Min, B.H.; Jeong, M.K.; Yoon, S.J.; Choi, M.R.; Sharma, S.P.; Jang, Y.J.; et al. Multistrain probiotics alleviate diarrhea by modulating microbiome-derived metabolites and serotonin pathway. Probiotics Antimicrob. Proteins 2024, 1–15, online ahead of print. [Google Scholar] [CrossRef]

| Items | CON | 5% SB | 10% SB | 15% SB |
|---|---|---|---|---|
| Corn | 42.195 | 43.045 | 44.005 | 44.875 |
| Soybean meal | 7.7 | 9.5 | 11.2 | 13.0 |
| Wheat bran | 22.9 | 20.2 | 17.5 | 14.8 |
| Soybean hulls | 15 | 10 | 5 | 0 |
| Sugarcane bagasse | 0 | 5 | 10 | 15 |
| Flour | 8 | 8 | 8 | 8 |
| Stone powder | 1.22 | 1.20 | 1.16 | 1.12 |
| Soybean oil | 1.0 | 1.0 | 1.0 | 1.0 |
| NaCl | 0.34 | 0.34 | 0.35 | 0.35 |
| CaHPO4 | 0.30 | 0.42 | 0.56 | 0.68 |
| L-Lysine H2SO4 | 0.27 | 0.23 | 0.19 | 0.15 |
| NaHCO3 | 0.20 | 0.20 | 0.20 | 0.20 |
| Mineral premix 1 | 0.20 | 0.20 | 0.20 | 0.20 |
| MgO | 0.20 | 0.20 | 0.20 | 0.20 |
| Choline chloride | 0.10 | 0.10 | 0.10 | 0.10 |
| Antifungal agent | 0.10 | 0.10 | 0.10 | 0.10 |
| L-Threonine | 0.09 | 0.08 | 0.06 | 0.05 |
| DL-Methionine | 0.05 | 0.05 | 0.04 | 0.04 |
| Vitamin premix 2 | 0.05 | 0.05 | 0.05 | 0.05 |
| Antioxidants | 0.04 | 0.04 | 0.04 | 0.04 |
| Xylanase | 0.03 | 0.03 | 0.03 | 0.03 |
| Phytase | 0.015 | 0.015 | 0.015 | 0.015 |
| Total (%) | 100 | 100 | 100 | 100 |
| Nutrient levels 3 | ||||
| NE (MJ/kg) | 9.21 | 9.21 | 9.21 | 9.21 |
| DM, % | 88.49 | 88.63 | 88.77 | 88.91 |
| CP, % | 12.98 | 13.01 | 12.99 | 13.01 |
| Ash, % | 5.03 | 5.03 | 5.03 | 5.00 |
| CF, % | 8.83 | 9.05 | 9.27 | 9.49 |
| NDF, % | 22.44 | 22.63 | 22.82 | 23.02 |
| ADF, % | 10.38 | 10.64 | 10.89 | 11.15 |
| Ca, % | 0.70 | 0.70 | 0.70 | 0.70 |
| TP, % | 0.50 | 0.50 | 0.50 | 0.50 |
| SID Lys, % | 0.60 | 0.60 | 0.60 | 0.60 |
| SID Met, % | 0.22 | 0.22 | 0.22 | 0.22 |
| SID Thr, % | 0.43 | 0.43 | 0.42 | 0.42 |
| Nutritional Indicators | Soybean Hulls | Wheat Bran | Sugarcane Bagasse |
|---|---|---|---|
| DM, % | 91.5 | 86.35 | 94.1 |
| Ash, % | 4.25 | 5.52 | 3.4 |
| CP, % | 9.5 | 15.8 | 1.38 |
| EE, % | 2.07 | 2.95 | 0.9 |
| CF, % | 37.5 | 10.3 | 42.1 |
| NDF, % | 60 | 40 | 81.3 |
| NE, Kcal/Kg | 1397 | 1492 | 1000 |
| Items | CON | 5% SB | 10% SB | 15% SB | p-Value |
|---|---|---|---|---|---|
| Back fat, mm | 14.23 ± 1.54 | 14.10 ± 1.84 | 14.20 ± 1.49 | 14.18 ± 1.45 | 0.996 |
| Days of gestation, d | 116.3 ± 1.10 ab | 116.9 ± 0.60 a | 116.4 ± 1.30 ab | 115.9 ± 1.20 b | 0.054 |
| Feed intake, kg | 2.39 ± 0.18 | 2.40 ± 0.14 | 2.48 ± 0.19 | 2.48 ± 0.14 | 0.274 |
| Litter size, n | 14.25 ± 2.43 | 14.63 ± 2.03 | 13.43 ± 2.61 | 14.94 ± 2.74 | 0.356 |
| Born alive, n | 13.31 ± 2.27 | 14.06 ± 1.95 | 12.75 ± 2.46 | 13.68 ± 2.60 | 0.436 |
| Healthy piglets, n | 11.25 ± 2.59 | 12.18 ± 2.17 | 10.88 ± 2.33 | 11.94 ± 2.89 | 0.278 |
| Weak piglets, n | 2.06 ± 2.52 | 1.88 ± 3.05 | 1.88 ± 2.58 | 1.75 ± 2.38 | 0.990 |
| Stillborn, n | 0.75 ± 1.18 | 0.50 ± 0.97 | 0.63 ± 1.02 | 1.06 ± 0.93 | 0.457 |
| Mummies, n | 0.19 ± 0.40 | 0.06 ± 0.25 | 0.06 ± 0.25 | 0.19 ± 0.40 | 0.532 |
| Litter birth weight, kg | 15.83 ± 2.61 b | 17.46 ± 1.76 a | 15.12 ± 2.15 b | 16.71 ± 2.10 ab | 0.036 |
| Birth weight/piglet, kg | 1.21 ± 0.27 | 1.26 ± 0.20 | 1.21 ± 0.22 | 1.22 ± 0.19 | 0.933 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, R.-H.; Zhang, B.-B.; Wang, J.; Zhao, W.; Huang, Y.-X.; Liu, Y.; Sun, L.-H.; Deng, Z.-C. Effect of Dietary Sugarcane Bagasse on Reproductive Performance, Constipation, and Gut Microbiota of Gestational Sows. Animals 2024, 14, 2523. https://doi.org/10.3390/ani14172523
Huang R-H, Zhang B-B, Wang J, Zhao W, Huang Y-X, Liu Y, Sun L-H, Deng Z-C. Effect of Dietary Sugarcane Bagasse on Reproductive Performance, Constipation, and Gut Microbiota of Gestational Sows. Animals. 2024; 14(17):2523. https://doi.org/10.3390/ani14172523
Chicago/Turabian StyleHuang, Rong-Hui, Bing-Bing Zhang, Juan Wang, Wei Zhao, Yu-Xuan Huang, Ying Liu, Lv-Hui Sun, and Zhang-Chao Deng. 2024. "Effect of Dietary Sugarcane Bagasse on Reproductive Performance, Constipation, and Gut Microbiota of Gestational Sows" Animals 14, no. 17: 2523. https://doi.org/10.3390/ani14172523






