Transcriptomic Characterization of Male Formosan Pangolin (Manis pentadactyla pentadactyla) Reproductive Tract and Evaluation of Domestic Cat (Felis catus) as a Potential Model Species
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
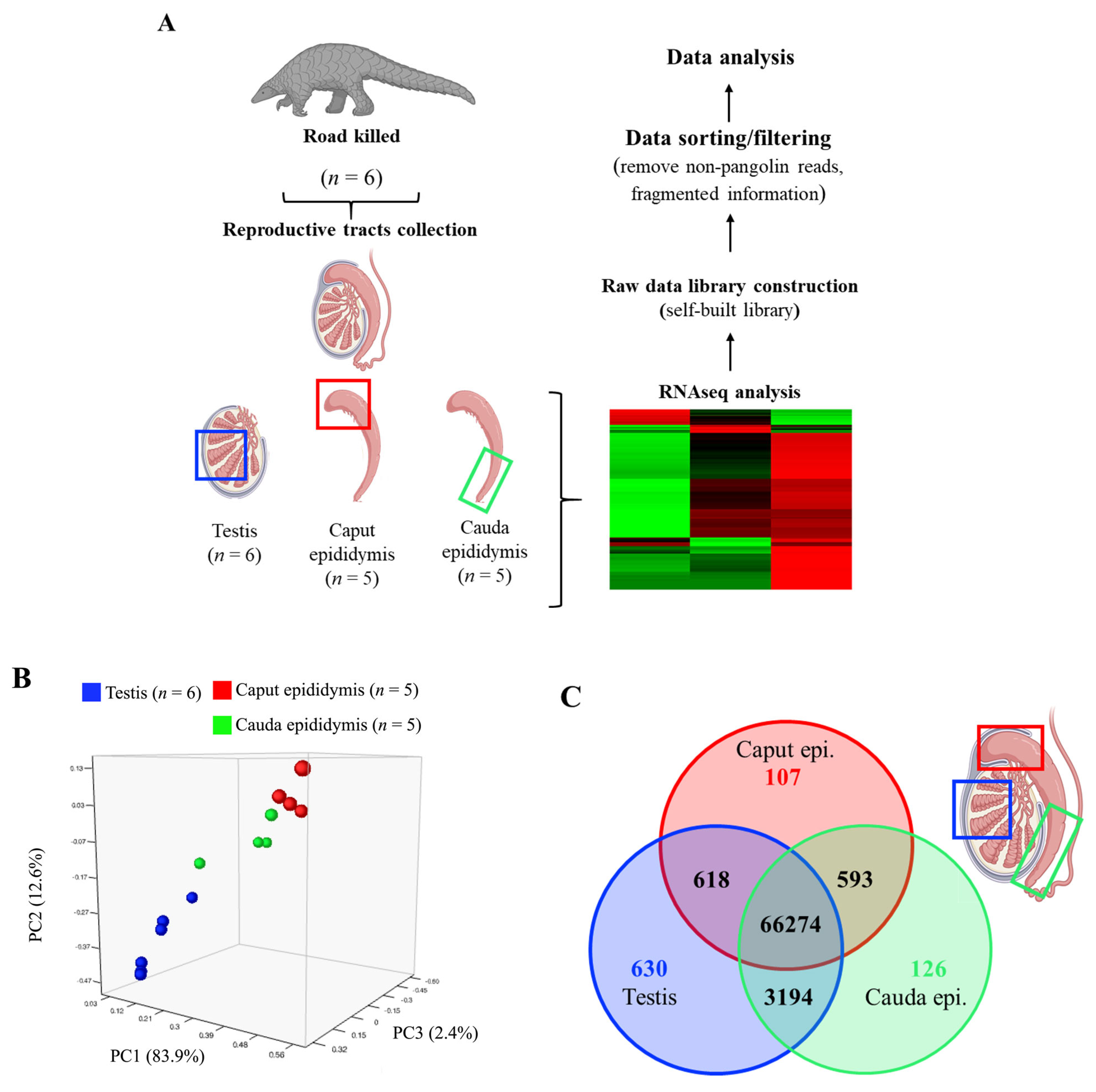
2.2. Reproductive Tissue Collection
2.3. RNA-Seq Library Construction and Bioinformatic Analyses
2.4. Data Sorting/Filtering
2.5. Inter-Species RNAseq Analysis
3. Results
3.1. RNAseq Analysis Reveals Tissue-Specific Gene Expressions of Pangolin Male Reproductive Tract
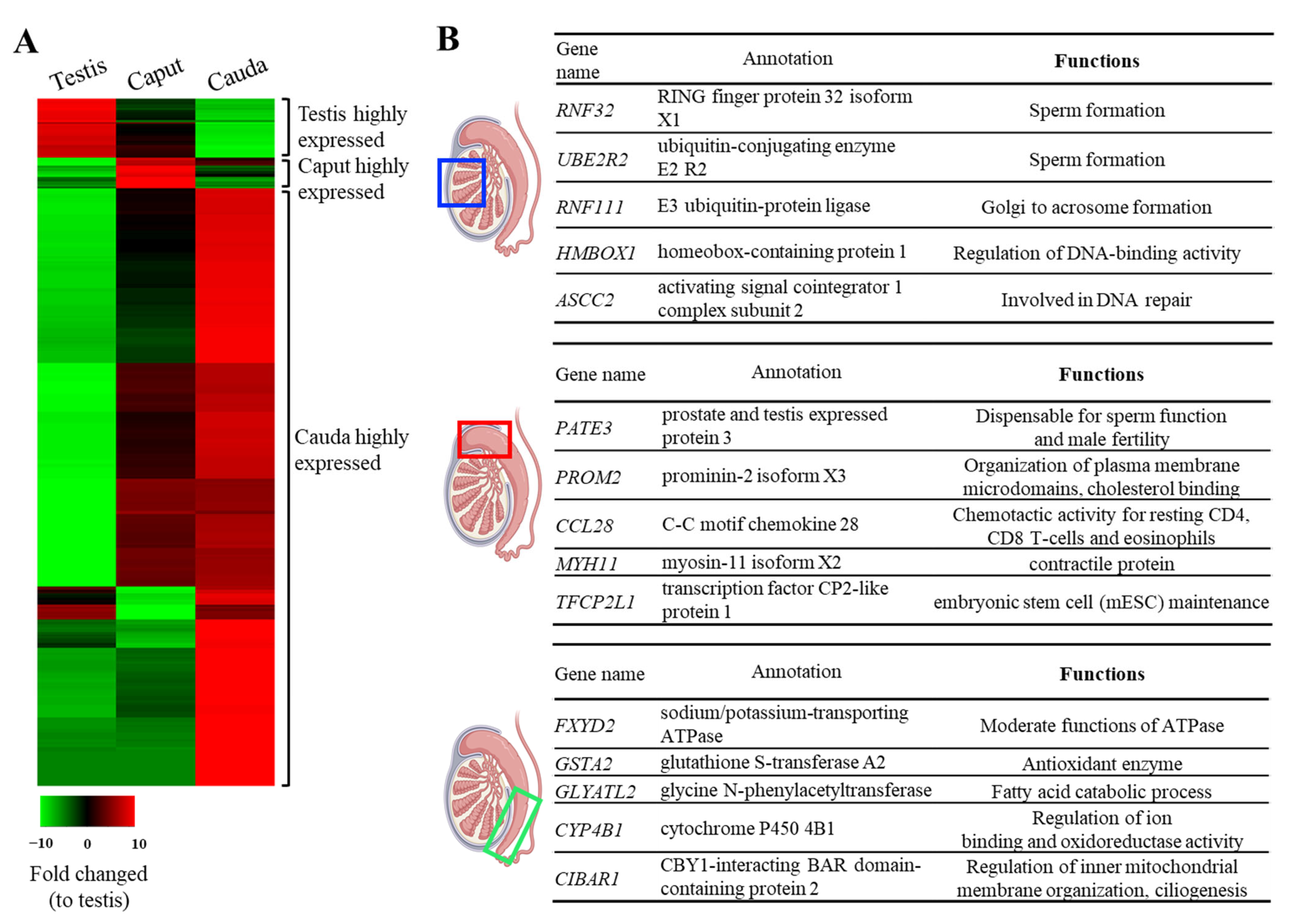
3.2. The Top 5 Highly Expressed Genes Identified in Each Region Support the Unique Biological Signatures of Each Reproductive Tract Section
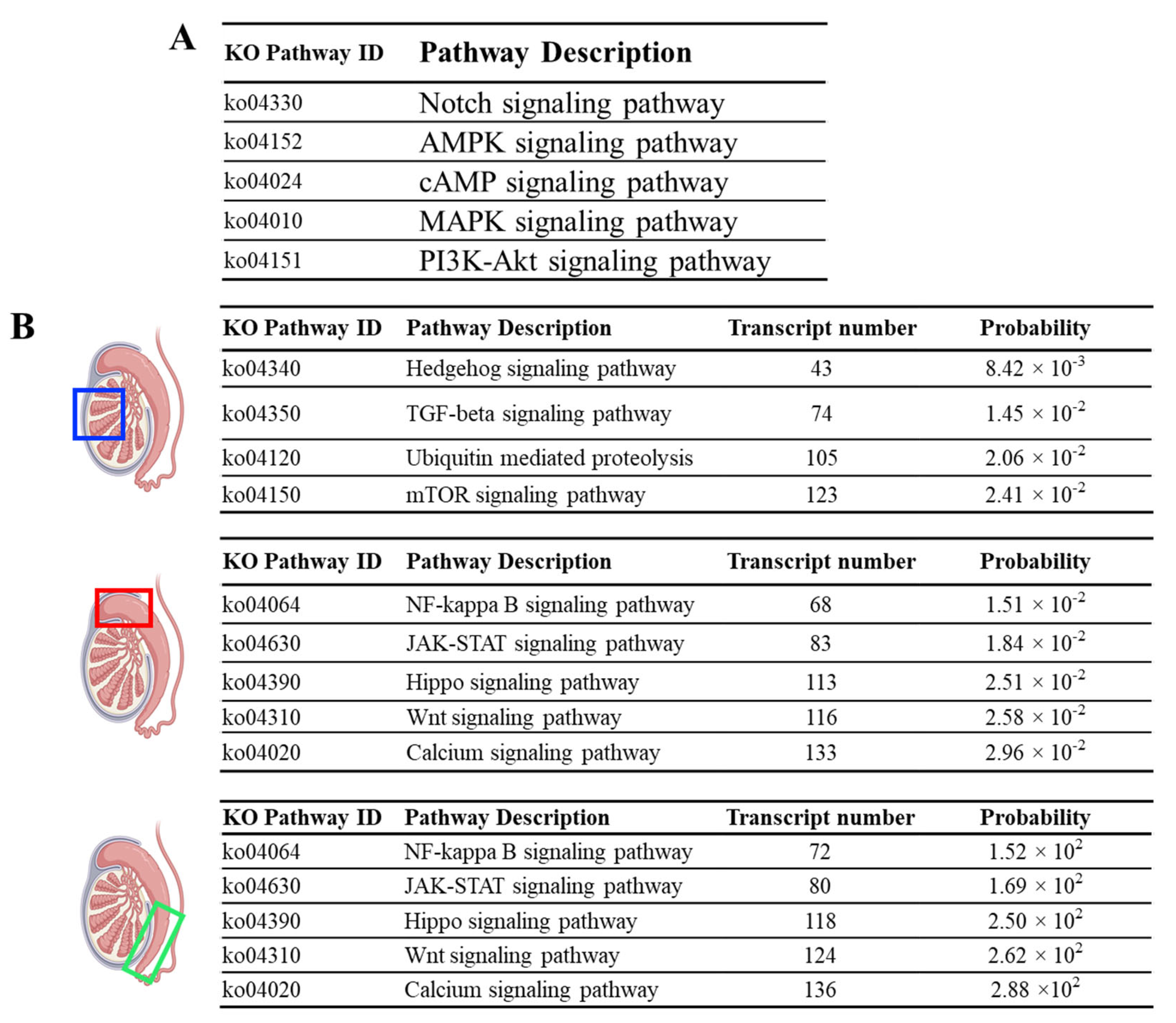
3.3. Pathway Enrichment Analysis Reveals Unique Pathways in the Testis but Common Signaling Pathways throughout the Epididymis
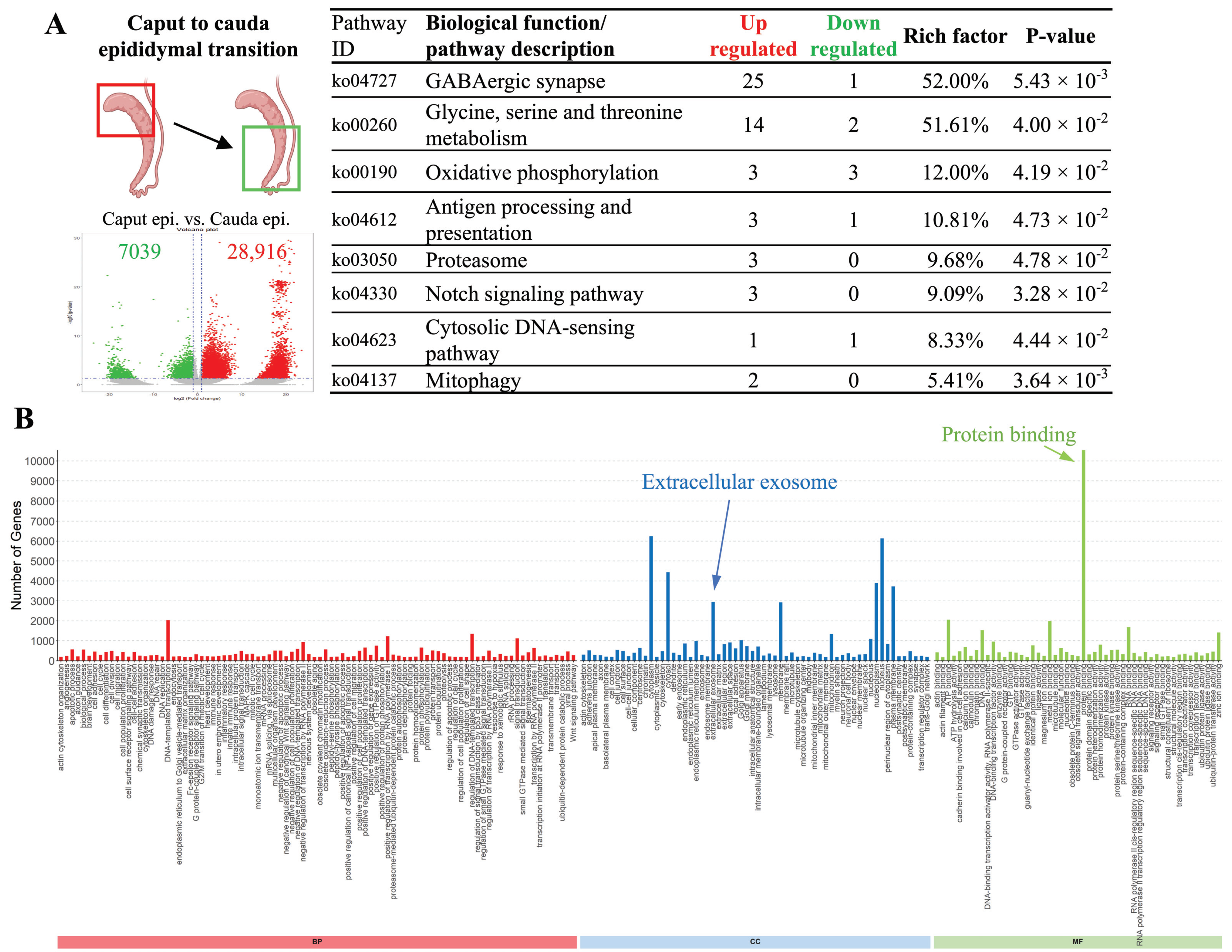
3.4. Specific Biological Activities Are Significantly Upregulated upon Epididymal Transition
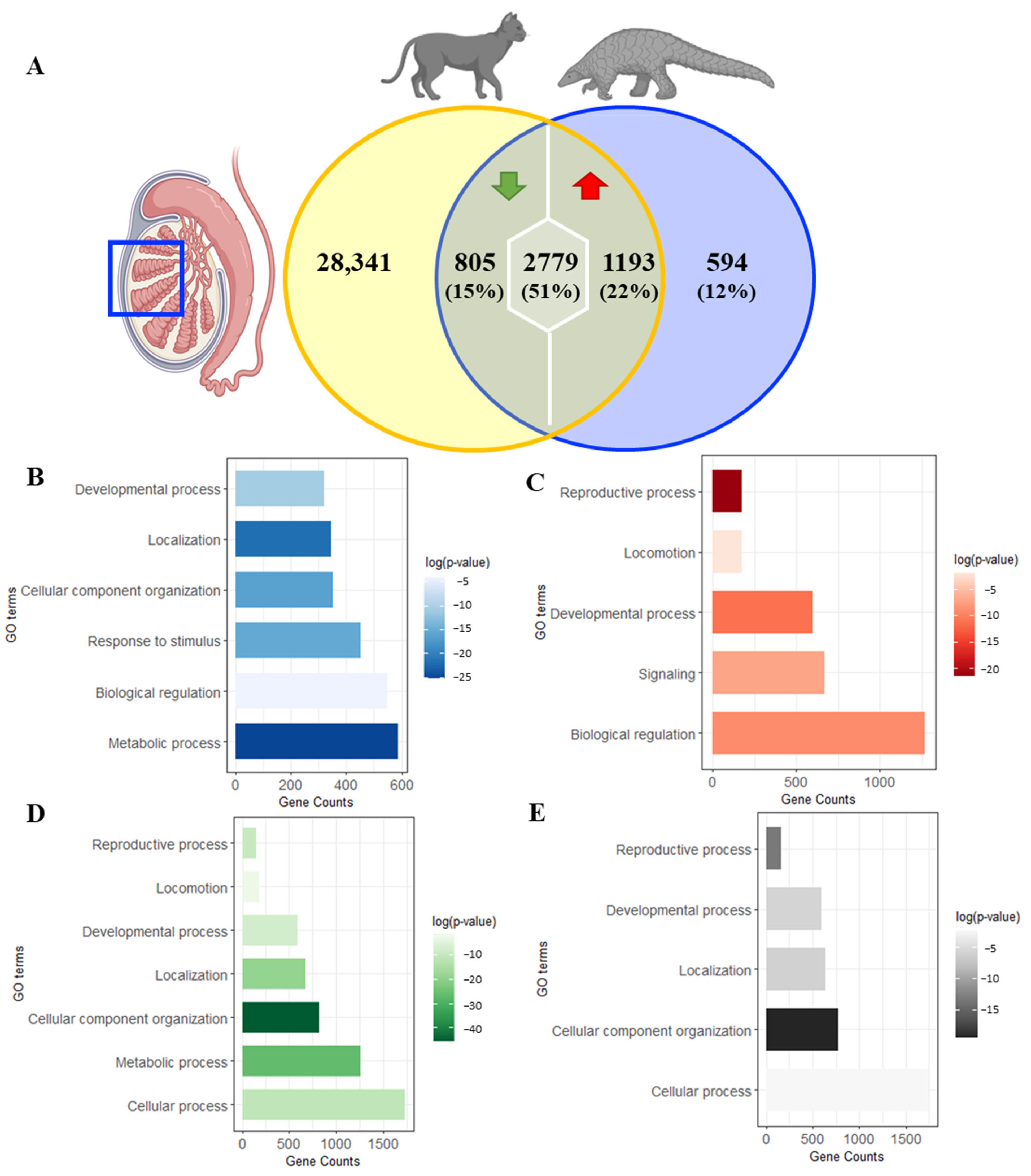
3.5. Cross-Species Comparisons Demonstrate 50% Transcriptome Variations between Pangolin and Domestic Cat Testes
3.6. In-Depth Analyses Suggests That Domestic Cats May Not Serve as an Ideal Universal Research Model Species for Pangolin
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Schlitter, D.A. Order Pholidota. In Mammal Species of the World: A Taxonomic and Geographic Reference, 2nd ed.; Wilson, D.E., Reeder, D.M., Eds.; Smithsonian Institution Press: Washington, DC, USA, 1993; p. 530. [Google Scholar]
- Challender, D.; Wu, S.; Kaspal, P.; Khatiwada, A.; Ghose, A.; Ching-Min Sun, N.; Mohapatra, R.K.; Laxmi Suwal, T. Manis pentadactyla. In The IUCN Red List of Threatened Species 2019; International Union for Conservation of Nature and Natural Resources: Gland, Switzerland, 2019. [Google Scholar] [CrossRef]
- Sun, N.C.; Arora, B.; Lin, J.S.; Lin, W.C.; Chi, M.J.; Chen, C.C.; Pei, K.J. Mortality and morbidity in wild Taiwanese pangolin (Manis pentadactyla pentadactyla). PLoS ONE 2019, 14, e0198230. [Google Scholar] [CrossRef] [PubMed]
- Chao, J.T.; Tsao, E.H.; Traylor-Holzer, K.; Reed, D.; Leus, K. Formosan Pangolin Population and Habitat Viability Assessment: Final Report; IUCN/SSC Conservation Breeding Specialist Group: Apple Valley, MN, USA, 2005. [Google Scholar]
- Wu, S.B.; Ma, G.Z.; Tang, M.; Chen, H.; Liu, N.F. The status and conservation strategy of pangolin resource in China. J. Nat. Resour. 2002, 17, 174–180. [Google Scholar]
- Zhang, F.; Yu, J.; Wu, S.; Li, S.; Zou, C.; Wang, Q.; Sun, R. Keeping and breeding the rescued Sunda pangolins (Manis javanica) in captivity. Zoo Biol. 2017, 36, 387–396. [Google Scholar] [CrossRef]
- Hua, L.; Gong, S.; Wang, F.; Li, W.; Ge, Y.; Li, X.; Hou, F. Captive breeding of pangolins: Current status, problems and future prospects. Zookeys 2015, 507, 99–114. [Google Scholar] [CrossRef]
- Zhang, F.; Wu, S.; Zou, C.; Wang, Q.; Li, S.; Sun, R. A note on captive breeding and reproductive parameters of the Chinese pangolin, Manis pentadactyla Linnaeus, 1758. Zookeys 2016, 618, 129–144. [Google Scholar] [CrossRef]
- Zhang, F.; Yu, Y.; Yu, J.; Wu, S.; Li, S.; Wang, Q.; Min, Y.; Sun, R. Reproductive behavior of the captive Sunda pangolin (Manis javanica Desmarest, 1822). Zoo Biol. 2020, 39, 65–72. [Google Scholar] [CrossRef] [PubMed]
- Chin, S.C.; Lien, C.Y.; Chan, Y.T.; Chen, C.L.; Yang, Y.C.; Yeh, L.S. Monitoring the gestation period of rescued Formosan pangolin (Manis pentadactyla pentadactyla) with progesterone radioimmunoassay. Zoo Biol. 2012, 31, 479–489. [Google Scholar] [CrossRef]
- Chen, D.Y.L.C.; Yu, C.B. Evaluation of pangolin parturient time. Res. Dev. Mark. 2000, 16, 287. [Google Scholar]
- Chang, Y.-C.; Yu, J.-F.; Wang, T.-E.; Chin, S.-C.; Wei, Y.-S.; Chen, T.-Y.; Tsai, P.-S. Investigation of epididymal proteins and general sperm membrane characteristics of Formosan pangolin (Manis pentadactyla pentadactyla). BMC Zool. 2020, 5, 15. [Google Scholar] [CrossRef]
- Perelman, P.L.; Beklemisheva, V.R.; Yudkin, D.V.; Petrina, T.N.; Rozhnov, V.V.; Nie, W.; Graphodatsky, A.S. Comparative chromosome painting in Carnivora and Pholidota. Cytogenet. Genome Res. 2012, 137, 174–193. [Google Scholar] [CrossRef]
- Khodadadian, A.; Darzi, S.; Haghi-Daredeh, S.; Sadat Eshaghi, F.; Babakhanzadeh, E.; Mirabutalebi, S.H.; Nazari, M. Genomics and Transcriptomics: The Powerful Technologies in Precision Medicine. Int. J. Gen. Med. 2020, 13, 627–640. [Google Scholar] [CrossRef] [PubMed]
- Su, A.I.; Cooke, M.P.; Ching, K.A.; Hakak, Y.; Walker, J.R.; Wiltshire, T.; Orth, A.P.; Vega, R.G.; Sapinoso, L.M.; Moqrich, A.; et al. Large-scale analysis of the human and mouse transcriptomes. Proc. Natl. Acad. Sci. USA 2002, 99, 4465–4470. [Google Scholar] [CrossRef] [PubMed]
- Jhun, H.; Lee, W.Y.; Park, J.K.; Hwang, S.G.; Park, H.J. Transcriptomic Analysis of Testicular Gene Expression in a Dog Model of Experimentally Induced Cryptorchidism. Cells 2022, 11, 2476. [Google Scholar] [CrossRef] [PubMed]
- Grabherr, M.G.; Haas, B.J.; Yassour, M.; Levin, J.Z.; Thompson, D.A.; Amit, I.; Adiconis, X.; Fan, L.; Raychowdhury, R.; Zeng, Q.; et al. Full-length transcriptome assembly from RNA-Seq data without a reference genome. Nat. Biotechnol. 2011, 29, 644–652. [Google Scholar] [CrossRef]
- Fu, L.; Niu, B.; Zhu, Z.; Wu, S.; Li, W. CD-HIT: Accelerated for clustering the next-generation sequencing data. Bioinformatics 2012, 28, 3150–3152. [Google Scholar] [CrossRef] [PubMed]
- Buchfink, B.; Xie, C.; Huson, D.H. Fast and sensitive protein alignment using DIAMOND. Nat. Methods 2015, 12, 59–60. [Google Scholar] [CrossRef]
- Chen, T.W.; Gan, R.C.; Wu, T.H.; Huang, P.J.; Lee, C.Y.; Chen, Y.Y.; Chen, C.C.; Tang, P. FastAnnotator—An efficient transcript annotation web tool. BMC Genom. 2012, 13 (Suppl. S7), S9. [Google Scholar] [CrossRef]
- Moriya, Y.; Itoh, M.; Okuda, S.; Yoshizawa, A.C.; Kanehisa, M. KAAS: An automatic genome annotation and pathway reconstruction server. Nucleic Acids Res. 2007, 35, W182–W185. [Google Scholar] [CrossRef] [PubMed]
- Kanehisa, M.; Sato, Y.; Kawashima, M.; Furumichi, M.; Tanabe, M. KEGG as a reference resource for gene and protein annotation. Nucleic Acids Res. 2016, 44, D457–D462. [Google Scholar] [CrossRef]
- Noda, T.; Sakurai, N.; Nozawa, K.; Kobayashi, S.; Devlin, D.J.; Matzuk, M.M.; Ikawa, M. Nine genes abundantly expressed in the epididymis are not essential for male fecundity in mice. Andrology 2019, 7, 644–653. [Google Scholar] [CrossRef] [PubMed]
- Pervouchine, D.D.; Djebali, S.; Breschi, A.; Davis, C.A.; Barja, P.P.; Dobin, A.; Tanzer, A.; Lagarde, J.; Zaleski, C.; See, L.H.; et al. Enhanced transcriptome maps from multiple mouse tissues reveal evolutionary constraint in gene expression. Nat. Commun. 2015, 6, 5903. [Google Scholar] [CrossRef] [PubMed]
- Redon, E.; Bosseboeuf, A.; Rocancourt, C.; Da Silva, C.; Wincker, P.; Mazan, S.; Sourdaine, P. Stage-specific gene expression during spermatogenesis in the dogfish (Scyliorhinus canicula). Reproduction 2010, 140, 57–71. [Google Scholar] [CrossRef]
- Nozawa, K.; Fujihara, Y.; Devlin, D.J.; Deras, R.E.; Kent, K.; Larina, I.V.; Umezu, K.; Yu, Z.; Sutton, C.M.; Ye, Q.; et al. The testis-specific E3 ubiquitin ligase RNF133 is required for fecundity in mice. BMC Biol. 2022, 20, 161. [Google Scholar] [CrossRef]
- Shen, C.; Xu, J.; Zhou, Q.; Lin, M.; Lv, J.; Zhang, X.; Wu, Y.; Chen, X.; Yu, J.; Huang, X.; et al. E3 ubiquitin ligase ASB17 is required for spermiation in mice. Transl. Androl. Urol. 2021, 10, 4320–4332. [Google Scholar] [CrossRef] [PubMed]
- Jahn, A.; Rane, G.; Paszkowski-Rogacz, M.; Sayols, S.; Bluhm, A.; Han, C.T.; Draškovič, I.; Londoño-Vallejo, J.A.; Kumar, A.P.; Buchholz, F.; et al. ZBTB48 is both a vertebrate telomere-binding protein and a transcriptional activator. EMBO Rep. 2017, 18, 929–946. [Google Scholar] [CrossRef]
- Jia, J.; Absmeier, E.; Holton, N.; Pietrzyk-Brzezinska, A.J.; Hackert, P.; Bohnsack, K.E.; Bohnsack, M.T.; Wahl, M.C. The interaction of DNA repair factors ASCC2 and ASCC3 is affected by somatic cancer mutations. Nat. Commun. 2020, 11, 5535. [Google Scholar] [CrossRef]
- Correia, B.; Sousa, M.I.; Ramalho-Santos, J. The mTOR pathway in reproduction: From gonadal function to developmental coordination. Reproduction 2020, 159, R173–R188. [Google Scholar] [CrossRef] [PubMed]
- Breton, S.; Smith, P.J.; Lui, B.; Brown, D. Acidification of the male reproductive tract by a proton pumping (H+)-ATPase. Nat. Med. 1996, 2, 470–472. [Google Scholar] [CrossRef]
- James, E.R.; Carrell, D.T.; Aston, K.I.; Jenkins, T.G.; Yeste, M.; Salas-Huetos, A. The Role of the Epididymis and the Contribution of Epididymosomes to Mammalian Reproduction. Int. J. Mol. Sci. 2020, 21, 5377. [Google Scholar] [CrossRef]
- Baskaran, S.; Panner Selvam, M.K.; Agarwal, A. Exosomes of male reproduction. Adv. Clin. Chem. 2020, 95, 149–163. [Google Scholar] [CrossRef]
- Rimmer, M.P.; Gregory, C.D.; Mitchell, R.T. The transformative impact of extracellular vesicles on developing sperm. Reprod. Fertil. 2021, 2, R51–R66. [Google Scholar] [CrossRef] [PubMed]
- Rowlison, T.; Cleland, T.P.; Ottinger, M.A.; Comizzoli, P. Novel Proteomic Profiling of Epididymal Extracellular Vesicles in the Domestic Cat Reveals Proteins Related to Sequential Sperm Maturation with Differences Observed between Normospermic and Teratospermic Individuals. Mol. Cell. Proteom. 2020, 19, 2090–2104. [Google Scholar] [CrossRef]
- Nixon, B.; De Iuliis, G.N.; Hart, H.M.; Zhou, W.; Mathe, A.; Bernstein, I.R.; Anderson, A.L.; Stanger, S.J.; Skerrett-Byrne, D.A.; Jamaluddin, M.F.B.; et al. Proteomic Profiling of Mouse Epididymosomes Reveals their Contributions to Post-testicular Sperm Maturation. Mol. Cell. Proteom. 2019, 18, S91–S108. [Google Scholar] [CrossRef]
- Barrachina, F.; Battistone, M.A.; Castillo, J.; Mallofré, C.; Jodar, M.; Breton, S.; Oliva, R. Sperm acquire epididymis-derived proteins through epididymosomes. Hum. Reprod. 2022, 37, 651–668. [Google Scholar] [CrossRef] [PubMed]
- Gatti, J.L.; Métayer, S.; Belghazi, M.; Dacheux, F.; Dacheux, J.L. Identification, proteomic profiling, and origin of ram epididymal fluid exosome-like vesicles. Biol. Reprod. 2005, 72, 1452–1465. [Google Scholar] [CrossRef] [PubMed]
- Lüpold, S.; Pitnick, S. Sperm form and function: What do we know about the role of sexual selection? Reproduction 2018, 155, R229–R243. [Google Scholar] [CrossRef] [PubMed]
- Shirakawa, H.; Ohsaki, Y.; Minegishi, Y.; Takumi, N.; Ohinata, K.; Furukawa, Y.; Mizutani, T.; Komai, M. Vitamin K deficiency reduces testosterone production in the testis through down-regulation of the Cyp11a a cholesterol side chain cleavage enzyme in rats. Biochim. Biophys. Acta 2006, 1760, 1482–1488. [Google Scholar] [CrossRef] [PubMed]
- Sanjo, H.; Yao, T.; Katagiri, K.; Sato, T.; Matsumura, T.; Komeya, M.; Yamanaka, H.; Yao, M.; Matsuhisa, A.; Asayama, Y.; et al. Antioxidant vitamins and lysophospholipids are critical for inducing mouse spermatogenesis under organ culture conditions. FASEB J. 2020, 34, 9480–9497. [Google Scholar] [CrossRef]
- Silva, A.F.; Escada-Rebelo, S.; Amaral, S.; Tavares, R.S.; Schlatt, S.; Ramalho-Santos, J.; Mota, P.C. Can we induce spermatogenesis in the domestic cat using an in vitro tissue culture approach? PLoS ONE 2018, 13, e0191912. [Google Scholar] [CrossRef]
| Non-Differentially Expressed Genes Domestic Cat–Pangolin (Reproductive Processes) | Strength | p Value | |
|---|---|---|---|
| Biological processes | Meiotic sister chromatid cohesion | 2.12 | 3.1 × 10−5 |
| Formin-nucleated actin cable assembly | 2.12 | 1.7 × 10−2 | |
| Luteinizing hormone secretion | 2.12 | 1.7 × 10−2 | |
| Activation of meiosis | 1.94 | 2.4 × 10−2 | |
| Establishment of meiotic spindle | 1.82 | 3.3 × 10−2 | |
| Polar body extrusion after meiotic divisions | 1.82 | 3.3 × 10−2 | |
| Male meiosis I | 1.52 | 1.4 × 10−4 | |
| Endocrine hormone secretion | 1.52 | 1.1 × 10−2 | |
| Tissue expression | Spermatocyte | 1.82 | 4.9 × 10−3 |
| Spermatozoon | 1.72 | 4.5 × 10−4 | |
| Spermatogonium | 1.69 | 8.8 × 10−3 | |
| Subcellular localization | P granule | 1.57 | 2.9 × 10−3 |
| Condensed nuclear chromosome | 1.19 | 4.8 × 10−3 | |
| Acrosomal vesicle | 1.05 | 4.8 × 10−3 | |
| Upregulated Genes Pangolin (Reproductive Processes) | Strength | p Value | |
|---|---|---|---|
| Biological processes | Acrosomal vesicle exocytosis | 1.81 | 4.8 × 10−3 |
| Acrosome reaction | 1.53 | 1.8 × 10−4 | |
| Sperm axoneme assembly | 1.46 | 3.8 × 10−5 | |
| Acrosome assembly | 1.41 | 3.6 × 10−3 | |
| Spermatid differentiation | 1.38 | 4.6 × 10−27 | |
| Spermatid development | 1.38 | 4.7 × 10−26 | |
| Flagellated sperm motility | 1.35 | 9.1 × 10−15 | |
| Germ cell development | 1.31 | 6.5 × 10−31 | |
| Tissue expression | Testis | 0.73 | 2.1 × 10−7 |
| Internal male genital organ | 0.66 | 5.3 × 10−7 | |
| Male reproductive system | 0.65 | 2.1 × 10−7 | |
| Subcellular localization | Sperm head plasma membrane | 2.08 | 1.9 × 10−3 |
| Acrosomal membrane | 1.48 | 2.8 × 10−2 | |
| Sperm flagellum | 1.15 | 1.9 × 10−3 | |
| Unique Genes Pangolin (Metabolic Processes) | Strength | p Value | |
|---|---|---|---|
| Biological processes | Vitamin K metabolic process | 1.68 | 6.4 × 10−3 |
| Keratan sulfate metabolic process | 1.55 | 1.2 × 10−3 | |
| Steroid hormone biosynthesis process | 1.49 | 1.5 × 10−5 | |
| Prostaglandin biosynthesis process | 1.48 | 1.98 × 10−3 | |
| Arachidonic acid metabolic process | 1.42 | 5.9 × 10−8 | |
| Triglyceride biosynthetic process | 1.38 | 3.1 × 10−2 | |
| Retinol metabolic process | 1.37 | 9.5 × 10−7 | |
| Retinoic acid metabolic process | 1.3 | 7.0 × 10−3 | |
| Phosphatidylserine metabolic process | 1.3 | 4.7 × 10−2 | |
| Unsaturated fatty acid metabolic process | 1.29 | 1.7 × 10−9 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Méar, L.O.; Tseng, I.; Lin, K.-S.; Hsu, C.-L.; Chen, S.-H.; Tsai, P.-S. Transcriptomic Characterization of Male Formosan Pangolin (Manis pentadactyla pentadactyla) Reproductive Tract and Evaluation of Domestic Cat (Felis catus) as a Potential Model Species. Animals 2024, 14, 2592. https://doi.org/10.3390/ani14172592
Méar LO, Tseng I, Lin K-S, Hsu C-L, Chen S-H, Tsai P-S. Transcriptomic Characterization of Male Formosan Pangolin (Manis pentadactyla pentadactyla) Reproductive Tract and Evaluation of Domestic Cat (Felis catus) as a Potential Model Species. Animals. 2024; 14(17):2592. https://doi.org/10.3390/ani14172592
Chicago/Turabian StyleMéar, Laura Orama, IShin Tseng, Kuei-Shien Lin, Chia-Lin Hsu, Szu-Hua Chen, and Pei-Shiue Tsai. 2024. "Transcriptomic Characterization of Male Formosan Pangolin (Manis pentadactyla pentadactyla) Reproductive Tract and Evaluation of Domestic Cat (Felis catus) as a Potential Model Species" Animals 14, no. 17: 2592. https://doi.org/10.3390/ani14172592






